Abstract
Gene therapy is a promising therapeutic strategy for genetic and acquired hematologic diseases. With the improvements in gene transfer and expression, factors affecting safety and efficacy of gene therapy can now be evaluated to establish the best clinical benefit-to-risk ratio. The induction of immune responses against gene therapy components is one of the potential limitations. We studied the occurrence of such event in 23 patients treated with donor lymphocyte infusions (DLIs), with lymphocytes transduced to express the HSV-TK suicide gene for relapse of hematologic malignancies occurring after allogeneic hematopoietic stem cell transplantation (HSCT). The suicide gene was used to selectively control graft-versus-host disease (GvHD). Seven patients given infusions late after HSCT developed an immune response against the transgene. Immunization involved appearance of thymidine kinase (TK)–specific CD8+ effectors and required a level of immunocompetence at the time of TK-DLI that can be achieved only several months after transplantation. This did not prevent graft-versus-leukemia (GvL) effect of the TK-DLI, since 5 of 7 immunized patients maintained the complete remission achieved prior to immunization. We suggest that appropriate study designs taking into account the immune suppression of the patient and time-kinetics of GvL mediated by TK-transduced donor lymphocytes may allow the full exploitation of TK-DLI.
Introduction
The induction of immunoreactivity against genetically modified cells is one of the most relevant factors that could potentially affect efficacy of gene therapy. Virtually every component of a gene therapy strategy may represent a foreign molecule, including the transgene products, viral components, or the genetically modified cells (GMCs), and could be the target of an immune response, ultimately leading to loss of efficacy. In addition to a large body of evidence in animal models, in the clinical setting immune reactions against vector components have been detected in gene therapy trials for adenosine deaminase (ADA)–deficient severe combined immunodeficiency1 (SCID) and hemophilia B2 ; moreover, cellular-mediated elimination of autologous genetically modified lymphocytes has been reported in an HIV trial.3 Although these observations have demonstrated the clinical relevance of this limitation, immunity to gene therapy components have not been properly evaluated in other clinical settings. In particular, in cancer gene therapy, immunogenicity of the GMCs has not been perceived as a major concern. Actually, one of the most common approaches to cancer gene therapy relies on induction of immunity against genetically modified tumor cells,4 where the risk of reduced survival of GMCs, secondary to immunity to transgene products, is acceptable, or even highly rewarded by the increased potency of the genetically modified vaccine. In the case of adoptive immunotherapy, however, immune responses against the GMCs may represent a major limitation by reducing survival of the effector cells. One of such approaches is based on donor lymphocyte infusions (DLIs), with lymphocytes transduced to express a suicide gene, the viral HSV-TK transgene, in the context of allogeneic hematopoietic stem cell transplantation (HSCT). The strategy aims at providing immune-mediated protection from neoplastic relapse and infectious mortality, while selectively controlling a devastating side effect of DLI, namely, graft-versus-host disease (GvHD). This approach proved to be one of the first successful clinical gene therapy approaches to cancer.5 In fact, despite extensive variability in clinical results, mainly due to the difficulties in identifying and standardizing T-cell culture conditions, all the clinical studies, now including more than 100 treated patients, have clearly demonstrated that this suicide machinery is highly effective in controlling GvHD, thus rendering allo-HSCT safer and potentially more effective in treating patients with blood cancer.5,6 Despite the potential immunogenicity of the HSV-TK transgene, the profound immunosuppression associated with the posttransplantation period proved to be sufficient to prevent immunity against GMCs in the majority of these studies. However, a recent report revealed that treatment of immunocompetent patients with TK-DLI, late after transplantation, results in the development of an immune response to TK-derived epitopes.7 Based on this observation, alternative suicide genes, potentially less immunogenic than HSV-TK, have been proposed.8,9 However, immune responses depend not only on the potential immunogenicity of the transgene, but also on the presence of a functional immune system, a condition often absent in the posttransplantation setting where DLIs are proposed.
To address these variables, we analyzed 23 patients treated with TK-DLI for disease relapse occurring after HSCT. Here we report that the development of an immune response leading to the elimination of TK-transduced donor lymphocytes is strictly related to the presence of a competent immune system at the time of infusion of GMCs. Furthermore, we show that an appropriate study design taking into account the relative time kinetics of graft-versus-leukemia (GvL) activity and anti-TK immunity allows full exploitation of the GvL activity of the TK-transduced donor lymphocytes. We conclude that highly immunosuppressive transplantation settings can fully benefit from TK-DLI.
Patients, materials, and methods
Transgene-immunized patients
This study has been approved in 1993 by the Institutional Ethics Committee of Istituto Scientifico H. S. Raffaele, and informed consent was obtained from donors and recipients, in accordance with the Declaration of Helsinki. The use of the HSV-TK technology in the context of HSCT to treat hematologic malignancies has been approved by the Italian Ministry of Health. Twenty-three patients with disease relapse after allogeneic HSCT were enrolled in this study. Patient characteristics have been reported by Ciceri et al, beginning on page 4698.10 Donor lymphocytes were genetically modified to express both a truncated form of the human low-affinity nerve growth factor receptor (ΔLNGFr), as a cell-surface selectable marker, and the HSV-TK suicide gene. Two different vectors were used, encoding the ΔLNGFr and either the entire HSV-TK (the SFCMM3 vector) or the HSV-TK/neo fusion protein (TN), a bifunctional protein displaying both the HSV-TK activity and the neomycin resistance (the SFCMM2 vector).11
Patients surviving more than 1 month after TK-DLI without ganciclovir treatment were evaluable for studying the development of immune responses against GMCs. Seventeen of the 23 treated patients met these criteria.10 Sixteen patients were studied; one patient was excluded due to lack of biologic material. GMCs were detected in the circulation of all the 16 evaluable patients, by both polymerase chain reaction (PCR) and fluorescence-activated cell sorting (FACS) analysis for the expression of the cell-surface marker. Seven of the 16 evaluable patients developed variable levels of GMC-specific immune responses, which were characterized in this study.
Stimulator and target cells
To characterize the immune response against GMCs, the following cell lines were used as stimulator and target cells. Activated T cells were obtained by cultivation of peripheral blood mononuclear cells (PBMCs), isolated by Lymphoprep (Nycomed, Oslo, Norway) gradients, in the presence of 1 μg/mL PHA (Boerhinger Mannheim, Mannheim, Germany) and 100 U/mL recombinant human IL-2 (Chiron, Milan, Italy). B-lymphoblastoid cell lines (LCLs) were derived by transformation of peripheral blood B lymphocytes with the B95-8 strain of EBV. All the cell lines were cultured in IMDM supplemented with 10% FCS. The Cos-7 cell line was kindly provided by Prof T. Boon (Ludwig Institute for Cancer Research, Brussels, Belgium) and maintained in DMEM supplemented with 10% FCS.
Retroviral transduction of stimulator and target cells
Genetically modified T cells used as stimulators or targets and LCLs, used only as target cells in the ex vivo studies, were transduced by the SFCMM2 and SFCMM3 vectors and selected for the expression of the ΔLNGFr cell-surface marker as previously described.5,11 In particular, the SFCMM2 vector, encoding the TN fusion protein, was used to transduced T cells from HSC donors of patients 2, 7, and 8; lymphocytes from HSC donors of patients 16, 17, 20, and 21 were transduced by the SFCMM3 vector coding for the wild-type HSV-TK protein. To characterize the transgene products target of the immune response, patients' LCLs were transduced with the LxSΔN vector encoding only the ΔLNGFr, to exclude recognition of the cell-surface marker, then with LΔNSNeo and LTSΔN (ie, SFCMM3) to demonstrate recognition of neo and HSV-TK gene products, respectively.
Ex vivo detection of transgene-specific immune responses
PBMCs or CD3+ lymphocytes from patients and healthy donors were cultured with irradiated (50 Gy) autologous SFCMM2/SFCMM3-transduced T lymphocytes or with allogeneic T cells in 24-well plates. On day 3, a final concentration of 10 U/mL IL-2 was added to each culture. Effector T lymphocytes were maintained in culture by weekly stimulations in the same conditions. The cytolytic activity was evaluated about 10 days after the stimulation as previously described.12 Lysis values were positive when higher than the minimum value plus 3 SDs. A culture was considered positive when at least 2 points of the effector-target (E/T) ratio titration were positive.
To perform semiquantitative analysis of the immune effectors, we stimulated the same number of CD3+ cells from samples collected at different time points after treatment both against allogeneic and SFCMM2 autologous T cells. Lytic activity of the cultures was tested after one round of stimulation, when it is still proportional to the number of T-cell precursors present in the starting samples.13 To avoid artifacts (eg, different viability of stored samples) and experimental variability (eg, immunologic status of the patients) the results of anti-TN responses were normalized by plotting the ratio of anti-TN and antiallogeneic (anti-allo) lytic activity. Lytic units were calculated as the number of effectors/107 cells required to obtain 30% target lysis, with 2000 labeled target cells being used in the assay.
Identification of an antigenic HSV-TK–encoded epitope
The eukaryotic expression vector pcDNA3.1/B*0701, encoding the HLA-B*0701 allele isolated from PBMCs of patient 2, was produced as previously described.12 The 5′-end subfragment of HSV-TK gene (622-bp fragments) was obtained by digestion of HSV-TK cDNA with ApaI and cloned into the pcDNA3.1 plasmid. Transfection of Cos-7 cells was performed by the DEAE-dextran-chloroquine method. Briefly, 1.5 × 104 Cos-7 cells were transfected with 100 ng plasmid pcDNA3.1/B*0701 and 100 ng of expression vectors containing the gene e of interest. Transfected Cos-7 cells were tested in an IFN-γ assay. After 48 hours, 5000 effector cells were added in 150 μL culture medium supplemented with 25 U/mL IL-2. Twenty-four hours later, 100 μL supernatant was harvested and the IFN-γ concentration was measured using an enzyme-linked immunosorbent assay (ELISA) kit (Genzyme, Cambridge, MA) according to the manufacturer's recommendations.
Antigenic peptide and IFN-γ release assay
The HSV-TK sequence was analyzed on a website of the National Institutes of Health (http://www-bimas.cit.nih.gov/molbio/hla_bind/), for the presence of potential HLA-B*0701-binding peptides. Lyophilized peptides, 90% pure as indicated by analytical high-performance liquid chromatography (HPLC), were purchased from Primm (Milan, Italy), dissolved at 10 mM concentration in DMSO and stored at −80°C. Then, 10 μM concentration of the peptides were added to LCL-B7 cells (104cells/well) in a 96-microwell plate. Following 1 hour of incubation at room temperature, 5000 effector cells/well were added. IFN-γ release was measured 18 hours later, as described.
PCR analysis
The presence of the SFCMM2/SFCMM3 proviral sequences was analyzed in gDNA (200 ng) extracted from PBMCs isolated from peripheral blood samples collected during follow-up of the treated patients, by conventional PCR using primers: TKs 5′-CCATAGCAACCGACGTACG-3′ and TKas 5′-GCGAATCGGGCCAGCATAGC-3′. PCR amplification was performed for 35 cycles (1 minute at 94°C, 1 minute at 61°C, and 1 minute at 72°C). PCR products were size-fractionated on a 1% agarose gel, stained with ethidium bromide, and visualized on a UV transilluminator. The sensitivity of this assay was determined by amplification of DNA extracted from mixtures of transduced and untransduced cells, and allowed the detection of one positive cell in 104 unmodified cells.
Results
Immunogenicity of TK genetically modified lymphocytes
We have previously reported on the safety and the therapeutic potential of an adoptive immuno-gene therapy approach for the treatment of leukemic relapse, after allogeneic HSCT.5,10,14 As part of this strategy, patients have been treated by the infusion of HSC-donor lymphocytes (DLI) engineered to express both a truncated form of the human low-affinity nerve growth factor receptor (ΔLNGFr) as a cell-surface selectable marker and the HSV-TK suicide gene to control the risk of GvHD associated with the treatment.5,10 Two different vectors were used in those studies, encoding the ΔLNGFr and either the entire HSV-TK (the SFCMM3 vector) or the HSV-TK/neo fusion protein (TN), a bifunctional protein displaying both the HSV-TK activity and the neomycin resistance (the SFCMM2 vector).11
During the follow-up, long-term survival of genetically modified donor lymphocytes was observed in the majority of patients, with variable levels of T-cell expansion, possibly related to antigenic stimulation.5 In the absence of ganciclovir administration, GMCs were detected at all studied time points, with the longest follow-up at 10 years after TK-DLI. As exception to this generalized finding, 7 patients (Table 1) revealed a decline in circulating TK-transduced lymphocytes below the level of PCR detection (< 10−4). Timing and kinetics of the disappearance suggested the development of TK-specific immune effectors responsible for clearance of the GMCs.
Detection of a transgene-specific immune response in the treated patients
| Patient no., infusion . | No. of GMCs infused, 106/kg . | Interval HSCT to infusion, wk . | CD4+ T cells at infusion, cells/μL . | Immune response . | Interval between GMC infusion and immune response evaluation, wk . | Vector . | Target antigen . | HLA restriction . |
|---|---|---|---|---|---|---|---|---|
| Patient 2 | ||||||||
| 1st | 0.1 | 41 | 210 | Negative | 8 | SFCMM2 | HSV-TK/neo | Bw60, B7 |
| 2nd | 0.5 | 49 | 93 | ND | — | SFCMM2 | HSV-TK/neo | Bw60, B7 |
| 3rd | 0.4 | 53 | 234 | Positive | 8 | SFCMM2 | HSV-TK/neo | Bw60, B7 |
| Patient 7 | ||||||||
| 1st | 0.1 | 38 | 229 | ND | — | SFCMM2 | HSV-TK | B7; Cw7 |
| 2nd | 1 | 41 | 165 | ND | — | SFCMM2 | HSV-TK | B7; Cw7 |
| 3rd | 10 | 73 | 107 | Positive | 47 | SFCMM2 | HSV-TK | B7; Cw7 |
| Patient 8 | ||||||||
| 1st | 5 | 8 | ND | IC | 10 | SFCMM2 | HSV-TK | ND |
| 2nd | 10 | 16 | 47 | IC | 11 | SFCMM2 | HSV-TK | ND |
| 3rd | 5 | 24 | 60 | Neg/pos* | 43/102* | SFCMM2 | HSV-TK | ND |
| Patient 16 | ||||||||
| 1st | 70 | 12 | 208 | Negative | 38 | SFCMM3 | HSV-TK | A1, B35, Cw4 |
| 2nd | 70 | 67 | 270 | Positive | 7 | SFCMM3 | HSV-TK | A1, B35, Cw4 |
| Patient 17 | ||||||||
| 1st | 47 | 52 | 442 | Positive | 14 | SFCMM3 | HSV-TK | B35, B51; Cw4 |
| Patient 20 | ||||||||
| 1st | 100 | 278 | 656 | Positive | 23 | SFCMM3 | HSV-TK | ND |
| Patient 21 | ||||||||
| 1st | 50 | 120 | 200 | Positive | 8 | SFCMM3 | HSV-TK | ND |
| Patient no., infusion . | No. of GMCs infused, 106/kg . | Interval HSCT to infusion, wk . | CD4+ T cells at infusion, cells/μL . | Immune response . | Interval between GMC infusion and immune response evaluation, wk . | Vector . | Target antigen . | HLA restriction . |
|---|---|---|---|---|---|---|---|---|
| Patient 2 | ||||||||
| 1st | 0.1 | 41 | 210 | Negative | 8 | SFCMM2 | HSV-TK/neo | Bw60, B7 |
| 2nd | 0.5 | 49 | 93 | ND | — | SFCMM2 | HSV-TK/neo | Bw60, B7 |
| 3rd | 0.4 | 53 | 234 | Positive | 8 | SFCMM2 | HSV-TK/neo | Bw60, B7 |
| Patient 7 | ||||||||
| 1st | 0.1 | 38 | 229 | ND | — | SFCMM2 | HSV-TK | B7; Cw7 |
| 2nd | 1 | 41 | 165 | ND | — | SFCMM2 | HSV-TK | B7; Cw7 |
| 3rd | 10 | 73 | 107 | Positive | 47 | SFCMM2 | HSV-TK | B7; Cw7 |
| Patient 8 | ||||||||
| 1st | 5 | 8 | ND | IC | 10 | SFCMM2 | HSV-TK | ND |
| 2nd | 10 | 16 | 47 | IC | 11 | SFCMM2 | HSV-TK | ND |
| 3rd | 5 | 24 | 60 | Neg/pos* | 43/102* | SFCMM2 | HSV-TK | ND |
| Patient 16 | ||||||||
| 1st | 70 | 12 | 208 | Negative | 38 | SFCMM3 | HSV-TK | A1, B35, Cw4 |
| 2nd | 70 | 67 | 270 | Positive | 7 | SFCMM3 | HSV-TK | A1, B35, Cw4 |
| Patient 17 | ||||||||
| 1st | 47 | 52 | 442 | Positive | 14 | SFCMM3 | HSV-TK | B35, B51; Cw4 |
| Patient 20 | ||||||||
| 1st | 100 | 278 | 656 | Positive | 23 | SFCMM3 | HSV-TK | ND |
| Patient 21 | ||||||||
| 1st | 50 | 120 | 200 | Positive | 8 | SFCMM3 | HSV-TK | ND |
The development of an immune response against the transgene products was monitored by ex vivo stimulation of PBMCs against autologous-transduced and allogeneic T cells. In the presence of an anti-allospecific immune response, the patient was considered not immunized (negative) or immunized (positive) depending on the anti-HSV-TK cytolytic activity detected. In the absence of an anti-allo immune response, the patient was considered immune compromised (IC).
ND indicates not done; —, not applicable.
Forty-three weeks after the 3rd infusion the immune-response was negative, but a positive response was detected after 102 weeks; in the meantime, ganciclovir was administered.
To document and characterize in vitro this immune response, PBMCs isolated from patients after disappearance of GMCs from circulation were stimulated in vitro by irradiated SFCMM2 or SFCMM3-transduced donor T cells (Table 1). In parallel, as positive control, specific immune response against allogeneic target cells was tested. Under these conditions, cytotoxic T cells with specificity for the transduced targets were obtained from all these patients (Figure 1). These effector cells were specific for a vector-encoded antigen, since untransduced HSC-donor T cells (□, ○) were not lysed. No such cytotoxic T cells could be established from appropriate controls including the HSC donors and unrelated individuals (data not shown).
Effector cells isolated from the infused patients specifically kill GMCs. PBMCs isolated from the patients listed in Table 1 at the indicated time to HSCT were stimulated with GMCs from the respective HSC donors (○, ●) and with allogeneic T cells (□, ■). Lytic activities of the responder lymphocytes were measured against stimulator cells (■, ●) and untransduced HSC donor T cells (○, □) in a standard chromium-release assay at the indicated E/T ratios. Detection of positive immune responses against GMCs with the appropriate controls were reported.
Effector cells isolated from the infused patients specifically kill GMCs. PBMCs isolated from the patients listed in Table 1 at the indicated time to HSCT were stimulated with GMCs from the respective HSC donors (○, ●) and with allogeneic T cells (□, ■). Lytic activities of the responder lymphocytes were measured against stimulator cells (■, ●) and untransduced HSC donor T cells (○, □) in a standard chromium-release assay at the indicated E/T ratios. Detection of positive immune responses against GMCs with the appropriate controls were reported.
Characterization of the transgene-specific immune response
To investigate whether the suicide or the marker gene products were targets of the immune response, effector cells derived from patient 2 were tested against autologous LCLs expressing single components of the SFCMM2 vector. LCLs expressing the suicide HSV-TK or the bacterial neo genes were lysed at comparable level (Figure 2A). On the other hand, target cells expressing the ΔLNGFr were not recognized (Figure 2A). Immune response against HSV-TK was detected in all the immunized patients studied, but we failed to obtain neo-specific effectors from patients other than patient 2 (Table 1), suggesting a different immunogenic potential of the 2 transgenes.
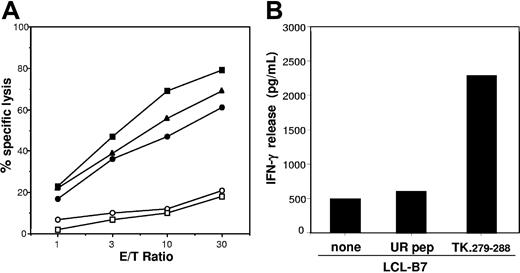
Vector-encoded proteins are the target antigens of the GMC-specific immune response: identification of an immunodominant HSV-TK epitope. (A) LCLs from patient 2 were transduced with retroviral vectors encoding single components of the SFCMM2 vector and used as target in a standard chromium release assay at the indicated E/T ratios. LCLs expressing the TN fusion protein (●), the wild-type HSV-TK (■), or the neo gene (▴) were recognized and killed at a similar level. Untransduced LCLs (○) and LCLs expressing the ΔLNGFr cell-surface marker15 (□) were not recognized. (B) HLA-B*0701+ LCLs (LCL-B7) were incubated with 10 μM peptide TK.279-288 and used as stimulator cells in an IFN-γ release assay. An unrelated peptide (UR pep) able to bind HLA-B*0701 molecule was used as control for specificity.
Vector-encoded proteins are the target antigens of the GMC-specific immune response: identification of an immunodominant HSV-TK epitope. (A) LCLs from patient 2 were transduced with retroviral vectors encoding single components of the SFCMM2 vector and used as target in a standard chromium release assay at the indicated E/T ratios. LCLs expressing the TN fusion protein (●), the wild-type HSV-TK (■), or the neo gene (▴) were recognized and killed at a similar level. Untransduced LCLs (○) and LCLs expressing the ΔLNGFr cell-surface marker15 (□) were not recognized. (B) HLA-B*0701+ LCLs (LCL-B7) were incubated with 10 μM peptide TK.279-288 and used as stimulator cells in an IFN-γ release assay. An unrelated peptide (UR pep) able to bind HLA-B*0701 molecule was used as control for specificity.
Recognition of the transgene products was HLA class I restricted as demonstrated by inhibition experiments performed with anti-HLA class I antibodies (data not shown). In some patients, the HLA-restricting alleles were identified by the use of vector-transduced cells sharing single HLA class I alleles with the patient (Table 1). Interestingly, an HLA-B7-restricted immune response against HSV-TK was detected in both patients 2 and 7 (ie, all the HLA-B7 patients analyzed), thus suggesting the existence of a HSV-TK immunodominant epitope. To characterize the antigenic peptide, a truncated fragment of the HSV-TK cDNA encoding the first 207 amino acids was transfected into Cos-7 cells along with a HLA-B*0701 construct. The transfectants were then used to stimulate effectors derived from patient 2. Only the full-length sequence proved positive, thus demonstrating that the antigenic peptide was encoded by the last 513 nucleotides of the HSV-TK open reading frame (data not shown). The amino acid sequence of this fragment was screened for peptides carrying the binding motif for HLA-B*0701.16 The peptide TK.279-288 (GPRPHIGDTL) carrying proline in position 2 and leucine in position 10 was identified, which sensitized an LCL expressing HLA-B*0701 (LCL-B7) to recognition by effectors from patient 2 (Figure 2B). LCL-B7 cells pulsed with an unrelated peptide (ie, UR pep) able to bind HLA-B*0701 were not recognized (Figure 2B).
This set of data confirmed that elimination of GMCs was directly related to T-cell recognition of HSV-TK determinants exclusively present in transduced lymphocytes.
Anti-TK immunity requires in vivo priming in immunocompetent patients
To investigate whether previous exposure to herpes virus could play a determinant role in the generation of immune response to GMCs, we stimulated 20 replicates, each containing 2 × 106 CD3+ lymphocytes obtained from the HSV-seropositive HSC donor of patient 2, in the conditions previously described. In this seropositive donor, only culture no. 6 (1 of 20) recognized, at a very low level, autologous SFCMM2-transduced cells (Figure 3), indicating the presence of a minimal frequency of cytotoxic T-cell precursors in the circulation,17 which is suggestive of a naive T-cell repertoire. On the contrary, transgene-specific effectors could be easily expanded from low numbers of PBMCs from the recipient patient 2, strongly suggesting that the newly generated immune response against TK is independent of the donor's previous exposure to the herpes virus, but is generated in vivo in the recipient by TK-transduced cell priming.
Transgene-specific effectors are present at low frequency in a healthy donor. PBMCs containing 2 × 106 CD3+ lymphocytes isolated from the donor of patient 2 were stimulated, in 20 independent microcultures, with autologous GMCs. On day 10, lytic activities of the lymphocytes were measured against SFCMM2-transduced (○) and untransduced (●) autologous GMCs as well as allogeneic T cells (□) in a standard chromium-release assay at the indicated E/T ratios. As shown, an immune response specific for the transduced cells was detected in microculture 6.
Transgene-specific effectors are present at low frequency in a healthy donor. PBMCs containing 2 × 106 CD3+ lymphocytes isolated from the donor of patient 2 were stimulated, in 20 independent microcultures, with autologous GMCs. On day 10, lytic activities of the lymphocytes were measured against SFCMM2-transduced (○) and untransduced (●) autologous GMCs as well as allogeneic T cells (□) in a standard chromium-release assay at the indicated E/T ratios. As shown, an immune response specific for the transduced cells was detected in microculture 6.
Effective in vivo priming requires the presence of a competent immune systems, as well as availability of the antigen presented in an immunogenic context. Therefore, we investigated the kinetic of the generation of transgene-specific immune effectors, with respect to the immune reconstitution level of the patients at the time of the infusion of GMCs. The delay between transplantation and the first infusion necessary to induce immunity was significantly longer (P < .01; as determined by the Wilcoxon rank test), median 95 weeks (Table 2), as compared to the posttransplantation interval present in nonimmunized patients, median 43 weeks (Table 2). This observation is in agreement with the concept that profound immune suppression lasts for a significant period of time after transplantation in patients recipients of an allogeneic HSCT. A direct correlation of the immunization event with the profile of immune reconstitution is revealed by the mean number of circulating CD4+ lymphocytes/μL observed at the time of infusion in patients who developed an immune response: 281 (Table 2), which differs significantly (P < .05 as determined by the Wilcoxon rank test) from the mean of 114 cells/μL in patients who did not respond to the transgene (Table 2).
Patient characteristics measured at the first infusion or at the infusion inducing immunity
| Patient no. . | Total number of GMCs infused, 106/kg . | Interval between HSCT and infusion, wk . | CD4+ cells at infusion, cells/μL . |
|---|---|---|---|
| Immunized | |||
| 2 | 1 | 53 | 234 |
| 7 | 11 | 73 | 107 |
| 8 | 20 | 24 | 60 |
| 16 | 140 | 67 | 270 |
| 17 | 47 | 52 | 442 |
| 20 | 100 | 278 | 656 |
| 21 | 50 | 120 | 200 |
| Mean | 53 | 95 | 281 |
| Not immunized | |||
| 3 | 0.5 | 276 | 400 |
| 1 | 1.5 | 9 | 122 |
| 5 | 4.9 | 15 | 55 |
| 10 | 1 | 18 | 0 |
| 11 | 110 | 12 | 0 |
| 12 | 24 | 7 | 0 |
| 13 | 10 | 6 | 0 |
| 22 | 110 | 15 | 400 |
| 23 | 120 | 26 | 50 |
| Mean | 43 | 43 | 114 |
| Patient no. . | Total number of GMCs infused, 106/kg . | Interval between HSCT and infusion, wk . | CD4+ cells at infusion, cells/μL . |
|---|---|---|---|
| Immunized | |||
| 2 | 1 | 53 | 234 |
| 7 | 11 | 73 | 107 |
| 8 | 20 | 24 | 60 |
| 16 | 140 | 67 | 270 |
| 17 | 47 | 52 | 442 |
| 20 | 100 | 278 | 656 |
| 21 | 50 | 120 | 200 |
| Mean | 53 | 95 | 281 |
| Not immunized | |||
| 3 | 0.5 | 276 | 400 |
| 1 | 1.5 | 9 | 122 |
| 5 | 4.9 | 15 | 55 |
| 10 | 1 | 18 | 0 |
| 11 | 110 | 12 | 0 |
| 12 | 24 | 7 | 0 |
| 13 | 10 | 6 | 0 |
| 22 | 110 | 15 | 400 |
| 23 | 120 | 26 | 50 |
| Mean | 43 | 43 | 114 |
The reported parameters were measured at the infusion inducing immunity for patients 2, 7, 8, and 16, and at the time of the first infusion for all the other patients.
This concept is further sustained by the course of 2 patients (patients 8 and 6), who received the first infusion of TK lymphocytes early after HSCT and did not immunize, but who did mount a TK-specific response subsequently when fully immunocompetent (Table 1), as consequence of new exposure to high antigen amounts. Patient 8 was treated for a tumor relapse with 3 infusions of SFCMM2-DLI when still immunodeficient (Table 15 ). Thirty-one weeks after HSCT, when CD4+ cells were 140/μL, the GMCs were still 6% of the circulating lymphocytes, the same value (range, 5%-7.4%) detected since the third infusion performed 7 weeks earlier. Moreover, anti-TK immune effectors were not detectable ex vivo (data not shown). The second patient, patient 16, was injected 86 days after HSCT and circulating transduced cells were detected by PCR, even when the immune system was fully reconstituted as documented by both the presence of 228 CD4+ cells/μL and by the ex vivo detection of an active anti-allo immune repertoire in the absence of an anti-TK response (data not shown). These data demonstrate that the infusion of GMCs in immunodeficient patients, allowed long-term survival of the transduced cells that persisted in the circulation even after immune reconstitution. Unresponsiveness against otherwise antigenic molecules (ie, HSV-TK and neo) may result from either ignorance or tolerization of T cells.18,19 In these patients, the transgene-specific T cells remained inducible, as demonstrated by the generation of a strong cytotoxic HSV-TK–specific response on the treatment of patient 8 with ganciclovir to control severe GvHD5 and of patient 16 with a second infusion of TK-DLI for an additional relapse. All together these results demonstrate that immunization occurs only when GMCs are infused or killed in an already immunocompetent environment.
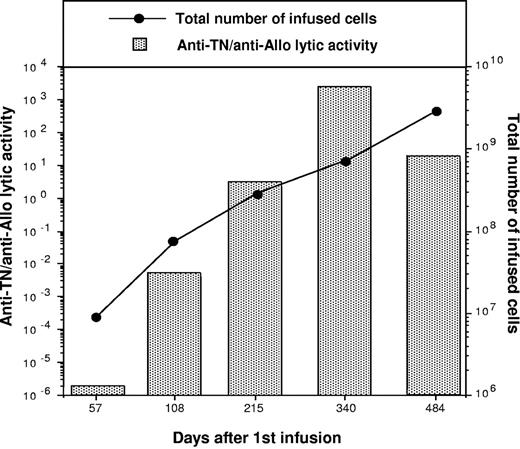
In addition to the immune profile of the infused patients, the total amount of available antigen may also play a decisive role in the immunization process. In this clinical setting, the total number per kilogram of infused GMCs resulting in immunization (median 53 × 106/kg; range, 1-140 × 106/kg; Table 2) does not significantly differ from the amount administered to patients who did not develop immunity (median, 43 × 106/kg; range, 0.5-120 × 106/kg; Table 2). In immune reconstituted patients, a single infusion of GMCs was sufficient for inducing immune response. Patients 17, 20, and 21 (Table 1) received a single administration of GMCs (47, 100, and 50 × 106 cells/kg, respectively) to treat a late relapse event (Table 1). GMCs were detected in peripheral blood and bone marrow of all the 3 patients 30 days after infusion by PCR analysis, but became undetectable 1 month later (data not shown); concomitantly a strong cytotoxic immune response against HSV-TK was observed (Figure 1). However, the amount of GMCs administered can influence the extent of the response. Indeed, the elicited immune effectors can be boosted by repeated infusions as documented by a semiquantitative analysis (see “Patients, materials, and methods”) of the frequency of transgene-specific effectors performed during the treatment of patient 2. As shown in Figure 4, lytic activity was undetectable on day 57 after the first infusion; then a continuous increase of the amount of transgene-reactive T cells that paralleled the total amount of GMCs infused, was observed. Remarkably, beyond the increasing number of injected cells, also the cross-presentation promoting-action of ganciclovir, which was administered at day 150 to treat GvHD,5 could have been responsible for the booster effect.
Multiple infusions of GMCs enhance the extent of the transgene-specific immune response. Samples 1 to 5 were collected from patient 2 at different times after HSCT. PBMCs containing 106 CD3+ lymphocytes from each sample were stimulated with allogeneic and autologous SFCMM2-transduced T cells and tested 10 days later for their lytic activity against the stimulators. Results are plotted as ratio of anti-TN lytic units/106 cells to anti-allo lytic units/106 cells (TN/allo ratio). The numbers of infused cells at the various time points are reported on the right ordinate axis.
Multiple infusions of GMCs enhance the extent of the transgene-specific immune response. Samples 1 to 5 were collected from patient 2 at different times after HSCT. PBMCs containing 106 CD3+ lymphocytes from each sample were stimulated with allogeneic and autologous SFCMM2-transduced T cells and tested 10 days later for their lytic activity against the stimulators. Results are plotted as ratio of anti-TN lytic units/106 cells to anti-allo lytic units/106 cells (TN/allo ratio). The numbers of infused cells at the various time points are reported on the right ordinate axis.
Clinical impact of transgene-specific immune responses
In this clinical setting, the control of viral infections and tumor growth correlated with the presence and expansion of circulating TK donor lymphocytes.10 Therefore, we investigated whether the generation of the immune response against suicide transgene had a direct impact on the clinical outcome. From the 6 complete remissions observed in the study, 5 occurred in the series of eventually immunized patients.10 Analysis of remission time and immunization development revealed that the clinical outcome strictly correlated with the level of remission obtained before immunization. Representative are the clinical histories of patients 20 and 17, who received a single infusion of GMCs late after transplantation, to treat disease persistence (in both cases, multiple myeloma). The infusions resulted in complete (patient 17) and partial (patient 20) clinical remission.10 A few months later a strong cytotoxic immune response against HSV-TK developed in both patients, leading to the elimination of GMCs from circulation (Table 1). The 2 patients experienced a different clinical outcome: patient 17 remained in complete remission for 2 years, whereas patient 20 dropped into a full hematologic relapse. These observations support the concept that immune elimination of GMCs resulted in either achievement of the full antitumor effect (patient 17) or disease progression (patient 20), depending on the status of disease at the time of the immune elimination of GMCs.
Discussion
Suicide gene transfer has been proposed as a tool for elimination of cells carrying an unwanted effect in both animal models and clinical studies. This includes the use of suicide genes for elimination of tumor cells,20 and more frequently as a fail-safe system.21 For this purpose, different suicide genes have been proposed, including the ganciclovir/thymidine kinase,22 the CD20/rituximab,8 the Fas/dimerizer,23 the caspase 9/dimerizer,9 and others. All of these proposed strategies present advantages and limitations. The HSV-TK/ganciclovir-based strategy is the most widely used and the only one for which extensive clinical experience is available with a solid record of safety and efficacy.5,6 Specifically in patients undergoing allogeneic bone marrow transplantation followed by TK-DLI, this is the only gene that proved to be able to abrogate severe GvHD both in allo-matched5,10,14 and allo-mismatched transplants,24 independently of the dose of genetically engineered donor lymphocytes infused. On the other hand, HSV-TK is a viral protein, thus potentially highly immunogenic. Our study and a similar observation confirm this possibility.7 However, this cannot be hastily used to simplistically propose the replacement of the TK system with other, yet untested, suicide genes.25 Rather, the role of TK as the suicide gene strategy to be used in association with DLI in allogeneic HSCT should include an in-depth analysis of the conditions and mechanisms required to generate a detrimental immune response against this transgene, and, consequently, the identification of which suicide gene could be more appropriate for the application in a clinical context where the profound immune suppression associated with the transplantation procedure should be more than adequate to prevent immunization.
Indeed, as it appears clear from our study, immunogenicity of the TK-engineered donor lymphocytes completely depends on the time of infusion after HSCT and, ultimately, on the level of immune reconstitution of the patient. Genetically modified lymphocytes were able to induce a transgene-specific immune response only when injected or suicided late after transplantation into immunocompetent recipients. Targets of this response were 2 proteins: the bacterial product of the neo gene, used as a positive selection marker in the large majority of gene therapy/marking studies that use retroviral vectors, and the product of the HSV-TK suicide gene. Cells expressing these foreign genes are targets of this immune response, whereas endogenous proteins (ie, ΔLNGFr) are not recognized, even if ectopically expressed in a context otherwise extremely immunogenic.
All the transgene-specific effectors isolated throughout the study were CD8+ HLA class I- restricted cytotoxic T lymphocytes. We never obtained CD4+ effectors. This result may reflect the actual situation occurring in vivo or may be a bias of the culture conditions and the read-out protocols used during the ex vivo studies. HSV-TK–specific CD4+ effectors have recently been described7 ; moreover, we have identified by a reverse immunology approach26 a TK-encoded epitope recognized by CD4+ T cells, whose role in the generation of in vivo immune responses is still under investigation.
The presence of an host active immune system is necessary for the generation of HSV-TK–specific effectors. In patients given infusions very late after HSCT, a single administration of GMCs was sufficient to induce a detrimental immune response, whereas patients given injections with a comparable number of transduced lymphocytes early after transplantation failed to develop a transgene-specific immune response. Unresponsiveness against an otherwise antigenic molecule (ie, HSV-TK and in some instance neo) may result from either ignorance or tolerization of newly developing donor T cells by the product of the transgene in the post-HSCT reconstitution phase. In several animal models, transplantation of stem cell progenitors expressing foreign gene products has been associated with the induction of tolerance,18,19 even if some exceptions do exist.27,28 Long-term persistence of cells expressing the neo-gene product has been reported by several gene-marking studies, after the infusion of EBV-specific T-cell population in patients who had undergone ablative chemotherapy and marrow grafting.29,30 Neo-tolerance has been observed also in ADA-SCID patients given injections with both genetically modified lymphocytes and marrow-derived cells.31 In this particular set of patients beside the long-term detection of GMCs, we analyzed ex vivo the transgene-specific immune responsiveness. We were unable to detect any significant level of cytotoxic response against the GMCs (C.T., unpublished data, March 1996), thus suggesting the existence of tolerance to the transgene product, which was maintained despite the repeated injections of GMCs. In the HSV-TK/DLI setting, the transgene-specific T-cell precursors present in the patients given injections early after transplantation were not tolerant and remained inducible; either a second injection of GMCs or the administration of ganciclovir, performed when patients were immunocompetent, abolished the peripheral unresponsiveness and resulted in T cell-mediated destruction of GMCs.
The cellular mechanism responsible for priming of transgene-specific effectors is still unclear. Some reports32 suggest that activated human T lymphocytes are able by themselves to induce a Th-independent cytolytic T-cell response in vitro. However, we were able to detect transgene-specific immune responses only on in vitro stimulation of already primed T cells derived from treated patients, thus suggesting that an in vivo cross-presentation pathway, mediated by resident antigen-presenting-cells, could be responsible for the induction of the transgene-specific immune response. This is consistent with the observation that immunity against the transgene products could be induced by partial cell death of the GMCs, mediated by ganciclovir administration or growth factor deprivation at the time of injection (ie, IL-233 ). This hypothesis has recently been confirmed, in a murine model (V.R., C.T., and C. Bordignon, manuscript submitted, October 2006).
Despite the fact that generation of transgene-specific immune responses and clearance of the transduced cells proved to be a relatively rare phenomenon in the clinical applications of gene therapy, it is clear that this event may occur. Therefore, the crucial variable to be determined relates to the occurrence and frequency of unwanted effects associated with this potentially detrimental consequence of the transgene immunogenicity. Since the effectiveness of the HSV-TK/ganciclovir suicide machinery in controlling even the most severe form of GvHD has been proven, the open issue remaining relates to the potential loss of GvL effect. Altogether the findings reported here and by Ciceri10 strongly suggest that immunogenicity of the TK suicide gene did not prevent the exploitation of the antitumor potential of the strategy. In our clinical setting, clearance of the GMCs resulted in either persistence of the antitumor effect, and therefore maintenance of disease remission, or loss of the therapeutic effect with tumor relapse, exclusively depending on the status of the disease at the onset of the transgene-specific immune response.10 Actually, 5 of the 7 patients in the study who developed an immune response to the transgene as a consequence of late exposure to the transgene product had previously achieved a complete remission of the disease due to the GvL effect associated to TK cell infusions. Such complete remissions were maintained even after immunization, indicating that the time kinetic of GvL effect is consistently more rapid as compared to the development of an anti-TK response.
In conclusion, selection of the best suitable suicide gene should include evaluation of the clinical setting in which the use of genetically modified donor lymphocytes is proposed, with TK most suitable for allogeneic HSCT from partially mismatched or unrelated donors, where the risk of severe GvHD is higher and protocols of ex vivo and in vivo T-cell depletion are more frequently used. Other less immunogenic transgenes should prove their efficacy first in those clinical contexts where faster immune reconstitution and less profound immunosuppression is predicted (ie, transplantation after reduced-intensity conditioning from allo-matched donors).
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Italian Association for Cancer Research (AIRC), the Ministry of Health, and by the European Union grant QLK3-CT-2001-01265.
Authorship
Contribution: C.T., C. Bonini, and C. Bordignon wrote the paper; C.T., S.M., Z.M., P.M., and C. Bonini performed laboratory research; C.T., S.M., V.R., F.C., C. Bonini, and C. Bordignon designed and discussed experiments; and S.M., F.C., C. Bonini, and C. Bordignon performed clinical research.
Conflict-of-interest disclosure: C. Bonini, F.C., and C. Bordignon have declared a financial interest in MolMed S.p.A., whose potential product (TK-DLI) was studied in the present work.
Correspondence: Claudio Bordignon, Istituto Scientifico San Raffaele, Via Olgettina 58, Milan, Italy; e-mail: c.bordignon@hsr.it.