Abstract
Mesenchymal stem cells (MSCs) are widespread in adult organisms and may be involved in tissue maintenance and repair as well as in the regulation of hematopoiesis and immunologic responses. Thus, it is important to discover the factors controlling MSC renewal and differentiation. Here we report that adult MSCs express functional Toll-like receptors (TLRs), confirmed by the responses of MSCs to TLR ligands. Pam3Cys, a prototypic TLR-2 ligand, augmented interleukin-6 secretion by MSC, induced nuclear factor κ B (NF-κB) translocation, reduced MSC basal motility, and increased MSC proliferation. The hallmark of MSC function is the capacity to differentiate into several mesodermal lineages. We show herein that Pam3Cys inhibited MSC differentiation into osteogenic, adipogenic, and chondrogenic cells while sparing their immunosuppressive effect. Our study therefore shows that a TLR ligand can antagonize MSC differentiation triggered by exogenous mediators and consequently maintains the cells in an undifferentiated and proliferating state in vitro. Moreover, MSCs derived from myeloid factor 88 (MyD88)–deficient mice lacked the capacity to differentiate effectively into osteogenic and chondrogenic cells. It appears that TLRs and their ligands can serve as regulators of MSC proliferation and differentiation and might affect the maintenance of MSC multipotency.
Introduction
Mesenchymal stem cells (MSCs) comprise an adult population that resides in many organs and exhibits multiple functions and phenotypes upon in vitro culture; MSCs can be induced to differentiate into mesodermal cell lineages,1,2 support and regulate hematopoiesis,3-7 regulate the stem-cell niche,8-12 and may participate in the repair of tissue damage inflicted by normal wear and tear, injury, or disease.13-16 MSCs comprise 0.01% to 0.001% of the bone marrow (BM)–nucleated cells and are obtained by expansion of the BM, plastic-adherent cell fraction.1,17-21 Under certain physiologic or experimental conditions, MSCs can be induced to differentiate in vitro into cells of the mesodermal lineage, specifically to osteocytes, adipocytes, chondrocytes, myocytes, tenocytes, myocardiocytes, and hematopoietic supportive stroma.1,17,19,22 MSCs are an attractive cell-based therapy tool for developmental defects; degenerating diseases; and bone, cartilage, muscle, and other mesodermal tissue injuries.23-30
Toll-like receptors (TLRs) are a class of molecules first discovered to play a role in body development31 and later found to play a role in body maintenance.32-36 The TLR family has been shown to be of importance in the innate immune system for the recognition of pathogen-associated molecular patterns (PAMPs) by immune cells, initiating a primary response toward invading pathogens and recruitment of the adaptive immune response.32,37-49 TLRs can be activated not only by pathogen components, but also by mammalian endogenous molecules such as heat-shock proteins and extracellular matrix breakdown products.50-52
In the steady state, during the generation of immune cells, as well as under pathologic conditions, there are intimate interactions between lymphocyte populations and the organ stroma mesenchyme. These interactions regulate cell growth and differentiation and control cell functions. It is possible therefore that lymphocytes and the stromal mesenchyme share regulatory mechanisms. To test this possibility we aimed, in the present study, to examine the expression and possible regulatory functions of TLRs in mesenchymal cells.
We explored the expression of TLRs by MSCs, the response of MSCs to known TLR activators, and the ability of a TLR-2 ligand to regulate MSC proliferation and differentiation. We show here that cultured MSCs express TLR molecules 1 to 8, but not TLR-9. Activation of MSCs by TLR ligands induced interleukin-6 (IL-6) secretion and nuclear factor κ B (NF-κB) nuclear translocation. Pam3Cys, a prototypic ligand for TLR-2, induced proliferation of MSCs and regulated their differentiation. Relatively little is known about the signals that regulate MSC proliferation, differentiation, and development.53,54 Our findings suggest that TLR signaling may play a role in restraining MSC differentiation and thus promote MSC renewal.
Materials and methods
Mice
C57BL/6J were purchased from Harlan Olac. Myeloid factor 88 (MyD88)–knockout mice were provided by Prof S. Akira (Osaka University, Osaka, Japan).
Cell culture
MSCs were grown in murine MesenCult Basal Media supplemented with 20% murine mesenchymal supplement (StemCell Technologies, Vancouver, Canada), 60 μg/mL penicillin, and 100 μg/mL streptomycin. MSCs from passage 12 to passage 16 were used in all experiments described.
Reagents
Human heat shock protein 60 (HSP60) was prepared as described.55 Endotoxin contamination of the preparations was determined using the kinetic-turbidmetric LAL test, performed by Biological Industries (Beit Haemek, Israel). The endotoxin content was less than 0.0001 EU/μg protein, corresponding to less than 0.01 pg lipopolysaccharide (LPS) equivalents per microgram of recombinant HSP60. Escherichia coli O55:B5 LPS, peptidoglycan of Staphylococcus aureus, and polyinosinic-polycytidylic acid were purchased from Sigma (Rehovot, Israel). Pam3Cys was purchased from EMC microcollections (Tübingen, Germany). Salmonella muenchen flagellin was purchased from Calbiochem (Darmstadt, Germany). CpG and GpC—the phosphorothioaete oligonucleotides—were synthesized at the Oligonucleotide Synthesis Unit of the Weizmann Institute of Science (Rehovot, Israel). The oligonucleotide CpG contains 2 9-mer segments. The control oligonucleotide GpC displays the same nucleotides with an inverted motif: oligonucleotide CpG, 5′-TCCATAACGTTGCAAACGTTCTG-3′; and oligonucleotide GpC, 5′-TCCATAAGCTTGCAAAGCTTCTG-3′.
Imiquimod (R837) and ssRNA40 were purchased from InvivoGen (San Diego, CA). The MOG p35-55 peptide sequence is MEVGWYRSPFSROVHLYRNGK.
The peptide used was synthesized using the F-MOC technique with an automatic multiple peptide synthesizer (AMS 422; ABIMED, Langenfeld, Germany). The purity of the peptides was analyzed by high-performance liquid chromatography (HPLC). For antibodies, polyclonal rabbit anti-ERK 1/2 was purchased from Sigma (Rehovot, Israel), polyclonal rabbit anti–NF-κB p65 was obtained from eBioscience (San Diego, CA), and monoclonal mouse antinucleolin was obtained from MBL (Nagoya, Japan).
BM cell extraction and MSC production
BM cells were obtained from 7- to 8-week-old C57BL/6 mice, resuspended in PBS and red blood cell lysis buffer (Sigma), and, after 5 minutes of incubation, subjected to an additional centrifugation. The cells were then seeded in 60-mm plates containing MSC medium. Half of the medium was replaced every 3 days; once a confluent layer was formed, the cells were removed using trypsin (0.05% EDTA, 0.25% trypsin; Biological Industries) and reseeded.
Cell sorting
Primary BM cells were incubated with antibodies specific to CD45.2 R-phycoerythrin (RPE; Southern Biotechnology Associates, Birmingham, AL) and CD11b/ Mac1 fluorescein isothiocyanate (FITC; Southern Biotechnology Associates) for 1 hour and then washed and suspended in PBS with 1% FCS. The cells were sorted using the FACSVANTAGE cell sorter (FACSVantage SE; Becton Dickinson Immunocytometry Systems, San Jose; CA). The double-negative cell population was collected and seeded in MSC medium.
Flow cytometry analysis
For flow cytometry analysis, the following antibodies were used: anti-CD11b–PE, anti-CD25–PE, anti-CD45.2–FITC, anti-CD31–FITC, and anti–SCA-1–PE were purchased from Southern Biotechnology Associates; anti–Ter-119–FITC, anti-MHC-I–FITC, anti-MHC-II–FITC, rat IgG2b isotype control–FITC, and rat IgG2a isotype control–RPE were purchased from eBioscience. MSCs were harvested and incubated with specific antibodies for 1 hour. Next, cells were subjected to flow cytometry analysis using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems). Cells were gated according to their high fluorescence intensity.
MSC proliferation
For cell count, MSCs at 6 × 103/cm2 were seeded in 24-well plates in MSC medium. Later (24 hours), MSC medium was replaced by Dulbecco modified Eagle medium (DMEM) containing 2% FCS. After an additional 24 hours, medium was replaced by DMEM containing 10% FCS with or without Pam3Cys. Cells were counted 24, 48, and 72 hours after Pam3Cys treatment. The mean cell number ± standard error was calculated for each triplicate or quadruplicate. For thymidine incorporation, MSCs at 8.5 × 103/cm2 were seeded in 96-well-plates in MSC medium. Later (24 hours), MSC medium was replaced by 2% FCS containing DMEM. After an additional 24 hours, medium was replaced by DMEM containing 10% FCS with or without Pam3Cys for 48 hours. Cells were pulsed with 0.037 MBq (1 μCi) 3H thymidine for 4 hours, and 3H thymidine incorporation was measured using a 96-well plate beta-counter. The mean counts per minute (cpm) plus or minus standard error was calculated for each triplicate or quadruplicate.
Evaluation of MSC differentiation
Adipogenesis.
Cells were seeded at a concentration of 2.5 × 104/cm2 in a 24-well plate. The next day, adipogenic medium containing 10 ng/mL insulin (Sigma) and 1 × 10−8 M dexamethasone (Sigma) was added either with or without Pam3Cys. The cells were grown for 1 to 3 weeks, with medium replacement twice a week. Adipogenesis was detected by Oil red O staining. For Oil red O quantification, 4% IGEPAL CA 630 (Sigma) in isopropanol was added to each well. Light absorbance by the extracted dye was measured in 492 nm.
Osteogenesis.
Cells were seeded at a concentration of 2.5 × 104/cm2 in a 24-well plate. The next day, osteogenic medium containing 50 μg/mL l-ascorbic acid-2 phosphate, 10 mM glycerol 2-phosphate disodium salt, and 1 × 10−8 M dexamethasone (all from Sigma) were added, either with or without Pam3Cys. The cells were grown for 3 to 4 weeks with medium replacement twice a week. Osteogenic differentiation was detected by Alizarin red staining. For Alizarin red quantification, 0.5 N HCl and 5% sodium dodecyl sulfate (SDS) were added to each well. Light absorbance by the extracted dye was measured in 405 nm. Alkaline phosphatase (ALP) activity was detected by BCIP/NBT substrate chromogen system (Dakocytomation, Glostrup, Denmark) according to the manufacturer's instructions.
Chondrogenesis.
Cells were grown in micromass culture supplied with chondrogenesis induction medium. Cells at 0.2 × 106/tube were centrifuged at 300g in conical polyproylane tubes. After centrifugation, the supernatant was gently removed and 1 mL chondrogenesis medium containing 0.1 mM l-ascorbic acid-2 phosphat, 10 ng/mL human transforming growth factor-β1 (TGF-β1; Peprotech/Cytolab, Rehovot, Israel) and 1 × 10−7 M dexamethasone was added either with or without Pam3Cys, with medium replacement twice a week. Chondrogenic differentiation was detected by Alcian blue staining. All phase-contrast micrographs of adipogenic and osteogenic differentiation in figures 1B, 6A,C, and 7B-D were taken with an Olympus IX71 microscope (Olympus America, Melville, NY) using a CplanFL 10×/0.20 PhC objective lens. Samples in water were photographed with a DP70 camera using DP Controler version 1.2.1.108, and were imported into DPManager software version 1.2.1.107 (Olympus) as TIFF or JPEG files. Micrographs were processed using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). Chondrogenic differentiation micrographs were taken by a Zeiss Axioplan microscope (Zeiss, Jena, Germany) using a Plan-NEOFLUAR 20×/0.50 objective lens. Samples were mounted with Eukitt Mounting Medium (EMS, Hatfield, PA). Micrographs were acquired and processed as described earlier in this paragraph.
MSCs, free of hematopoietic cells, express Sca-1 and differentiate into osteocytes, adipocytes, and chondrocytes. MSCs were stained with antibodies against surface markers or control antibodies and subjected to flow cytometry analysis (A). Thick black lines represent specific antibody staining, thin black lines represent nonstained cells, and dashed lines represent staining with control antibody. (B) MSCs were cultured with (ii, iv, vi, and viii) or without (i, iii, v, and vii) induction media for 2 to 3 weeks to induce cell differentiation. Differentiation into osteocytes was detected by Alizarin red staining (i-ii) and by ALP activity assay (iii-iv). Adipogenesis was detected by Oil red O staining (v-vi). Differentiation into chondrocytes was detected by Alcian blue staining (vii-viii). Original magnifications: × 10 for Bi-vi; × 20 for Bvii-viii.
MSCs, free of hematopoietic cells, express Sca-1 and differentiate into osteocytes, adipocytes, and chondrocytes. MSCs were stained with antibodies against surface markers or control antibodies and subjected to flow cytometry analysis (A). Thick black lines represent specific antibody staining, thin black lines represent nonstained cells, and dashed lines represent staining with control antibody. (B) MSCs were cultured with (ii, iv, vi, and viii) or without (i, iii, v, and vii) induction media for 2 to 3 weeks to induce cell differentiation. Differentiation into osteocytes was detected by Alizarin red staining (i-ii) and by ALP activity assay (iii-iv). Adipogenesis was detected by Oil red O staining (v-vi). Differentiation into chondrocytes was detected by Alcian blue staining (vii-viii). Original magnifications: × 10 for Bi-vi; × 20 for Bvii-viii.
Pam3Cys treatment of MSC cell cultures
Cells were seeded at the concentration of 2.5 × 104/cm2 in a 24-well plate. The next day, medium was added either with or without Pam3Cys. The cells were grown for 3 weeks, with medium replacement twice a week. Cells were then washed with PBS, fixated with PFA for 15 minutes, and stained either with Oil red O, Alizarin red, or by the BCIP/NBT substrate chromogen system.
RT-PCR
Total RNA was isolated from confluent MSCs using TRI reagent (MRC, Cincinnati, OH). Contaminating DNA was removed using DNase I treatment. Expression of mRNAs for mouse TLR-1 to TLR-9 as well as GAPDH were assessed by first-strand cDNA synthesis from 5 μg of total RNA by extension of oligo(dT) primers with 200 U Moloney murine leukemia virus reverse transcriptase (MMLV-RT; Promega, Madison, WI). Primer sequences are shown in Table 1 All polymerase chain reaction (PCR) products were size fractionated by 1% agarose gel electrophoresis, and DNA bands were visualized by ethidium bromide staining.
TLR-specific primers
| TLR . | Primer pairs . | Tm0 . | Product size, bp . | |
|---|---|---|---|---|
| Sense . | Antisense . | |||
| TLR-1 | GCGAGCAGAGGCAATTGTGGA | GACAGAGCCTGTAAGCATATTCG | 51 | 428 |
| TLR-2 | CGGTCAGAAAACAACTTACCGAA | TACCCAGCTCGCTCACTACGT | 60 | 975 |
| TLR-3 | TTGTCTTCTGCACGAACCTG | CGCAACGCAAGGATTTTATT | 53 | 207 |
| TLR-4 | CAAGAACATAGATCTGAGCTTCAACCC | GCTGTCCAATAGGGAAGCTTTCTAGAG | 57 | 280 |
| TLR-5 | ACTGAATTCCTTAAGCGACGTA | AGAAGATAAAGCCGTGCGAAA | 51 | 428 |
| TLR-6 | GTACCGTCAGTGCTGGAAATA | CCAGGAAAGTCAGCTTCGTC | 47 | 543 |
| TLR-7 | TTCCGATACGATGAATATGCACG | TGAGTTTGTCCAGAAGCCGTAAT | 51 | 404 |
| TLR-8 | CGTTTTACCTTCCTTTGTCT | CATTTGGGTGCTGTTGTTTG | 51 | 342 |
| TLR-9 | GGAGAATCCTCCATCTCCCAA | CCAGGAAGGTTCTGGGCTCA | 54 | 386 |
| GAPDH | AACTTTGGCATTGTGGAAGG | ACACATTGGGGGTAGGAACA | 55 | 225 |
| TLR . | Primer pairs . | Tm0 . | Product size, bp . | |
|---|---|---|---|---|
| Sense . | Antisense . | |||
| TLR-1 | GCGAGCAGAGGCAATTGTGGA | GACAGAGCCTGTAAGCATATTCG | 51 | 428 |
| TLR-2 | CGGTCAGAAAACAACTTACCGAA | TACCCAGCTCGCTCACTACGT | 60 | 975 |
| TLR-3 | TTGTCTTCTGCACGAACCTG | CGCAACGCAAGGATTTTATT | 53 | 207 |
| TLR-4 | CAAGAACATAGATCTGAGCTTCAACCC | GCTGTCCAATAGGGAAGCTTTCTAGAG | 57 | 280 |
| TLR-5 | ACTGAATTCCTTAAGCGACGTA | AGAAGATAAAGCCGTGCGAAA | 51 | 428 |
| TLR-6 | GTACCGTCAGTGCTGGAAATA | CCAGGAAAGTCAGCTTCGTC | 47 | 543 |
| TLR-7 | TTCCGATACGATGAATATGCACG | TGAGTTTGTCCAGAAGCCGTAAT | 51 | 404 |
| TLR-8 | CGTTTTACCTTCCTTTGTCT | CATTTGGGTGCTGTTGTTTG | 51 | 342 |
| TLR-9 | GGAGAATCCTCCATCTCCCAA | CCAGGAAGGTTCTGGGCTCA | 54 | 386 |
| GAPDH | AACTTTGGCATTGTGGAAGG | ACACATTGGGGGTAGGAACA | 55 | 225 |
Tm0 indicates melting temperature in degrees Celsius.
IL-6 ELISA
MSCs at 6 × 104/cm2 were seeded in MSC medium in 96-well plates. Later (24 hours), the medium was replaced with DMEM (Gibco/BRL, Gaithersburg, MD) and supplemented with 10% FCS with or without TLR ligands. IL-6 concentration in culture media was determined by enzyme-linked immunosorbent assay (ELISA) for IL-6 (OptiEIA kit; BD Pharmingen, San Diego, CA) according to the manufacturer's instructions. Standard curves were established using mouse recombinant IL-6. The assay detection limit was 16 to 32 pg/mL.
TLR-2–blocking experiments
MSCs were seeded in MSC medium in 96-well plates at a concentration of 3 × 104/cm2. Later (24 hours), the medium was replaced with DMEM supplemented with 10% FCS containing 60 μg/mL penicillin, 100 μg/mL streptomycin, and 50 mg/L kanamycin, either with 50 μg/mL anti–TLR-2 neutralizing antibody or control antibody (functional grade clone T2.5 and isotype control purchased from eBioscience) for 30 minutes at 37°C. LPS or Pam3cys at a concentration of 100 ng/mL was added to the cells for an additional 4 hours. Conditioned media from cell cultures were assayed for IL-6 secretion.
In vitro “wound healing”
For this assay, 8 × 105 MSCs were plated in a 6-well plate. Upon confluence, cells were removed from a round area of 5 mm in diameter by gently rotating the flat round rubber end of a syringe nozzle onto the plate surface to create an area empty of cells. This procedure is a modification of our previously reported method56 and yields uniform size “wounds.” Five days later, cells were fixed with May-Grunwald and stained with Giemsa to detect the growth of the MSCs into the “wound.” Photomicrographs of dry cultures were taken using an Olympus IX71 microscope as described, or using an Szx12 microscope (Olympus) equipped with a DP PALPO 1×/0.111 PF lens (Figure 5Bi,ii). Micrographs were acquired and processed as described. The average “wound” diameter was measured using Adobe Photoshop 7.0 software.
Immunosuppression assay
MSCs were treated with Pam3Cys for 48 hours. The cells were then washed, trypsinized, counted, and plated at different concentrations into round 96-well plates in medium containing RPMI-1640 supplemented with 2.5% FCS, 100 U/mL penicillin, 100 μg/mL streptomycin, 50 μM 2β-ME, and 2 mM l-glutamine. We used 12 × 104 cells of the T-cell line specific to MOG p35-55 and 5 × 105 irradiated 30 Gy (3000 rad) spleen cells, and 10 μg/mL of MOG p35-55 were added to each well. After 72 hours, the T cells were pulsed with 0.037 MBq (1 μCi) [3H] thymidine, specific activity 135 GBq/mmol (5.0 Ci/mmol), for 16 hours, and [3H] thymidine incorporation was measured using a 96-well plate beta-counter. The mean cpm plus or minus standard error was calculated for each triplicate or quadruplicate.
Western blot analysis
Cell lysates were microcentrifuged at 950g for separation of nuclear (pellet) and cytoplasmic (supernatant) fraction. The nuclear fraction was then lysed in a buffer containing 30 mM HEPES, 450 mM NaCl, 25% glycerol, 0.5 mM EDTA, 12 mM MgCl2, 6 mM DTT, 1 mM PMSF, and protease and phosphatase inhibitors. Equal amounts of protein were loaded and electrophoresed on a 12% SDS–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and blocked and treated overnight with a mouse monoclonal antinucleolin, rabbit polyclonal anti–ERK 1/2, or rabbit polyclonal anti–NF-κB in PBS containing 0.05% tween (PBST) with 1% BSA. Following washing, the membranes were incubated with horseradish peroxidase–conjugated anti–mouse or anti–rabbit IgG antibody in PBST with 2.5% skimmed milk. Electrochemiluminescence (ECL) detected the immunoreactive protein. Autoradiographs were scanned and quantified using the National Institutes of Health (NIH) Image 1.62 program (http://rsb.info.nih.gov/nih-image/).
Statistical analysis
The InStat 2.01 program (Graph Pad Software, San Diego, CA) was used for statistical analysis by using the Welch t test, 2-sided. Differences were considered statistically significant with a P value less than .05.
Results
MSCs express TLR-1 to TLR-8, but not TLR-9, mRNA
We isolated and propagated plastic adherent, hematopoietic-cell–depleted stromal cells from mouse BM. The isolated stromal cell population was negative for expression of CD45, CD11b, CD31, CD34, Ter119, or MHC-II, and positive for MHC-I and stem cell antigen-1 (Sca-1) expression (Figure 1A). The cell-surface marker expression analysis showed no contamination of the stromal cell culture with hematopoietic cells. To establish that the stromal cell cultures contained MSCs, the cells were cultured under various conditions to assess their capacity to differentiate into mesodermal lineages.1,2 Differentiation into osteocytes, following supplementation of the cell culture media with osteogenic induction medium, was detected by Alizarin red staining of the cells (Figure 1Bi-ii), and by ALP substrate hydrolysis (Figure 1Biii-iv). Adipogenic differentiation induced with adipogenic induction medium was detected by Oil red O staining (Figure 1Bv-vi). Cells grown in micromass culture supplied with chondrogenic induction media resulted in chondrogenic differentiation detected by Alcian blue staining (Figure 1Bvii-viii). As shown in Figure 1B, the isolated, purified stromal cells were able to differentiate into chondrocytes, adipocytes, and osteocytes by incubation with specific induction media, suggesting that these isolated stromal cultures indeed contained MSCs.
TLR mRNA expression by MSCs was examined by RT-PCR. Specific primers were used to amplify sequences from TLR-1 to TLR-9, and the identity of the fragments was confirmed by sequencing. The results presented in Figure 2 show that MSCs expressed TLR-1 through TLR-8 but not TLR-9 mRNA. Since TLR-1, TLR-5, and TLR-7 genomic sequences do not contain introns, we verified that the RNA samples do not contain genomic DNA contamination by performing PCR of non–reverse transcribed RNA, which indeed did not result in PCR products (data not shown). To confirm the lack of TLR-9, we examined its expression under different induction conditions. TLR-9 mRNA was not detected by PCR analysis in MSCs incubated with TLR-9 ligand CpG for 48 hours, in MSCs differentiated into adipocytes or osteocytes, or in MSCs grown for 3 weeks in the presence of Pam3Cys (data not shown).
TLR mRNA expression by MSCs. MSC total RNA was subjected to RT-PCR and amplified with TLR-1 to TLR-9 specific primers. RAW 264.7 cell line cDNA was used as positive control for PCR amplification.
TLR mRNA expression by MSCs. MSC total RNA was subjected to RT-PCR and amplified with TLR-1 to TLR-9 specific primers. RAW 264.7 cell line cDNA was used as positive control for PCR amplification.
TLR ligands induce MSC IL-6 secretion and NF-κB nuclear translocation
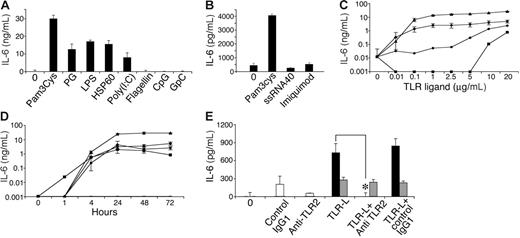
In immune cells, TLR activation leads to secretion of a variety of cytokines.43,48,50,51,57-60 We therefore tested conditioned media from MSCs following culture with various known TLR ligands by ELISA to detect the presence of several cytokines, including IL-4, TGF-β, IL-10, IL-6, IL-12, IFN-γ, IL-1β, and tumor necrosis factor-α (TNF-α). As expected, all TLR ligands tested activated the RAW 264.7 cell line to secrete IL-12 (data not shown), verifying the potency of the ligands used and the validity of the assays. Among the TLR ligands, only some induced IL-6 secretion by MSC cultures (Figure 3A-B), including TLR-2 ligands peptidoglycan (PG) and Pam3Cys, TLR-3 ligand poly (I:C), and TLR-4 ligands LPS and HSP60. The TLR-5 ligand flagellin, the TLR-7/8 ligand ssRNA40, the TLR-7 ligand imiquimod (R837), and the TLR-9 ligand CpG did not induce IL-6 secretion by MSCs. IL-6 secretion induced by Pam3Cys, PG, LPS, or Poly(I:C) increased depending on dose (Figure 3C) and time (Figure 3D). MSC IL-6 secretion induced by Pam3Cys could be completely blocked by TLR-2–neutralizing antibodies (Figure 3E), confirming that the IL-6 secretion depended on TLR-2 signaling.
MSCs secrete IL-6 in response to TLR ligands. MSCs were plated in a 96-well plate. Later (24 hours), MSC medium was replaced with 10% FCS in DMEM containing 10 μg/mL of either Pam3Cys, PG, LPS, HSP60, Poly(I:C), flagellin, CpG, or GpC, or 20 μg/mL of ssRNA40 and imiquimod (R837). Conditioned media were collected from the cultures after 72 hours (A) or 24 hours (B) and assayed by ELISA for the presence of IL-6. IL-6 secretion by MSCs was measured in response to different (10 ng/mL-20 μg/mL) doses of Pam3Cys (▴), PG (•), LPS (♦), or Poly(I:C) (▪), or without TLR ligands for 7 days (—) (C), and at different time points (0-72 hours) in response to 20 μg/mL of the TLR ligands (D). MSCs were plated in a 96-well plate. Later (24 hours), MSCs were preincubated with anti–TLR-2 antibody, control antibody, or medium alone, followed by addition of 100 ng/mL Pam3Cys (▪), LPS (⊡), or none (□) for 4 hours. Conditioned media from cultures were assayed by ELISA for IL-6 secretion (E). The results represent the means ± SE of triplicate wells. *P < .05, Pam3Cys treatment versus with or no antibody addition, by the 2-tailed Welch t test.
MSCs secrete IL-6 in response to TLR ligands. MSCs were plated in a 96-well plate. Later (24 hours), MSC medium was replaced with 10% FCS in DMEM containing 10 μg/mL of either Pam3Cys, PG, LPS, HSP60, Poly(I:C), flagellin, CpG, or GpC, or 20 μg/mL of ssRNA40 and imiquimod (R837). Conditioned media were collected from the cultures after 72 hours (A) or 24 hours (B) and assayed by ELISA for the presence of IL-6. IL-6 secretion by MSCs was measured in response to different (10 ng/mL-20 μg/mL) doses of Pam3Cys (▴), PG (•), LPS (♦), or Poly(I:C) (▪), or without TLR ligands for 7 days (—) (C), and at different time points (0-72 hours) in response to 20 μg/mL of the TLR ligands (D). MSCs were plated in a 96-well plate. Later (24 hours), MSCs were preincubated with anti–TLR-2 antibody, control antibody, or medium alone, followed by addition of 100 ng/mL Pam3Cys (▪), LPS (⊡), or none (□) for 4 hours. Conditioned media from cultures were assayed by ELISA for IL-6 secretion (E). The results represent the means ± SE of triplicate wells. *P < .05, Pam3Cys treatment versus with or no antibody addition, by the 2-tailed Welch t test.
In immune cells, TLR activation induces nuclear translocation of NF-κB from the cell cytosol, which results in NF-κB–dependent gene expression.61 We therefore investigated NF-κB activity in MSCs after exposure to TLR ligands. As shown in Figure 4, Pam3Cys and LPS both induced NF-κB translocation to the nucleus within 15 minutes, further substantiating the functional status of TLRs expressed by MSCs.
Pam3Cys and LPS induce NF-κB nuclear translocation in MSCs. MSCs were plated in MSC medium. When the cells reached confluence, 10 μg/mL Pam3Cys or LPS was added in 10% FCS containing DMEM. MSCs were harvested at 15 minutes, 1 hour, or 4 hours, and nuclear and cytpalsmic proteins were extracted. The nuclear (A,C) and cytoplasmic (B,D) extracts were quantified, run on SDS-PAGE gel, and blotted with anti–NF-κB p65, antinucleolin, or anti–total ERK antibodies. The autoradiographs were quantified by densitometry (C-D).
Pam3Cys and LPS induce NF-κB nuclear translocation in MSCs. MSCs were plated in MSC medium. When the cells reached confluence, 10 μg/mL Pam3Cys or LPS was added in 10% FCS containing DMEM. MSCs were harvested at 15 minutes, 1 hour, or 4 hours, and nuclear and cytpalsmic proteins were extracted. The nuclear (A,C) and cytoplasmic (B,D) extracts were quantified, run on SDS-PAGE gel, and blotted with anti–NF-κB p65, antinucleolin, or anti–total ERK antibodies. The autoradiographs were quantified by densitometry (C-D).
The TLR-2 ligand Pam3Cys augments spontaneous MSC proliferation, inhibits migration, and does not affect immunosuppressive activity
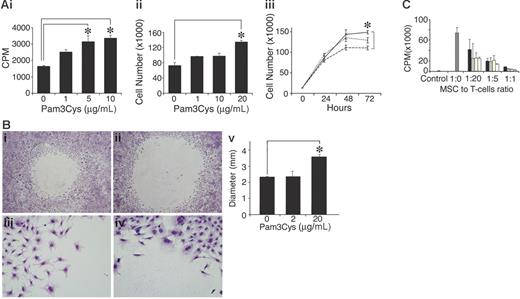
To examine the effect of Pam3Cys on MSC proliferation, MSCs were cultured with Pam3Cys for several days, and were then counted or pulsed with 3H thymidine. As shown in Figure 5, Pam3Cys enhanced MSC proliferation and thymidine incorporation 48 to 72 hours after its addition to the culture. The increase in thymidine incorporation was 2-fold (Figure 5Ai), while cell counts were elevated by 40% (Figure 5Aii-iii). To test whether Pam3Cys affects MSC migration, confluent MSC cultures were subjected to an in vitro “wound healing” assay. MSC were removed from a 5-mm diameter round circle by gentle rubbing of the plate surface. The cells were then incubated with fresh medium containing Pam3Cys for 5 days. As shown in Figure 5B, MSC cultures treated with Pam3Cys migrated to a lesser extent into the empty area compared with the control, suggesting that Pam3Cys inhibits MSC basal motility. We next examined the effect of Pam3Cys on MSC immunosuppressive activity. A T-cell line specific to MOG p35-55 was activated in the presence of MSCs treated with different doses of Pam3Cys. As shown in Figure 5C, MSC preincubation with Pam3Cys did not affect their ability to inhibit T-cell proliferation in vitro.
Pam3Cys promotes MSC proliferation, inhibits in vitro “wound healing,” and does not affect the MSC's ability to inhibit T-cell response. MSCs were plated in MSC medium and starved with 2% serum-containing media for 24 hours following 10% FCS-containing media with Pam3Cys. MSC proliferation was measured after 48 hours by 3H thymidine incorporation (Ai) and by cell count (Aii). Time response (Aiii) of an experiment similar to that in panel Aii, where MSCs were treated with medium alone (dashed line), 1 μg/mL Pam3Cys (dotted line), or 10 μg/mL Pam3Cys (solid line) were counted every 24 hours for 3 days. For migration assay, a round 5-mm diameter space was made in confluent MSC cultures and DMEM containing 10% FCS without (Bi,iii) or with (Bii,iv) 20 μg/mL Pam3Cys was added. Five days later, cells were fixed and stained. The diameters of the circles were measured and quantified in panel Bv. Original magnifications: × 10 (Biii-iv); × 6.3 (Bi-ii). The results represent the means ± SD of a total of 5 “wounds” in duplicate wells. For the immunosuppression assay, MSCs were incubated for 48 hours with 1 μg/mL Pam3Cys (□), 10 μg/mL Pam3Cys (yellow bars), 20 μg/mL Pam3Cys (⊡), or without Pam3Cys (▪), washed, and added to a T-cell line activated by its cognate antigen in different ratios. After 72 hours, cells were pulsed with 0.037 MBq (1 μCi) 3H thymidine and measured for 3H thymidine incorporation (C). The results represent the means ± SD of triplicate wells. *P < .05, Pam3Cys treatment versus medium alone, by 2-tailed Welch t test.
Pam3Cys promotes MSC proliferation, inhibits in vitro “wound healing,” and does not affect the MSC's ability to inhibit T-cell response. MSCs were plated in MSC medium and starved with 2% serum-containing media for 24 hours following 10% FCS-containing media with Pam3Cys. MSC proliferation was measured after 48 hours by 3H thymidine incorporation (Ai) and by cell count (Aii). Time response (Aiii) of an experiment similar to that in panel Aii, where MSCs were treated with medium alone (dashed line), 1 μg/mL Pam3Cys (dotted line), or 10 μg/mL Pam3Cys (solid line) were counted every 24 hours for 3 days. For migration assay, a round 5-mm diameter space was made in confluent MSC cultures and DMEM containing 10% FCS without (Bi,iii) or with (Bii,iv) 20 μg/mL Pam3Cys was added. Five days later, cells were fixed and stained. The diameters of the circles were measured and quantified in panel Bv. Original magnifications: × 10 (Biii-iv); × 6.3 (Bi-ii). The results represent the means ± SD of a total of 5 “wounds” in duplicate wells. For the immunosuppression assay, MSCs were incubated for 48 hours with 1 μg/mL Pam3Cys (□), 10 μg/mL Pam3Cys (yellow bars), 20 μg/mL Pam3Cys (⊡), or without Pam3Cys (▪), washed, and added to a T-cell line activated by its cognate antigen in different ratios. After 72 hours, cells were pulsed with 0.037 MBq (1 μCi) 3H thymidine and measured for 3H thymidine incorporation (C). The results represent the means ± SD of triplicate wells. *P < .05, Pam3Cys treatment versus medium alone, by 2-tailed Welch t test.
The TLR-2 ligand Pam3Cys reduces spontaneous MSC adipogenesis
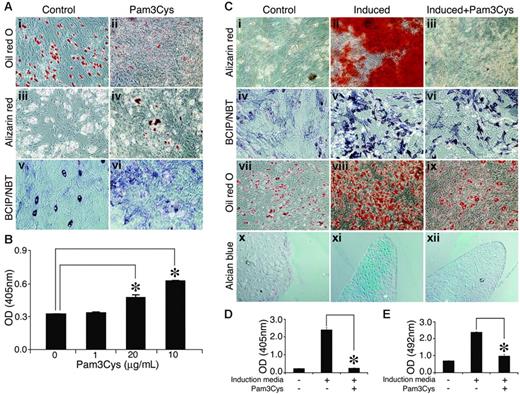
In untreated MSC cultures, some cells showed ALP activity and spontaneous adipogenic differentiation under overconfluence conditions (Figure 6Ai, Aiii, Av). Despite ALP expression, however, no calcium deposition was observed. Adding Pam3Cys to the MSC cultures resulted in a reduction of spontaneous adipogenic differentiation in the overconfluent cultures (Figure 6Ai-ii), and the cultures manifested sporadic, small calcium deposits (Figure 6Aiii-iv, B) and increased ALP activity (Figure 6Av-vi). Thus, in noninduced MSC culture, Pam3Cys promoted some osteogenic differentiation of MSCs and at the same time inhibited adipogenic differentiation.
Pam3Cys modulates MSC differentation. (A) Pam3Cys inhibits spontaneous adipogenesis, induces calcium deposition, and increases ALP activity in noninduced MSCs. MSCs were plated in MSC medium. Later (48 hours), the medium was replaced with DMEM containing 10% FCS with 20 μg/mL (Aii), 10 μg/mL (Aiv), and 5 μg/mL (Avi) or without (Ai,iii,v) Pam3Cys. Later (2 to 3 weeks), the cells were stained with Oil red O (Ai-ii), Alizarin red (Aiii-iv), or ALP substrate (Av-vi) to examine possible spontaneous differentiation. Original magnifications: × 10 for all micrographs. Alizarin red stain from experiment shown in panels Aii and Aiii was extracted from the cell culture and quantified by light absorbance in 405 nm (B). Pam3Cys reduces MSC-induced differentation into adipogenic, osteogenic, and chondrogenic pathways. MSCs were induced to differentiate into osteocytes (Cii-iii, v-vi), adipocytes (Cviii-ix), or chondrocytes (Cxi-xii) with 5 μg/mL (Ciii, vi, xii) and 10 μg/mL (Aviii) or without (Cii, v, viii, and xi) Pam3Cys. After 1 week (adipogenic differentiation) or 3 weeks (osteogenic and chondrogenic differentiation), cell cultures were fixed and stained with Alizarin red (Ci-iii), ALP substrate (Civ-vi), Oil red O (Cvii-ix) or Alcian blue (Cx-xii). Original magnifications: × 10 (Ci-ix); × 20 (Cx-xii). MSCs incubated with osteogenic induction media with or without 1 μg/mL Pam3Cys for 3 weeks were stained with Alizarin red, which was then extracted and measured for light absorbance at 405 nm (D). MSCs incubated with adipogenic induction media with or without 10 μg/mL Pam3Cys for 1 week were fixed and stained with Oil red O, which was then extracted and measured for light absorbance at 492 nm (E). The results represent the means ± SE of duplicate wells. *P < .04, Pam3Cys versus untreated control by the 2-tailed Welch t test.
Pam3Cys modulates MSC differentation. (A) Pam3Cys inhibits spontaneous adipogenesis, induces calcium deposition, and increases ALP activity in noninduced MSCs. MSCs were plated in MSC medium. Later (48 hours), the medium was replaced with DMEM containing 10% FCS with 20 μg/mL (Aii), 10 μg/mL (Aiv), and 5 μg/mL (Avi) or without (Ai,iii,v) Pam3Cys. Later (2 to 3 weeks), the cells were stained with Oil red O (Ai-ii), Alizarin red (Aiii-iv), or ALP substrate (Av-vi) to examine possible spontaneous differentiation. Original magnifications: × 10 for all micrographs. Alizarin red stain from experiment shown in panels Aii and Aiii was extracted from the cell culture and quantified by light absorbance in 405 nm (B). Pam3Cys reduces MSC-induced differentation into adipogenic, osteogenic, and chondrogenic pathways. MSCs were induced to differentiate into osteocytes (Cii-iii, v-vi), adipocytes (Cviii-ix), or chondrocytes (Cxi-xii) with 5 μg/mL (Ciii, vi, xii) and 10 μg/mL (Aviii) or without (Cii, v, viii, and xi) Pam3Cys. After 1 week (adipogenic differentiation) or 3 weeks (osteogenic and chondrogenic differentiation), cell cultures were fixed and stained with Alizarin red (Ci-iii), ALP substrate (Civ-vi), Oil red O (Cvii-ix) or Alcian blue (Cx-xii). Original magnifications: × 10 (Ci-ix); × 20 (Cx-xii). MSCs incubated with osteogenic induction media with or without 1 μg/mL Pam3Cys for 3 weeks were stained with Alizarin red, which was then extracted and measured for light absorbance at 405 nm (D). MSCs incubated with adipogenic induction media with or without 10 μg/mL Pam3Cys for 1 week were fixed and stained with Oil red O, which was then extracted and measured for light absorbance at 492 nm (E). The results represent the means ± SE of duplicate wells. *P < .04, Pam3Cys versus untreated control by the 2-tailed Welch t test.
Pam3Cys inhibits induced differentiation of MSCs into mesodermal derivatives
We further examined the effects of Pam3Cys on the induction of MSC differentiation into adipocytes, osteocytes, and chondrocytes. MSCs were incubated with osteogenic (Figure 6Cii-iii, Cv-vi), adipogenic (Figure 6Cviii-ix) or chondrogenic (Figure 6Cxi-xii) induction media, with (Figure 6Ciii, Cvi, Cix, and Cxii) or without (Figure 6Cii, Cv, Cviii, and Cxi) Pam3Cys, and were then allowed to differentiate. As shown in Figure 7, the presence of Pam3Cys in the osteogenic medium inhibited calcium deposition (Figure 6Ciii), and reduced ALP activity (Figure 6Cvi). The presence of Pam3Cys in adipogenic medium significantly reduced MSC differentiation into adipocytes; however, a few lipid-accumulating cells could still be detected (Figure 6Cix). The presence of Pam3Cys in chondrogenic medium significantly inhibited proteoglycan secretion into the extracellular matrix (Figure 6Cxii). MSC cultures, induced to differentiate into osteogenic or adipogenic lineages in the presence of Pam3Cys, were stained with Oil red O or Alizarin red, respectively; then, the stains were extracted and measured by light absorbance (Figure 6D-E). Thus, in MSC cultures induced to differentiate, the effect of Pam3Cys was pronounced and uniform; it reduced MSC differentiation into osteoblasts, adipocytes, and chondrocytes.
MyD88-deficient MSCs secrete IL-6 in response to poly(I:C) and differentiate into adipocytes but lack osteogenic and chondrogenic capacities. (A) Wild-type (▪) and 2 independent strains of MyD88-deficient MSCs (□, ⊡) were plated in MSC medium. Two days later, MSCs were treated with 20 μg/mL of TLR ligands. Conditioned media were collected from the cultures after 24 hours and assayed by ELISA for the presence of IL-6. The results represent the means ± SE of triplicate wells. Wild-type and 2 different batches of MyD88-deficient MSCs were induced to differentiate into adipocytes (Bii,iv,vi), osteocytes (Cii,iv,vi), and chondrocytes (Dii,iv,vi). After 3 weeks, cell cultures were fixed and stained with Oil red O (Bi-vi), Alizarin red (Ci-vi), and Alcian blue (Di-vi). Original magnifications: × 10 (B-C); × 20 (D).
MyD88-deficient MSCs secrete IL-6 in response to poly(I:C) and differentiate into adipocytes but lack osteogenic and chondrogenic capacities. (A) Wild-type (▪) and 2 independent strains of MyD88-deficient MSCs (□, ⊡) were plated in MSC medium. Two days later, MSCs were treated with 20 μg/mL of TLR ligands. Conditioned media were collected from the cultures after 24 hours and assayed by ELISA for the presence of IL-6. The results represent the means ± SE of triplicate wells. Wild-type and 2 different batches of MyD88-deficient MSCs were induced to differentiate into adipocytes (Bii,iv,vi), osteocytes (Cii,iv,vi), and chondrocytes (Dii,iv,vi). After 3 weeks, cell cultures were fixed and stained with Oil red O (Bi-vi), Alizarin red (Ci-vi), and Alcian blue (Di-vi). Original magnifications: × 10 (B-C); × 20 (D).
MSCs from MyD88-deficient mice exhibit incomplete differentiation potential
MyD88 is an adaptor protein that signals downstream from most TLR molecules,62 while only TLR-3 and TLR-4 have MyD88-independent pathways.63,64 The expression of functional TLRs in MSCs raised the question of whether these receptors are of importance in the development of MSCs. We generated 2 independent stains of MSCs from MyD88-deficient mouse BM. Fluorescence-activated cell sorter (FACS) analysis showed that the 2 strains were free of hematopoietic stem cells and positive for Sca-1 (not shown). MyD88-deficient MSCs did not secrete IL-6 in response to LPS, PG, Pam3Cys, or imiquimod. However, IL-6 secretion in response to poly(I:C) was detected in both MyD88-deficient strains of MSCs (Figure 7A), thereby implicating poly(I:C) signaling in a MyD88-independent manner, inducing IL-6 secretion. We then examined the ability of MSCs from MyD88-deficient mice to differentiate into adipocytes, osteocytes, and chondrocytes. As shown in Figure 7B-D, MyD88-deficient MSCs effectively differentiated into adipocytes but failed to differentiate into osteocytes and chondrocytes, as examined by Alizarin red and Alcian blue staining, respectively. These results implicate TLR signaling in the acquisition or maintenance of MSC multipotency.
Discussion
The natural role of MSCs is not yet clear; however, they are presumed to maintain regeneration of mesodermal tissue throughout the lifetime of an individual. This function is dependent on the balance between the ability of the stem cell population to self-renew and the ability of the cells to differentiate into specialized cell types. The balance between self-renewal and differentiation must be tightly regulated; overdifferentiation might cause stem cell depletion, and excess self-renewal might induce large numbers of proliferating progenitors, leading to mutations and tumorigenesis. MSCs exhibit multiple functions and are considered to be important for prospective cell-based therapy. Understanding the factors and mechanisms regulating their ability to differentiate, self-renew, participate in injury repair, and suppress an ongoing immune response are crucial, and could allow us to manipulate MSCs for therapeutic use. We show here that functional TLRs are expressed in adult MSCs and that their activation by specific ligands regulates MSC functions. This, to the best of our knowledge, is the first demonstration of involvement of TLRs and their ligands in the regulation of cytokine release and multilineage differentiation of MSCs.
Toll receptors were first identified in Drosophila,36 where they are required both for immunity against pathogens and for embryonic development; in the Drosophila immune system, Toll is required for protection against fungal infection,35 while during embryonic development, the different members of the toll family are essential for morphogenesis and embryonic development.31 Recently, TLR expression and activation were demonstrated during hematopoietic stem cell differentiation and in human adipose tissue–derived mesenchymal progenitors.65,66 Here, we extend TLR signaling in mammalian systems beyond its known immune functions and implicate its regulation in the differentiation of an adult stem cell, the MSC. These findings point to a developmental role for TLRs in mammalian systems.
On the mRNA level, MSCs expressed several TLRs, with the exclusion of TLR-9. Expression on the mRNA level does not necessarily mean that MSCs bear functional receptors. However, our findings indicate the functional status of TLR-2 in MSCs. Neutralizing antibodies to TLR-2 abrogated the secretion of IL-6 by the TLR-2 ligand Pam3Cys. IL-6 was also secreted from MSCs incubated with PG, LPS, poly (I:C), and HSP60, but not with flagellin, ssRNA40, imiquimod (R837), or CpG. The lack of TLR-9 transcripts may account for unresponsiveness to CpG. Our findings are in accord with data obtained for synovial fibroblasts, where TLR-2 is expressed and its activation leads to IL-6 secretion, while TLR-9 is expressed at a low level and its activation does not induce IL-6 secretion.67 IL-6 is a multifunctional cytokine involved in the regulation of many systems. It plays a major role in regulation of both inflammatory responses and hematopoiesis. During pathogen infection, IL-6 is released from immune cells following TLR and other innate receptor activation,68,69 and during hematopoiesis, IL-6 regulates the differentiation and function of lymphoid and hematopoietic cells.70-73 In addition, IL-6 overproduction is associated with the pathology of autoimmune diseases such as rheumatoid arthritis (RA) and Crohn disease.74
Pam3Cys, a synthetic lipopeptide, was shown to be the most potent IL-6 inducer of the TLR ligands used in this study, inducing 5-fold higher IL-6 levels then PG, LPS, or Poly(I:C). PG and Pam3Cys are both TLR-2 ligands; however, they do not share the same signaling molecules. In macrophages, Pam3Cys induces TLR-2–TLR-1 heterodimerization,75 while PG induces TLR-2–TLR-6 heterodimerization,76 thus recruiting different signaling pathways. This can account for the different magnitudes of IL-6 secretion by Pam3Cys and PG. The suppression of MSC differentiation by the TLR-2 ligand further substantiates the functional status of TLR-2 in MSCs; addition of the TLR-2 ligand, Pam3Cys, to chondrogenic, osteogenic, and adipogenic induction media reduced MSC differentiation into chondrocytes, osteocytes, and adipocytes, demonstrating a regulatory role for TLRs in MSCs. Inhibition of MSC differentiation by Pam3Cys did not involve MSC death, as assessed by cell proliferation and by cell-cycle analysis (Figure 5; data not shown).
Pam3Cys induced IL-6 secretion and blocked MSC responsiveness to differentiation factors. The question was therefore raised as to whether IL-6 or other mediators secreted by MSCs, in response to Pam3Cys, are responsible for the observed inhibition of differentiation. However, recombinant IL-6 by itself or conditioned medium of MSCs induced by Pam3Cys failed to block the induction of MSC differentiation (results not shown).
MSC cultures incubated with Pam3Cys without any additional inducing factors still showed reduced spontaneous adipogenesis, but exhibited an increase in ALP activity and de novo formation of extracellular calcium deposition. This osteogenic differentiation was mild and sporadic compared with MSCs induced to differentiate by osteogenic induction media, though clearly apparent. Possible explanations for the disparate consequences of the TLR-2 ligand relate to the heterogeneity of primary MSC populations or to the cell state of lineage commitment in culture, inducing osteoblastic differentiation on a minor cell population within the bulk of the MSC culture. This effect could be masked by the overwhelming response to differentiation inducers to which most cells in the culture react. This latter assumption is substantiated by the opposite responses of clonal populations of BM-derived stromal cells to TLR ligands; while Pam3Cys promoted an MSC-like cell line MBA-15 to differentiate into osteocytes, it inhibited, under the same conditions, the differentiation of the cell line MBA-13, which is biased to the osteogenic lineage (data not shown). Future studies should identify the specific cell population undergoing osteogenic differentiation after incubation with Pam3Cys and the mechanisms underlying this response. IL-6 secreted by Pam3Cys-treated MSCs might be involved in osteogenic differentiation. IL-6 promotes terminal osteogenic differentiation by committed osteoprogenitors, but not by MSCs.77 Thus, IL-6 secreted by MSCs, in response to Pam3Cys, might promote differentiation of preosteocytes into ALP-expressing, calcium-depositing osteocytes in the cell culture, leading to sporadic differentiation of the osteoprogenitors. Pam3Cys increased MSC proliferation and inhibited differentiation in induced MSC cultures. This might indicate a shift of MSCs to self-renewal rather than differentiation. Whether TLR-activated MSCs maintain their self-renewal is to be further explored.
NF-κB is a transcription factor that regulates a large number of genes in response to many cellular stimuli, including inflammation.78,79 These stimuli induce phosphorylation and degradation of the NF-κB–sequestering IκB, thus leading to the release of NF-κB, and its translocation into the nucleus where it exerts its transcriptional regulator functions. As shown in Figure 4, activation of MSCs by LPS and Pam3Cys induced NF-κB nuclear translocation, suggesting that at least TLR-2 and TLR-4 signaling in MSC induce NF-κB–dependent signaling. Interestingly, NF-κB activation was shown to inhibit the induced differentiation of MSCs, in particular, into adipocytes,80,81 osteoblasts,82,83 chondrocytes, and myocytes.84 This appears to result in down-regulating specific lineage transcription-factor functions that are required for differentiation. These findings offer a molecular mechanism for the inhibition of MSC differentiation by the TLR-2 ligand Pam3Cys through the NF-κB pathway.
MSCs residing in the BM and in peripheral tissues may encounter molecular infectious agents, resulting in TLR activation. Activation of TLRs by endogenous molecules such as heat-shock proteins and extracellular matrix–breakdown products might occur as well.50-52 These endogenous TLR ligands might regulate MSC function by endogenous stimuli during sterile inflammation and tissue repair found at sites of tissue injury and cell necrosis.57 MSCs isolated from patients with osteoarthritis, in which chronic synovitis occurs, show decreased adipogenesis and chondrogenesis compared with control subjects.85 This indicates the attenuation of MSC function by the inflammatory environment. Whether such occurrences characterize inflammatory processes in general remains to be determined. The link between MSCs and the immune response has been implicated by several lines of evidence.86-91 MSCs were shown to localize in sites of inflammation,15,92 and have recently been shown to suppress T-cell, natural killer (NK)–cell, and B-cell responses in vitro.93 We suggest an additional level of cross-talk between the MSC compartment and the immune response, namely the direct activation of MSCs via TLR signaling. In our study, Pam3Cys did not alter the ability of MSCs to inhibit T-cell activation. However, Pam3Cys did inhibit MSC basal migration, which might indicate MSC detainment in inflammatory sites and subsequently the down-regulation of immune responses.
To examine whether TLR signaling might be involved in MSC development, we first examined the incidence of colony-forming unit fibroblasts (CFU-Fs), but found no statistically significant differences between normal and MyD88-deficient mice. Furthermore, we generated MSCs from MyD88-deficient mice. These MSCs secreted IL-6 only in response to poly(I:C), a TLR-3 ligand, in a MyD88-independent manner. The other TLR ligands did not induce the secretion of IL-6, though TLR-4 ligands have been shown to have a MyD88-independent signaling pathway as well.63,64 The MyD88–/– MSCs expressed Sca-1, as would be expected from intact MSCs. However, they lacked the ability to differentiate into osteocytes or chondrocytes under the conditions that were permissive for differentiation of their normal counterparts. These results imply that TLR signaling may be required for acquisition of multipotency by MSCs. It cannot be excluded, at this point, that MyD88 is required for a pathway unrelated to TLR activation. Based on these findings, we propose that TLRs and their ligands may be part of the tissue-regulatory mechanisms that limit the capacity of MSCs to express in full their differentiation potential. Further characterization of TLR-activated MSCs using in vivo experimental systems is needed to establish the physiologic role for TLRs in regulation of adult stem cell functions.
Authorship
Contribution: the experiments reported herein were designed and executed by M.P.-F. following an initial proposal of M.C.-S. that TLRs may be significant in stem cell biology. L.R.-N. derived the MSCs used in this study, V.M. designed the conditions for MSC differentiation, and A.Z.-Z. set the conditions for the NF-κB assay. S.C. generated the T-cell line used for the immunosuppression assay. I.R.C. and D.Z. are heads of 2 independent research groups and directed this collaborative study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dov Zipori, Department of Molecular Cell Biology, Weizmann Institute of Science, Rehovot 76100, Israel; e-mail: dov.zipori@weizmann.ac.il.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Shizuo Akira (Osaka University, Osaka, Japan) for kindly providing MyD88-deficient mice. We thank Dr Ron Apte (Ben Gurion University of the Negev, Beer Sheva, Israel) for providing the IL-1β ELISA results for us. We thank Dr Tamara Berkutzki from the Department of Veterinary Resources of the Weizmann Institute for excellent assistance in the preparation of histologic sections.
This work was supported by the 6th platform of the European Union and the Center for the Study of Emerging Diseases (I.R.C.) and by The Charles and David Wolfson Charitable Trust and Ruth Zeigler Trust grants for Stem Cell Research at the Weizmann Institute of Science, and by the Gabrielle Rich Center for Transplantation Biology (D.Z.).





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal