Abstract
FLT3–internal tandem duplications (FLT3-ITDs) comprise a heterogeneous group of mutations in patients with acute leukemias that are prognostically important. To characterize the mechanism of transformation by FLT3-ITDs, we sequenced the juxtamembrane region (JM) of FLT3 from 284 patients with acute leukemias. The length of FLT3-ITDs varied from 2 to 42 amino acids (AAs) with a median of 17 AAs. The analysis of duplicated AAs showed that in the majority of patients, the duplications localize between AAs 591 to 599 (YVDFREYEY). Arginine 595 (R595) within this region is duplicated in 77% of patients. Single duplication of R595 in FLT3 conferred factor-independent growth to Ba/F3 cells and activated STAT5. Moreover, deletion or substitution of the duplicated R595 in 2 FLT3-ITD constructs as well as the deletion of wild-type R595 in FLT3-ITD substantially reduced the transforming potential and STAT5 activation, pointing to a critical role of the positive charge of R595 in stabilizing the active confirmation of FLT3-ITDs. Deletion of R595 in FLT3-WT nearly abrogated the ligand-dependent activation of FLT3-WT. Our data provide important insights into the molecular mechanism of transformation by FLT3-ITDs and show that duplication of R595 is important for the leukemic potential of FLT3-ITDs.
Introduction
Mutations in the FMS-like tyrosine-kinase 3 (FLT3) are one of the most frequently found genetic alterations in patients with acute myeloid leukemia (AML),1-13 myelodysplastic syndromes (MDSs;10%-15%),2,14 and acute lymphoblastic leukemia (ALL; 1%-3%).9,16,17 FLT3 belongs to the class III of receptor tyrosine kinases, which are characterized by the presence of an extracellular immunoglobulin-like domain, a transmembrane and the cytoplasmic juxtamembrane (JM) domain, and the tyrosine kinase domain (TKD).18 The class III receptors also include KIT, CSF-1, PDGFRA, and PDGFRB.19,20
Activation of FLT3 by FLT3 ligand (FL) leads to receptor oligomerization and transphosphorylation of specific tyrosine residues,21 which activates the downstream signaling pathways including STAT5, Ras/mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3-kinase (PI3K)/AKT.22-25 FLT3 is highly expressed in CD34+ hematopoietic progenitor cells and plays an important role in normal hematopoiesis.26-29 Three distinct activating mutations of FLT3 in hematologic malignancies have been reported: point mutations (FLT3-JM-PM)30,31 and internal tandem duplications (FLT3-ITD) in the JM domain and mutations in the tyrosine-kinase domain (FLT3-TKD).1,8,9,12,16,17,32
The crystal structure of FLT3 has shown that the JM domain acts as an autoinhibitory domain in the inactive state.33 The JM domain is highly conserved across all members of class III RTKs. Hence many tumors in humans show activating mutations of JM in class III RTKs.34-37 FLT3-ITDs, found in a majority of acute leukemia patients, are in-frame duplications of a fragment of the JM domain. FLT3-ITDs are highly heterogeneous and vary in length from 2 to 68 AAs. These duplications are thought to disrupt the autoinhibitory mechanism and result in constitutive activation of the catalytic domain of FLT3. Activated FLT3 mutants promote cell proliferation and inhibit apoptosis, leading to factor-independent growth of murine hematopoietic cells in vitro and a myeloproliferative phenotype in vivo.38
FLT3-ITDs are present in the leukemic blasts of 20% to 30% of all AML patients. Recent studies have also shown that FLT3-ITDs are found in the leukemic stem cells.39 Furthermore, the presence of a FLT3-ITD has been recognized as an independent poor prognostic factor in AML and is associated with a decreased survival due to an increased relapse rate.8,9,11,12,40-43 Several factors influence the poor prognosis seen in AML patients harboring FLT3-ITDs (eg, a high FLT3-ITD/wild-type ratio).9,44 A recent study has reported that the detection of FLT3-ITD mutation in less mature progenitor populations, for example, CD34+/CD33−, might be associated with drug resistance.43
In the present study, we asked whether any common duplicated motif exists in AML patients carrying FLT3-ITDs, which might be responsible for the transforming potential. To address this question, we sequenced and analyzed the JM region of FLT3 from 284 patient samples with acute leukemias carrying FLT3-ITDs. We found that the length of FLT3-ITDs varied from 2 to 42 amino acids (AAs) with a median of 17 AAs. Duplications were localized in the AA stretch from 591 to 599 (YVDFREYEY) of FLT3. Arginine 595 (R595), within this region, is duplicated in 77% of patients. In vitro studies showed that both wild-type and duplicated R595 are critical for the transforming potential of the FLT3-ITDs. These data provide important insight into the molecular mechanisms of malignant transformation by FLT3-ITDs.
Patients, materials, and methods
Patients and samples
Two hundred eighty-four patients diagnosed with acute leukemias and carrying FLT3-ITD listed in the patient data bank of the Laboratory for Leukemia Diagnostics, University Clinic of Grosshadern, Munich were analyzed in this study. All patients gave informed consent before entering the study. The study design adhered to the principles of the Declaration of Helsinki and was approved by the ethics committees of the participating institutions. Clinical and laboratory data of patients analyzed in this study are given in Table 1.
Clinical and laboratory data of the patients analyzed in this study
| . | N . |
|---|---|
| No. of patients | 284 |
| Age, y | |
| Range | 18-89 |
| Median | 60.5 |
| Sex | |
| Female | 163 |
| Male | 121 |
| FAB class | |
| AUL | 2 |
| Biphenotypic | 3 |
| ALL | 4 |
| AML M0 | 6 |
| AML M1 | 63 |
| AML M2 | 49 |
| AML M3 | 28 |
| AML M4 | 39 |
| AML M5 | 21 |
| AML M6 | 4 |
| MDS | 3 |
| AML with unknown FAB | 52 |
| Patients without clinical data | 10 |
| WBC count, 109/L | |
| Median | 50 |
| Range | 12-16 |
| Cytogenetic abnormality | |
| Favorable | 29 |
| Intermediate | 185 |
| Adverse | 11 |
| Unknown | 59 |
| . | N . |
|---|---|
| No. of patients | 284 |
| Age, y | |
| Range | 18-89 |
| Median | 60.5 |
| Sex | |
| Female | 163 |
| Male | 121 |
| FAB class | |
| AUL | 2 |
| Biphenotypic | 3 |
| ALL | 4 |
| AML M0 | 6 |
| AML M1 | 63 |
| AML M2 | 49 |
| AML M3 | 28 |
| AML M4 | 39 |
| AML M5 | 21 |
| AML M6 | 4 |
| MDS | 3 |
| AML with unknown FAB | 52 |
| Patients without clinical data | 10 |
| WBC count, 109/L | |
| Median | 50 |
| Range | 12-16 |
| Cytogenetic abnormality | |
| Favorable | 29 |
| Intermediate | 185 |
| Adverse | 11 |
| Unknown | 59 |
Favorable, intermediate, and unfavorable cytogenetic abnormality has been defined according to published data.64
AUL indicates acute undifferentiated leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; and WBC, white blood cell.
Reagents and cell lines
Low passage murine Ba/F3 cells were obtained from the DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany) and were maintained in RPMI-1640 medium with 10% fetal bovine serum (FBS) and 10% WEHI conditioned medium as a source of murine IL-3 when indicated. PKC412 was kindly provided by Novartis Pharma (Basel, Switzerland).
Cell proliferation of Ba/F3 cells
Cells were seeded at a density of 4 × 104/mL for short-term proliferation and in the presence or absence of IL-3 and inhibitor as indicated. Viable cells were counted at the indicated time points in a standard hemacytometer after staining with trypan blue. Figures show mean values and standard deviations from 3 independent experiments unless otherwise indicated.
The following antibodies were used: anti-FLT3 antibody (sc-480; Santa Cruz, Heidelberg, Germany), anti–phospho-STAT5-Tyr694 (New England Biolabs, Frankfurt, Germany), and anti-STAT5 (sc-835; Santa Cruz).
DNA constructs and vectors
The FLT3-ITD-W51 construct contains a 7–amino acid–duplicated sequence (REYEYDL) inserted between AAs 601/602 of human FLT3-WT, and the FLT3-ITD-NPOS contains a 28-AA–duplicated sequence (CSSDNEYFY-DFREYEYDLKWEFPRENL) inserted between AAs 611/612 of FLT3-WT. All FLT3 constructs were subcloned in the MSCV-IRES-EYFP/EYFP retroviral expression vector (kindly provided by R. K. Humphries, The Terry Fox Laboratory, Vancouver, University of British Columbia).
In vitro mutagenesis
The mutants FLT3-WT-ins595R, FLT3-WT-ins597EY, FLT3-WT-ins596RE, FLT3-WT-ins597REY, FLT3-WT-ins591YFY, FLT3-WT-ins602KWE, and FLT3-WT-Δ595 in the FLT3-WT; FLT3-W51-R602A, FLT3-W51-R602E, FLT3-W51-R602K, FLT3-W51-ΔR602, FLT3-W51-ΔE603, and FLT3-W51-ΔR595 in the FLT3-ITD-W51; and FLT3-NPOS-ΔR623 in the FLT3-ITD-NPOS were created using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The correct sequence of all constructs was confirmed by nucleotide sequencing.
Transient transfection
Transient transfection of 293 cells and stable transduction of Ba/F3 cells were performed as previously described.45
Western blot analysis
Western blot analysis was performed as previously described.45
Statistical analysis
Western blot analysis of duplicated amino acids was performed by PERL-programs (Version 5; http://www.perl.com). Sequences were parsed with regular expressions. Sequence patterns were analyzed as frequency tables of individual amino acids by position as well as frequency tables of subsequences by position. All subsequences of length 1 to 30 were extracted from the data set and sorted by frequency.
Results
Internal tandem duplications are located in the common motif YVDFREYEY and include R595 in 77% of patients
The most common form of activating FLT3 mutation is an internal tandem duplication, which occurs in 20% to 25% of patients with AML1,3,4 and 5% to 10% of patients with MDS.1,3,4 Since these duplications are of variable length and location, we aimed to identify a common duplicated motif in the FLT3-JM region.
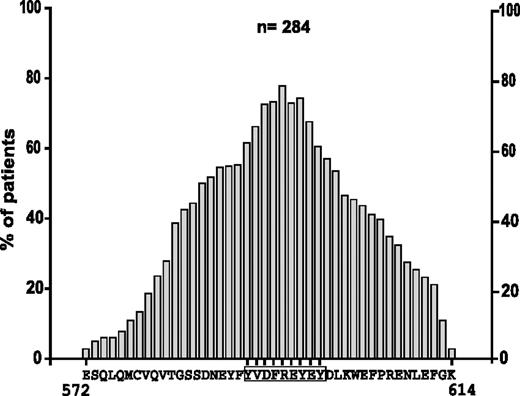
The cDNA of 284 unselected patient samples carrying FLT3-ITDs was analyzed. Nucleotide sequencing of the JM region showed that 118 patients expressed pure tandem duplications, whereas 166 patients carried additional insertions always maintaining the reading frame (data not shown). The length of duplications varied from 2 AAs to 42 AAs, with the median length being 17 AAs. In 95% of the patients, at least one AA within the stretch Y591 to Y599 (YVDFREYEY) was duplicated (data not shown). Analysis of the frequency of single AAs in the duplicated region revealed that arginine 595 was the most frequently duplicatedsingle AA in 77%, followed by Y597 in 74%, and F594 and E596 in 73% of all patients (Figure 1).
Analysis of the AA composition in the duplicated region in FLT3-ITDs. Frequency of single AAs by position in the duplicated region is provided. For each position, the most frequent single AA was selected.
Analysis of the AA composition in the duplicated region in FLT3-ITDs. Frequency of single AAs by position in the duplicated region is provided. For each position, the most frequent single AA was selected.
Next, we analyzed the frequency of AA combinations, ranging in length from 1 to 30 AAs within the duplicated region. As shown in Figure 2A, the single R595 is subsequently followed by the combination of amino acids EY (AA596-597) and REY (AA595-597) in 70% of all patients.
Duplications located in the motif YVDFREYEY and include R595 in 77% of patients. (A) Most frequent AA combinations within the duplicated region, sorted by length from 1 to 30 AAs. (B) Panel showing the duplicated sequences of 25 patients ranging from 2 to 8 AAs.
Duplications located in the motif YVDFREYEY and include R595 in 77% of patients. (A) Most frequent AA combinations within the duplicated region, sorted by length from 1 to 30 AAs. (B) Panel showing the duplicated sequences of 25 patients ranging from 2 to 8 AAs.
These findings point to a commonly duplicated motif that centers around R595 within the Y-rich stretch from AAs 591 to 599 (YVDFREYEY). We hypothesized that this region might play an important role for the transforming activity of FLT3-ITDs. To confirm this hypothesis, we analyzed the patients that carried the shortest ITDs. Figure 2B shows the duplicated sequences of 25 patients ranging in length from 2 to 8 AAs. The patient with the shortest duplication (2 AAs) showed insertion of R595 and E596. All but 4 patients (21/25 = 84%) had duplications of R595. Moreover, all patients had duplications of at least one amino acid of the protein stretch REYEY (AAs 595 to 599).
Taken together, our data show that a common duplicated AA stretch can be defined in patients with FLT3-ITD. This region includes R595, which is duplicated in 77% of all patients and supports the hypothesis that this residue might play an important role for the transforming potential of FLT3-ITDs.
Insertion of a single arginine between AAs 595 and 596 in FLT3-WT confers IL-3–independent growth to Ba/F3 cells and activation of STAT5
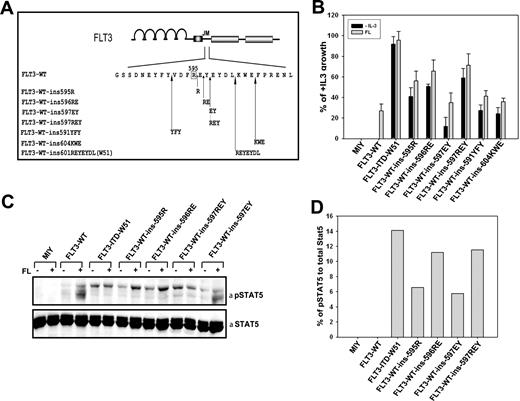
The acquisition of FLT3-ITD mutations in the FLT3 gene was shown to induce constitutive activation of the receptor and ligand-independent cell growth in different cell lines.5,16,32,46 To validate our hypothesis that duplication of R595 plays an important role for the transforming activity of the receptor, we introduced an arginine in the FLT3-WT cDNA between positions 595 and 596 (FLT3-ins595R). The second and third most frequently duplicated AA combinations E596/Y597 (FLT3-ins597EY) and R595/E596/Y597 (FLT3-ins597REY), and also the shortest duplication found in patients (ie, AA combination R595/E596 [FLT3-ins596RE]) were generated by in vitro mutagenesis (Figure 3A). Furthermore, we analyzed if tandem duplication of AAs outside the region of AA592-599 can confer factor-independent growth. Hence, we generated 2 different hypothetical ITDs that have duplications outside the AAs 592-595 stretch (FLT3-WT-ins591YFY and FLT3-WT-ins602KWE in FLT3-WT) using in vitro mutagenesis (Figure 3A).
Duplication of R595 in FLT3 induces IL-3–independent growth in Ba/F3 cells. (A) Localization of insertion mutants of FLT3 generated by duplication of 1 to 3 AAs of the stretch between 3 AA regions: 595 to 597, 589 to 591, and 602 to 604. (B) Ba/F3 cells stably transduced with FLT3-WT, FLT3-ITD-W51, FLT3-ins595R, FLT3-ins596RE, FLT3-ins597EY, FLT3-ins597REY, FLT3-WT-ins591YFY, FLT3-WT-ins602KWE, or mock-transduced cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3 or FL (60 ng/mL). Viable cells were counted after 72 hours by trypan blue exclusion. The growth of cells with IL-3 was defined as 100% (control). Standard error of the mean calculated from 3 independent experiments is shown. (C) Western blot showing the autoactivation of STAT5 in the mutants FLT3-ins595R, FLT3-ins597EY, FLT3-ins597REY, and FLT3-ITD-W51 when compared to FLT3-WT in unstimulated cells. FLT3-WT-ins595R, FLT3-WT-ins597EY, FLT3-WT-ins597REY, FLT3-WT-ins596RE, FLT3-ITD-W51, FLT3-WT, or mock-transduced cell lines were starved for 24 hours in the presence of 0.3% FBS and stimulated with 60 ng FL/mL for 5 minutes. Crude cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on a nitrocellulose membrane. Blots were then incubated with anti–phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody. (D) Densitometric analysis of the Western image in panel C was used to quantify the ratio of phospho-STAT5 to total STAT5.
Duplication of R595 in FLT3 induces IL-3–independent growth in Ba/F3 cells. (A) Localization of insertion mutants of FLT3 generated by duplication of 1 to 3 AAs of the stretch between 3 AA regions: 595 to 597, 589 to 591, and 602 to 604. (B) Ba/F3 cells stably transduced with FLT3-WT, FLT3-ITD-W51, FLT3-ins595R, FLT3-ins596RE, FLT3-ins597EY, FLT3-ins597REY, FLT3-WT-ins591YFY, FLT3-WT-ins602KWE, or mock-transduced cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3 or FL (60 ng/mL). Viable cells were counted after 72 hours by trypan blue exclusion. The growth of cells with IL-3 was defined as 100% (control). Standard error of the mean calculated from 3 independent experiments is shown. (C) Western blot showing the autoactivation of STAT5 in the mutants FLT3-ins595R, FLT3-ins597EY, FLT3-ins597REY, and FLT3-ITD-W51 when compared to FLT3-WT in unstimulated cells. FLT3-WT-ins595R, FLT3-WT-ins597EY, FLT3-WT-ins597REY, FLT3-WT-ins596RE, FLT3-ITD-W51, FLT3-WT, or mock-transduced cell lines were starved for 24 hours in the presence of 0.3% FBS and stimulated with 60 ng FL/mL for 5 minutes. Crude cell lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on a nitrocellulose membrane. Blots were then incubated with anti–phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody. (D) Densitometric analysis of the Western image in panel C was used to quantify the ratio of phospho-STAT5 to total STAT5.
We stably transduced the pro-B-cell line Ba/F3 with the FLT3-WT-ins595R/ins596RE/ins597EY/ins597REY/ins591YFY/ins602KWE constructs. In addition, Ba/F3 cells expressing FLT3-WT and FLT3-ITD-W51 were generated. Expression of all FLT3 constructs was confirmed by CD135 antibody staining and fluorescence-activated cell sorting (FACS) analysis as well as Western blotting (data not shown).
Overexpression of the mutant FLT3-ins595R, but not FLT3-WT, induced IL-3–independent growth in Ba/F3 cells (Figure 3B). In detail, the growth rate of FLT3-ins595R was 40% compared to cells expressing the FLT3-ITD-W51 construct, which served as a positive control. Similar results were obtained for cells expressing FLT3-ins597RE (50.7%) and FLT3-ins597REY (58.9%). When expressed in Ba/F3 cells, the FLT3-insEY construct induced a 15% growth rate compared to FLT3-ITD-W51 (Figure 3B). The cells expressing the constructs FLT3-WT-ins591YFY and FLT3-WT-ins602KWE also showed factor-independent growth of 28% and 24%, respectively, when compared to FLT3-ITD-W51 (Figure 3B), but again the transforming potential was weaker when compared to the cells expressing FLT3-ins595R, FLT3-ins596RE, and FLT3-ins597REY. To analyze whether the autonomous growth of these mutants might be further stimulated by exogenous ligand, all these mutant cell lines were grown in the presence of 60 ng FLT3 ligand (FL)/mL, and viable cells were counted after 72 hours. All mutants showed a growth rate which was 1.3 to 3 times higher, than that of FLT3-WT cells grown under identical conditions (Figure 3B). These data clearly indicate that the duplication of R595 is sufficient to activate the transforming potential of FLT3. Introduction of an ITD outside the AA stretch 591 to 595 resulted in weaker transforming potential compared to constructs carrying R595 duplication.
STAT5 is the downstream target of the constitutively activated FLT3 receptor probably responsible for the transforming potential of the FLT3 receptor in vitro and in vivo.46-48 To investigate the activation of the STAT5 signaling pathway, we prepared crude cell lysates of serum-starved Ba/F3 cells transduced with either vector control (MIY) or FLT3WT, FLT3-ITD-W51, FLT3-ins595R, FLT3-ins597EY, FLT3-ins597RE, and FLT3-ins597REY. Lysates were analyzed by immunoblotting with a specific antibody against phospho-STAT5. We could clearly demonstrate that the expression of FLT3-ins595R, FLT3-ins597EY, FLT3-ins597RE, and FLT3-ins597REY led to increased phosphorylation of tyrosine at position 694 of STAT5 compared to FLT3-WT in accordance with the proliferation data; the FLT3-ins597EY mutant showed a significantly weaker STAT5 activation (Figure 3C-D)
Deletions or substitutions of R595 with alanine or glutamic acid in the duplicated region of FLT3-ITD reduce the proliferation rate of FLT3-ITD–transformed cells
Having shown that duplication of R595 can activate the transforming activity of FLT3, we asked whether R595 is necessary for the oncogenic potential of FLT3-ITD mutants.
For this purpose, we substituted the positively charged duplicated AA arginine (R602) with the neutral AA alanine (FLT3-W51-R602A) and also with the negatively charged AA glutamic acid (FLT3-W51-R602E) (Figure 4A). Furthermore, we generated a deletion mutant of duplicated R595 (FLT3-W51ΔR602) (Figure 4A) and the deletion of neighboring AA glutamic acid (FLT3-W51ΔE603) (Figure 4A). Ba/F3 cells were stably transduced with these different FLT3-ITD-W51 mutants and with FLT3-WT and FLT3-ITD-W51 as controls.
The positive charge of duplicated arginine 595 is critical for the transforming potential of FLT3-ITDs. (A) Shown are 3 different substitution and 2 deletion mutants of duplicated R595 generated in 2 different FLT3-ITDs (W51 and NPOS). The deletion mutant of duplicated E596 in FLT3-ITD-W51 is also shown. (B) Ba/F3 cells stably transduced with FLT3-WT, FLT3-ITD-W51, FLT3-W51-R602A, FLT3-W51-R602E, FLT3-W51-ΔR602, FLT3-W51-ΔE603, or mock-transduced cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3. Viable cells were counted after 72 hours. The growth of cells with IL-3 was defined as 100% (control). Standard error of the mean calculated from 3 independent experiments is indicated. (C,D) The mutants FLT3-NPOSΔR623 and FLT3-W51-R602K were expressed in Ba/F3 cells and analyzed as described in panel B.
The positive charge of duplicated arginine 595 is critical for the transforming potential of FLT3-ITDs. (A) Shown are 3 different substitution and 2 deletion mutants of duplicated R595 generated in 2 different FLT3-ITDs (W51 and NPOS). The deletion mutant of duplicated E596 in FLT3-ITD-W51 is also shown. (B) Ba/F3 cells stably transduced with FLT3-WT, FLT3-ITD-W51, FLT3-W51-R602A, FLT3-W51-R602E, FLT3-W51-ΔR602, FLT3-W51-ΔE603, or mock-transduced cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3. Viable cells were counted after 72 hours. The growth of cells with IL-3 was defined as 100% (control). Standard error of the mean calculated from 3 independent experiments is indicated. (C,D) The mutants FLT3-NPOSΔR623 and FLT3-W51-R602K were expressed in Ba/F3 cells and analyzed as described in panel B.
In proliferation assays, FLT3-W51ΔR602–, FLT3-W51-R602E–, and FLT3-W51-R602A–expressing cells showed a growth reduction of approximately 55% to 70% when compared to FLT3-W51 cells (Figure 4B), with the deletion mutant showing the maximum growth reduction of 70%. Next, we generated a deletion mutant of duplicated R595 (FLT3-NPOS-Δ623R) in a structurally different FLT3-ITD construct, FLT3-ITD-NPOS, that contains a 28-AA–duplicated sequence (CSSDNEYFY-DFREYEYDLKWEFPRENL) inserted between AAs 611/612 of FLT3-WT (Figure 4A). Expression of the deletion mutant (FLT3-NPOS-Δ623R) showed a similar phenotype compared to FLT3-W51ΔR602 with a reduction of approximately 70% of its proliferative capacity when compared to the FLT3-ITD-NPOS construct (Figure 4C). The deletion mutant of neighboring AA (FLT3-W51ΔE603) did not result in a significant growth reduction (Figure 4B).
Substitution of duplicated R595 with lysine did not alter the proliferation rate of the FLT3-ITD mutants
Having shown that the positively charged arginine plays an important role in the transforming potential of FLT3-ITDs, we asked if the duplicated arginine can be replaced with another positively charged amino acid. Hence, we substituted the duplicated arginine with the positively charged AA lysine (FLT3-W51-R602K) (Figure 4A). Overexpression of FLT3-W51-R602K in Ba/F3 cells induced factor-independent growth and did not show any reduction of the transforming potential when compared to FLT3-ITD-W51 (Figure 4D). These data clearly show that the positive charge of the AA (arginine or lysine) plays a crucial role in the transforming potential of FLT3-ITDs.
FLT3-ITD–duplicated R595 substitution/deletion mutants show a reduced capacity to activate STAT5 compared with FLT3-ITDs
To investigate the activation of the STAT5 signaling pathway, we prepared crude cell lysates of serum-starved Ba/F3 cells, transduced with either vector control (MIY) or FLT3-WT, FLT3-ITD-W51, FLT3-ITD-NPOS, FLT3-W51-R602A, FLT3-W51-R602E, FLT3-W51-R602K, FLT3-W51-ΔR602, FLT3-W51-ΔE603, and FLT3-NPOS-ΔR623. All the mutants FLT3-W51-R602A, FLT3-W51-R602E, FLT3-W51-ΔR602, and FLT3-NPOSΔ623R in Ba/F3 cells reduced the STAT5 activation compared to nonmanipulated FLT3-ITD constructs (Figure 5). STAT5 activation of FLT3-W51-R602K was similar to the FLT3-ITDs. Thus, the activation of the most important signaling pathway downstream of FLT3, STAT5, showed reduced phosphorylation in FLT3-W51-R602A, FLT3-W51-R602E, FLT3-W51Δ602R, and FLT3-NPOSΔ623R, but not in FLT3-W51-R602K–expressing cells, which correlates with IL-3–independent growth.
The constitutive STAT5 activation is reduced in substitution/deletion mutants of duplicated R595. FLT3-WT–, FLT3-ITD-W51–, FLT3-ITD-NPOS–, FLT3-W51-R602A–, FLT3-W51-R602E–, FLT3-W51-R602K–, FLT3-W51-ΔR602–, FLT3-W51-ΔE603–, and FLT3-NPOS-ΔR623–expressing cells were starved for 24 hours in the presence of 0.3% FBS. Blots were incubated with anti–phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody.
The constitutive STAT5 activation is reduced in substitution/deletion mutants of duplicated R595. FLT3-WT–, FLT3-ITD-W51–, FLT3-ITD-NPOS–, FLT3-W51-R602A–, FLT3-W51-R602E–, FLT3-W51-R602K–, FLT3-W51-ΔR602–, FLT3-W51-ΔE603–, and FLT3-NPOS-ΔR623–expressing cells were starved for 24 hours in the presence of 0.3% FBS. Blots were incubated with anti–phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody.
Deletion of wild-type R595 reduces the transforming potential and STAT5 activation of FLT3-ITD mutants
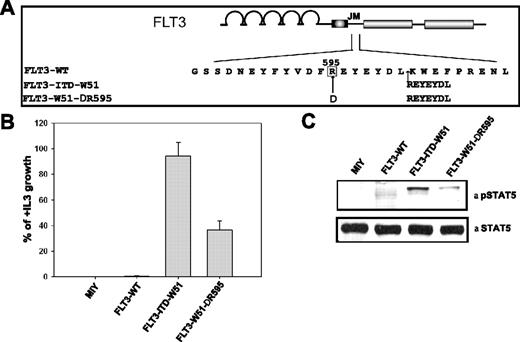
Since the duplicated arginine plays an important role in the transforming potential of the FLT3-ITDs, we asked whether wild-type R595 also has a critical role in the transforming capacity of FLT3-ITDs. We therefore created a deletion mutant of R595 in FLT3-ITD-W51 (FLT3-W51-ΔR595) (Figure 6A).
Deletion of wild-type R595 in FLT3-ITD-W51 results in reduced transforming potential and STAT5 activation in Ba/F3 cells. (A) Schematic representation of wild-type R595 (FLT3-W51-ΔR595) deletion mutant in the FLT3-ITD-W51 construct. (B) Ba/F3 cells expressing the FLT3-WT, FLT3-W51-ΔR595, FLT3-ITD-W51, and mock-transduced cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3. Viable cells were counted after 72 hours by trypan blue exclusion. The growth of cells with IL-3 was defined as 100% (control). The standard error of the mean calculated from 3 independent experiments is indicated. (C) Western blot image showing the activation of STAT5 in cells expressing FLT3-W51-ΔR595 and FLT3-ITD-W51, when compared to FLT3-WT or mock-transduced cells. Cells were starved in the presence of 0.3% FBS for 24 hours. Blots were incubated with anti–phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody.
Deletion of wild-type R595 in FLT3-ITD-W51 results in reduced transforming potential and STAT5 activation in Ba/F3 cells. (A) Schematic representation of wild-type R595 (FLT3-W51-ΔR595) deletion mutant in the FLT3-ITD-W51 construct. (B) Ba/F3 cells expressing the FLT3-WT, FLT3-W51-ΔR595, FLT3-ITD-W51, and mock-transduced cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3. Viable cells were counted after 72 hours by trypan blue exclusion. The growth of cells with IL-3 was defined as 100% (control). The standard error of the mean calculated from 3 independent experiments is indicated. (C) Western blot image showing the activation of STAT5 in cells expressing FLT3-W51-ΔR595 and FLT3-ITD-W51, when compared to FLT3-WT or mock-transduced cells. Cells were starved in the presence of 0.3% FBS for 24 hours. Blots were incubated with anti–phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody.
Overexpression of the wild-type arginine deletion mutant FLT3-W51-ΔR595 reduced the IL-3–independent growth by 64% when compared to FLT3-ITD-W51 (Figure 6B). These proliferation data were supported by the measurement of STAT5 activation (Figure 6C). STAT5 activation was significantly lower in the cells expressing FLT3-W51-ΔR595, when compared to FLT3-ITD-W51. These data suggest that not only the duplicated R595 but also the wild-type R595 plays an important role in the transforming potential and STAT5 activation of FLT3-ITD-W51.
Deletion of R595 in FLT3-WT nearly abrogates the growth of cells upon FL stimulation
As wild-type R595 had a significant effect on the transforming properties of FLT3-ITDs, we analyzed the role of the wild-type arginine 595 in the signaling properties of the FLT3-WT receptor. Hence, we created a deletion mutant of R595 in FLT3-WT (FLT3-WT-ΔR595) (Figure 7A) and expressed it in Ba/F3 cells.
Deletion of wild-type R595 in FLT3-WT abrogates the ligand-dependent activation of FLT3-WT in Ba/F3 cells. (A) Schematic representation of wild-type R595 (FLT3-WT-ΔR595) deletion mutant in the FLT3-WT construct. (B) Ba/F3 cells expressing the FLT3-WT, FLT3-WT-ΔR595, FLT3-ITD-W51, and mock-transduced cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3 and FL (60 ng/ml). Viable cells were counted after 72 hours by trypan blue exclusion. The growth of cells with FL was defined as 100% (control). The standard error of the mean calculated from 3 independent experiments is indicated. (C) Western blot image showing the abrogation of activation of STAT5 in cells expressing FLT3-WT-ΔR595, when compared to FLT3-ITD-W51 and FLT3-WT, upon stimulation of FL (60 ng/mL). Cells were starved in the presence of 0.3% FBS for 24 hours. Blots were incubated with anti–phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody.
Deletion of wild-type R595 in FLT3-WT abrogates the ligand-dependent activation of FLT3-WT in Ba/F3 cells. (A) Schematic representation of wild-type R595 (FLT3-WT-ΔR595) deletion mutant in the FLT3-WT construct. (B) Ba/F3 cells expressing the FLT3-WT, FLT3-WT-ΔR595, FLT3-ITD-W51, and mock-transduced cells were seeded at a density of 4 × 104 cells/mL in the absence or presence of IL-3 and FL (60 ng/ml). Viable cells were counted after 72 hours by trypan blue exclusion. The growth of cells with FL was defined as 100% (control). The standard error of the mean calculated from 3 independent experiments is indicated. (C) Western blot image showing the abrogation of activation of STAT5 in cells expressing FLT3-WT-ΔR595, when compared to FLT3-ITD-W51 and FLT3-WT, upon stimulation of FL (60 ng/mL). Cells were starved in the presence of 0.3% FBS for 24 hours. Blots were incubated with anti–phospho-STAT5 antibody, stripped, and reblotted with anti-STAT5 antibody.
Overexpression of FLT3-WT-ΔR595 did not show any IL-3–independent growth, but cell proliferation was almost abrogated in the presence of FL, when compared to FLT3-WT (Figure 7B). In detail there was a reduction of approximately 90% in the proliferation of cells expressing FLT3-WT-ΔR595 after stimulation with FL, when compared to cells expressing FLT3-WT (taken as 100%)
To check the signaling pathway of STAT5, we prepared crude cell lysates of serum-starved Ba/F3 cells transduced with MIG, FLT3-WT, and FLT3-WT-ΔR595 and FLT3-ITD-W51 acting as positive control. Lysates were analyzed by immunoblotting with a specific antibody against phospho-STAT5. We detected very slight STAT5 activation in the FLT3-WT-ΔR595 in the presence of FL, when compared to FLT3-WT–expressing cells (Figure 7C).
The arginine substitution/deletion mutants of FLT3-ITDs are sensitive to the FLT3 PTK inhibitor PKC12
PKC412 (Novartis Pharma), an inhibitor initially discovered as an inhibitor of protein kinase C, was found to block the phosphorylation and activity of FLT3-WT and mutant FLT3 receptors.49,50 The inhibitory activity of PKC412 against the arginine mutants of FLT3-ITDs was analyzed by treating the FLT3-W51-R602A–, FLT3-W51-R602E–, FLT3-W51Δ602R–, and FLT3-NPOSΔ623R–expressing cells with different concentrations of PKC412 ranging from 1 to 100 nM. PKC412 showed a strong growth inhibitory effect on FLT3-W51R602A, FLT3-W51-R602E, FLT3-W51-R602K, FLT3-W51Δ602R, and FLT3-NPOSΔ623R receptors expressing cells in the absence but not in the presence of IL-3. The IC50 of PKC412 was significantly lower in arginine substitution/deletion mutants (0.5-1 nM) compared to FLT3-ITD mutants (4 nM) (Table 2).
IC50 of PKC412 in different FLT3-ITD arginine mutants
| Mutant . | IC50, nM . |
|---|---|
| FLT3-ITD(W51/NPOS) | 4.0 |
| FLT3-W51-R602A | 0.9 |
| FLT3-W51-R602E | 0.8 |
| FLT3-W51-R602K | 4.0 |
| FLT3-W51-ΔR602 | 0.6 |
| FLT3-NPOS-ΔR623 | 0.7 |
| Mutant . | IC50, nM . |
|---|---|
| FLT3-ITD(W51/NPOS) | 4.0 |
| FLT3-W51-R602A | 0.9 |
| FLT3-W51-R602E | 0.8 |
| FLT3-W51-R602K | 4.0 |
| FLT3-W51-ΔR602 | 0.6 |
| FLT3-NPOS-ΔR623 | 0.7 |
Ba/F3 cells expressing FLT3-WT, FLT3-ITD-W51, FLT3-ITD-NPOS, FLT3-W51-R602A, FLT3-W51-R602E, FLT3-W51-R602K, FLT3-W51-ΔR602, and FLT3-NPOS-ΔR623 seeded at a density of 4 × 104 cells/mL in the absence or presence of different concentrations of PKC412 (0 to 100 nM) and counted after 72 hours. All cell lines were also cultured in the presence of IL-3 and 100 nM PKC412 to confirm nontoxicity of PKC412 to Ba/F3 cells. The IC50 was calculated from 3 independent experiments (IC50 is defined as the concentration of inhibitor required to induce a growth reduction of 50% compared to the cells grown in the absence of inhibitor).
Discussion
FLT3-ITDs are found in 20% to 25% of AML patients and are associated with an adverse prognosis. Recently, 2 different studies have reported conflicting results, stating the importance of the length of the duplication for the prognostic significance of the mutation. One study showed that longer duplications are associated with an unfavorable prognosis in patients with AML, whereas another study found the opposite.51,52 Given the substantial structural heterogeneity of FLT3-ITD mutations, no common motif has been identified so far that is duplicated in all patients. We sequenced the JM region of FLT3 in 284 patients with acute leukemias carrying internal tandem duplications in order to look for a common signature in these ITDs.
Our results confirm previous data showing that internal tandem duplications are heterogeneous with respect to length. The analysis of single residues within the duplicated region showed that R595 was the single most frequently duplicated AA in 77% of the patients, followed by Y597 in 73%, and F594 and E596 in 71% of all patients. A detailed statistical analysis of AA combinations from 1 to 30 AAs in the duplicated regions showed that, although there is no central motif common in all patients, the site of insertion (mutational hotspot) is frequently located in or around the Y-rich stretch region from AAs 591 to 599 (YVDFREYEY) of FLT3. R595 is duplicated in 77% of the patients followed by a combination of EY (E596 and Y597) in 70% of patients. In vitro studies showed that duplication of R595 in FLT3 is able to confer factor-independent growth to Ba/F3 cells and led to activation of STAT5. Substitution of the duplicated R595 with alanine (R602A) or glutamic acid (R602E) in a representative FLT3-ITD-W51 construct showed a significant reduction in the transforming potential of FLT3-ITD-W51. In contrast, substitution of the duplicated R595 with the positively charged AA (R602K) did not alter the transforming potential of cells compared to FLT3-ITD-W51 construct.
Statistical analysis of our data showed that R595 is the most frequently duplicated single AA. Moreover, the analysis of patients with the shortest duplications showed that R595 was duplicated in 84% of these patients. Of interest, the shortest duplication found in our cohort was an R595/E596 duplication. This prompted us to check if a single R595 duplication was sufficient to confer factor-independent growth to Ba/F3 cells. In vitro, duplication of a single AA, R595 (FLT3-ins595R), was sufficient to activate the transforming potential of FLT3. The weak transforming phenotype of FLT3-ins595R could be attributed to a slight disturbance in the autoinhibitory conformation of the JM region caused by a single amino acid insertion. Similar results were reported by our group on point mutations in the JM region found in AML patients that have a weak transforming potential, when compared to FLT3-ITDs.31 Addition of AAs next to arginine (FLT3-ins596RE, FLT3-ins597REY) increased the transforming potential but only slightly when compared to FLT3-ins595R. Moreover, FLT3-ITD mutant lacking the R595 duplication (ie, FLT3-ins595EY or FLT3-WT-ins591YFY/FLT3-WT-ins602KWE, which carry duplications outside AAs 592-599 stretch) showed a weaker transforming potential when compared to FLT3-ins595R (40.9%), FLT3-ins596RE (50.7%), FLT3-ins597REY (58.9%), and FLT3-ITD-W51 (92%). These data clearly show that the duplicated R595 plays an important role for the higher transforming potential of FLT3-ITDs. Ba/F3 cells expressing FLT3-ins595R, FLT3-ins597EY, FLT3-ins596RE, FLT3-ins597REY, FLT3-WT-ins591YFY, and FLT3-WT-ins602KWE showed hyperresponsiveness to FLT3 ligand, with a 1.3 to 3 times higher proliferation rate compared to FLT3-WT cells. This experimental setting probably reflects the in vivo situation, as it has been found that FL is coexpressed in AML blasts.54
FLT3-ITDs mutants are strong activators of STAT5,47,55 and STAT5 has been shown to be activated in blasts of 20% to 80% of patients with AML.56-59 Previous studies have also shown that STAT5 regulates the expression of early cytokine genes, such as PIM-1,60 which are responsible for cell survival and growth, and lead to tumor development and progression.61 In accordance with the proliferation data, all the mutants FLT3-ins595R, FLT3-ins596RE, FLT3-ins597REY, and FLT3-ins597EY showed activation of STAT5, but to a lesser degree compared to the FLT3-ITD-W51 mutant.
Determination of the crystal structure of FLT3-WT has indicated that the JM can be divided into 3 parts.33 The JM binding motif (JM-B) acts as the autoinhibitory domain, by preventing the rotation of the N lobe toward the C lobe of the tyrosine kinase domain (TKD) to generate the activated kinase fold, whereas the JM switch motif (JM-S) that lies next to JM-B provides a rigid and properly oriented scaffold for the interposition of tyrosines 589 and 591 between the JM-S and the C lobe of the kinase. FLT3-ITDs are frequently found in the JM zipper region (JM-Z) that aligns and maintains the JM-S in the proper orientation during and after the transition between activated and inactive states of FLT3. It is hard to predict the mechanisms by which these FLT3-ITDs change the conformation of the FLT3 protein, but it is hypothesized that the duplications offset the position of the JM-S in the FLT3 structure. This probably results in disturbing or preventing the optimal orientation of JM-S as it tries to position the JM-B in its binding site. Since FLT3-ins597EY showed a transforming potential 2 to 4 times lower than FLT3-ins595R, FLT3-ins596RE, and FLT3-ins597REY, we hypothesized that the positive charge of R595 might be involved in forming crucial interactions with other AAs. These new interactions might not only disrupt the autoinhibitory loop formed by JM-B, but also promote higher tyrosine phosphorylation by rendering the JM domain more accessible for autophosphorylation.
To analyze whether the positive charge of the duplicated R595 has any role for the oncogenic potential of FLT3-ITDs, we generated substitution mutants of the duplicated R595 with an aliphatic AA, alanine (FLT3-W51-R602A), and a negatively charged AA, glutamic acid (FLT3-W51-R602E), as well as a deletion mutant of the duplicated R595 (FLT3-W51-ΔR602) in the strongly transforming FLT3-ITD-W51. All mutants showed factor-independent growth in Ba/F3 cells, but the transforming potential of these mutants was reduced by 55% to 70% compared to FLT3-ITD-W51. The same degree of reduction was seen after deletion of the duplicated R595 in a different FLT3-ITD (FLT3-NPOS-ΔR623) construct. Of interest, there was no effect on the transforming potential when the duplicated R595 was replaced by another positively charged AA (eg, lysine; FLT3-W51-R602K). Deletion of the AA adjacent to arginine in FLT3-ITD-W51 (FLT3-W51ΔE603) did not significantly reduce the transforming potential of FLT3-ITD-W51, confirming that the positive charge of the duplicated R595 plays an important role in transforming potential of FLT3-ITDs. This hypothesis is further supported by the finding that deletion of WT-R595 in FLT3-ITD-W51 (FLT3-W51ΔR595) reduces the growth rate of Ba/F3 cells by 64% compared to the FLT3-ITD-W51 construct. All deletion and substitution mutants of wild-type and duplicated R595 in FLT3-ITDs (W51 and NPOS) showed a reduction in the activation of STAT5 except for the FLT3-W51-R602K, suggesting that the positive charge of wild-type and duplicated R595 has an essential role in activation of STAT5.
As the positive charge of both duplicated and wild-type R595 was critical to the transforming potential of FLT3-ITDs, we analyzed the role of R595 in the ligand-dependent activation of FLT3-WT. Deletion of R595 in FLT3-WT (FLT3-WT-ΔR595) nearly abrogated the growth of cells expressing FLT3-WT-ΔR595 upon stimulation with FL when compared to cells expressing FLT3-WT. This was further confirmed by the analysis of STAT5 signaling. Cells expressing the FLT3-WT-ΔR595 showed very weak activation of STAT5 when compared to FLT3-WT. These data clearly show that R595 regulates the mitogenic activity of FLT3-WT and its constitutively activated receptor mutants.
The importance of FLT3 for the survival and proliferation of AML blasts, and its mutation and overexpression in a large cohort of AML patients, has led to development of selective FLT3 protein tyrosine kinase inhibitors such as PKC412 and CEP-701. These inhibitors block the FLT3 kinase activity, thereby inducing apoptosis in FLT3-expressing cell lines, and cause cytotoxicity in primary ALL and AML blasts.49,62-64 PKC412, which was originally developed as an inhibitor of PKC, has also been found to inhibit FLT3 phosphorylation in vitro and in vivo.49,50,62 We tested the effect of PKC412 on the described substitution and deletion mutants of duplicated R595 in FLT3-ITD compared to nonmanipulated FLT3-ITD constructs as a control. The IC50 of PKC412 in FLT3-W51-R602A–, FLT3-W51-R602E–, FLT3-W51-ΔR602–, and FLT3-NPOS-ΔR623–expressing Ba/F3 cells was approximately 5 times lower in Ba/F3 cells expressing nonmanipulated FLT3-ITDs. In contrast, there was no difference in the IC50 of PKC412 for FLT3-W51-R602K–expressing Ba/F3 cells compared to FLT3-ITD-W51 cells. The high sensitivity of FLT3-W51-R602A, FLT3-W51-R602E, FLT3-W51-ΔR602, and FLT3-NPOS-ΔR623 toward PKC412 might be due to their weak transforming potential as seen in our study for point mutants in the JM region of FLT3.31
Our results clearly show that R595 is duplicated in most patients carrying FLT3-ITDs and plays a critical role in the transforming potential of FLT3-ITD mutants and ligand-dependent activation of FLT3-WT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 684/A12).
We would like to thank Bianka Ksienzyk for help in sorting of the cell lines. We would also like to thank Stefan Bohlander, Tobias Kohl, Aniruddha Deshpande, and Rob Chapman for critical reading of and suggestions in the preparation of the paper.
Authorship
Contribution: S.V. designed and performed research and wrote the paper; C.R. performed research and edited the paper; S.K.K., R.K., and G.M. performed research; T.M. and M.D. performed statistical analysis; S.S. performed research; W.H. designed research; and K.S. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: K. Spiekermann, Department of Medicine III, University of Munich-Grosshadern, Clinical co-operative group, “Leukemia,” GSF-National Research Center for Environment and Health, Marchioninistr 25, 81377 Munich, Germany; e-mail: karsten.spiekermann@med.uni-muenchen.de.