Abstract
Kit receptor and its ligand stem cell factor (SCF) are critical regulators of mast cell production, proliferation, degranulation, and chemotaxis. In this study, we investigated how Fyn kinase regulates chemotaxis of mast cells toward SCF. On β1-integrin engagement, Fyn-deficient (fyn−/−) mast cells displayed a striking defect in cell spreading and lamellipodia formation compared to wild-type mast cells. The hematopoietic-specific Src family kinases (Lyn/Fgr/Hck) were not required for initial SCF-induced cell spreading. Reduced SCF-induced activation of Rac1 and Rac2 GTPases, p38 mitogen-activated protein kinase, and filamentous actin polymerization was observed in fyn−/− mast cells compared to wild-type mast cells. Retroviral-mediated expression of Fyn, constitutively active forms of Rac2 or phosphatidylinositol 3-kinase (PI3K) in fyn−/− mast cells rescued defects in SCF-induced cell polarization and chemotaxis of Fyn-deficient mast cells. Thus, we conclude that Fyn kinase plays a unique role upstream of PI3K and Rac GTPases to promote the reorganization of the cytoskeleton during mast cell spreading and chemotaxis.
Introduction
Mast cells play important protective roles during immune responses, particularly to helminth and bacterial infections.1 Immature mast cells are released from bone marrow, then migrate to target tissues to undergo terminal differentiation and perform their biologic functions.2 The White-spotting (W) locus in mice encodes Kit, a type III receptor protein tyrosine kinase (PTK)3,4 Kit is required for development of erythrocytes, melanocytes, germ cells, mast cells, and interstitial cells of Cajal (ICCs). Stem cell factor (SCF) is the ligand for Kit and is produced in both soluble and membrane-bound forms.5 In the absence of SCF, the juxtamembrane domain and activation loop region of Kit repress kinase activity.6 Mutations within the extracellular, juxtamembrane, and kinase domains of Kit that lead to SCF-independent activation have been identified in human cancers derived from mast cells and germ cells, and in gastrointestinal stromal tumors (GISTs) derived from ICCs.7 Mutations in Kit are frequent in GISTs (85%) and mostly involve juxtamembrane mutations that correlate with poor prognosis.8 Imatinib mesylate blocks Kit-mediated growth of GIST cell lines and has recently been approved for treatment of unresectable and metastatic GISTs.9
Activation of Kit by SCF results in recruitment of many SH2 domain-containing proteins (for a review, see Roskoski10 ). Mutational analyses of individual docking sites on Kit have demonstrated the critical involvement of Src family kinases (SFKs) and phosphatidylinositol 3-kinase (PI3K) for growth and migration responses.11–13 Juxtamembrane tyrosines (Y567/Y569 in mouse; Y568/Y570 in human) of Kit are potential recruitment sites for SFKs, Csk-homology kinase (Chk), and Shc adaptor protein.14,15 In mast cells, there is evidence for roles of 2 SFKs, Fyn and Lyn, in signaling from juxtamembrane tyrosines of Kit.14,16 Knock-in of Y567F/Y569F mutations of the kit allele in mice causes postnatal lethality and failure to produce melanocytes and mast cells in vivo.17 This occurs despite the detection of normal numbers of mast cell precursors in the bone marrow (BM) and the ability to generate ex vivo cultures of BM-derived mast cells (BMMCs). Because individual SFK knock-out mouse lines do not have defects in mast cell production,18 it is likely that redundancy exists between SFKs for Kit-mediated signals for migration or survival of mast cells in vivo.
The p85 subunit of class IA PI3K can be recruited directly to phosphorylated Y719 of Kit or indirectly via the adaptor protein Grb2-associated binding-2 (Gab2). Gab2 is phosphorylated following SCF-induced recruitment of Shc adaptor protein to pY567 of Kit. Shc binds a Grb2-Gab2 complex, a process that leads to phosphorylation of Gab2 on multiple sites that bind the SH2 domains of p85 and Shp2 PTP (PTPN11).19,20 SCF-induced Gab2 phosphorylation requires Y567 and, therefore, may involve Fyn PTK, as previously shown downstream of the IgE receptor (FcϵRI) on mast cells.21,22 However, another study indicates that Syk is the primary kinase for Gab2 downstream of FcϵRI.23 Down-regulation of Shp2 expression in mature BMMCs was recently shown to result in reduced GTP-loading of Ras and Rac1/Rac2 GTPases, defects in Jnk mitogen-activated protein kinase (MAPK) activation, and impaired SCF-induced proliferation.20 This suggests a prominent role for Shp2 in mediating c-Kit signaling following its recruitment to Gab2 and dephosphorylation of currently unidentified substrates. SWAP-70 is a guanine nucleotide exchange factor (GEF) that promotes GTP-loading of Rac1 and Rac2.24 SWAP-70–deficient BMMCs are defective in SCF-induced Rac activation and chemotaxis of mast cells.25 SWAP-70 also interacts with filamentous actin (F-actin)26 and may target activated Rac1 and Rac2 to the cell periphery during mast cell activation.
Mast cells express β1- and β7-integrin receptors that modulate mast cell homing and function.2 Cross-talk between Kit and α4β1, and to a lesser extent α5β1, leads to elevated integrin-mediated adhesion to fibronectin and haptotactic migration.27,28 Coordinated activation of Kit and α4β1 signaling is key for mast cells to reorganize their F-actin cytoskeleton and to adopt a polarized cell shape with distinct signaling events at leading and trailing edges.29 Integrin signaling promotes formation of F-actin–containing filopodia and lamellipodia, which require activation of Rho GTPases Cdc42 and Rac1/Rac2, respectively.30 Whereas Rac1 is widely expressed, Rac2 expression is restricted to hematopoietic cells and has a distinct localization pattern compared to Rac1.31,32 Rac2−/− mice have defects in multiple cell types including mast cells, neutrophils, and T cells.33–36 In hematopoietic progenitors and mast cells, Rac2 plays a key role in F-actin–based processes (eg, adhesion, migration, degranulation)35 and also regulates gene expression via the Jnk MAPK pathway.37
In this study, we show that BMMCs from fyn knock-out (fyn−/−) mice display defects in SCF-induced cell spreading on fibronectin and lamellipodia formation compared to wild-type BMMCs. This correlated with reduced SCF-induced Rac1 and Rac2 activation in fyn−/− BMMCs compared to wild-type BMMCs. Colocalization of Rac2 with F-actin–containing structures near the cell periphery was also greatly reduced in Fyn-deficient mast cells. Defects in Rac activation and colocalization with F-actin were restored on expression of Fyn in fyn−/− BMMCs by retroviral transduction. Interestingly, defects in cell spreading, polarization, and chemotaxis of SCF-treated fyn−/− BMMCs could also be rescued by expression of constitutively active forms of PI3K and Rac2. Taken together, our findings suggest that Fyn is required for Kit and integrin-mediated activation of PI3K/Rac2-dependent pathways involved in F-actin reorganization, polarization, and chemotaxis of mast cells.
Materials and methods
Materials
Wild-type and fyn knock-out (fyn−/−) mice38 were maintained on a mixed background (C57/BL6-129/SvJ, 37.5%-62.5%). Mice were housed under specific pathogen-free conditions at Queen's University Animal Care Services according to Canadian Council on Animal Care regulations. The MSCV-based39 retroviral rescue plasmid for Fyn was described previously.40 For MSCVpuro-Rac2V12 construction, using polymerase chain reaction (PCR) we amplified Myc-epitope–tagged mouse Rac2V12 (pRK5-Rac2V12-myc; kindly provided by Dr M. Dinauer, Herman B. Wells Center for Pediatric Research and Department of Pediatrics) with primers conferring XhoI and EcoRI sites (5′ GACCTCGAGCATGGAGCAGAAGCTGATCTCC 3′; 5′ GCTTCTGCAGGAATTCGGTACCC 3′). Following digestion of MSCVpuro and the PCR product with XhoI/EcoRI, and ligation with T4 DNA ligase, the resulting plasmid was obtained and sequence verified. For MSCVpuro-p110CAAX construction, we subcloned a 3.2-kb BamHI fragment from pSG5/5′MycTp110αCAAX41 (kindly provided by Dr J. Downward, Imperial Cancer Research Fund, London, United Kingdom) into the BglII site of MSCVpuro and verified the resulting plasmid by sequencing.
BMMC cultures
Femurs were isolated aseptically from 4- to 8-week-old wild-type and fyn−/− mice, and BM cells were isolated by repeated flushing with BMMC medium (IMDM + 10% fetal bovine serum, 1% antimicrobial-antimycotic solution [Gibco BRL, Grand Island, NY], 1 mM sodium pyruvate [Gibco BRL], 1% nonessential amino acids [Gibco BRL], 1% to 2% conditioned medium from X63-IL-3 cells, 50 μM α-monothioylglycolate [Sigma, St Louis, MO]). Cultures were maintained at 0.5 to 1.5 × 106 cells/mL for nonadherent cells, with adherent cells being discarded. After more than 4 weeks of culturing, the purity of BMMCs was monitored by flow cytometry. For the detection of FcϵRI, 106 BMMCs were incubated overnight with antibody to (α-) dinitrophenyl (DNP)–IgE (1 μg/mL σ or in some cases 10% [vol/vol] conditioned medium from SPE-7 cells), washed, and then labeled with α-IgE–fluorescein isothiocyanate (FITC; Southern Biotechnology Associates, Birmingham, AL) and α-Kit-PE (Caltag Labs, Burlingame, CA), or with isotype controls: rat IgG1-FITC (Caltag Labs) and rat IgG2b-PE (Caltag Labs) and analyzed by flow cytometry.
BMMC stimulation and harvesting
BMMCs were starved overnight in starvation medium (Iscove modified Dulbecco medium, 10% [vol/vol] fetal bovine serum, 1% [vol/vol] antimicrobial-antimycotic solution, 50 μM α-monothioylglycolate) with or without α-DNP–IgE 10% [vol/vol] conditioned medium from SPE-7 cells). Cells were washed in starvation medium, resuspended in prewarmed Tyrode buffer (10 mM HEPES, pH 7.4, 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 0.1% bovine serum albumin) with or without recombinant mouse SCF (100 ng/mL; PeproTech, Rocky Hill, NJ), and incubated for various times at 37°C. Cells were then placed on ice, pelleted by centrifugation at 4°C, and washed in phosphate-buffered saline/0.1 mM sodium orthovanadate. Cells were then pelleted and solubilized in kinase lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% [vol/vol] Nonidet P-40, 0.5% [wt/vol] sodium deoxycholate, 10 μg aprotinin/mL, 10 μg leupeptin/mL, 1 mM vanadate, 100 μM phenylmethyl sulfonyl fluoride) for 30 minutes at 4°C on a platform rotator. Insoluble material was removed by centrifugation at 12 000g to generate soluble cell lysates (SCLs).
Immunoblotting
SCLs (equivalent to 5 × 106 cells/condition) were subjected to immunoblotting with the following antibodies: α-Rac1 mouse monoclonal (1:1000, Upstate Biotechnology, Lake Placid, NY), α-Rac2 rabbit polyclonal (1:1000, initially provided by Gary Bokoch, subsequently the same antisera was purchased from Upstate Biotechnology), α-phosphorylated p38 (α-pp38; Thr180/Tyr182) rabbit polyclonal (1:1,000; Cell Signaling Technology, Beverly, MA), α-p38 rabbit polyclonal (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), α-phosphorylated Akt rabbit monoclonal (1:1,000; Cell Signaling Technology), and α-Akt mouse monoclonal (1:200; Santa Cruz Biotechnology). Antibody complexes were detected with either horseradish peroxidase-conjugated sheep anti–mouse immunoglobulin (1:5000; Amersham Pharmacia Biotech, Piscataway, NJ) or horseradish peroxidase-conjugated goat anti–rabbit immunoglobulin (1:10 000; Amersham Pharmacia Biotech) and revealed by enhanced chemiluminescence (ECL).
Rac activation assays
The Cdc42/Rac-interactive binding (CRIB) domain of p21-activated kinase (PAK1) was amplified by PCR and subcloned in pGEX to create an in-frame fusion with glutathione-S-transferase (GST). The construct was sequence verified prior to recombinant protein expression in BL21, and purification on glutathione-Sepharose beads (Amersham Pharmacia Biotech.). Aliquots of GST-CRIB–bound Sepharose beads were snap-frozen and stored in aliquots containing 10 μg GST-CRIB at −80°C. BMMCs (107/time point) were starved and SCF-treated (as described in “BMMC stimulation and harvesting”), prior to lysis in MLB (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.25% sodium deoxycholate, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM EDTA, 1 mM sodium orthovanadate, 10 μg/mL aprotinin, 10 μg/mL leupeptin). Lysates were incubated with GST-CRIB beads for 60 minutes at 4°C on a platform rotator. For positive and negative controls, 10% of the input lysates were incubated with either 0.1 mM GTPγS or 1 mM GDP in the presence of 1 mM EDTA at 30°C for 15 minutes with agitation. Reaction was terminated by addition of 60 mM MgCl2 and incubated with GST-CRIB beads for 30 minutes at 4°C. All beads were washed 3 times with MLB before addition of 2 × SDS sample buffer, boiled, and subjected to SDS-15% polyacrylamide gel electrophoresis (PAGE) and immunoblotting.
Imaging of BMMCs
BMMCs (106/condition) were starved, placed in wells containing glass coverslips coated with 20 μg/mL fibronectin, and treated with SCF (10 ng/mL) for the times indicated in the figure legends. BMMCs were fixed using 4% paraformaldehyde, permeabilized using PBS/0.1% Triton X-100, stained with TRITC-phalloidin (1:200; Sigma-Aldrich, St Louis, MO), and analyzed using a Nikon TE-2000 inverted fluorescent microscope (Nikon Canada, Mississauga, ON, Canada). For immunofluorescence analysis of Rac2, we fixed and permeabilized BMMCs as described above, blocked with 5% normal goat serum, and incubated with anti-Rac2 polyclonal antibody (1:100) overnight at 4°C. Following 5 washes in PBS/0.1% Triton X-100, coverslips were incubated with Alexa488-antirabbit immunoglobulin secondary antibody (1:100; Molecular Probes, Eugene, OR) and TRITC-phalloidin. Subsequent analysis was performed by confocal microscopy (Leica TCS SP2 multiphoton; PL APO 100×/1.40 oil-immersion UV objective, Leica Microsystems, Richmond Hill, ON, Canada) in the Queen's University Protein Function Discovery facility.
Retroviral transduction of fyn−/− BM progenitors
fyn−/− mice were injected with 5-fluorouracil (5-FU; Sigma-Aldrich; 100 mg/kg intraperitoneally) to promote expansion of BM progenitor cells. After 4 days, BM was harvested and cultured in a cytokine cocktail (10 ng/mL IL-3, 10 ng/mL IL-6, and 100 ng/mL SCF; PeproTech) for 2 days. VSV-pseudotyped retroviruses were collected and filtered (0.2 μm pore) 48 hours after transfection of HEK293T cells (growing on 100-mm plates) using Lipofectamine (Invitrogen, Carlsbad, CA). Each transfection contained 2.5 μg EcoPAK, 2.5 μg LVSV-G, 15 μg MSCV (or MSCV-Fyn, MSCV-Rac2V12, MSCV-p110CAAX). BM progenitors growing in cytokine cocktail were incubated twice with retroviral supernatants in media containing 10 μg/mL Polybrene (Sigma) for 24 hours. Cells were cultured in BMMC media for 2 days, followed by selection with puromycin (1 μg/mL; Sigma). Transduced cells were grown in BMMC media for an additional 3 to 4 weeks and analyzed by flow cytometry as described in “BMMC cultures.” Surface expressions of Kit and FcϵRI were indistinguishable from BMMCs derived from untreated mice.
BMMC migration assays
BMMC migration assays were essentially done as previously described.42 Briefly, to prepare Transwell chambers (3-μm pore size; Becton Dickinson, San Jose, CA), the undersides of the filters were coated with 20 μg/mL bovine fibronectin (Roche Diagnostics, Indianapolis, IN) for 2 hours at 37°C, rinsed once with PBS, and used for the migration assays with BMMCs starved of IL-3 overnight. Lower chambers contained 500 μL migration media (IMDM + 0.5% FBS) supplemented with or without SCF (10 ng/mL). BMMCs were rinsed and resuspended in migration media (2 × 106 cells/mL), and 4 × 105 cells were placed in the upper well of a Transwell chamber. Following incubation at 37°C for 4 hours, the nonmigratory cells on the upper membrane surface were removed with a cotton swab, and migrated cells attached to the bottom surface of the membrane were fixed (2% paraformaldehyde) and stained (0.1% crystal violet/0.1 M borate, pH 9.0/2% ethanol) for 15 minutes at room temperature. The numbers of BMMCs in the lower wells were scored, and data from 4 independent experiments are shown relative to migration of wild-type BMMCs.
Results
Defective cell spreading and membrane ruffling of Fyn-deficient mast cells on SCF-induced integrin activation
We have previously shown that Fyn is required for maximal SCF-evoked chemotaxis of BMMCs using fibronectin-coated Transwell filters.43 Consistent with our findings, another group recently reported defects in SCF-induced chemotaxis of fyn−/− BMMCs.44 To better understand the involvement of Fyn in SCF-induced chemotaxis, we examined early events in the response of BMMCs to SCF, including adhesion and cell spreading assays on fibronectin. Consistent with previous work,45 no defects in adhesion of Fyn-deficient mast cells were observed (data not shown). However, there were clear defects in cell spreading ability of fyn−/− compared to fyn+/+ BMMCs on SCF treatment visualized by F-actin staining and fluorescence microscopy (Figure 1A). Wild-type BMMCs display extensive F-actin–containing projections within 15 minutes of SCF treatment. In contrast, fyn−/− BMMCs display mostly a rounded phenotype with only a cortical ring of F-actin. This defect was corrected on Fyn expression in fyn−/− BMMCs by retroviral transduction (MSCV-Fyn). We classified the morphologies of SCF-treated BMMCs plated on fibronectin using light microscopy and found that Fyn-deficient BMMCs displayed a significant increase in cells that had failed to spread, and a dramatic decrease in cells with lamellipodia, compared to wild-type BMMCs (Figure 1B; P < .01). These defects are due to Fyn because they were corrected in MSCV-Fyn BMMCs. Significant differences in cell size were also observed, reflecting the defect in cell spreading in Fyn-deficient mast cells (Figure 1C; P < .01). These results together with all morphologic analysis showed that in SCF-treated BMMCs, Fyn kinase is involved in cell spreading and promoting formation of F-actin–containing projections. Since these BMMCs were plated on the β1-integrin substrate fibronectin, Fyn may also be important for β1-integrin–dependent signaling.
Defective SCF-induced cell spreading of fyn−/− BMMCs on fibronectin. (A) fyn+/+, fyn−/− BMMCs, and fyn−/− BMMCs transduced with MSCV-Fyn were plated on fibronectin in the presence of SCF (20 ng/mL) for 15 or 45 minutes prior to fixation and F-actin staining as described in “Materials and methods.” Representative images obtained by fluorescence microscopy are shown. (B) Cell spreading and lamellipodia formation of SCF-treated BMMCs (20 ng/mL for 45 minutes) plated on fibronectin-coated wells were analyzed by light microscopy. Significant differences in cell spreading and lamellipodia formation in fyn−/− BMMCs (indicated by an asterisk, P < .01, n > 100; error bars indicate standard deviations [SD]) with those of fyn+/+ BMMCs. (C) fyn−/− BMMCs showed significantly lower total cell spread area compared to wild-type (indicated by an asterisk; P < .01, n = 50/genotype; mean ± SD), as quantified using Image Pro Plus software (Media Cybernetics, Silver Spring, MD).
Defective SCF-induced cell spreading of fyn−/− BMMCs on fibronectin. (A) fyn+/+, fyn−/− BMMCs, and fyn−/− BMMCs transduced with MSCV-Fyn were plated on fibronectin in the presence of SCF (20 ng/mL) for 15 or 45 minutes prior to fixation and F-actin staining as described in “Materials and methods.” Representative images obtained by fluorescence microscopy are shown. (B) Cell spreading and lamellipodia formation of SCF-treated BMMCs (20 ng/mL for 45 minutes) plated on fibronectin-coated wells were analyzed by light microscopy. Significant differences in cell spreading and lamellipodia formation in fyn−/− BMMCs (indicated by an asterisk, P < .01, n > 100; error bars indicate standard deviations [SD]) with those of fyn+/+ BMMCs. (C) fyn−/− BMMCs showed significantly lower total cell spread area compared to wild-type (indicated by an asterisk; P < .01, n = 50/genotype; mean ± SD), as quantified using Image Pro Plus software (Media Cybernetics, Silver Spring, MD).
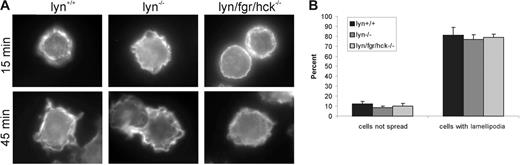
Early SCF-induced cell spreading and lamellipodia formation does not require Lyn/Fgr/Hck kinases
To examine the potential involvement of hematopoietic-specific SFKs in β1-integrin–mediated adhesion and cytoskeletal reorganization induced by SCF, BMMCs were generated from lyn+/+, lyn−/−, and lyn−/−/fgr−/−/hck−/− mice. No striking defects in F-actin staining of membrane projections was observed during cell spreading of BMMCs between genotypes (Figure 2A). The morphologies of BMMCs of each genotype were classified on plating on fibronectin-coated tissue culture plates, and no significant differences in cells that fail to spread or those with lamellipodia were observed between genotypes (Figure 2B). This suggests that Lyn, Fgr, or Hck are not required for these early cytoskeletal reorganization events. As Fyn PTK remains active in these BMMCs, this early event after β1-integrin engagement is likely Fyn-dependent. However, in chemotaxis assays we observed an approximate 50% reduction in SCF-induced migration of lyn−/− and lyn−/−/fgr−/−/hck−/− compared to lyn+/+ BMMCs (data not shown). These results are consistent with a previous study of lyn−/− BMMCs16 and suggest that although early SCF-induced cytoskeletal reorganization is independent of Lyn, it does contribute to SCF-induced chemotaxis of mast cells.
SCF-induced cell spreading of BMMCs on fibronectin is independent of Lyn/Fgr/Hck kinases. (A) BMMCs from lyn+/+, lyn−/−, lyn/fgr/hck−/− mice were plated on fibronectin-coated coverslips in the presence of SCF (20 ng/mL) for 15 or 45 minutes. Cells were fixed, permeabilized, and stained with TRITC-phalloidin. Representative fluorescence microscopy images are shown. (B) Cell spreading and lamellipodia formation of SCF-treated BMMCs (20 ng/mL for 45 minutes; mean ± SD; n = 100) plated on fibronectin-coated wells were analyzed by light microscopy.
SCF-induced cell spreading of BMMCs on fibronectin is independent of Lyn/Fgr/Hck kinases. (A) BMMCs from lyn+/+, lyn−/−, lyn/fgr/hck−/− mice were plated on fibronectin-coated coverslips in the presence of SCF (20 ng/mL) for 15 or 45 minutes. Cells were fixed, permeabilized, and stained with TRITC-phalloidin. Representative fluorescence microscopy images are shown. (B) Cell spreading and lamellipodia formation of SCF-treated BMMCs (20 ng/mL for 45 minutes; mean ± SD; n = 100) plated on fibronectin-coated wells were analyzed by light microscopy.
Fyn promotes activation of Rac GTPases and p38 MAPK in SCF-treated mast cells
Given the importance of Rac GTPases in regulating Kit- and integrin-mediated cell spreading and chemotaxis,36 we measured GTP loading of Rac1 and Rac2 using the CRIB domain pull-down assay in SCF-treated fyn+/+ and fyn−/− BMMCs. Our findings showed a clear reduction in activation of Rac1 and Rac2 on SCF treatment of fyn−/− BMMCs compared to wild-type cells (Figure 3A). Preloading of lysates (10% of protein used for SCF treatment) with excess GDP and GTPγS served as negative and positive controls for the CRIB pull-down assay, respectively. Similar defects in Rac2 activation were noted in Fyn-deficient BMMCs, and this was corrected on Fyn expression (MSCV-Fyn; Figure 3B). The apparent elevation in Rac2 levels in lysates from fyn−/− BMMCs was not due to the genotype and was not consistently observed. These findings suggest that Fyn plays a significant role in activation of Rac GTPases downstream of Kit. To address potential signaling changes relevant to Fyn signaling from Kit and β1 integrins, we compared SCF-induced signaling in fyn+/+ and fyn−/− BMMCs plated on fibronectin. No overt changes in profiles of tyrosine phosphorylated proteins were observed between genotypes, with the possible exception of an approximate 35-kDa protein (indicated by an arrow) that was more highly expressed or phosphorylated in Fyn-deficient cells (Figure 3C). No substantial differences in SCF-induced phosphorylation of Akt were observed, whereas phosphorylation of p38 was reduced, particularly at 15 minutes after plating. These results suggest that Fyn may function upstream of Rac GTPases and p38 MAPK in mast cells activated via Kit and β1 integrins.
SCF-induced activation of Rac1 and Rac2 GTPases and p38 MAPK are coordinated through Fyn kinase. (A) fyn+/+ and fyn−/− BMMCs were activated with SCF (50 ng/mL) for 0, 5, 15, or 30 minutes. CRIB pull-down assays were used to detect activated Rac GTPases (RacGTP), as described in “Materials and methods.” SCLs and pull-down samples were immunoblotted (IB) with anti-Rac1 and anti-Rac2 antibodies. In all experiments, 10% of input was used for loading of GTPases with excess GDP or GTPγS to serve as controls. (B) fyn−/− BMMCs transduced with MSCV or MSCV-Fyn were analyzed as described. (C) fyn+/+ and fyn−/− BMMCs were plated on fibronectin-coated wells and treated with SCF (50 ng/mL) for 0, 5, or 15 minutes. SCLs were prepared and IB with: antiphosphotyrosine (pY), antiphospho-Akt (pAkt), anti-Akt, antiphospho-p38 (pp38), or anti-p38. Arrow at right indicates a phosphoprotein that appears to be elevated in fyn−/− BMMCs.
SCF-induced activation of Rac1 and Rac2 GTPases and p38 MAPK are coordinated through Fyn kinase. (A) fyn+/+ and fyn−/− BMMCs were activated with SCF (50 ng/mL) for 0, 5, 15, or 30 minutes. CRIB pull-down assays were used to detect activated Rac GTPases (RacGTP), as described in “Materials and methods.” SCLs and pull-down samples were immunoblotted (IB) with anti-Rac1 and anti-Rac2 antibodies. In all experiments, 10% of input was used for loading of GTPases with excess GDP or GTPγS to serve as controls. (B) fyn−/− BMMCs transduced with MSCV or MSCV-Fyn were analyzed as described. (C) fyn+/+ and fyn−/− BMMCs were plated on fibronectin-coated wells and treated with SCF (50 ng/mL) for 0, 5, or 15 minutes. SCLs were prepared and IB with: antiphosphotyrosine (pY), antiphospho-Akt (pAkt), anti-Akt, antiphospho-p38 (pp38), or anti-p38. Arrow at right indicates a phosphoprotein that appears to be elevated in fyn−/− BMMCs.
Fyn regulates F-actin reorganization in SCF-treated mast cells
Previous studies have provided evidence for a unique role of Rac2 in Kit- and integrin-mediated cell spreading and chemotaxis.36 To determine whether Fyn regulates Rac2 localization in mast cells, we used confocal microscopy to compare F-actin and Rac2 staining of SCF-treated fyn+/+ and fyn−/− BMMCs plated on fibronectin. The fyn+/+ BMMCs showed a clear ringlike localization of Rac2 within 1 minute after SCF-induced adhesion to fibronectin (Figure 4). Extensive colocalization of Rac2 with F-actin–containing structures was also observed. Whereas no significant differences in Rac2 localization was observed in fyn−/− BMMCs, the amount of F-actin–containing structures within the cytosol was greatly reduced. F-actin polymerization at the cell periphery and within the cytoplasm was elevated in fyn−/− BMMCs transduced with MSCV-Fyn. Likewise, expression of constitutively active Rac2 (MSCV-Rac2V12) and PI3K (MSCV-p110CAAX) resulted in a dramatic increase in F-actin/Rac2-containing structures. Together with our findings showing defective Rac activation in Fyn-deficient mast cells, these results imply that F-actin polymerization depends on Fyn/PI3K/Rac2 following SCF-induced integrin engagement.
Involvement of Fyn, PI3K, and Rac2 in F-actin remodeling in response to SCF-induced integrin engagement. fyn+/+, fyn−/−, and fyn−/− transduced with either MSCV-Fyn, MSCV-Rac2V12, or MSCV-p110CAAX BMMCs were plated on glass coverslips, previously coated with fibronectin. Cells were then activated with SCF for 1 minute at 37°C, prior to fixation and Rac2 and F-actin staining, as described in “Materials and methods.” Images were acquired by confocal microscopy, and representative images are shown.
Involvement of Fyn, PI3K, and Rac2 in F-actin remodeling in response to SCF-induced integrin engagement. fyn+/+, fyn−/−, and fyn−/− transduced with either MSCV-Fyn, MSCV-Rac2V12, or MSCV-p110CAAX BMMCs were plated on glass coverslips, previously coated with fibronectin. Cells were then activated with SCF for 1 minute at 37°C, prior to fixation and Rac2 and F-actin staining, as described in “Materials and methods.” Images were acquired by confocal microscopy, and representative images are shown.
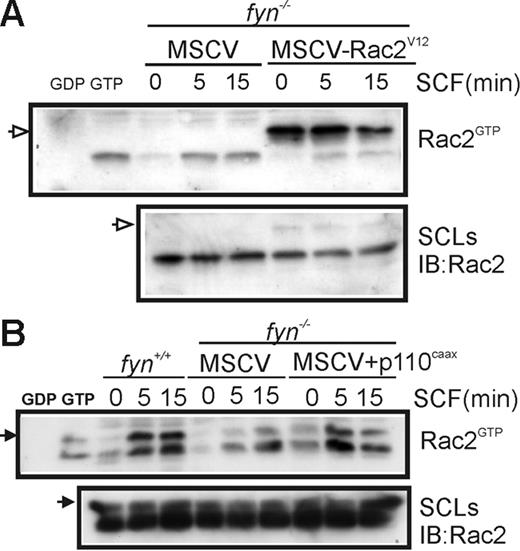
To further confirm the importance of the PI3K/Rac pathway in SCF-induced chemotaxis of BMMCs, we measured GTP loading of Rac2 in fyn+/+ and fyn−/− BMMCs transduced with MSCV-Rac2V12 or MSCV-p110CAAX. As expected, GTP loading of Myc-tagged Rac2V12 (indicated by open arrow) was independent of SCF treatment, whereas activation of endogenous Rac2 was unaffected (Figure 5A). Expression of p110CAAX in fyn−/− BMMCs resulted in elevated GTP loading of Rac2 that was less dependent on SCF treatment (Figure 5B). The faster migrating species may reflect partial proteolysis of Rac2 in these lysates.
Effects of constitutively active PI3K and Rac2 on SCF-induced Rac activation. (A) fyn−/−MSCV and fyn−/−MSCV-Rac2V12 BMMCs were activated with SCF for 0 or 5 minutes. CRIB pull-down assays were carried out to detect Rac2GTP (endogenous and Myc-tagged) and total Rac2 in SCLs. Position of Myc-Rac2V12 is indicated by an open arrow on the left. (B) fyn+/+ and fyn−/− transduced with either MSCV or MSCV-p110CAAX BMMCs were subjected to CRIB pull-down assays as described. Position of Rac2 is indicated on the left with an arrow.
Effects of constitutively active PI3K and Rac2 on SCF-induced Rac activation. (A) fyn−/−MSCV and fyn−/−MSCV-Rac2V12 BMMCs were activated with SCF for 0 or 5 minutes. CRIB pull-down assays were carried out to detect Rac2GTP (endogenous and Myc-tagged) and total Rac2 in SCLs. Position of Myc-Rac2V12 is indicated by an open arrow on the left. (B) fyn+/+ and fyn−/− transduced with either MSCV or MSCV-p110CAAX BMMCs were subjected to CRIB pull-down assays as described. Position of Rac2 is indicated on the left with an arrow.
Constitutively active PI3K and Rac2 can replace the role of Fyn in SCF-induced polarization and chemotaxis of mast cells
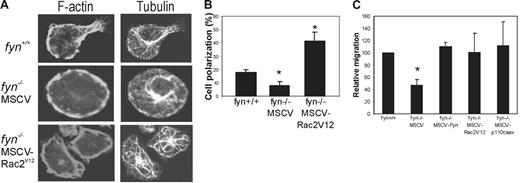
We next used confocal microscopy to compare the polarization and reorganization of F-actin and microtubules in SCF-treated fyn+/+, fyn−/−MSCV, fyn−/−MSCV-Rac2V12 BMMCs plated on fibronectin. Interestingly, the majority of fyn+/+ and fyn−/−MSCV-RacV12 BMMCs displayed polarized cell morphologies (Figure 6A) that were similar to those described in other leukocytes.29 Fyn-deficient BMMCs retain a round cell shape with little evidence for dynamic regulation of the F-actin cytoskeleton or microtubules. We scored the percentage of polarized BMMCs following SCF treatment and plating on fibronectin and observed a significant decrease in fyn−/− compared to fyn+/+ (Figure 6B; P < .05). This defect was rescued in fyn−/−MSCV-RacV12 BMMCs (Figure 6B; P < .01).
Constitutively active PI3K or Rac2 can compensate for Fyn kinase during SCF-induced polarization and chemotaxis of mast cells. (A) Status of F-actin and microtubule reorganization during chemotaxis of SCF-treated (20 ng/mL) BMMCs (fyn+/+ and fyn−/− transduced with either MSCV or MSCV-Rac2V12) plated on fibronectin-coated coverslips. Representative confocal images for tubulin immunostaining/TRITC-phalloidin staining are shown. (B) SCF-treated (20 ng/mL) BMMCs from fyn+/+ and fyn−/− transduced with either MSCV or MSCV-Rac2V12 were plated on fibronectin-coated tissue culture plates for 45 minutes. The percentages of polarized cells are depicted for 3 separate fields of view (n = 50; mean ± SD). Fyn-deficient cells displayed a significant decrease in polarization (P < .05) that was rescued by Rac2V12 expression (P < .01). (C) BMMCs from fyn+/+ and fyn−/− transduced with either MSCV, MSCV-Rac2V12, or MSCV-p110CAAX BMMCs were subjected to Transwell chemotaxis assays as described in “Materials and methods.” Numbers of BMMCs that had migrated to the bottom chamber were counted. Migration of BMMCs from the various genotypes was compared to numbers of fyn+/+ BMMCs that had migrated (set at 100%). Results from 3 independent experiments are shown (mean ± SD). Asterisk indicates a significant difference (P < .05) between fyn−/− and fyn+/+ and fyn−/− transduced with MSCV-Rac2V12 or MSCV-p110CAAX BMMCs.
Constitutively active PI3K or Rac2 can compensate for Fyn kinase during SCF-induced polarization and chemotaxis of mast cells. (A) Status of F-actin and microtubule reorganization during chemotaxis of SCF-treated (20 ng/mL) BMMCs (fyn+/+ and fyn−/− transduced with either MSCV or MSCV-Rac2V12) plated on fibronectin-coated coverslips. Representative confocal images for tubulin immunostaining/TRITC-phalloidin staining are shown. (B) SCF-treated (20 ng/mL) BMMCs from fyn+/+ and fyn−/− transduced with either MSCV or MSCV-Rac2V12 were plated on fibronectin-coated tissue culture plates for 45 minutes. The percentages of polarized cells are depicted for 3 separate fields of view (n = 50; mean ± SD). Fyn-deficient cells displayed a significant decrease in polarization (P < .05) that was rescued by Rac2V12 expression (P < .01). (C) BMMCs from fyn+/+ and fyn−/− transduced with either MSCV, MSCV-Rac2V12, or MSCV-p110CAAX BMMCs were subjected to Transwell chemotaxis assays as described in “Materials and methods.” Numbers of BMMCs that had migrated to the bottom chamber were counted. Migration of BMMCs from the various genotypes was compared to numbers of fyn+/+ BMMCs that had migrated (set at 100%). Results from 3 independent experiments are shown (mean ± SD). Asterisk indicates a significant difference (P < .05) between fyn−/− and fyn+/+ and fyn−/− transduced with MSCV-Rac2V12 or MSCV-p110CAAX BMMCs.
We performed transwell chemotaxis assays with fyn+/+, fyn−/−MSCV, fyn−/−MSCV-fyn, and fyn−/−MSCV-Rac2V12 and fyn−/−MSCV-p110CAAX (Figure 6C). Interestingly, we found that compared to Fyn-deficient BMMCs transduced with empty vector (MSCV) that displayed a significant decrease in chemotaxis (P < .05), all other transduced BMMCs (fyn−/−MSCV-Fyn, fyn−/−MSCV-Rac2V12, and fyn−/−MSCV-p110CAAX) displayed similar SCF-induced chemotaxis to that observed for fyn+/+ BMMCs. These findings suggest that Fyn plays an important role in a PI3K/Rac2-dependent pathway controlling F-actin reorganization and chemotaxis of mast cells toward SCF.
Discussion
SCF signaling through Kit receptor PTK is essential for mast cell development and also regulates proliferation, chemotaxis, and degranulation of mature mast cells.46 Previous studies have implicated signals emanating from juxtamembrane tyrosines (Y567/Y569) of Kit as important regulators of cell proliferation, chemotaxis, and mast cell differentiation.27,47 Although the SFK Fyn was previously shown to bind Y567 of Kit following SCF treatment,12 there was no evidence for a function of Fyn in Kit signaling. In an initial characterization of Kit signaling in Fyn-deficient BMMCs, we observed defects in SCF-induced Shp2 phosphorylation, p38 and Jnk2 MAPKs, and chemotaxis.43 In the current study, we have identified a unique role for Fyn in SCF-induced cell spreading and lamellipodia formation in mast cells. This correlated with reduced SCF-induced Rac1 and Rac2 activation in fyn−/− BMMCs. Defects in F-actin polymerization and chemotaxis were largely corrected on expression of Fyn, constitutively active Rac2, or PI3K in fyn−/− BMMCs. Based on these results and our previous study, we provide evidence that Fyn functions upstream of Shp2, PI3K, and Rac GTPases in SCF-treated BMMCs. This unique role for Fyn in mast cells is potentially related to the finding that Fyn is involved in the strengthening of αvβ3 cytoskeleton connections during cell spreading in fibroblasts.48 Also, a recent study shows that palmitoylation of Fyn, which does not occur in Src, is key for Fyn localization to the leading edge of motile cells and leads to initial reinforcement of focal contacts.49 Receptor protein tyrosine phosphatase α (PTPα) is also implicated in αvβ3-mediated activation of SFKs and for cell spreading.48,50 Phosphorylation of the SFK substrate p130Cas serves to recruit additional regulators of downstream signaling cascades (eg, Rac, p38 MAPK).51 Interestingly, PTPα is both a substrate of SFKs (Y789 in the C-terminus of PTPα) and also functions in activating SFKs by dephosphorylation of Y529 in Src (and analogous residues in Fyn and Yes PTKs).52,53 Another recent study examining α4β1-mediated migration implicates PTPα, SFKs, and p130Cas as key for early signaling events.54 Future studies will be required to test whether this model also holds true for mast cell activation by β1 integrins and whether PTPα, Fyn, and p130Cas play a role in SCF-induced cell spreading and migration of mast cells.
The Gab2 adaptor protein was recently shown to be essential for SCF-induced phosphorylation of Shp2, activation of Rac GTPases, and mast cell proliferation.15 Also, genetic evidence is provided for parallel pathways leading to PI3K activation via recruitment to Gab2 and Y719 of Kit, which contribute to mast cell development. Interestingly, Gab2 was previously shown to be a substrate of Fyn PTK downstream of the high-affinity IgE receptor FcϵRI in mast cells.21,22 However, a more recent study has implicated Syk PTK as the principal Gab2 kinase downstream of FcϵRI in mast cells.23 They also observed that Syk activity was dependent on Fyn, and this may explain the defects in Gab2 tyrosine phosphorylation in Fyn-deficient mast cells. Although Syk activation downstream of Kit has not been described in BMMCs, it was identified in a proteomics screen for signaling proteins involved in oncogenic Kit in human mast cell leukemia cells.55 It is possible therefore to reconcile our results with the Gab2/Shp2 study by postulating that Fyn regulates SCF-induced Shp2 activation directly via phosphorylation of Shp2, by phosphorylation of Shp2 recruitment sites on Gab2, or via activation of Syk PTK downstream of Kit. Future studies will be required to address the PTKs required for Shp2 activation in SCF-treated mast cells, and the downstream substrates of Shp2 that regulate SCF-induced Rac activation and proliferation.
Another potential mechanism for Fyn-dependent regulation of chemotaxis of mast cells involves the regulated production and autocrine/paracrine actions of sphingosine-1-phosphate (S1P). S1P is produced and secreted from mast cells on activation of sphingosine kinases 1 and 2 (SphK1 and SphK2) downstream of Kit or FcϵRI.44 In this study, Fyn-deficient BMMCs were shown to be defective in chemotaxis toward SCF or antigen (following sensitization with IgE). Antigen-induced migration of fyn−/− BMMCs was partially rescued on addition of exogenous S1P. S1P1 (or EDG-1) is a Gi protein-coupled S1P receptor expressed on mast cells that promotes lamellipodia formation and chemotaxis of mast cells.56,57 Although S1P is a weak chemotactic factor for mast cells on its own, production of S1P following activation of Kit and FcϵRI contributes to mast cell migration.44 Interestingly, SCF-induced activation of SphK1 and SphK2 was significantly reduced in fyn−/− BMMCs. Membrane recruitment of ShpK1 and SphK2 and access to its substrate sphingosine is dependent on Fyn in mast cells activated by FcϵRI. However, several questions remain to be addressed, including the amounts of SCF-induced S1P production in mast cells, whether this significantly differs between wild-type and fyn−/− BMMCs, and, finally, whether addition of exogenous S1P can fully rescue the role of Fyn in promoting SCF-induced Rac activation, cell spreading, and chemotaxis.
Chemotaxis is a complex process that requires coordination of localized F-actin polymerization.58,59 Initiation of F-actin polymerization, polarization, and cellular migration are mainly coordinated through Rac GTPases.35 Activation of Rac GTPases are linked with activation of Kit receptor.27 Potential GEFs for Rac activation include Vav1 and SWAP-70.25,60 Recruitment of Rac GEFs to activate Rac GTPases requires the PI3K product phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 accumulates at the leading edge of polarized, motile cells, which leads to formation of pseudopodia or the cellular leading process that promotes migration of cells.61 Rac2 is necessary for migration of neutrophils, hematopoietic stem cells, T cells, and B cells.35,62 It was previously shown that p85−/− and Rac2−/− BMMCs display defects in SCF-induced chemotaxis.27 Cross-talk between Kit and α4β1 integrin has been linked to activation of class IA PI3K and Rac GTPases in BMMCs.27 It is clear that PI3K is important in the migration of mast cells to tissue targets, such as dermis and intestine because PI3K−/− mice showed greatly reduced tissue-specific mast cells.63,64 Therefore, migration of mast cells in vitro and in vivo are coordinated through Rac GTPases, which are regulated through the PI3K pathway.27 Future studies will be required to determine if Fyn signals upstream of Vav1 or SWAP-70 or other unknown regulators of Rac GTPases in Kit or integrin-mediated signaling in mast cells.
The importance of SCF-induced chemotaxis of human mast cells has been linked to accumulation of mast cells in bronchi of patients with asthma. Bronchoalveolar lavage fluid from asthmatic patients during pollen season contains chemotactic activity toward mast cells, which was partially blocked using anti-SCF antibody.65 Similar results were found for nasal lavage fluid of patients with allergic rhinitis on allergen provocation.66 Mutations in the “activation loop” of the Kit kinase domain (eg, D816V) are typically found in systemic mastocytosis and mast cell leukemias.67 This Kit variant is not inhibited by imatinib,68 and therefore other inhibitors are being developed.69 Systemic mastocytosis is a broad-spectrum disorder that is characterized by tissue infiltration and inflammatory mediator release by mast cells leading to a variety of symptoms (eg, skin rash, anaphylaxis, gastrointestinal symptoms, etc).70 Mast cell leukemias are the most aggressive form of systemic mastocytosis, with malignant mast cells accounting for more than 20% of BM and less than 10% of circulating leukocytes. Currently, there are no effective treatments for mast cell leukemia and most patients die of the disease within 12 months of diagnosis. Thus, understanding how Kit promotes cell growth and migration will be key for developing new treatments for these Kit-driven cancers.
Authorship
Contributions: L.A.S. and A.W.B.C designed and performed the research, analyzed the data, and wrote the paper; R.K. contributed vital reagents, helped analyze the data, and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrew W. B. Craig, Department of Biochemistry, Queen's University, Kingston, ON K7L 3N6, Canada; e-mail: ac15@post.queensu.ca
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grant 14312 from National Cancer Institute of Canada (A.W.B.C), with funds from the Terry Fox Foundation, a Canadian Institutes for Health Research (CIHR) Pilot Project for New Investigators in Infection and Immunity (NIP 79925; A.W.B.C.), and by the National Institutes of Health, National Heart, Lung and Blood Institute grant R01 HL075816 (R.K.). A.W.B.C is supported by a New Investigator Award from CIHR.
We thank members of the laboratory for their assistance during the course of this work, Xiaolong Yang for providing access to his fluorescence microscope, Gary Bokoch for Rac2 antisera, and Mary Dinauer and Julian Downward for providing Rac2V12 and p110CAAX plasmids, respectively.

![Figure 1. Defective SCF-induced cell spreading of fyn−/− BMMCs on fibronectin. (A) fyn+/+, fyn−/− BMMCs, and fyn−/− BMMCs transduced with MSCV-Fyn were plated on fibronectin in the presence of SCF (20 ng/mL) for 15 or 45 minutes prior to fixation and F-actin staining as described in “Materials and methods.” Representative images obtained by fluorescence microscopy are shown. (B) Cell spreading and lamellipodia formation of SCF-treated BMMCs (20 ng/mL for 45 minutes) plated on fibronectin-coated wells were analyzed by light microscopy. Significant differences in cell spreading and lamellipodia formation in fyn−/− BMMCs (indicated by an asterisk, P < .01, n > 100; error bars indicate standard deviations [SD]) with those of fyn+/+ BMMCs. (C) fyn−/− BMMCs showed significantly lower total cell spread area compared to wild-type (indicated by an asterisk; P < .01, n = 50/genotype; mean ± SD), as quantified using Image Pro Plus software (Media Cybernetics, Silver Spring, MD).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/9/10.1182_blood-2006-11-057315/4/m_zh80090700310001.jpeg?Expires=1765887078&Signature=mktf-zcAMvwNW0Hf~k~W~AFCGNwHg4qhDB4jekp5HY0KrlJ~hyjMVITDvZzxDfs459K~acz8yUucIxfUFXSItQE8qAMCYfCy2jm2-nm3CjKcwW~0WhlzrKsssFJGorDAbXErCZl5YAx6cBIGgFwARAjx8b7Mu4dF231eyvNbU-rwxaDKOu5G4Ueu22ILfj1aq4wFmRIFWpILo-NfTBHbw5ENuRfEGU~HrkCMQD7DKbSWgGcRIX81BnZrl4HMIulMeKc7EbyZbHFHXoYVo89IjdHgy9T2jFuo8tm4obf34QE8~tDkVBftxkLOtwXMFOdfyvNIFNpCio7C6XxsCal5FQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal