Although hematopoietic cell transplantation (HCT) is generally accomplished using a single donor, multiple donors have been used to enhance the speed of engraftment, particularly in the case of umbilical cord blood grafts. Here we posed the question in the canine HCT model whether stable dual-donor chimerism could be established using 2 DLA-identical donors. We identified 8 DLA-identical littermate triplets in which the marrow recipients received 2 Gy total body irradiation followed by marrow infusions from 2 donors and postgrafting immunosuppression. All 8 dogs showed initial “trichimerism,” which was sustained in 5 dogs, while 2 dogs rejected one of the allografts and remained mixed chimeras, and 1 dog rejected both allografts. Immune function in one trichimeric dog, as tested by mixed leukocyte culture response and antibody response to sheep red blood cells, was found to be normal. Five dogs received kidney grafts from one of their respective marrow donors at least 6 months after HCT without immunosuppressive drugs, and grafts in 4 dogs are surviving without rejection. In summary, following nonmyeloablative conditioning, simultaneous administration of marrow grafts from 2 DLA-identical littermates could result in sustained trichimerism, and immunologic tolerance could include a kidney graft from one of the marrow donors.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is an established treatment for a variety of malignant and nonmalignant hematologic diseases. Generally, cells from single suitably human leukocyte antigen (HLA)–matched donors are transplanted. However, in 1963, before the advent of human histocompatibility testing, Mathé et al1 treated a patient with pooled marrow from 6 family members in hopes that one or more of these donors might be compatible with the patient. While engraftment occurred, the blood genetic marker techniques available at the time did not permit identification of the engrafting donor(s).
Recently, umbilical cord blood (UCB) has been used as a source of hematopoietic cells. This is due in part to the advantages UCB possesses such as, availability2 and lessened risk of graft-versus-host disease (GVHD) despite donor and recipient major histocompatibility complex (MHC) mismatch.3,4 Characteristically, in the case of UCB where the units are partially mismatched, one hematopoietic donor eventually prevails.5 However, for the issue of hematopoietic chimerism, dominance is less clear when bone marrow from 2 MHC mismatched donors is used for HCT. Using a murine model, Lee et al6 recently showed that 2 different kinds of bone marrow cells from BALB/c and CBA donor mice established mixed chimerism in host C57BL/6 mice receiving conditioning with busulfan and treatment with co-stimulatory molecule blockade (anti-CD40L and anti-CD45RB). Conversely, using the canine model, Sandmaier et al7 demonstrated that a regimen of postgrafting immunosuppression, cyclosporine (CSP), and mycophenolate mofetil (MMF) following 4.5 Gy total body irradiation (TBI) in DLA-haploidentical littermate recipients usually resulted in either complete donor hematopoietic engraftment or graft rejection with subsequent autologous recovery.
We have described a model of successful DLA-identical marrow transplantation using conditioning with only 2 Gy TBI and postgrafting immunosuppression with CSP and MMF.8 This approach has been successfully translated into the clinical setting.9 Interestingly, the relatively young canine recipients tended to be mixed donor/host hematopoietic chimeras, a finding that was replicated in human patients with nonmalignant genetic diseases10 but not in generally older patients with malignant diseases and a history of chemotherapy, in whom recipient hematopoiesis tended to disappear at a median of 6 months after HCT.11,12
Here we showed that simultaneous transplantation of marrow from 2 DLA-identical donors after 2 Gy TBI could result in sustained coexistence of 3 hematopoietic systems, including cells from both donors and their recipient.
Materials and methods
Dogs
Litters of beagles and beagle/mini-mongrel mixes were raised at the Fred Hutchinson Caner Research Center (FHCRC), assessed for disease, and enrolled in a veterinary preventive medicine program against worms, distemper, parvovirus, adenovirus type 2, para-influenza virus, corona virus, rabies, and canine papilloma virus. Dogs were 7 to 9 (median 8) months old and weighed 7.2 to 12.3 kg (median 8.5 kg) at the initiation of the study. The study was approved by the Institutional Animal Care and Use Committee at the FHCRC, which has been accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Selection of donors and recipient triplets included typing of the litters and parents for highly polymorphic microsatellite markers within DLA class I and class II regions,13 which was confirmed by DLA-DRB1 sequencing.14 The occurrence of litters with 3 or more DLA-identical pups was high and litter size dependent in our breeding facility. In evaluating 121 recent litters (705 dogs), 53% of the litters had 3 or more DLA-identical pups.
HCT
On day 0, recipients were treated with 2 Gy TBI and subsequent intravenous infusion of donor marrow as previously described.8 Marrow grafts contained a median of 4.1 (range, 2.0-7.0) × 108 nucleated cells/kg. Dogs were given supportive postgrafting care. Immunosuppression consisted of oral CSP, 15 mg/kg twice daily from day −1 to day 35, and MMF, 10 mg/kg twice daily injected subcutaneously from day 0 to day 27. MMF dosing was adjusted according to clinical toxicity which consisted of gastrointestinal distress.
Chimerism analysis
The contributions of recipient and donor cells to peripheral blood and other hematopoietic tissues were quantified by fluorescent VNTR (Variable Number Tandem Repeat) analysis, as described15,16 with the following modifications. Twenty-microliter PCR reactions contained 50-100 ng DNA, 0.4 μl Advantage II polymerase mix (Clontech, Mountain View, CA), 1X Advantage II buffer, dNTPs at 200 μM each, and human primers, at 50 nM each. PCR conditions included a hold at 94°C for 5 minutes, cycling parameters of 20 seconds at 94°C, 30 seconds at 57°C, and 60 seconds at 68°C, followed by a 2-minute hold at 68°C. Products were separated and documented by capillary electrophoresis on an ABI Prism 310 Genetic Analyzer, and quantitated with GeneScan Analysis Software 3.1 (Applied Biosystems, Foster City, CA). Since each recipient received cells from 2 donors, multiple loci were analyzed to fully distinguish all 3 individuals in each transplant. Recipient G513 was analyzed at loci, FH2001, FH2609, and FH2634 as a multiplex PCR at 30 cycles. Recipient G664 was analyzed at loci, FH2607 (30 cycles), and FH2001 (26 cycles) as separate PCRs. Recipient G643 was analyzed at loci FH2199, and FH2318 as a multiplex PCR at 28 cycles. Titrated mixtures of pretransplant donor and recipient DNAs were used to determine the limits of detection of microchimerism and to standardize the quantitative readout of the VNTR in each case. In cases where chimerism declined below the detectability threshold, it was designated as zero. In cases where DNA was limiting, amplification was done at higher cycles. Primer pairs used for this analysis are listed in Table 1.
Primer pairs for VNTR-PCR analysis of hematopoietic chimerism
| Locus . | Forward primer (5′-3′) . | Reverse primer (5′-3′)* . |
|---|---|---|
| FH2001 | TCCTCCTCTTCTTTCCATTGG | [6-FAM]TGAACAGAGTTAAGGATAGACACG |
| FH2199 | GCTGAGCACTGGGTATTGTATG | [6-FAM]TGTTACAAATTAATGTGAAATGGC |
| FH2318 | TGAGTTCAAATGCCAGCAATC | [6-FAM]CAAGTCTGAGATGAGGCTTGG |
| FH2607 | CCAACATTCCCACATGTCAG | [6-FAM]GTTACAGGCCCAAACCCTCT |
| FH2609 | TATATTGCTTTGGCCTAAAGGA | [6-FAM]GGAGTTGAAATGGAAAGAAAGA |
| FH2634 | AAAGATTGTCTTGACACGCTG | [6-FAM]GAAGGAAGGAAGGAAGAAAAGA |
| Locus . | Forward primer (5′-3′) . | Reverse primer (5′-3′)* . |
|---|---|---|
| FH2001 | TCCTCCTCTTCTTTCCATTGG | [6-FAM]TGAACAGAGTTAAGGATAGACACG |
| FH2199 | GCTGAGCACTGGGTATTGTATG | [6-FAM]TGTTACAAATTAATGTGAAATGGC |
| FH2318 | TGAGTTCAAATGCCAGCAATC | [6-FAM]CAAGTCTGAGATGAGGCTTGG |
| FH2607 | CCAACATTCCCACATGTCAG | [6-FAM]GTTACAGGCCCAAACCCTCT |
| FH2609 | TATATTGCTTTGGCCTAAAGGA | [6-FAM]GGAGTTGAAATGGAAAGAAAGA |
| FH2634 | AAAGATTGTCTTGACACGCTG | [6-FAM]GAAGGAAGGAAGGAAGAAAAGA |
Reverse primers labeled at 5′ end with 6-FAM
Monoclonal antibodies and FACS
Cell separations were performed using monoclonal antibodies and a fluorescence-activated cell sorter (FACS) (Vantage FACS, Becton Dickinson, San Jose, CA). Bone marrow was first treated with ammonium chloride lysing solution (155 mM ammonium chloride, 10 mM sodium bicarbonate, 0.1 mM EDTA), marrow nucleated cells were washed in phosphate-buffered saline (PBS) containing 2% horse serum and stained with monoclonal antibodies. Lymph node cells were isolated from popliteal lymph nodes by mechanical disruption through a stainless steel sieve, washed in PBS and 2% horse serum, and stained with monoclonal antibodies. Monoclonal antibodies generated by our laboratory consisted of mouse anticanine CD34 (2E9; bone marrow progenitor cell antigen), DM5 (DM5F; canine myeloid antigen). Additional monoclonals consisting of CD45 (CA12.10C12; common leukocyte antigen), CD3 (CA17.6F9; canine T-cell antigen) and CD21 (CA2.1D6; canine B-cell antigen) were obtained as gifts from Dr Peter Moore (UC-Davis, Davis CA).
Kidney transplantation
Kidney allografts from one of the marrow donors to the respective recipient were performed as described.17 Briefly, one donor dog from each set of DLA-identical triplets was anesthetized, a midline laparotomy was performed, and the left kidney was exposed. The ureter, renal vein, and renal artery were tied and cut. The kidney was removed from the body cavity and perfused with saline containing 10 U/mL heparin. The kidney was maintained in cold heparinized saline while the recipient was prepared for surgery and the donor dog's incision closed. The kidney was then transplanted into the recipient dog's right inguinal region.18 Dogs did not receive immunosuppressive therapy. The next day, the health of the kidney was confirmed by blood flow via ultrasound. At least 2 biopsies of transplanted kidneys were done using a percutaneous trucut biopsy needle; the tissue was fixed in 10% buffered formalin and stained using hematoxylin and eosin for evaluation by microscopy.
Immune responses
Mixed leukocyte reactions (MLRs) were done as described.19 Briefly, mononuclear cells were collected from blood, which was overlaid on ficoll (density 1.074), and centrifuged at 350 × g for 40 minutes. Cells were washed in PBS and resuspended in 45% Waymouth medium (GibCo, Grand Island, NY), 45% Iscove modified Dulbecco medium (GibCo), 10% heat-inactivated dog serum, sodium pyruvate, nonessential amino acids and l-glutamine (all except serum from GibCo). Stimulator cells were irradiated with 22 Gy (cesium irradiator). Cells were incubated for 7 days with 3H-thymidine added on day 6. Antibody responses to sheep red blood cells (SRBCs) were determined as described.8
Results
Following 2 Gy TBI and marrow grafts from 2 donor dogs, the mean platelet nadir was 20.25 × 109/L and occurred on day 10. The platelet counts recovered to near pretransplant levels by day 30. The mean cell counts and nadirs for mononuclear cells were 295 cells/μL at day 9 and for granulocytes were 1.76 × 109/L at day 10. Complete recoveries for both cell types occurred at approximately day 40 (data not shown). The day to mean granulocyte recovery was comparable to that seen for single donor mixed chimerism results in this model, suggesting the presence of an additional hematopoietic donor did not alter the speed of hematopoietic recovery.8
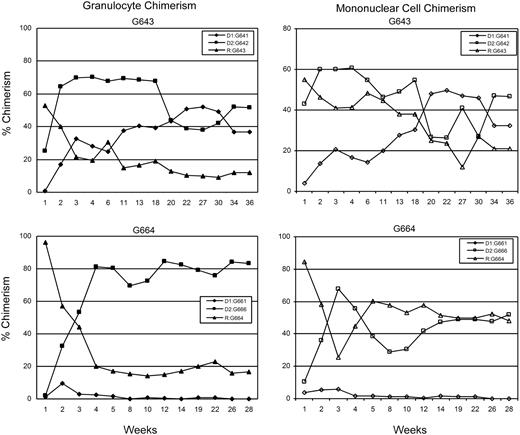
All recipients had initial engraftment of hematopoietic cells from both donors. Stable long-term (greater than 26 weeks) trichimerism for mononuclear cells was established in 5 of 8 recipient dogs: G158, G362, G513, G551, G643 (Table 2). Chimerism levels for donor 1 ranged from 5% to 39%, donor 2 levels ranged from 1% to 55%, and recipient levels ranged from 17% to 94%. Two dogs (G193, G664) rejected one of the donor grafts by weeks 16 and 6, respectively, and became “bi-chimeric”; while one dog (G631) rejected both donor grafts by week 10. Although donor (G661) chimerism was initially only 10% in recipient G664, the other dogs that rejected one (G193) or both (G631) donor grafts had transient donor chimerism levels above 20% before eventual rejection (data not shown). Microchimerism was confirmed in G158 by the indefinite acceptance of skin grafts from each of the donor dogs and rejection of a skin graft from an unrelated dog. Complete mononuclear cell and granulocyte chimerism analyses of one representative trichimeric dog and one “bichimeric” dog are shown in Figure 1.
Mononuclear cell chimerism of dogs receiving marrow transplants from 2 donors
| Recipient . | Nucleated marrow cells × 108/kg . | Duration of engraftment (wks) . | GVHD . | Status: Last Observation (week) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor 1 . | Donor 2 . | Donor 1 (% chimerism) . | Donor 2 (% chimerism) . | Recipient (% chimerism) . | |||||||
| G158 | 3.8 | 2.0 | >47 (5) | >47 (1) | (94) | No | Trichimeric | E1 | (54) | ||
| G193 | 3.7 | 4.8 | >52 (61) | 16 (0) | [R] | (39) | No | Bichimeric | E1 | (52) | |
| G362 | 3.6 | 6.1 | >47 (28) | >47 (55) | (17) | No | Trichimeric | E1 | (47) | ||
| G513 | 4.8 | 2.7 | >56 (39) | >56 (34) | (27) | Yes | Trichimeric | E1 | (56) | ||
| G551 | 7.0 | 7.2 | >17 (35) | >17 (20) | (45) | No | Trichimeric | E1 | (37) | ||
| G631* | 4.9 | 4.3 | 10 (4.4) | [R] | 10 (4.0) | [R] | (92) | No | Host | E2 | (10) |
| G643 | 4.6 | 3.8 | >36 (33) | >36 (46) | (21) | No | Trichimeric | Alive | (36) | ||
| G664 | 2.8 | 2.0 | >28 (52) | 26 (0) | [R] | (48) | No | Bichimeric | Adopted | (28) | |
| Recipient . | Nucleated marrow cells × 108/kg . | Duration of engraftment (wks) . | GVHD . | Status: Last Observation (week) . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor 1 . | Donor 2 . | Donor 1 (% chimerism) . | Donor 2 (% chimerism) . | Recipient (% chimerism) . | |||||||
| G158 | 3.8 | 2.0 | >47 (5) | >47 (1) | (94) | No | Trichimeric | E1 | (54) | ||
| G193 | 3.7 | 4.8 | >52 (61) | 16 (0) | [R] | (39) | No | Bichimeric | E1 | (52) | |
| G362 | 3.6 | 6.1 | >47 (28) | >47 (55) | (17) | No | Trichimeric | E1 | (47) | ||
| G513 | 4.8 | 2.7 | >56 (39) | >56 (34) | (27) | Yes | Trichimeric | E1 | (56) | ||
| G551 | 7.0 | 7.2 | >17 (35) | >17 (20) | (45) | No | Trichimeric | E1 | (37) | ||
| G631* | 4.9 | 4.3 | 10 (4.4) | [R] | 10 (4.0) | [R] | (92) | No | Host | E2 | (10) |
| G643 | 4.6 | 3.8 | >36 (33) | >36 (46) | (21) | No | Trichimeric | Alive | (36) | ||
| G664 | 2.8 | 2.0 | >28 (52) | 26 (0) | [R] | (48) | No | Bichimeric | Adopted | (28) | |
Fluorescent VNTR-PCR analysis was used to determine percent donor chimerism from 2 donors in a single recipient. Percent chimerism was determined for the week indicated for the duration of donor 1 and donor 2 engraftment. G551 long-term trichimerism was confirmed at week 37 using bone marrow cells. GVHD was established by serially following clinical signs and symptoms and liver enzyme chemistries in peripheral blood of the recipient and tissue biopsy histological confirmation. GVHD was treated with CSP.
G631 had to be euthanized before donor 1 and donor 2 hematopoietic chimerism was completely rejected.
D1 indicates donor 1; D2, donor2; E1, euthanized at end of study; E2, euthanized, thrombocytopenia; and [R], rejection.
Percent donor chimerism for granulocytes and mononuclear cells for dog G643 showing stable long-term trichimeric engraftment and dog G664 showing initial trichimerism with subsequent rejection of one donor 4 weeks after TBI. Data points were determined by VNTR-PCR analysis.
Percent donor chimerism for granulocytes and mononuclear cells for dog G643 showing stable long-term trichimeric engraftment and dog G664 showing initial trichimerism with subsequent rejection of one donor 4 weeks after TBI. Data points were determined by VNTR-PCR analysis.
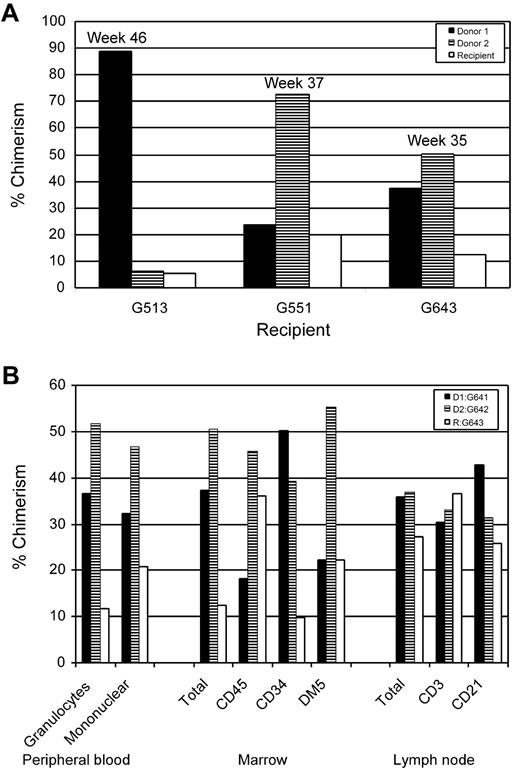
Bone marrow hematopoietic chimerism was evaluated in 3 recipients. In one of the recipients (G643), chimerism also was evaluated at 35 weeks among lymphocytes isolated from peripheral blood and cells isolated from a popliteal lymph node (Figure 2). The data revealed that hematopoietic trichimerism was established throughout the hematopoietic system, including lymph node T and B cells (CD3+ and CD21+, respectively) and marrow CD34+ cells, CD45+ cells and DM5+ myeloid cells, and granulocytes.
Trichimerism in cells derived from hematopoietic cells from peripheral blood, bone marrow, and lymph node. (A) Examples of marrow trichimerism in 3 of the dogs given marrow transplants from 2 DLA-identical donors determined at the weeks after HCT shown. (B) Chimerism analysis for dog G643, 34 weeks after HCT, of granulocytes and mononuclear cells from peripheral blood cells; CD45+ (leukocytes), CD34+ (progenitor stem cells), and DM5+ (myeloid cells) from bone marrow; CD3+ (T-cell antigen) and CD21 + (B-cell antigen) from lymph node cells. Cells were labeled with antibodies indicated above and sorted by FACS before determining chimerism.
Trichimerism in cells derived from hematopoietic cells from peripheral blood, bone marrow, and lymph node. (A) Examples of marrow trichimerism in 3 of the dogs given marrow transplants from 2 DLA-identical donors determined at the weeks after HCT shown. (B) Chimerism analysis for dog G643, 34 weeks after HCT, of granulocytes and mononuclear cells from peripheral blood cells; CD45+ (leukocytes), CD34+ (progenitor stem cells), and DM5+ (myeloid cells) from bone marrow; CD3+ (T-cell antigen) and CD21 + (B-cell antigen) from lymph node cells. Cells were labeled with antibodies indicated above and sorted by FACS before determining chimerism.
As shown in Table 2, GvHD was observed in one trichimeric recipient (G513), and was controlled by CSP, 7.5 mg/kg BID, from day 62 through day 111 with taper to day 162. Diagnosis of liver GvHD was based upon significant elevations in serum alkaline phosphatase, gamma GT, alanine aminotransferase, and aspartate aminotransferase (reaching 48-, 22-, 67-, and 6-fold normal values, respectively). In addition, a liver biopsy revealed triaditis and bile duct lesions (the latter typical of GvHD), while swab cultures of the liver were negative for viral, fungal, and bacterial isolation. Despite GvHD, this dog remained a trichimera with 27% recipient hematopoiesis at 56 weeks, and 39% and 34% hematopoiesis from the respective marrow donors.
Establishment of long-term chimerism in this model may be dependent on the total number of nucleated marrow donor cells given to the DLA-identical recipient after 2 Gy TBI. The median number of donor cells injected was 4.1 (range, 2.0-7.0) × 108 total nucleated cells/kg (Table 2). Although dog G631 received nearly equal numbers of marrow cells from both donors that were above the median value for all donors, both grafts were rejected. Analysis using the Spearman rank correlation coefficient failed to find a significant correlation between allograft acceptance and the number of cells injected into the recipient.
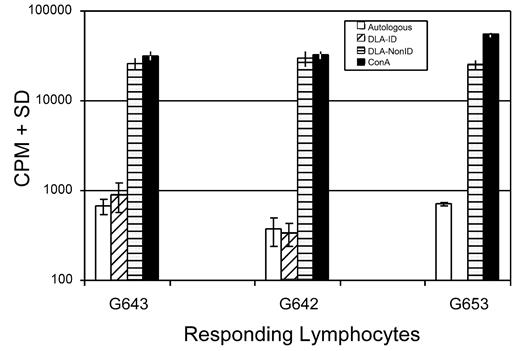
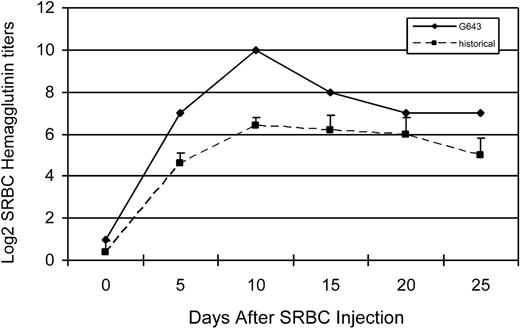
The immune responsiveness of one of the trichimeric recipients was evaluated. To assess T-cell responsiveness, an MLR was done comparing the response of the trichimeric recipient (G643) with one DLA-identical donor (G642) and a DLA-nonidentical unrelated dog (G653) 7 months after HCT. As shown in Figure 3, the trichimeric recipient cells (G643) responded equally well to the unrelated DLA-nonidentical stimulator cells (G653) as did the donor G642. Cells from all 3 dogs responded similarly to the positive control ConA, while cells from neither dog responded to cells from the respective DLA-identical littermate. SRBC hemaglutinin titers were evaluated in the same trichimeric recipient (G643) injected with a single dose of SRBC (Figure 4). Titers rose to a maximum response by day 10 and decreased thereafter, suggesting that the B-cell response was robust in this trichimeric dog. The kinetics of the anti-SRBC response were similar to those previously reported for normal dogs.8
Mixed leukocyte culture reactivity of lymphocytes from a trichimeric recipient (dog# G643) and one of its DLA-identical donors (G642) and an unrelated DLA-nonidentical (G653) in response to stimulation with irradiated autologous lymphocytes and lymphocytes from the DLA-identical littermates and an unrelated dog. Blood samples for the MLR were collected from donor and recipient 7 months after HCT. Values shown are counts per minute (CPM) ± standard deviation of triplicate samples. Concanavalin A (ConA) served as the positive control.
Mixed leukocyte culture reactivity of lymphocytes from a trichimeric recipient (dog# G643) and one of its DLA-identical donors (G642) and an unrelated DLA-nonidentical (G653) in response to stimulation with irradiated autologous lymphocytes and lymphocytes from the DLA-identical littermates and an unrelated dog. Blood samples for the MLR were collected from donor and recipient 7 months after HCT. Values shown are counts per minute (CPM) ± standard deviation of triplicate samples. Concanavalin A (ConA) served as the positive control.
Log 2 hemaglutinin titers in serum from dog G643 (diamonds) in response to sheep red blood cell (SRBC) injection. Control values (boxes) were obtained from historical data of 5 dogs.8
Log 2 hemaglutinin titers in serum from dog G643 (diamonds) in response to sheep red blood cell (SRBC) injection. Control values (boxes) were obtained from historical data of 5 dogs.8
Tolerance to kidney allografts transplanted from one marrow donor into the respective trichimeric recipients was followed for a minimum of 3 months in dogs G158, G362, G513, and G643. The kidneys were transplanted at least 6 months after HCT into the recipients' inguinal regions. Lack of rejection as indicated by cellular infiltrate was determined by kidney biopsy collected up to 90 days after transplant (data not shown). Dog G158 had microchimerism for donor 2 by week 47, but neither skin nor kidney allografts from donor 2 were acutely rejected at 11 and 16 weeks, respectively, after transplant, suggesting establishment of stable hematopoietic chimerism. A fifth dog, G664, received a kidney from donor 2 at 26 weeks after HCT (Table 2). However, 2 weeks after the kidney transplant, the kidney was rejected as confirmed by histopathology of a biopsy. VNTR-PCR analysis of peripheral blood leukocytes after kidney rejection confirmed the rejection of hematopoiesis from the kidney donor. Thus, we observed kidney allograft acceptance in a total of 4 of 4 dogs with sustained chimerism that received a kidney transplant.
Discussion
In the present study, we used the same 2 Gy TBI conditioning and postgrafting immunosuppression regimens in DLA-identical dogs that reliably established single-donor long-term mixed hematopoietic chimerism in previous studies.8 Two Gy TBI was nonmyeloablative, since dogs given 2 Gy TBI and no marrow infusion recovered marrow function and survived.20 We hypothesized that immune tolerance in canine mixed chimeras was maintained by an active suppressor cell population,21 and we speculated that the mechanism for trichimeric tolerance was similarly controlled by Treg cells.
In addition, we demonstrated stable multidonor engraftment in 5 of 8 attempts as confirmed by microsatellite polymorphism and, in some cases, skin or kidney transplantation. In 2 of 8 dogs, one graft dominated without rejecting residual host hematopoiesis, and in one case, host hematopoiesis dominated with rejection of both donor grafts shortly following completion of postgrafting immunosuppression. Sustained engraftment was not correlated with marrow cell doses. We defined long-term hematopoietic engraftment as trichimerism exceeding 26 weeks; historically, among recipients of a single DLA-identical littermate bone marrow graft, rejections beyond that time point have not been observed.
Our results of stable trichimerism in a majority of dogs given DLA-identical marrow grafting differed from chimerisms reported for unrelated umbilical cord blood transplantation (UCBT). After UCBT, one unit generally dominated over the other(s), which might be due to the numbers of total nucleated cells infused, CD34+ cells, CD3+ cells infused, or the HLA mismatch.22 Also, recipient cells were generally not observed to persist, due to the use of myeloablative conditioning. In a recent clinical study, 23 patients with high-risk hematologic malignancies were treated with combined transplantation of 2 partially HLA-matched UCB. By day 100, a single unit predominated in76% of the patients.5 The reasons one unit predominated were unclear; however, Barker et al5 reported that the dominant unit contained a higher percentage of CD3+ cells over the rejected unit.
In the canine model, a regimen of postgrafting immunosuppression (CSP and MMF) following 4.5 Gy TBI in DLA-haploidentical littermate recipients usually resulted in either complete donor hematopoietic engraftment or complete autologous recovery.7 In contrast, the DLA-identities of current double marrow grafts permitted trichimerism in a majority of recipients and bichimerism in 2 of the dogs, with only one animal rejecting both grafts and surviving with autologous recovery. The respective numbers of transplanted nucleated marrow cells neither predicted the levels of donor 1 versus donor 2 chimerisms nor which of the grafts were rejected. While we did not study the number of transplanted CD3+ cells in the current study, canine marrow CD3+ cell content was low (less than 5%). A previous study from our laboratory showed that of 9 dogs that were given a marrow transplant, the mean CD3+ cell number was 2.9 ± 1.4 (SD) × 107/kg.23 Taken together, with effective postgrafting immunosuppression consisting of combined CSP and MMF, trichimerism was possible, and within the limitations of the experiment, its stability did not depend on the numbers of transplanted cells.
GvHD was seen in 1 of 8 recipients and was controlled and eventually eliminated with CSP. GvHD was generally not seen in dogs receiving 2 Gy TBI and single donor DLA-identical littermate grafts but has been noted in dogs given 4.5 Gy lymph node irradiation.24 Tolerance to 3 hematopoietic systems did not impair the recipients' cellular immune responses to cells from unrelated dogs in MLR, to Con A or to SRBC. The kinetics of the anti-SRBC response were similar to those shown for dogs receiving a single donor HCT.8
Kidney allografts from one of the marrow donors to 4 of 5 of the respective recipients studied showed that the mutual tolerance to hematopoietic cells extended to include the kidney alloantigens. One additional dog that rejected the kidney allograft apparently had both insufficient and disappearing hematopoietic engraftment from the kidney donor to maintain tolerance to the kidney graft. This result suggested that transient very low-level donor hematopoietic chimerism was insufficient for solid organ graft tolerance. Although acceptance of vascularized kidney allografts previously has been shown in canine mixed hematopoietic chimeras,17 it was of interest that 3-directional tolerance did not alter this observation. In fact, the lack of tolerance to a kidney allograft in one of the kidney allograft recipients was likely due to loss of hematopoietic microchimerism from the kidney donor in the recipient dog.
In conclusion, we have demonstrated that hematopoietic cells from 2 donors could stably engraft in a DLA-identical recipient following nonmyeloablative conditioning and a short course of postgrafting immunosuppression. The study also showed that the immunological tolerance established under these conditions could extend to the acceptance of a kidney allograft from one of the marrow donors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are grateful to Michele Spector, DVM, Alix Joslyn, Brian Steinmetz and the Fred Hutchinson Cancer Research Center Animal Laboratory Research Technicians for assistance in animal care; to David Mathes, MD, for assistance in kidney transplantation; to Patrice Stroup for the DLA typing; to Drs Beard, Kiem, Mielcarek, Nash, Parker, Thakar, Venkataraman, and Wang, who participated in weekend treatments; and to Bonnie Larson and Helen Crawford for manuscript preparation. The authors are grateful to Dr Elizabeth Squires, SangStat Medical Corporation, Freemont, CA, for the gift of cyclosporine; and to Dr Sabine Hadulco, Roche, Palo Alto, CA, for the gift of mycophenolate mofetil.
This work was supported by National Institutes of Health grants No. CA 78902 and CA15704. The laboratory also was supported through a prize from the Joseph Steiner Krebsstiftung, Bern, Switzerland, and The Lupin Foundation, Metairie, LA, awarded to R.S.
National Institutes of Health (NIH)
Authorship
Contribution: W.H., S.G., R.S., and G.G. designed the study. S.G., W.H., and R.D. conducted animal experiments. C.K., S.G., and S.W.B. performed animal surgeries. M.H. developed and supervised the trichimerism VNTR-PCR analyses. E.Z. supervised the typing and assigned the dogs to the study. C.J. and B.S. provided suggestions and interpretations of the study. S.G. and R.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott S. Graves, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1–100, PO Box 19024, Seattle, WA 98109-1024; e-mail: sgraves@fhcrc.org.