Abstract
The UKALLXII/ECOG2993 international study for adults with acute lymphoblastic leukeamia (ALL) included a separate, single-arm study (still open) to evaluate the role of etoposide/TBI conditioned allogeneic bone marrow transplantation (BMT) in patients with Philadelphia chromosome positive (Ph pos) disease. Patients received 2 phases of induction, intensification with high dose methotrexate and BMT using unrelated donor stem cells where a matched sibling was not available. 267 patients with Ph pos ALL were treated from 1993 onwards, in the pre-imatinib era. The study was amended in 2003 to add imatinib 600mg per day following induction and after BMT for 2 years or until relapse. A subsequent amendment at the end of 2005, added imatinib 600mg per day also to phase 2 of induction. 153 patients have now been treated on the imatinib-containing protocol − 89 received imatinib following induction and 64 started imatinib with phase 2 induction.
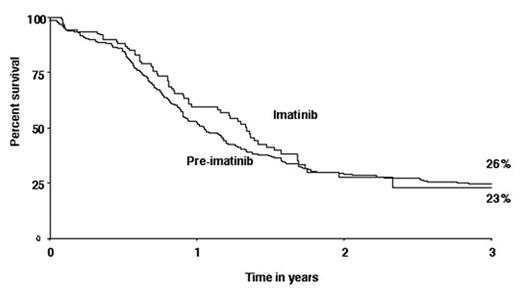
Complete remission (CR) rate at the end of 2 phases of induction was 91% with imatinib started in induction, 81% with imatinib started in consolidation only & 83% in the pre imatinib era. 128 of the 153 patients treated with any imatinib were eligible for BMT. Of the 98 patients with over 6 months follow-up, 57 (58%) actually received BMT. This transplant rate does not differ from the 62% in the pre-imatinib era although the data pertain mostly to those whose imatinib started later during therapy. Overall survival of the imatinib-treated group as a whole is 23% at 3 years, which does not differ from the 26% pre-imatinib rate as shown in the figure. Hence, imatinib started post-induction does not appear to improve survival. Imatinib given with induction may improve the CR rate, but there is insufficient follow up to assess transplant or survival rates. Earlier imatinib may be optimal but overall, our study does not provide evidence yet that imatinib alters the outcome of this disease.
Figure
Patient characteristics
| . | Pre-imatinib (N=267) . | Post-imatinib (N=153) . |
|---|---|---|
| Gender: Male | 150 (56%) | 96 (63%) |
| WBC (x 10 ^9/l) (2 unknown): <30 | 141(53%) | 86 (56%) |
| ≥ 30 | 124 (47%) | 67 (44%) |
| Median (range) | 26.8 (1.5–438) | 21.0 (0.5–491) |
| Age: median (range) | 40 (15–60) | 42 (16–64) |
| CNS disease (82 unknown) | 14 (8%) | 4 (3%) |
| . | Pre-imatinib (N=267) . | Post-imatinib (N=153) . |
|---|---|---|
| Gender: Male | 150 (56%) | 96 (63%) |
| WBC (x 10 ^9/l) (2 unknown): <30 | 141(53%) | 86 (56%) |
| ≥ 30 | 124 (47%) | 67 (44%) |
| Median (range) | 26.8 (1.5–438) | 21.0 (0.5–491) |
| Age: median (range) | 40 (15–60) | 42 (16–64) |
| CNS disease (82 unknown) | 14 (8%) | 4 (3%) |
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal