Abstract
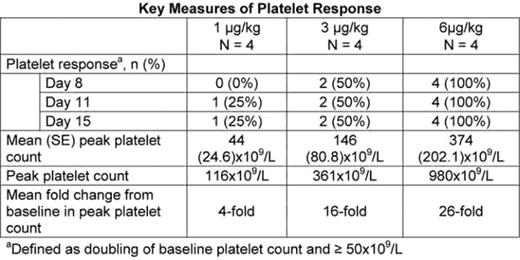
AMG 531 is a novel thrombopoiesis-stimulating peptibody that is being studied for its ability to increase platelet production by stimulating the thrombopoietin (TPO) receptor. This phase 2 study was conducted to identify the appropriate starting dose of AMG 531 for treatment of chronic ITP in adult Japanese patients. The study consisted of 2 phases: a 2-week cohort dose-escalation phase to determine the starting dose for subsequent phase 3 evaluation, and a treatment continuation phase (that lasted until completion of the dose-escalation phase) which provided continuation of treatment for those with a platelet response. Patients in the continuation phase received the dose of AMG 531 they had received in the dose-escalation phase with the option for subsequent dose adjustments. Twelve patients were enrolled with a mean platelet count of 11.8x109/L and a median age of 60.5 years (range 32 to 63); 8 were female. Four patients enrolled into each of 1μg/kg, 3μg/kg, or 6μg/kg dose cohorts and received AMG 531 by subcutaneous injection on days 1 and 8 with no dose adjustments. Cohort dose escalation was to be stopped in the event of an observed platelet count >1000x109/L. Comparison of dose cohorts showed that the proportion of patients achieving a platelet response (doubling of baseline counts and ≥50x109/L) was greater in cohorts receiving higher doses of AMG 531 (see Table). A dose response was also observed in the mean peak platelet counts, the mean fold changes from baseline in peak platelet counts, and the maximum platelet count. Because one patient in the 6μg/kg cohort had an excessively high platelet count (980x109/L), this dose was eliminated from consideration as the starting dose in phase 3 and further dose escalation to 10μg/kg dose cohort was stopped. Five of 7 eligible patients (3μg/kg, 1/4; 6μg/kg, 4/4) elected to enter the treatment continuation phase. There were no study withdrawals, and no serious adverse events (AEs) were reported during the study in either phase. The most common treatment-related AE in the cohort phase was headache (25%), and in the treatment continuation phase were arthralgia, contact dermatitis, and malaise (each 20%). No patients received rescue medications during the entire treatment period. No antibodies against either AMG 531 or endogenous TPO were detected. AMG 531 was well-tolerated and produced a dose-responsive increase in platelet counts. The phase 3 study in Japanese patients with ITP will be initiated with a starting dose of 3μg/kg based on the outcome of this study.
Author notes
Disclosure: Employment: YSonehara, MO, TO, JN: Amgen. Research Funding: YShirasugi, KA, SH, TN, YKurata, YKishimoto, KI: Amgen.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal