To the editor:
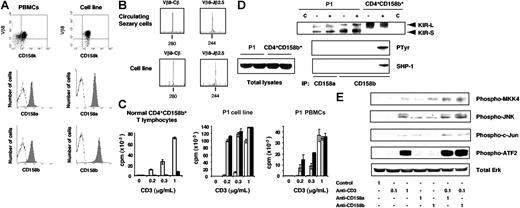
Sézary syndrome (SS) is an aggressive leukemic and erythrodermic variant of cutaneous T-cell lymphomas characterized by the presence of a clonal T-lymphocyte population in the skin, lymph nodes, and peripheral blood.1 We recently identified KIR3DL2/CD158k as the first cell-specific marker for the evaluation of the circulating tumoral burden and for the follow-up of patients with SS.2–4 We next investigated the expression of additional killer cell Ig-like receptors (KIRs) on the peripheral blood mononuclear cells (PBMCs) of patients with SS and detected the simultaneous expression of CD158a and CD158b on all malignant cells from a unique patient (P1; Figure 1A, left panel).
Expression and functional characterization of CD158a and CD158b on P1 malignant CD4+ T cells and an in vitro–derived T-cell clone. (A) PBMCs and the P1 cell line were subjected to a double immunostaining using a PE-conjugated anti–TCR-Vβ8 mAb, and an anti-CD158k mAb plus FITC-conjugated goat anti–mouse IgM antibodies (top panel). Alternatively, cells were stained with a PE-conjugated anti-CD158a (middle panel) or anti-CD158b (bottom panel) mAb. (B) The CDR3 size analysis of the TCR-Vβ8 transcript from P1 PBMCs (top panel) or derived cell line (bottom panel) was performed. Following total RNA extraction and reverse transcription, cDNA were amplified by PCR with Vβ8- and Cβ-specific primers. The unlabeled amplification products were elongated using a nested fluorescent Cβ and Jβ2.5 primers. The samples were subjected to electrophoresis and analyzed on an automated sequencer. (C) Expanded normal CD4+CD158b+ lymphocytes, the P1-derived cell line, or PBMCs were activated by incubation with an anti-CD3 mAb together with an isotype-matched anti-CD16 (white histogram), anti-CD158a (gray) or anti-CD158b (black) mAb. When necessary, the anti-CD16 mAb was used instead of the anti-CD3 mAb (concentration “0”) alone, or in combination with an anti-KIR mAb. Concentrations of the anti-CD3 mAb used are indicated. Results are expressed as the mean of triplicates ± SD. (D) Sorted and expanded CD4+CD158b+ T cells from a healthy donor, or the P1-derived cell line, were surface biotinylated and left untreated (−) or incubated in the presence of vanadate (+). An aliquot of each NP40 cell lysate, corresponding to 5 × 105 cell equivalent, was collected, and immunoprecipitations were performed on the remaining samples using an anti-CD16 (C), anti-CD158a, or anti-CD158b mAb. The immunoprecipitates were separated by SDS–8% PAGE and transferred onto a nitrocellulose membrane. The immunoprecipitated receptors were first revealed by incubation of the blot with streptavidin-peroxidase, and an ECL detection system (top panel). The position of the short (KIR-S) and long (KIR-L) isoforms is indicated. After a dehybridization step, the membrane was reprobed with the antiphosphotyrosine mAb 4G10 (middle panel), stripped, and incubated with the purified anti–SHP-1 polyclonal antibodies (bottom panel). Total cell lysates from control or activated cells were similarly probed with the anti–SHP-1 antibodies to ensure protein expression. (E) Cells were incubated in the presence of control murine IgG, anti-CD3 and/or anti-CD158a or anti-CD158b mAb. Following activation, cell lysates were prepared and subjected to gel electrophoresis. Immunoblotting was then performed sequencially using the indicated antiphosphoprotein antibodies. Equal protein loading was verified by detection of total Erk1/2. The concentrations of the antibodies used for cell activation are given in micrograms per milliliter.
Expression and functional characterization of CD158a and CD158b on P1 malignant CD4+ T cells and an in vitro–derived T-cell clone. (A) PBMCs and the P1 cell line were subjected to a double immunostaining using a PE-conjugated anti–TCR-Vβ8 mAb, and an anti-CD158k mAb plus FITC-conjugated goat anti–mouse IgM antibodies (top panel). Alternatively, cells were stained with a PE-conjugated anti-CD158a (middle panel) or anti-CD158b (bottom panel) mAb. (B) The CDR3 size analysis of the TCR-Vβ8 transcript from P1 PBMCs (top panel) or derived cell line (bottom panel) was performed. Following total RNA extraction and reverse transcription, cDNA were amplified by PCR with Vβ8- and Cβ-specific primers. The unlabeled amplification products were elongated using a nested fluorescent Cβ and Jβ2.5 primers. The samples were subjected to electrophoresis and analyzed on an automated sequencer. (C) Expanded normal CD4+CD158b+ lymphocytes, the P1-derived cell line, or PBMCs were activated by incubation with an anti-CD3 mAb together with an isotype-matched anti-CD16 (white histogram), anti-CD158a (gray) or anti-CD158b (black) mAb. When necessary, the anti-CD16 mAb was used instead of the anti-CD3 mAb (concentration “0”) alone, or in combination with an anti-KIR mAb. Concentrations of the anti-CD3 mAb used are indicated. Results are expressed as the mean of triplicates ± SD. (D) Sorted and expanded CD4+CD158b+ T cells from a healthy donor, or the P1-derived cell line, were surface biotinylated and left untreated (−) or incubated in the presence of vanadate (+). An aliquot of each NP40 cell lysate, corresponding to 5 × 105 cell equivalent, was collected, and immunoprecipitations were performed on the remaining samples using an anti-CD16 (C), anti-CD158a, or anti-CD158b mAb. The immunoprecipitates were separated by SDS–8% PAGE and transferred onto a nitrocellulose membrane. The immunoprecipitated receptors were first revealed by incubation of the blot with streptavidin-peroxidase, and an ECL detection system (top panel). The position of the short (KIR-S) and long (KIR-L) isoforms is indicated. After a dehybridization step, the membrane was reprobed with the antiphosphotyrosine mAb 4G10 (middle panel), stripped, and incubated with the purified anti–SHP-1 polyclonal antibodies (bottom panel). Total cell lysates from control or activated cells were similarly probed with the anti–SHP-1 antibodies to ensure protein expression. (E) Cells were incubated in the presence of control murine IgG, anti-CD3 and/or anti-CD158a or anti-CD158b mAb. Following activation, cell lysates were prepared and subjected to gel electrophoresis. Immunoblotting was then performed sequencially using the indicated antiphosphoprotein antibodies. Equal protein loading was verified by detection of total Erk1/2. The concentrations of the antibodies used for cell activation are given in micrograms per milliliter.
To study the relevance of CD158a and CD158b expression by P1 malignant cells, a long-term cell line was generated. The identity of the in vitro–derived clone with the circulating tumoral clone was assessed by characterizing the CDR3-size Vβ distribution and the T-cell receptor (TCR) Vβ/Jβ junction (Figure 1B). Cell immunolabeling indicated that the derived T-cell line corresponded to the major TCR-Vβ8+CD158k+ circulating clone, and similarly expressed CD158a and CD158b (Figure 1A, right panel).
The influence of KIRs on the proliferation of a control CD4+CD158b+ T-cell line and P1 cells was evaluated. On control T cells, CD158b engagement led to a dramatic inhibition of their CD3-induced proliferation. In contrast, coligation of CD3 and CD158a or CD158b on the P1 cell line or on PBMCs resulted in an increased proliferation when compared with CD3 triggering alone (Figure 1C).
Further expression analysis demonstrated that both CD158a and CD158b were expressed under their inhibitory (KIR-L) and activating (KIR-S) isoforms in P1 cells, while only CD158b KIR-L was found on normal CD4+CD158b+ T cells (Figure 1D). It has been established that inhibitory KIRs exerted their activity, when phosphorylated, through an interaction with a protein tyrosine phosphatase.5 We observed that CD158b became efficiently tyrosine-phosphorylated in activated normal CD4+CD158b+ T cells, and consequently interacted with SHP-1. In contrast, no tyrosine-phosphorylated CD158a or CD158b, nor coprecipitated SHP-1, were detected in activated patient cells (Figure 2D). Note that equal levels of each isoform were recovered regardless of the cell activation status (Figure 1D, top panel). Thus, no inhibitory signaling was apparently generated following CD158a or CD158b triggering of P1 cells.
Stimulatory receptors usually interact with adaptor molecules to promote the downstream recruitment of Syk family protein tyrosine kinases.5 However, expression of the regular adaptor proteins DAP10 and DAP12 was undetectable in P1 cells, and no ζ was found associated with CD158a or CD158b in activated Sézary cells (not shown). In CD4+ T cells, CD158j/KIR2DS2 was identified as a costimulatory molecule using the DAP12-independent JNK pathway.6 We observed that CD158a- or CD158b-mediated stimulation of P1 cells resulted in phosphorylation of MKK4 and JNK, and to a lesser extent, of c-Jun and ATF2 (Figure 1E). In addition, while a suboptimal CD3 activation of the cells did not lead to protein phosphorylation, the coengagement of CD158a or CD158b resulted in the detection, for all proteins tested, of phosphorylation levels equivalent to that reached upon optimal CD3-mediated activation (Figure 1E).
In conclusion, we showed that CD158a and CD158b could act as costimulatory receptors on Sézary cells through the recruitment of the DAP12-independent JNK pathway. The delivery of coactivation signals through both KIRs, specific for all HLA-C alleles, might therefore contribute to Sézary cell clonal outgrowth in vivo.
Authorship
Correspondence: Anne Marie-Cardine, INSERM U841 (Equipe 02), Faculté de Médecine, 8 rue du Général Sarrail, 94010 Créteil Cedex, France; e-mail: anne.marie-cardine@creteil.inserm.fr.
Contribution: A.M.-C., D.H., N.O., N.R., and S.LG. performed the experiments; A.M.-C., A.B., and M.B. designed the research and analyzed the data; A.M.-C. and A.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A.M.-C. and D.H. contributed equally to this work.
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), Paris XII University, Société Française de Dermatologie, Société de Recherche Dermatologique, and the Association pour la Recherche sur le Cancer (ARC) (M.B.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal