Abstract
CD137 has long been recognized as a costimulatory receptor for growth and functional maturation of recently activated T cells in the presence of T-cell receptor signal. In this report, we present the fact that, in the absence of MHC and antigen, triggering of CD137 by an agonist monoclonal antibody induces vigorous growth of both CD8+ and CD4+ T cells with memory phenotype, whereas it does not affect naive T cells. Moreover, T cells with memory phenotype accumulate progressively in transgenic mice overexpressing CD137 ligand. CD137-mediated proliferation of memory T cells is directly through CD137 on T cells and does not require IL-15 and IFN-γ. Our results define a new role of CD137 signal in the growth of memory T cells.
Introduction
A hallmark of memory T cells is their capacity to rapidly expand in response to repeated antigen assaults to generate effector T cells as a means to protect the host from infection and malignancies.1,2 Yet to be fully clarified is whether molecular mechanisms underlie proliferation of memory T cells and maturation of effector functions. Using specific gene knockout (KO) mice, several recent studies suggest that IL-7 and IL-15 are essential for the growth and maintenance of CD8+ memory T cells in a steady number in hosts,3 with IL-15 required for antigen-independent cell proliferation and IL-7 for survival.4–6 Mechanisms for the persistence of CD4+ memory T cells, however, are less clear with possible involvement of both TCR and IL-7 signaling.7
CD137 (also called 4-1BB, ILA, TNFSFR9) is an inducible receptor of the tumor necrosis factor (TNF) receptor superfamily. It has been shown that CD137 is a potent cosignal or costimulatory molecule that is found on activated T cells, NK cells, monocytes, and dendritic cells.8 Its natural ligand, CD137L, is constitutively expressed on a fraction of dendritic cells and is inducible on macrophages, B cells, and T cells.9 In the presence of TCR engagement, signaling through CD137 by either CD137L or agonist monoclonal antibodies (mAbs) triggers T-cell expansion, cytokine production, and prevention of activation-induced death of effector T cells.9 Recent studies demonstrate that CD137 signal is also critical in the prevention and reversal of established T-cell tolerance and anergy in vivo.10,11 Agonistic CD137 mAbs augment T cell-mediated immune responses against cancer and viral infection in animal models.12,13 Surprisingly, the same mAbs are also effective in ameliorating autoimmune diseases in experimental animal models, but the mechanism underlying these observations is not well defined.14,15–17
The role of CD137 signal in the regulation of memory T-cell responses has been implicated in several studies. In a mouse model of lymphocytic choriomeningitis virus (LCMV) infection, although CD137L KO mice generated effective primary antiviral CD8+ T-cell responses to eliminate the infection, the number of LCMV-specific T cells after contraction was 2- to 3-fold lower than wild-type (WT) CD137L mice.18 As a result, recall of memory CD8+ T-cell responses to LCMV was impaired. In an influenza virus infection model, there was no difference in the early contraction of the CD8+ T cells in the spleen. However, CD137L KO mice showed decreased virus-specific CD8+ T-cell number late in the primary response (days 21-38) as well as a defect in the secondary response to influenza.19 These data are consistent with a recent report showing that CD137 signal is required in priming stage for memory T cells to respond to antigen recall.20 Although these studies support a role of CD137 in the generation of memory T cells during T-cell priming, a direct role of CD137 signal in proliferation in the absence of antigen signal has not been tested.21
In this study, we present a rather unexpected finding that signal through CD137 receptor alone induces a highly selective, antigen-independent signal for proliferation of T cells with memory but not naive phenotypes.
Materials and methods
Mice
Six- to 8-week-old C57BL/6 (B6), C3H/HeJ, B6/Thy1.1, and B6/IFN-γ KO mice were obtained from the Jackson Laboratory (Bar Harbor, ME). B6 H-2Kb KO, IL-15 KO, and OT-1 x RAG-1 KO mice were purchased from Taconic Farms (Germantown, NY). CD137L transgenic mice were generated as described before.22 All mice were housed under specific pathogen-free conditions in the Johns Hopkins animal facility with all protocols approved by the Institutional Animal Care and Use Committee (IACUS). To generate CD137-deficient mice, a 5.1-kb DNA fragment upstream of exon 1 and a 4.8-kb DNA fragment downstream of exon 6 of murine CD137 gDNA were polymerase chain reaction (PCR) amplified from a 129SvJ bacterial artificial chromosome (BAC) library (Invitrogen, Carlsbad, CA). The fragments were cloned into vector pKOscrambler NTKV-1907 (Stratagene, La Jolla, CA) to generate a targeting plasmid resulting in removing 6 exons from the CD137 gene. The targeting fragment containing the 5′ arm and 3′ arm of CD137, a positive selection marker NEO, and a negative selection marker TK was transfected into embryonic stem (ES) cells of 129Sv background. Southern blots were used to confirm gene targeting of positive clones. Chimeric mice were produced by injection of targeted ES cells into blastocysts of B6 hosts. Heterozygous mice were obtained from breeding chimeric mice with B6 mice. Homozygous mice were produced by back-crossing to B6 for more than 5 generations.
Antibodies
The following antibodies were purchased from PharMingen (San Diego, CA): CD8-Cy-Chrome, CD4-Cy-Chrome, Thy1.2-fluorescein isothiocyanate (FITC), CD44-phycoerythrin (PE), CD62L-FITC, CD122-PE, PD-1-PE, CD137-PE, and FITC BrdU flow kit. SIINFEKL/H-2Kb-PE tetramer (OT-1 tetramer) was bought from Beckman Coulter (Hialeah, FL). The generation and purification of CD137 mAb (clone 2A) was described previously.12
Cell division measurement in vivo
Mice were injected with 100 μg CD137 mAb or rat IgG (Sigma, St Louis, MO) on day 0 and 2. On day 3, treated mice were given BrdU (Sigma) in drinking water at a concentration of 0.8 mg/mL. On day 7, spleen and liver lymphocytes were prepared as previously described.23 All samples were preincubated for 15 minutes with anti-CD32 mAb and subsequently stained for 30 minutes at 4°C with antibodies. After cell-surface staining, intracellular BrdU was used following the manufacturer's instruction with a BrdU-FITC flow kit.
Preparation of memory T cells in vivo
The first method was described previously with small modifications.24 Briefly, 1 × 106/mL OT-1 cells were incubated with irradiated CD80/EG7, an EL4 mouse tumor line transfected to express chicken ovalbumin (OVA) and murine CD80 (Koji Tamada and L.C., unpublished data, May 2000) in RPMI medium at a 4:1 ratio for 48 hours. Live cells were isolated using Lympholyte-M (Cedarlane Labs, Hornby, ON, Canada) and incubated in medium containing 20 IU recombinant IL-2 for additional 3 days. Cells were isolated and 1 to 2 × 107 cells were transferred into naive B6 mice. More than 40 days after transfer, the OT-1 cells were purified and used as memory T cells. In some experiment, CD8+ T cells containing about 20% to 30% memory OT-1 cells were purified by a CD8+ T-cell isolation kit (Miltenyi Biotec, Auburn, CA) and were labeled with CFSE. The labeled cells in HBSS were transferred into H-2Kb KO mice, followed with CD137 mAb or control mAb injection on day 0 and day 2. Spleen cells were harvested and gated for CD8, OT-1 tetramer. Cell division was traced by CFSE dilution analysis. In some experiments, memory OT-1 cells were generated by published methods.25,26 B6 mice received 1 to 3 × 106 OT-1 cells and were immunized intraperitoneally with 2.5 mg OVA together with 150 μg poly I:C (Pharmacia Biotech, San Diego, CA). More than 50 days later, mice were used for experiments.
T-cell homeostatic proliferation assay
Spleens and lymph nodes (LNs) were harvested from OT-1 x RAG1 KO mice and CD8+ T cells were purified using CD8+ T-cell isolation kit (Miltenyi Biotec). The donor cells were labeled with CFSE as previously described.27 Briefly, cells were suspended in PBS at 2 × 107/mL and incubated in 5 μM CFSE solution for 15 minutes at 37°C. Cells were harvested and further incubated in RPMI 1640 medium for 30 minutes at 37°C. After incubation, donor cells were washedtwice with HBSS. Then, 1 × 106 labeled cells in 0.5 mL HBSS were transferred into B6 hosts that had been irradiated with 600 cGy. Mice were injected intraperitoneally with 100 μg CD137 mAb or control mAb on day 0 or day 7 after adoptive transfer. Spleen cell were harvested and analyzed by flow cytometry 6 days after antibody injection.
Results
CD137 signal stimulates the proliferation of memory T cells in naive mice
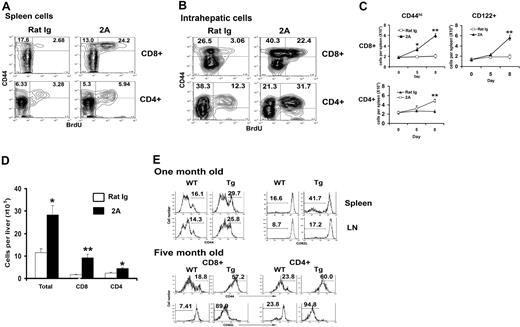
Although the role of CD137 in costimulating adaptive immunity has been studied extensively, its effect on T cells in the absence of antigen is unclear. As a first step to test this, naive B6 mice were given injections with a CD137 agonist mAb (clone 2A12 ), and subsequently fed with BrdU in drinking water continuously for 5 days to mark dividing cells. Division of T cells in spleens was then determined by the BrdU incorporation in flow cytometry analysis. Whereas only a small fraction of CD44hi cells (< 5%) underwent division in control mAb-treated mice, CD137 mAb treatment induced significant increases of CD44hi cells that undergo division (Figure 1A). This applies to both CD8+ and CD4+ T cells although the effect on CD8+ T cells was more profound. We did not observe significant change in T-cell apoptosis up to 7 days during CD137 mAb treatment (data not shown), suggesting that the increase in CD44hi T cells was mainly caused by enhanced proliferation. In sharp contrast, CD44lo T cells did not have significant division, albeit a small increase of dividing cells was observed in CD4+ subsets in some experiments (Figure 1A). We gave 2 doses of the mAb in initial experiments, but additional studies demonstrated that a single injection of CD137 mAb is sufficient to induce these changes (Y.Z. et al, data not shown). In addition to proliferation of spleen T cells, we found that injection of CD137 mAb also induced a significant increase of intrahepatic CD44hi T cells in both CD8+ and CD4+ subsets (Figure 1B), indicating that CD137 stimulation has a systemic effect on memory T cells. Kinetic analysis of memory T-cell responses to CD137 mAb using both CD44 and CD122 (IL-2 receptor γ) mAb indicates that memory T cells in both CD4+ and CD8+ subsets increased progressively. On day 8, the percentage (data not shown) and absolute number (Figure 1C) of these cells in spleens more than doubled. CD137 mAb had only minimal effect in CD44lo or CD122− cells both in the spleen and liver (data not shown). Of intrahepatic T cells, both CD8+ and CD4+ subsets increased dramatically in absolute numbers with more vigorous expansion in CD8+ subset (Figure 1D). We also observed a similar effect of CD137 mAb in the proliferation of CD44hi T cells in C3H/HeJ mice (data not shown). This observation excludes the effect of lipopolysaccharides (LPSs)28 because this mouse strain is genetically defective at the Toll-like receptor 4 (TLR4) locus so as to be nonresponsive to LPS. Furthermore, the effect of CD137 mAb is specific for CD137 because the mAb has no effect on T cells from CD137 KO mice. We conclude that memory T cells respond vigorously to CD137 stimulation in vivo.
CD137 stimulation selectively stimulates proliferation of memory T cells. B6 mice were injected with 100 μg control rat IgG or 2A on day 0 and 2 and fed with drinking water with 0.8 mg/mL BrdU from day 3 to 7. Splenocytes (A) or intrahepatic lymphocytes (B) were harvested on day 7 and stained for CD8, CD4, CD44, and BrdU by flow cytometry. Data were presented by gating on CD8 or CD4. Total numbers of CD44hi or CD122+ cells in CD8+ or CD4+ T-cell subsets in spleens (C) are shown on days 5 and 8, respectively, after antibody treatment. Error bars indicate SD. The number of total intrahepatic lymphocytes as well as CD4+ and CD8+ subsets on day 7 are also shown (D). The results are from one representative of 3 independent experiments with similar results. (E) Accumulation of memory phenotype T cells in CD137L transgenic mice. The phenotype of spleen or LN T cells from 1-month-old and 5-month-old CD137L transgenic together with age-matched littermate mice was determined by flow cytometry. Data shown are gated on CD3+ (1-month-old), CD8+, or CD4+ (5-month-old). The percentages of CD44hi and CD62Llo cells are indicated. Results are one representative of at least 3 independent experiments. *P < .05, **P < .001.
CD137 stimulation selectively stimulates proliferation of memory T cells. B6 mice were injected with 100 μg control rat IgG or 2A on day 0 and 2 and fed with drinking water with 0.8 mg/mL BrdU from day 3 to 7. Splenocytes (A) or intrahepatic lymphocytes (B) were harvested on day 7 and stained for CD8, CD4, CD44, and BrdU by flow cytometry. Data were presented by gating on CD8 or CD4. Total numbers of CD44hi or CD122+ cells in CD8+ or CD4+ T-cell subsets in spleens (C) are shown on days 5 and 8, respectively, after antibody treatment. Error bars indicate SD. The number of total intrahepatic lymphocytes as well as CD4+ and CD8+ subsets on day 7 are also shown (D). The results are from one representative of 3 independent experiments with similar results. (E) Accumulation of memory phenotype T cells in CD137L transgenic mice. The phenotype of spleen or LN T cells from 1-month-old and 5-month-old CD137L transgenic together with age-matched littermate mice was determined by flow cytometry. Data shown are gated on CD3+ (1-month-old), CD8+, or CD4+ (5-month-old). The percentages of CD44hi and CD62Llo cells are indicated. Results are one representative of at least 3 independent experiments. *P < .05, **P < .001.
Accumulation of T cells with memory phenotype in CD137L transgenic mice
An important issue is whether natural ligand of CD137 (CD137L) could also promote memory T-cell proliferation as well as agonist mAb. We previously described the generation of CD137L transgenic mice under the control of MHC class II Eα promoter, which allows the expression of CD137L exclusively on antigen-presenting cells (APCs).22 The transgenic mice behaved normally when they were young; however, the older mice showed massive splenomegaly and selective depletion of B220+ B cells resulted in low levels of circulating IgG and defective humoral responses to antigen challenge.22 Here we found that both LNs and spleens of the transgenic mice had an accumulation of T cells with effector/memory phenotype (CD44hi CD62Llo) at approximately 1 month of age (Figure 1E). T cells from CD137L transgenic mice did not show any increasing expression of T-cell activation markers, such as CD69 and CD25 (data not shown). The accumulation of effector/memory T cells in spleens and LNs increased when mice aged. As shown in Figure 1E in 5-month-old mice, the majority of splenic CD4+ and CD8+ were CD44hiCD62lo, resulting in a nearly complete diminishment of the naive T-cell population in aged CD137L transgenic mice. These data further support that, similar to agonist CD137 mAb, overexpression of CD137L is capable of increasing the size of memory T-cell pool.
Generation and characterization of CD137 KO mice
To further dissect the role of CD137 in the stimulation of memory T cells, we generated CD137 KO mice by homologous recombination in 129 ES cells. Our gene-targeting vector replaced exon 1-6 in the endogenous CD137 allele with a Neo-resistance cassette, thereby deleting the sequences encoding the signal peptide, the entire extracellular and transmembrane region of CD137 (Figure 2A). Mice were bred to C57BL/6 background and further backcrossed for at least 5 generations before use in this study. Experiments involving lymphocyte transferring were particularly done by using CD137 KO mice backcrossed for more than 10 generations. Southern blot analysis demonstrated the deletion of gDNA of CD137 (Figure 2B). To confirm the absence of the CD137 protein in these mice, Con A-activated spleen cells from CD137 KO mice were stained for CD137. As expected, CD137 was detected in WT but not KO cells, whereas these cells expressed normal level of PD-1 (Figure 2C), a T-cell activation marker.29
Generation and characterization of CD137 KO mice. (A) The targeting map of the CD137 genomic locus. The signal peptide with the ATG starting code and first 6 exons encoding extracellular and transmembrane regions of murine CD137 were replaced with the Neo cassette. A short open bar labeled “Probe” indicates the position of the 3′ end probe for screening of ES cells, and PCR indicates the position of PCR products in screening of CD137-deficient mice using primers. Shaded boxes represent exons within the murine CD137 open reading frame. (B) Southern blotting of heterozygous and homozygous CD137 mutants in the gDNA from targeted ES cells. The upper band (6691 bp) shows the targeted fragment, and the lower one (6147 bp) represents the one from the normal genome. (C) Splenocytes from WT B6 or CD137 KO mice were activated by Con A for 24 hours, and live cells were stained for CD137 or PD-1 gated on CD3+ cells by specific mAbs and subsequently analyzed by flow cytometry. Shaded histograms indicate isotype control; open histograms, anti-CD137 or anti-PD1. (D) Whole splenocytes or purified T cells from CD137KO or WT control mice and were activated by Con A or plate-bound CD3 mAb at the indicated concentrations. [3H]TdR was included in the cultures 16 hours before harvesting. The results are from one representative of 2 independent experiments with similar results. (E) Splenocytes from untreated WT or CD137 KO mice were stained for CD44 and CD62L, gated on CD8+ or CD4+ cells, respectively. The results are from one representative of 2 independent experiments with similar results.
Generation and characterization of CD137 KO mice. (A) The targeting map of the CD137 genomic locus. The signal peptide with the ATG starting code and first 6 exons encoding extracellular and transmembrane regions of murine CD137 were replaced with the Neo cassette. A short open bar labeled “Probe” indicates the position of the 3′ end probe for screening of ES cells, and PCR indicates the position of PCR products in screening of CD137-deficient mice using primers. Shaded boxes represent exons within the murine CD137 open reading frame. (B) Southern blotting of heterozygous and homozygous CD137 mutants in the gDNA from targeted ES cells. The upper band (6691 bp) shows the targeted fragment, and the lower one (6147 bp) represents the one from the normal genome. (C) Splenocytes from WT B6 or CD137 KO mice were activated by Con A for 24 hours, and live cells were stained for CD137 or PD-1 gated on CD3+ cells by specific mAbs and subsequently analyzed by flow cytometry. Shaded histograms indicate isotype control; open histograms, anti-CD137 or anti-PD1. (D) Whole splenocytes or purified T cells from CD137KO or WT control mice and were activated by Con A or plate-bound CD3 mAb at the indicated concentrations. [3H]TdR was included in the cultures 16 hours before harvesting. The results are from one representative of 2 independent experiments with similar results. (E) Splenocytes from untreated WT or CD137 KO mice were stained for CD44 and CD62L, gated on CD8+ or CD4+ cells, respectively. The results are from one representative of 2 independent experiments with similar results.
CD137 KO mice displayed normal numbers and ratios of CD4+CD8+ double-positive, and CD4+ and CD8+ single-positive T cells in the thymus. T-cell populations in the spleens and LNs also appeared in normal numbers. These results are consistent with a previously published report,30 indicating that CD137 signal does not affect T-cell development (data not shown). Activation of T cells from the KO mice by Con A or anti-CD3 mAb did not show any significant altered proliferation (Figure 2D). There was also no evidence of defect in endogenous memory T cells, judged by normal levels of CD62L and CD44 expression on CD4+ and CD8+ T cells in the KO mice (Figure 2E). Our result indicates that CD137 deficiency does not affect development and polyclonal activation of T cells.
CD137 on T cells is essential and sufficient for CD137 mAb-induced memory T-cell proliferation
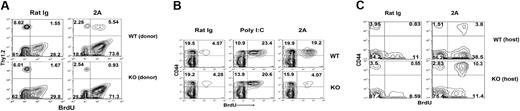
To determine the role of T cell-associated CD137, we transferred purified T cells from CD137 KO mice (Thy1.2) into congenic Thy1.1 B6 mice and subsequently treated the mice with CD137 mAb. Mice were fed with BrdU as described. In this system, donor T cells could be specifically traced by anti-Thy1.2 mAb. As expected, CD137 mAb treatment induced significant proliferation of WT donor Thy1.2+CD8+CD44hi cells (from 1.55% to 5.54%; Figure 3A). As an internal control, recipient CD8+CD44hi cells also proliferated significantly (from 28.2% to 73.6%). In sharp contrast, the effect of CD137 mAb in the expansion of CD137 KO donor memory T cells was completely eliminated (from 1.67% to 0.93%), whereas recipient CD8+CD44hi cells proliferated normally (Figure 3A). The failure of CD137 KO memory T cells to respond to CD137 mAb was not caused by their intrinsic defect because CD137KO memory T cells responded normally to poly I:C (Figure 3B), a potent inducer of IL-15 growth factor for CD8+ memory T cells.31 Although injection of CD137 mAb into WT mice induced vigorous proliferation of CD44hi memory T cells in comparison with that treated with control mAb (rat immunoglobulin; 4.57% versus 19.2%), similar treatment did not have effect in CD137KO mice (4.28% versus 4.07%; Figure 3B). Our results thus support that T cell-associated CD137 is essential for the effect of CD137 mAb on memory T-cell expansion.
CD137 on T cells is essential and sufficient for the anti-CD137 mAb effect. (A) Purified T cells from WT or CD137KO mice were adoptively transferred into WT B6/Thy1.1 mice. Mice treated with rat IgG or 2A (shown) were fed with BrdU as described. The data are gated on spleen CD8+ CD44hi cells and show the BrdU incorporation from host versus donor origin distinguished by Thy1.2 staining. The results are from one representative of 2 independent experiments with 3 mice each. (B) WT B6 or CD137KO mice injected with PBS, poly I:C or 2A on day 0 were fed with BrdU. Spleen cells were harvested and stained for CD8, CD44, and anti-BrdU. Data shown were gated on CD8+ cells. The results are from one representative of 2 independent experiments with 3 mice. (C) Purified T cells from WT B6/Thy1.1 mice were transferred into WT or CD137KO mice. Mice were treated as described in panel A. The data are gated on spleen CD8+ CD44hi cells and show the BrdU incorporation from host versus donor origin distinguished by Thy1.1 staining.
CD137 on T cells is essential and sufficient for the anti-CD137 mAb effect. (A) Purified T cells from WT or CD137KO mice were adoptively transferred into WT B6/Thy1.1 mice. Mice treated with rat IgG or 2A (shown) were fed with BrdU as described. The data are gated on spleen CD8+ CD44hi cells and show the BrdU incorporation from host versus donor origin distinguished by Thy1.2 staining. The results are from one representative of 2 independent experiments with 3 mice each. (B) WT B6 or CD137KO mice injected with PBS, poly I:C or 2A on day 0 were fed with BrdU. Spleen cells were harvested and stained for CD8, CD44, and anti-BrdU. Data shown were gated on CD8+ cells. The results are from one representative of 2 independent experiments with 3 mice. (C) Purified T cells from WT B6/Thy1.1 mice were transferred into WT or CD137KO mice. Mice were treated as described in panel A. The data are gated on spleen CD8+ CD44hi cells and show the BrdU incorporation from host versus donor origin distinguished by Thy1.1 staining.
To evaluate the effect of CD137 on non-T cells, we tested whether CD137 on T cells is sufficient for CD137 mAb-triggered memory T-cell proliferation. Purified WT Thy1.1+ CD8+ T cells were transferred into CD137 KO mice; mice were treated with CD137 mAb or control antibody and subsequently fed with BrdU. In this system, only transferred T cells can express CD137. WT mice transferred with Thy1.1+ T cells were used as a positive control. As shown on Figure 3C, WT CD44hi T cells transferred into CD137 KO mice still can respond to CD137 mAb greatly and proliferate vigorously as well as those transferred into WT mice. It suggested that CD137 on non-T cells is not required for the effect of CD137 mAb on memory T cells. Taken together, our data suggested that CD137 on T cells is essential and sufficient for CD137 mAb-induced memory T-cell proliferation.
CD137 signal alone promotes the proliferation of memory T cells, but not naive T cells
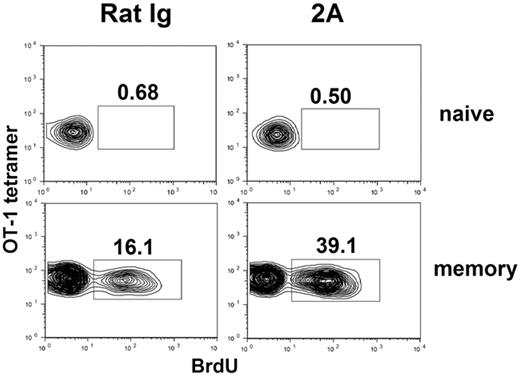
Although our experiments support that CD44hi memory-like T cells selectively expand on CD137 mAb stimulation, it is still possible that increased CD44hi may represent an acquired phenotype of naive T cells on CD137 mAb treatment. To exclude this possibility, we used an adoptive transfer system, in which naive CD8+ OT-1 TCR transgenic T cells were transferred into naive B6 mice and monitored by specific tetramer for their responses to CD137 mAb. OT-1 transgenic mice are in RAG-1 KO background to guarantee that all T cells are naive (CD44lo and CD122−). As shown in Figure 4 (upper panel), CD137 mAb treatment did not increase the incorporation of BrdU into transferred OT-1 cells as detected by OT-1 tetramer. As an internal positive control, we did observe a significant increase of BrdU incorporation into OT-1 tetramer-negative cells, presumably due to expansion of memory T cells of recipient origin (data not shown). Our results thus indicate that naive T cells do not respond to CD137 mAb stimulation in vivo.
CD137 stimulation induces proliferation of memory but not naive T cells. In vitro activated OT-1 cells were adoptively transferred into naive B6 mice and rested for more than 40 days to generate memory OT-1 cells. B6 mice containing naive (upper panels) or memory OT-1 × RAG1KO TCR transgenic T cells (lower panels) were subsequently treated with rat IgG or 2A. The mice were fed with BrdU-containing drinking water for 5 days. Spleen cells were harvested and stained for CD8, OT-1 tetramer, and anti-BrdU. Data shown were gated on CD8+ cells. The results are from one representative of 2 independent experiments with similar results.
CD137 stimulation induces proliferation of memory but not naive T cells. In vitro activated OT-1 cells were adoptively transferred into naive B6 mice and rested for more than 40 days to generate memory OT-1 cells. B6 mice containing naive (upper panels) or memory OT-1 × RAG1KO TCR transgenic T cells (lower panels) were subsequently treated with rat IgG or 2A. The mice were fed with BrdU-containing drinking water for 5 days. Spleen cells were harvested and stained for CD8, OT-1 tetramer, and anti-BrdU. Data shown were gated on CD8+ cells. The results are from one representative of 2 independent experiments with similar results.
To extend our finding to bona fide memory T cells, OT-1 T cells were activated in vitro and subsequently transferred into naive B6 mice to rest more than 40 days. This method allows the generation of memory T cells from effector T cells, a normal process of memory T-cell development.24 Flow cytometry analysis using OT-1 tetramer shows that all OT-1 cells in spleen express high levels of CD44 and more than 80% of these cells are also CD62L+, suggesting they are mainly central memory T cells (data not shown). CD137 mAb treatment induced a significantly higher level of cell division in OT-1 memory cells than that by control mAb (39.1% versus 16.1%; Figure 4 lower panel). As expected, CD137 mAb had no effect on division of naive OT-1 cells. Collectively, our results provide direct evidence that CD137 engagement selectively triggers memory but not naive T-cell proliferation in vivo.
CD137 stimulation promotes the growth of homeostatic proliferation-induced memory T cells
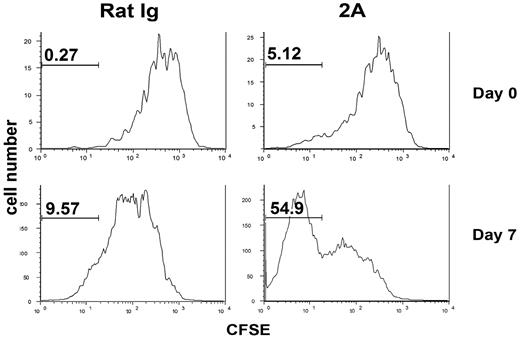
Naive T cells, on transfer into lymphopenic mice, will increase division and acquire memory T-cell phenotype, a phenomenon called homeostatic proliferation.32–35 To test whether CD137 stimulation also promotes this process, CFSE-labeled OT-1 x RAG-1 KO T cells were transferred into sublethally irradiated B6 mice and treated with CD137 mAb in the same day. On day 6 after treatment, spleen cells were harvested and CFSE dilution/cell division was examined by flow cytometry. The majority of transferred OT-1 cells had divided more than one cycle and the pattern of cell division between CD137 mAb-treated and control group was overall similar (Figure 5 upper panel). However, CD137 mAb-treated mice consistently contained a small population of OT-1 cells that underwent more than 5 divisions (Figure 5 upper panel). Consistent with previously published data,32,33 we demonstrated that, during T-cell homeostasis, the levels of memory T-cell markers (CD44 and CD122) increased progressively over cell division; only those cells experiencing more than 5 cell divisions acquired clear memory T-cell markers (CD44hiCD122+; data not shown). It is thus possible that CD137 mAb might be effective only on those T cells that have acquired memory phenotype. Based on this observation, we delayed the treatment by CD137 mAb starting at day 7 after OT-1 transfer and examined cell division 6 day later. Interestingly, more than 50% of transferred OT-1 cells underwent more than 7 divisions with CD137 mAb treatment, resulting in complete loss of CFSE staining. In contrast, only about 10% of OT-1 T cells had divided more than 7 divisions in control mice (Figure 5 lower panel). Taken together, our results suggest that CD137 mAb promotes proliferation of T cells that acquire memory phenotype during homeostatic proliferation.
Effects of CD137 mAb on naive T-cell homeostasis in lymphopenic mice. CFSE-labeled naive OT-1 × RAG1 KO T cells (1 × 106) were adoptively transferred into sublethally irradiated B6 mice. Then, 100 μg rat IgG or 2A was injected intraperitoneally on day 0 (upper panels) or day 7 (lower panels) after cell transfer. Spleen cells were prepared at day 6 after antibody treatment and analyzed by flow cytometry. Histogram plots of CFSE intensity of transferred OT-1 cells (gated on CD8+ OT-1 tetramer-positive cells) in the spleen are shown. The results are from one representative of 3 independent experiments with similar results.
Effects of CD137 mAb on naive T-cell homeostasis in lymphopenic mice. CFSE-labeled naive OT-1 × RAG1 KO T cells (1 × 106) were adoptively transferred into sublethally irradiated B6 mice. Then, 100 μg rat IgG or 2A was injected intraperitoneally on day 0 (upper panels) or day 7 (lower panels) after cell transfer. Spleen cells were prepared at day 6 after antibody treatment and analyzed by flow cytometry. Histogram plots of CFSE intensity of transferred OT-1 cells (gated on CD8+ OT-1 tetramer-positive cells) in the spleen are shown. The results are from one representative of 3 independent experiments with similar results.
Effect of CD137 stimulation on memory T-cell proliferation is MHC independent
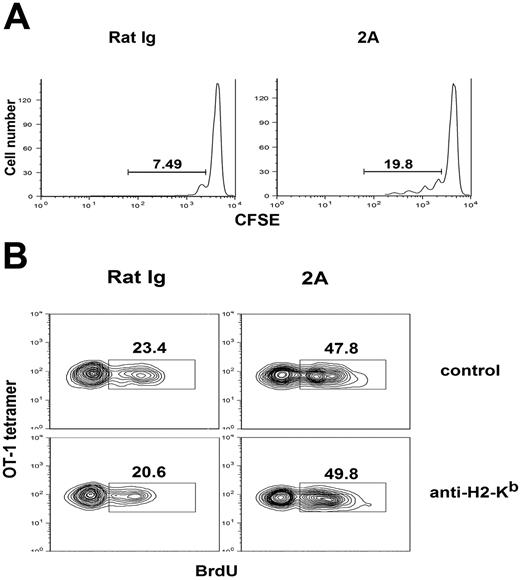
CD137 mAb could drive proliferation of memory OT-1 T cells without supply of OVA antigen (Figure 4). Although these data could be interpreted that CD137-triggered proliferation of memory T cells is independent of antigen, an alternative interpretation is that CD137 mAb-induced proliferation of memory T cells is still dependent on the interaction between TCR and MHC/self-antigen, which could cross-react with OT-1 TCR. To exclude this, we tested the effect of CD137 mAb in H-2Kb KO mice after transfer of memory OT-1 T cells, which is H-2Kb-restricted. As previously shown, we first generated memory T cells by transfer of in vitro fully activated OT-1 cells into naive B6 mice to rest more than 40 days to generate memory T cells. CD8+ T cells were purified (> 95%) by negative selection using magnetic-activated cell sorting (MACS) beads, CFSE-labeled and adoptively transferred into H-2Kb KO mice, which was followed with CD137 mAb or control mAb treatment. Cell division of OT-1 memory cells was traced on day 7 by triple staining of CD8, OT-1 tetramer, and CFSE. Memory OT-1 cells in CD137 mAb-treated mice have significantly more cell divisions than seen in control mAb (19.8% versus 7.49%), as shown by the dilution of the CFSE intensity (Figure 6A). This result suggests that the effect of CD137 mAb does not require the recognition of MHC recognition. To further validate this finding, we tested the effect of an H-2Kb-blocking mAb (clone AF6.88.5) in CD137 mAb-induced proliferation of memory T cells. This H-2Kb-specific mAb could efficiently inhibit naive OT-1 T-cell homeostasis in lymphopenic B6 mice (data not shown), which is believed to be a self MHC-dependent process.35 The treatment by CD137 mAb of B6 mice, which were transferred with naive OT-1 cells, immunized with OVA antigen, and rested for 50 days, led to clearly increased BrdU incorporation of memory OT-1 cells, regardless of the presence of H-2Kb-blocking mAb (Figure 6B). Taken together, our results indicate that CD137 stimulation triggers memory T-cell division in a self MHC-independent fashion, and the interaction between TCR and MHC is not required for CD137-induced proliferation of memory T cells.
Memory T-cell proliferation induced by CD137 stimulation is antigen and MHC independent. (A) Memory OT-1 cells generated as described Figure 4 were labeled with CFSE and adoptively transferred into H-2Kb KO mice and subsequently treated with CD137 mAb or control antibody on day 1 and 3. On day 7, spleen cells were harvested and stained for CD8, OT-1 tetramer. CFSE intensity of transferred memory OT-1 cells was shown by flow cytometry gated on CD8 and OT-1 tetramer-positive cells. (B) B6 mice transferred with naive OT-1 T cells were preimmunized with OVA plus poly I:C to generate memory OT-1 cells. Fifty days later, mice were injected with anti–H-2Kb blocking mAb on day −1 and 2. On day 0 and day 2, mice were treated with CD137 mAb or control mAb, respectively, and fed with PBS containing BrdU as shown previously. Spleen cells were prepared on day 7 after treatment and analyzed by flow cytometry. Histogram plots of CFSE intensity of transferred OT-1 cells (gated on CD8+ OT-1 tetramer-positive cells) in the spleen are shown. The results are from one representative of 2 independent experiments with 3 mice in each group.
Memory T-cell proliferation induced by CD137 stimulation is antigen and MHC independent. (A) Memory OT-1 cells generated as described Figure 4 were labeled with CFSE and adoptively transferred into H-2Kb KO mice and subsequently treated with CD137 mAb or control antibody on day 1 and 3. On day 7, spleen cells were harvested and stained for CD8, OT-1 tetramer. CFSE intensity of transferred memory OT-1 cells was shown by flow cytometry gated on CD8 and OT-1 tetramer-positive cells. (B) B6 mice transferred with naive OT-1 T cells were preimmunized with OVA plus poly I:C to generate memory OT-1 cells. Fifty days later, mice were injected with anti–H-2Kb blocking mAb on day −1 and 2. On day 0 and day 2, mice were treated with CD137 mAb or control mAb, respectively, and fed with PBS containing BrdU as shown previously. Spleen cells were prepared on day 7 after treatment and analyzed by flow cytometry. Histogram plots of CFSE intensity of transferred OT-1 cells (gated on CD8+ OT-1 tetramer-positive cells) in the spleen are shown. The results are from one representative of 2 independent experiments with 3 mice in each group.
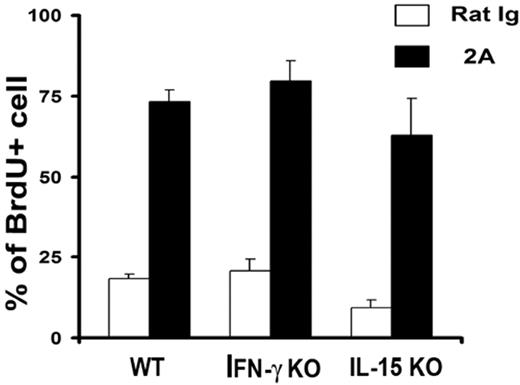
Effect of CD137 stimulation on memory T-cell proliferation does not require IL-15 and IFN-γ
IL-15 is a critical cytokine for the proliferation of CD8+ memory T cells.3 It is thus possible that the effect of CD137 mAb is mediated by the production of IL-15. In addition, CD137 mAb was found to induce IFN-γ secretion on engagement of TCR and the majority of CD137 mAb effects on T-cell responses are dependent on IFN-γ. To exclude these possibilities, IL-15 KO and IFN-γ KO mice were treated with CD137 mAb and subsequently fed with BrdU as shown previously. Six days after treatment, CD8+CD44hi cells were gated and BrdU+ cells were calculated. As shown in Figure 7, CD137 mAb stimulated memory T-cell proliferation in comparable levels to that of control mAb in both IL-15 and IFN-γ KO mice. Our results indicate that both IL-15 and IFN-γ are not required for CD137 mAb-triggered memory T-cell proliferation.
IL-15 and IFN-γ are not required for CD137-induced memory T-cell proliferation. IL-15 KO and IFN-γ KO mice were treated with the indicated mAb and fed with BrdU as shown in Figure 1. Data shown are percentages of BrdU+ cells gated on the CD8+CD44hi portion. The results are from one representative of 2 independent experiments with similar results. Error bars indicate SD. No significant difference was found among groups (P > .05).
IL-15 and IFN-γ are not required for CD137-induced memory T-cell proliferation. IL-15 KO and IFN-γ KO mice were treated with the indicated mAb and fed with BrdU as shown in Figure 1. Data shown are percentages of BrdU+ cells gated on the CD8+CD44hi portion. The results are from one representative of 2 independent experiments with similar results. Error bars indicate SD. No significant difference was found among groups (P > .05).
Discussion
CD137 is an important costimulatory molecule in the development of optimal effector T-cell immunity and the regulation of T-cell tolerance, which are explicitly dependent on simultaneous engagement of TCR. Our results demonstrate for the first time that antigen-MHC signal is not required for the effect of CD137 signal in the growth of T cells with memory phenotype. Therefore, selective triggering of CD137 signal to expand memory T cells may be used as a means to enhance memory T cell-mediated immunity.
It is well-established that CD137 engagement provides a strong costimulatory signal in the context of antigen-MHC for recently primed T cells and plays a critical role in the regulation of tolerance and the development of optimal effector T-cell immunity.8 These effects of CD137, however, are explicitly dependent on prior or simultaneous engagement of TCR signal.9 Our data indicate that agonist CD137 mAb, a mAb previously shown to be costimulatory for primed T cells,12 does not activate naive T cells when administered into naive mice. In sharp contrast, T cells with memory phenotype in naive mice proliferate vigorously. This effect is not restricted to a certain clone of CD137 mAb, because we found that, 3H3, another commonly used CD137 agonistic mAb, has similar effect on memory T cells (data not shown). Consistent with these findings, CD137L transgenic mice also exhibited an accumulation of memory-phenotype T cells in lymphoid organs (Figure 1E). Our CD137 mAb can promote proliferation of memory OT-1 T cells, which are specific for known nonself antigen in an antigen-free environment. In addition, blockade of specific MHC class I does not change this effect. Consistent with these findings, naive T cells that undergo homeostatic proliferation and acquire memory phenotype also respond to CD137 signal. All together, our findings support that CD137 signal provides a unique growth signal for memory T cells.
Expression of CD137 could be detected in non-T hematopoietic cells as well as parenchyma cells and diverse functions of CD137 on these cells have been implicated.9 It is thus possible that CD137 signaling on other cells may contribute to memory T-cell growth. By transfer of CD137KO T cells, the effect of CD137 in the proliferation of CD44hi cells was completely eliminated. This effect is not caused by intrinsic defect of memory T cells because CD137KO memory T cells respond normally to poly I:C-induced inflammation. Furthermore, proliferation of memory T cells in CD137KO mice was in the normal range (Figure 3B). Our results thus support that the effect of CD137 agonistic mAb is mediated through direct binding to memory T cells. Memory T cells express a minimal level of CD137 on the cell surface. However, this expression could be up-regulated after injection of CD137 mAb (Y.Z, unpublished data, February 2006). Because all effects of CD137 mAb could be achieved by a single injection, it is possible that the expression of CD137 on memory T cells is induced by CD137 mAb in vivo during the first few days and further engagement of the induced CD137 executes the effect. Recently it has been shown that homeostatic proliferation in lymphopenic hosts triggers naive T cells to acquire the phenotypic and functional properties of memory cells without transition through the effector cell stage.32–34 Consistent with this observation, we also found that small numbers of naive OT-1 cells, which were transferred into sublethally irradiated mice, increased CD44 and CD122 but not CD25 and CD69 expression (data not shown). In our experiments, injection of CD137 mAb had only a minimal effect on division of naive T cells. However, on day 7 as the majority of transferred T cells divided and acquired phenotypes of memory T cells, CD137 mAb treatment clearly enhanced the T-cell division. It appears that CD8+ T cells respond to CD137 mAb better than CD4+ T cells (Figure 1). The underlying mechanism for this difference is unknown. Because both CD4+ and CD8+ memory T cells do not constitutively express CD137, this may reflect a distinct intrinsic program between these cell subsets. Our findings support that T cells with memory phenotype either initiated from antigen stimulation or derived from homeostatic proliferation share a common pathway to respond to CD137 stimulation for growth.
During preparation of this manuscript, a study was published supporting a role of endogenous CD137 signaling in memory T-cell survival.21 When in vitro-generated memory OT-1 T cells were transferred into WT or CD137L KO mice, the lack of CD137L resulted in a 2- to 3-fold decrease of memory cell recovery by 3 weeks after transfer. The division rate of transferred cells was similar in both WT and CD137L KO hosts, indicating that endogenous CD137-CD137L interaction may be important for survival of CD8+ memory T cells.21 In our system, we did not observe significant changes in T-cell survival up to 7 days during CD137 mAb treatment (data not shown). It is possible that CD137 signaling by our mAb provides a strong proliferating signal, whereas weak CD137 signaling delivered by natural CD137-CD137L interaction supports survival. In the bone marrow of naive CD137L KO mice, there was significantly lower frequency of CD8+ memory phenotype cells.21 However, we did not observe any significant defect in endogenous memory T cells in the spleen from CD137 KO mice (Figure 2E), which is consistent with reported phenotypes in CD137L KO mice.21 The accumulation of memory T cells in our CD137L transgenic mice suggests that, similar to CD137 mAb, overexpression of CD137L could also promote memory T-cell proliferation (Figure 1E). In this regard, it is interesting to see that the majority of described effects by CD137 mAb could be reproduced by CD137 ligand, though at a lower level. These effects include promotion of tumor rejection, graft-versus-host disease, graft-versus-leukemia effect, and antiviral effect.36–39
The mechanism of CD137 stimulation on growth of memory T cells is yet to be elucidated. By transfer of CD137-deficient T cells into WT mice, we demonstrate that the lack of CD137 on T cells completely eliminates the effect of CD137 mAb, supporting a direct role of CD137 on memory T cells. It has been shown that CD137 receptor engagement can transmit signals through TNF receptor-associated factor (TRAF) 1, 2 and 39,40 and p38 mitogen-activated protein kinase25 in the presence of TCR signal. It is unclear, however, whether or not these signaling pathways are independent of or interconnected to TCR signal. Our findings may provide a unique opportunity to examine CD137 signal without interference by TCR signaling. IL-15, a cytokine critical for the proliferation of memory T cells, does not seem to be responsible for the effect because comparable memory T-cell proliferation to CD137 mAb treatment was also observed in IL-15 KO mice (Figure 7). Since our CD137 mAb can promote the proliferation of CD4+ memory T cells, on which IL-15 has no effect, it is not surprising to see that IL-15 is not involved in the effect of CD137 mAb on memory T cells. Our results thus suggest that the expression of CD137 on memory T cells in vivo is IL-15 independent. In contrast, Pulle et al suggested that IL-15 is involved in the effect of CD137 on memory T cells, all based on the observations that in vitro IL-15 can induce CD137 preferentially on CD8+ memory phenotype T cells.21 There is no evidence showing that IL-15 is the only cytokine or factor capable of inducing CD137 expression in vivo. Therefore, it is possible that the effect of IL-15 on memory T cells can be enhanced by inducing CD137 expression; however, the relative importance of IL-15 to the effect of the CD137 pathway on memory T-cell maintenance is yet to be determined.
Taken together, our data indicate a new method distinct from antigen and IL-15 to stimulate growth of memory T cells. Our findings may have important implications in development of new approaches to treat diseases with memory T-cell deficiency such as in chronic viral infection and some human malignancies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants CA106861 and CA85721.
We thank Jennifer Osborne for manuscript editing.
National Institutes of Health
Authorship
Conflict-of-interest disclosure: The authors declare that they have no competing financial interests.
Y.Z. and G.Z. contributed equally to this work.
Correspondence: Lieping Chen, Johns Hopkins Medicine, 1550 Orleans St, 209 David H. Koch Cancer Research Bldg, Baltimore, MD 21231; e-mail: lchen42@jhmi.edu.

![Figure 2. Generation and characterization of CD137 KO mice. (A) The targeting map of the CD137 genomic locus. The signal peptide with the ATG starting code and first 6 exons encoding extracellular and transmembrane regions of murine CD137 were replaced with the Neo cassette. A short open bar labeled “Probe” indicates the position of the 3′ end probe for screening of ES cells, and PCR indicates the position of PCR products in screening of CD137-deficient mice using primers. Shaded boxes represent exons within the murine CD137 open reading frame. (B) Southern blotting of heterozygous and homozygous CD137 mutants in the gDNA from targeted ES cells. The upper band (6691 bp) shows the targeted fragment, and the lower one (6147 bp) represents the one from the normal genome. (C) Splenocytes from WT B6 or CD137 KO mice were activated by Con A for 24 hours, and live cells were stained for CD137 or PD-1 gated on CD3+ cells by specific mAbs and subsequently analyzed by flow cytometry. Shaded histograms indicate isotype control; open histograms, anti-CD137 or anti-PD1. (D) Whole splenocytes or purified T cells from CD137KO or WT control mice and were activated by Con A or plate-bound CD3 mAb at the indicated concentrations. [3H]TdR was included in the cultures 16 hours before harvesting. The results are from one representative of 2 independent experiments with similar results. (E) Splenocytes from untreated WT or CD137 KO mice were stained for CD44 and CD62L, gated on CD8+ or CD4+ cells, respectively. The results are from one representative of 2 independent experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/11/10.1182_blood-2006-10-043463/4/m_zh80110704310002.jpeg?Expires=1767699861&Signature=Pns8etI9uKlXJAHOT2fWTBVYaB5sIg760gL4kfgtSWzIUyxEAlDa2j22qC7pzMSnyt--Q3uDt8pZYW2bqpUI-xau-wbajYdufRQBLNk85J6BP-822SFzCe-Y9LQD~7Lg6dHINKtoBuYEQkfAl7LxBhYejWwD1P1NCaRpBA~AKTnVb6pj8LldtjXHDWCLzZMdXwZrNvUNOffc54WpiX6uHPh9UALj1n7U1swhS3dnNLnbAKbTl0s-KaqndiBwnEt-Kn~zP-OycdWWihn3BjH4rC7nAVKHxx1ONgF6vSkS14qNvGOjG2Q6hxrErMH4UhvMMbDt72o~7sKX6qrtF0~eYQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal