Anaplastic large-cell lymphoma (ALCL) was initially recognized on the basis of morphologic features and the consistent expression of CD30. It then became evident that the majority of these tumors are derived from lymphoid cells of T or null immunophenotype. The subsequent finding that t(2;5)(p23;q35) occurs in 40% to 60% of ALCL patients established a distinct clinicopathologic entity. This chromosomal translocation induces the formation of the chimeric protein nucleophosmin–anaplastic lymphoma kinase (NPM-ALK), which possesses significant oncogenic potential resulting from the constitutive activation of the tyrosine kinase ALK. In addition to its specific pathophysiologic events, NPM-ALK–expressing lymphoma presents with consistent clinical manifestations. Only 13 years after the identification of NPM-ALK, tremendous progress has been made in our understanding of this molecule because of the relentless efforts of multiple investigators who have dissected its biologic roles using in vitro and in vivo experimental models. Several upstream modulators, cross-reacting oncogenes, and downstream effectors of NPM-ALK have been identified and characterized. Understanding these interacting oncogenic systems is expected to facilitate the design of new therapeutic strategies and agents. In this review, we briefly discuss ALCL and focus on NPM-ALK.
Introduction
Anaplastic large-cell lymphoma (ALCL) is a relatively uncommon tumor. It was first recognized by Stein et al1 in 1985, who reported the consistent expression of the Ki-1 antigen (later designated CD30) in tumors with frequent cohesive proliferation of large pleomorphic cells. Most of these tumors were labeled “histiocytic malignancies.”1 The Ki-1 monoclonal antibody was originally described by the same group and was used to identify a novel antigen in the Hodgkin lymphoma cell line L428.2 Subsequent immunophenotyping and gene rearrangement studies showed that the vast majority of ALCL tumors are derived from lymphoid cells of T or null immunophenotype.3
Histologically, several ALCL variants have been described. Of these variants, the common, lymphohistiocytic, and small-cell are the most frequently encountered. The “horseshoe” or “wreath” cell is considered the cytologic hallmark of this disease.4 ALCL occurs as 2 distinct clinical entities, as a widespread systemic disease, or as a localized cutaneous disease. Systemic ALCL comprises 2% to 8% of non-Hodgkin lymphomas in adults and 10% to 15% of these lymphomas in children.5 The frequency of ALCL increases to 30% to 40% of non-Hodgkin lymphomas in children when only cases with large-cell morphology are included.
ALK
The recognition of t(2;5)(p23;q35) established the molecular definition of a subset of ALCL tumors that harbors this translocation.6,–8 In 1994, 2 independent groups cloned the genes involved in this translocation and illustrated the fusion of the nucleophosmin (NPM) gene on chromosome 5q35 to the previously unidentified gene anaplastic lymphoma kinase (ALK) gene on 2p23.9,10 This chromosomal translocation leads to the generation of the chimeric protein NPM-ALK. Identifying these tumors with this translocation became clinically feasible after the production of antibodies that specifically interact with chimeric NPM-ALK and full-length ALK proteins.11 Tumors harboring t(2;5)(p23;q35) and expressing ALK soon became a distinct clinicopathologic entity known as ALK-positive (ALK+) ALCL, which is included in the current World Health Organization classification system.12 Several other chromosomal aberrations involving ALK were subsequently identified, including t(1;2)(q21;p23) [chimeric protein TPM3-ALK], inv2(p23q35) [ATIC-ALK], t(2;3)(p23;q21) [TFG-ALK], t(2;17)(p23;q23) [CLTC-ALK], t(2;19)(p23;q13.1) [TPM4-ALK], and t(2;X)(p23;q11-12) [MSN-ALK].13 Immunohistochemical staining has shown that ALK expression is both cytoplasmic and nuclear in tumors with t(2;5)(p23;q35) but is strictly cytoplasmic in most of the other variants.14
All the ALK chromosomal aberrations lead to the expression and constitutive activation of ALK. This transmembrane receptor tyrosine kinase belongs to the insulin receptor superfamily. ALK expression in humans is normally limited to cells of neural origin. In mice embryos, it is widely expressed in the nervous system, but its expression level decreases significantly at birth.15,16 In Drosophila melanogaster, ALK is widely expressed in the gut.17 These observations suggest that ALK plays an important role in the development of these systems. Full-length ALK has been detected in neuroblastoma and rhabdomyosarcoma.18,19 Full-length ALK protein expression has been described not only in nonhematologic tumors but also in rare cases of diffuse large B-cell lymphoma.20
Whereas full-length ALK is not detected in normal hematopoietic cells, low levels of NPM-ALK and ATIC-ALK fusion gene mRNA have been identified in these cells.21,22 The expression of ALK in hematologic neoplasms is largely limited to ALCL tumors of T-cell or null-cell immunophenotype, in which 40% to 60% of these tumors express ALK.18 In 80% of these cases, ALK expression results from t(2;5)(p23;q35). Rare cases of large B-cell lymphoma have been found to have ALK rearrangements.23,24 ALK rearrangements and CLTC-ALK, TPM3-ALK, and TPM4-ALK chimeric proteins have also been detected in inflammatory myofibroblastic tumors and neuroblastoma.25,26
How ALK is physiologically activated is not completely known. In D melanogaster, ALK is activated by the Jelly belly (Jeb) protein, and the Jeb/ALK pathway appears to play an important role in the development of the gut system.27,28 In humans, the heparin-binding growth factors pleiotrophin and midkine have been reported to be the ligands binding and activating ALK,29,30 but this idea is still controversial.31
ALK+ ALCL tends to affect children and young adults and has a striking male predominance. The majority of ALK+ ALCL cases present as advanced stage (III or IV) systemic disease with generalized lymphadenopathy and extranodal involvement; particularly of the skin and soft tissue.32 Compared with ALK-negative ALCL, ALK+ ALCL demonstrates a significantly favorable prognosis.33 In this review, we specifically discuss ALK+ ALCL and focus on the pathobiology of NPM-ALK.
NPM-ALK
To understand the oncogenic role of NPM-ALK, we need to understand the physiologic functions of the ALK and NPM proteins. We discussed the physiologic functions of ALK in the previous section. In contrast to ALK, NPM is a ubiquitously expressed RNA-binding nucleolar phosphoprotein.34 Its physiologic functions include the shuttling of ribonucleoproteins between the nucleus and cytoplasm. NPM carries the oligomerizing motif that drives the homodimerization of NPM-ALK, which leads to the constitutive activation of ALK tyrosine kinase.35
Numerous studies have proven that NPM-ALK is oncogenic, and its transforming ability has been repeatedly demonstrated in vitro.35,36 Transgenic mouse models have shown that the enforced expression of NPM-ALK leads to the development of malignant lymphoma. A significant number of these lymphomas were of plasmablastic or B-cell immunoblastic morphology and phenotype, even when NPM-ALK expression was driven by T-cell–associated antigen promoters.37,,,,–42 Furthermore, several of these lymphomas were restricted to the mediastinum and they lacked CD30 antigen expression.
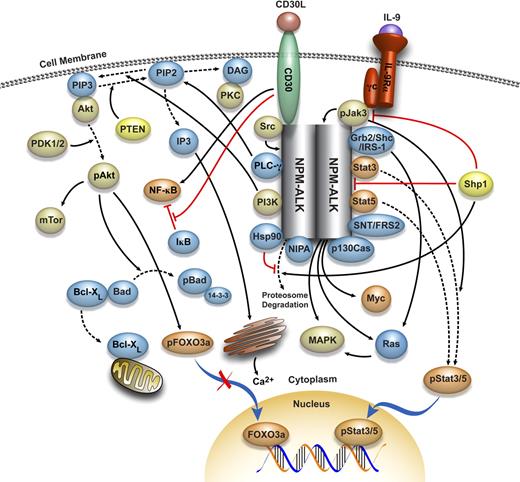
These important observations indicate that despite the universally accepted role of NPM-ALK in promoting lymphomagenesis, it is probably not the only factor that configures the known morphologic, immunophenotypic, and clinical features of NPM-ALK–expressing ALCL as we know it in humans. Most probably, NPM-ALK interacts with other biologic and molecular factors to result in the development of NPM-ALK–expressing lymphoma in humans with its characteristic features. NPM-ALK has been shown to interact with a wide range of oncogenic molecules (Figure 1), several of which are discussed in the subsequent sections.
Molecular network interacting with NPM-ALK. A complex network of protein kinases, protein phosphatases, transcription factors, apoptosis and cell-cycle regulators, adaptor proteins, and other molecules has been proposed to interact with NPM-ALK. The association and interaction between NPM-ALK and the majority of these molecules have been documented and briefly discussed in this review article. This model provides a rationale for designing specific and selective therapeutics that can target, individually or synergistically, members of this network and disrupt their oncogenic effects.
Molecular network interacting with NPM-ALK. A complex network of protein kinases, protein phosphatases, transcription factors, apoptosis and cell-cycle regulators, adaptor proteins, and other molecules has been proposed to interact with NPM-ALK. The association and interaction between NPM-ALK and the majority of these molecules have been documented and briefly discussed in this review article. This model provides a rationale for designing specific and selective therapeutics that can target, individually or synergistically, members of this network and disrupt their oncogenic effects.
Oncogenic molecules that interact with NPM-ALK
Jak/Stat
Signal transducers and activators of transcription (Stats) comprise a family of 7 latent transcription factors. Three major mechanisms contribute to the phosphorylation and subsequent activation of these proteins.43 The first is via a family of 4 cytokine receptor-associated tyrosine kinases known as Janus kinases (Jaks). The second mechanism of Stat activation is related to its interaction with receptor tyrosine kinases such as receptors for platelet-derived and epidermal growth factors. The third mechanism is related to its interaction with cytoplasmic tyrosine kinases such as Src and Abl. The Jak/Stat signaling pathway is one of the most extensively studied oncogenic mechanisms in NPM-ALK–expressing ALCL.
On cytokine/receptor engagement, Jaks undergo autophosphorylation at specific tyrosine residues. Some of these phosphotyrosine residues serve as docking sites where Stats bind to the receptor and undergo phosphorylation by Jaks. The activation of Stats is followed by their translocation to the nucleus where they regulate the transcription of a number of genes known to be directly involved in apoptosis, the cell cycle, and cell growth. This signaling pathway plays pivotal roles in normal cell differentiation and development.44 The activation status of Stats is under strict regulation, which involves the proper functioning of the activation and inhibition pathways.
Mounting evidence suggests that constitutively activated Stat3 is oncogenic and is found in a wide range of human cancers.45 In most experimental models, the net result of Stat3 activation is enhanced cell survival and proliferation. Blockade of Stat3 signaling has been shown to suppress tumorigenesis in vitro and in vivo. Constitutive activation of Stat3 is a highly consistent finding in ALK+ ALCL, and Stat3 activation significantly contributes to the pathogenesis of this lymphoma.46,–48 Transfection of a Stat3 dominant-negative construct into ALK+ ALCL cells has been shown to induce cell-cycle arrest and apoptosis.49
In parallel to the fact that Stat3 is activated by multiple mechanisms, the constitutive activation of Stat3 in ALK+ ALCL is multifactorial.50 NPM-ALK has been shown to physically bind to and phosphorylate Stat3, which results in its activation.51 In addition, Jak3, a physiologic activator of Stat3, is expressed and highly activated in ALK+ ALCL cell lines and primary tumors.51,–53 Unlike the activation of other members of the Jak family of kinases, the activation of Jak3 is restricted to a relatively small number of cytokines that possess the IL-2 common cytokine receptor γ-chain in their respective receptors.54,55 In addition to IL-2, this family includes IL-4, IL-7, IL-9, IL-15, and IL-21. Because Jak3 is a major physiologic activator of Stat3, it is not surprising that Jak3 contributes to the activation of Stat3 in ALK+ ALCL cells.52 Jak3 also appears to be bound to NPM-ALK,52,56 and inhibition of Jak3 decreases the tyrosine kinase activity of NPM-ALK.52 In addition, we recently identified the autocrine release of IL-9 by ALK+ ALCL cells as an upstream modulator of the Jak3/Stat3 signaling system in these cells.57 Consistent with the selective inhibition of Jak3, an anti–IL-9 neutralizing antibody was shown to downregulate phosphorylated Jak3 and Stat3 and to decrease NPM-ALK kinase activity.57 It is possible that the interactions between Jak3 and NPM-ALK are similar to the recently described interactions between Src and each of NPM-ALK and Bcr-Abl. Cussac et al58 and Meyn et al59 demonstrated that Src kinases are capable of phosphorylating NPM-ALK and Bcr-Abl, respectively.58,59
Another mechanism of Stat3 constitutive activation in ALK+ ALCL occurs via downregulation of SH2 domain-containing protein tyrosine phosphatase-1 (Shp1), which is a nontransmembrane tyrosine phosphatase predominantly expressed in hematopoietic cells.60 Shp1 plays important roles in regulating the growth and differentiation of hematopoietic cells. It has also been shown to be a major negative regulator of a wide range of cytokine-mediated signaling pathways.61 Shp1 modulates Jak/Stat signaling by binding to Jak via the SH2-binding motif, and dephosphorylating crucial tyrosine sites on Jak.61 Shp1 also has direct inhibitory effects on NPM-ALK via dephosphorylation of this protein.62 Loss of Shp1 expression is a relatively common defect in hematologic malignancies.63 In a series of ALK+ ALCL tumors derived from children and adult patients, Shp1 expression was lost in 50% and 86% of the tumors, respectively.62,64 Evidence of Shp1 gene methylation was detectable in a significant number of these cases.64 Loss of Shp1 is important to the pathogenesis of NPM-ALK–expressing lymphoma because restoration of Shp1 expression in NPM-ALK–expressing ALCL cell lines decreases Stat3 and Jak3 activation and enhances proteosome degradation of NPM-ALK and Jak3.65,66
Other negative regulatory systems of Jak/Stat, including the suppressors of cytokine signaling and protein inhibitors of activated Stat, have not been extensively investigated in NPM-ALK–expressing ALCL. Few studies have demonstrated that suppressors of cytokine signaling-3 is highly expressed in NPM-ALK–expressing cell lines and tumors, which might represent a biologic attempt to counterbalance the constitutively active Jak3/Stat3 signaling.67
Whereas the role of Jak3/Stat3 has been extensively explored in ALK+ ALCL, relatively few studies have addressed the role of other members of the Jak/Stat signaling family. These studies illustrated that in ALK+ ALCL, Jak2 and Stat5 exert mechanistic interactions and pathogenetic effects somehow reminiscent to those executed by Jak3 and Stat3. Nieborowska-Skorska et al68 found that NPM-ALK constitutively activates Stat5 and that this activation is essential to lymphomagenesis. However, the constitutive activation of Stat5 in these cells appears not to be restricted to NPM-ALK. Ruchatz et al69 demonstrated that Jak2 also contributes to the activation of Stat5 in these cells. In addition, Jak2 and NPM-ALK were shown to be physically associated and inhibition of Jak2 by a pharmacologic agent or dominant negative construct decreased NPM-ALK–mediated proliferation.69
PI3K/Akt
The class-Ia phosphoinositide 3-kinase (PI3K) is a heterodimer comprising a catalytic subunit (p110) and a regulatory subunit (p85).70 Activation of PI3K occurs as a sequel to receptor and nonreceptor tyrosine kinase activation. PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) at the 3′ position on its inositol ring, which leads to the production of the lipid second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3). Thereafter, PIP3 contributes to the recruitment to the plasma membrane of a wide range of downstream targets, including the serine/threonine protein kinase Akt (protein kinase B), via binding to their pleckstrin-homology domains.70 At the plasma membrane, another pleckstrin-homology domain-containing serine/threonine protein kinase, named 3-phosphoinositide–dependent protein kinase-1 phosphorylates Akt on Thr308.71 However, maximal activation of Akt requires additional phosphorylation at Ser473 by 3-phosphoinositide–dependent protein kinase-2.72 The level of PIP3 in the cell is strictly regulated by several lipid phosphatases such as the phosphatase and tensin homologue, which converts PIP3 back to PIP2.
The PI3K/Akt signaling pathway regulates a wide array of cellular processes critical for tumorigenesis, including proliferation, survival, growth, and motility.73 A major antiapoptotic effect of activated Akt resides in its ability to sequester the proapoptotic protein Bad and promote its binding to the cytoplasmic 14-3-3 proteins, which leads to increased free Bcl-XL.74 In various experimental models, activated Akt has also been shown to inhibit the activity of caspase 9, prevent nuclear localization of the Forkhead family of transcription factors (FOXO1 [FKHR], FOXO3a [FKHRL1], and AFX [FOXO4]), decrease transcription of Fas ligand, increase the activity of nuclear factor κB (NF-κB) and promote the degradation of the NF-κB inhibitor, IκB, increase the production of nitric oxide by enhancing the activity of endothelial nitric oxide synthase, and induce activation of the serine/threonine protein kinase mammalian target of rapamycin.73
The contribution of the PI3K/Akt signaling pathway to human cancer including ALK+ ALCL has been extensively investigated. Bai et al75 and Slupianek et al75 showed that NPM-ALK can activate this pathway. PI3K pharmacologic inhibitors have been shown to induce apoptosis in NPM-ALK–expressing lymphoma cells.75,76 An important finding is that dominant-negative PI3K and Akt mutants suppressed the proliferation of BaF3 cells transfected with NPM-ALK.76 In addition, transfection of NPM-ALK salvages Cos-1 cells from Bad-induced apoptotic cell death.75
PI3K/Akt signaling appears to induce cell-cycle progression in NPM-ALK–expressing lymphoma cells. This effect is mediated, at least partially, via the downregulation of the cyclin-dependent kinase inhibitor p27.77,78 Gu et al79 showed that Akt activation due to forced expression of NPM-ALK in BaF3 cells induced the phosphorylation and nuclear exclusion of FOXO3a (FKHRL1). This effect was associated with the upregulation of cyclin D2 and the downregulation of p27 and Bim-1.79 In further support of the role of PI3K/Akt signaling in NPM-ALK–expressing lymphoma, it has been recently shown that overexpression of activated Akt by using an adenoviral vector into NPM-ALK–expressing cell lines induces activation of mammalian target of rapamycin signaling proteins.80 Nonetheless, another recent study demonstrated that NPM-ALK can induce the activation of mammalian target of rapamycin signaling pathway primarily via a mitogen-induced extracellular kinase/extracellular signal-regulated kinase–dependent pathway, and to a much lesser extent through the PI3K/Akt signaling.81
CD30
CD30 is a member of the tumor necrosis factor receptor superfamily.82 The recognition of ALCL as a distinct clinicopathologic entity was primarily based on the consistent expression of CD30 by these tumors,1 although this protein is also detected in activated B- and T-lymphocytes, Reed-Sternberg cells, other types of malignant lymphomas, and rare solid tumors.83 Overexpression of CD30 in malignant lymphoma, including the NPM-ALK–expressing lymphoma, can be attributed to a constitutively active extracellular signal-regulated kinase/mitogen-activated protein kinase (MAPK)/JunB signaling cascade, which maintains high level of activity of the CD30 promoter.84,85 In addition, NPM-ALK has been shown to sustain the expression of CD30 and to be associated with its cytoplasmic domain.85,86 However, other studies showed that NPM-ALK can abrogate CD30 signaling.87 It is possible that the differences between these studies might be attributable to the experimental conditions as well as to the complexity of the biologic roles of CD30.
The physiologic function of CD30 is largely unknown. Depending on the cell type, activation of CD30 either enhances cell proliferation or induces apoptosis and cell growth arrest.88 Despite the similar mechanisms leading to CD30 overexpression in ALCL and Hodgkin lymphoma, the outcome of CD30 signaling differs significantly between the 2 diseases. Activation of CD30 decreases the proliferation and induces cycle arrest and apoptosis of NPM-ALK–expressing cells but not Hodgkin lymphoma cells.86,89,,–92 The cell-cycle arrest has been shown to be associated with the expression of p21 and the hypophosphorylation of the retinoblastoma protein.90,91
Contradictory findings have surrounded the role of NF-κB in CD30-mediated regulation of the cell cycle and apoptotic cell death in ALK+ ALCL. This role appears to be related, at least in part, to the mechanism of CD30 stimulation. Earlier studies demonstrated that activation of CD30 via cross-linking with an anti-CD30 antibody fails to induce NF-κB activation in these cells.86,89 In contrast, a subsequent study showed that an anti-CD30 antibody appears to indirectly activate NF-κB by decreasing IκB levels, which has been associated with upregulation of the cellular inhibitors of apoptosis (cIAP1 and cIAP2).93 A more recent study also showed that CD30 stimulation via interaction with its ligand (CD30L) induces activation of NF-κB in ALK+ ALCL cells.92 This study showed that the biologic outcome of this effect is time-dependent. Short, physiologic periods of stimulation induce apoptosis, and the surviving cells exhibit a high level of activation of both the canonical and alternative NF-κB pathways.92 The same study also demonstrated that longer durations of stimulation of NPM-ALK–expressing cells by CD30L induce upregulation of p21 and cell-cycle arrest, which is predominantly dependent on the activated canonical NF-κB pathway.92
PLC-γ
Activation of phospholipase C-γ (PLC-γ) through its interaction with receptor tyrosine kinases leads to hydrolysis of phophatidylinositol-4,5-biphosphate (PIP2) to soluble inositol-1,4,5-triphosphate (IP3) and membrane-bound diacylglycerol (DAG). IP3 and DAG act as secondary messengers in cellular signaling transduction.94 Whereas IP3 stimulates the release of Ca2+ from the endoplasmic reticulum to the cytosol, DAG binds and activates the serine/threonine protein kinase C (PKC).95
Bai et al36 demonstrated that NPM-ALK and PLC-γ are physically associated and identified Tyr664 residue on NPM-ALK as the binding site with PLC-γ. Replacement of Tyr664 with a phenylalanine residue abrogated the transforming potential of NPM-ALK; this finding supports an important role for PLC-γ in NPM-ALK oncogenic signaling.36 However, studies to further dissect the mechanisms by which PLC-γ transduces its mitogenic signaling in ALK+ ALCL are lacking. A recent gene array study showed that PKC is highly expressed in ALK+ ALCL.96 However, this study also showed that PKC is also overexpressed in ALK-negative ALCL cells, implying that PKC overexpression might not be directly associated with NPM-ALK.96
Grb2/Shc/insulin receptor substrate-1/Ras
Ras is a small GTPase with significant transforming potential through the modulation of MAPK.97 Activation of Ras by upstream tyrosine kinases is mediated via a number of widely expressed SH2 and SH3 domain-containing adaptor proteins such as growth factor receptor–bound protein 2 (Grb2), Src homology and collagen (Shc), and insulin receptor substrate-1.98,99
Simonitsch et al100 showed that NPM-ALK cooperates with Ras in inducing cellular transformation and that NPM-ALK is not, by itself, sufficient to transform rat embryonic cells without exogenously transfected Ras. In a more recent study, Turner et al101 demonstrated that NPM-ALK can induce the activation of Ras and the phosphorylation of extracellular signal-regulated kinase/MAPK. The physical association between NPM-ALK and Grb2, Shc, or insulin receptor substrate-1 has been documented, and these associations suggest a role for these molecules in transducing the oncogenic effects of NPM-ALK.35,36,102 Nonetheless, NPM-ALK mutants defective in the binding sites of Shc or insulin receptor substrate-1 (Tyr567 and Tyr156, respectively) were able to induce transforming effects. It is of note that the association of these mutants with Grb2 was still observed, which suggests that interaction of NPM-ALK with Grb2, but not Shc or insulin receptor substrate-1, is important for cell transformation.36,102 However, the exact binding site on NPM-ALK to Grb2 has yet to be identified. Regardless, few studies have investigated these signaling mechanisms in NPM-ALK–expressing cells, and thus a definitive role of these proteins has not been completely confirmed. Another caveat lies in the fact that these studies were performed using artificial models in which fibroblasts were transfected with NPM-ALK.
Myc
Myc is a transcription factor with potent oncogenic effects. It was originally identified as the cellular homolog of the product of the v-Myc oncogene of the avian myelocytomatosis virus.103 The Myc gene promoter is targeted by multiple signal transduction pathways including Jak/Stat and Ras/Raf/MAPK. Myc proteins drive the expression of a wide range of genes associated with cell proliferation and death.104 Tumor-associated biologic alterations result in the overexpression of Myc and the deregulation of its target genes. NPM-ALK induces its tumor-stimulatory effects, at least partially, via Myc. Transfection of rat fibroblasts with NPM-ALK results in elevated expression of Myc.105 Raetz et al106 used immunohistochemical staining to report that Myc expression was detectable in all 15 ALK+ ALCL tumors from pediatric patients, but in none of the ALK-negative tumors. The exact role of Myc and its interactions with upstream modifiers, including NPM-ALK, and downstream effectors is an area that needs further investigation.
Src
NPM-ALK associates with and activates the Src kinase pp60src; this association appears to be mediated via Tyr418 of NPM-ALK.58 Most probably, the interaction between NPM-ALK and pp60src is important for NPM-ALK–mediated oncogenesis, because loss of this interaction or direct inhibition of pp60src leads to inhibition of NPM-ALK–mediated proliferation.58 It is of note that pp60src has also been proposed to maintain the phosphorylation of NPM-ALK,58 similar to the recently identified mechanism for Bcr-Abl phosphorylation by Src.59 However, it is not yet clear which candidate targets are downstream of NPM-ALK-pp60src interactions. A recent study identified α-diacylglycerol kinase as a possible downstream target and discovered that its phosphorylation by NPM-ALK is dependent on pp60src.107 More studies are needed to delineate the role of Src kinases in ALK+ ALCL lymphoma. For example, Src is a major activator of Stat3, which is known to have an important role in the pathogenesis of ALK+ ALCL. It will be interesting to find out whether Src is capable of activating Stat3, independent of NPM-ALK, in this lymphoma.
Hsp90
Bonvini et al108 reported that pharmacologic blockade of heat shock protein (Hsp) 90 mediates effective apoptosis in ALK+ ALCL cell lines. This study highlighted the fact that NPM-ALK is unstable, and it undergoes proteosome degradation relatively rapidly. Most probably, Hsp90 protects NPM-ALK by slowing down its proteosome degradation. Targeting Hsp90 prevents its complex formation with NPM-ALK, probably by promoting the association of the latter with Hsp70 chaperone in an E3 ubiquitin ligase carboxyl terminus Hsp70-interacting protein (CHIP)–dependent manner.109
SNT/FRS2
Suc1- and Suc2-associated neurotrophic factor-induced phosphorylated target/fibroblast receptor substrates (SNT-1/FRS2α and SNT-2/FRS2β) are membrane-anchored docking proteins. Evidence supports a role for SNT-1 and SNT-2 in mediating signaling from receptors such as fibroblast growth factor receptor and the nerve growth factor receptor TrkA to Ras and MAPK.110 Recently, Chikamori et al111 demonstrated by the immunoprecipitation technique that NPM-ALK is physically associated with SNT-1 and SNT-2. This association was also detected when kinase-negative NPM-ALK mutant was used. The interaction between NPM-ALK and these adaptor proteins occurred via Tyr156, Tyr567, and a 19-amino-acid sequence of NPM-ALK. When these binding sites were mutated, the transforming potential of NPM-ALK was markedly diminished. A surprising finding was that these mutant constructs were still capable of binding molecules previously shown to contribute to the oncogenic effects of NPM-ALK, including PLC-γ and PI3K. This observation indicates that the contribution of these molecules may not be as important as was previously thought or that there is redundancy in the effects of the different oncogenic signaling pathways interacting with NPM-ALK. This area still requires more investigation.
NIPA
Using a yeast 2-hybrid screen, Ouyang et al112 identified the nuclear interacting partner of anaplastic lymphoma kinase (NIPA) as a novel NPM-ALK–interacting protein. NIPA is widely expressed in human tissues and it contains a nuclear localization signal in its carboxyl terminus. The interaction between NIPA and ALK is not limited to NPM-ALK, because other ALK chimeric proteins can also induce NIPA phosphorylation.112 NPM-ALK induces the phosphorylation of NIPA at several serine and tyrosine residues, particularly Ser354. Most probably, NIPA plays a role in NPM-ALK–mediated oncogenic effects by inhibiting apoptotic cell death. Mutations of the nuclear translocation signal or the Ser354 phosphorylation site block the antiapoptotic effects of NIPA. A more recent study identified an important role of NIPA in controlling mitotic entry and the cell cycle.113 In light of these findings, further analysis of the role of NIPA in NPM-ALK–mediated oncogenic effects is needed.
p130Cas
The mechanisms by which NPM-ALK induces its transforming effects have been relatively well characterized. Less is known about its effects on the cell shape. Transfection of NPM-ALK into fibroblasts or lymphoma cells induces notable morphologic changes.87,114 Depending on the cell type, NPM-ALK induces morphologic changes that make the cells similar to ALCL cells or induces neurite outgrowth.87,114 Using mass spectrometry–based screening of proteins interacting with NPM-ALK and involved in the cytoskeleton morphology, Ambrogio et al115 identified p130 Crk–associated substrate (p130Cas) protein and demonstrated that NPM-ALK is able to bind and phosphorylate this protein. They also showed that p130Cas plays a role in NPM-ALK–mediated modification and transformation of the cell shape.115
Current and future therapeutic approaches for ALK+ ALCL
Despite significant progress in delineating the molecular mechanisms of ALK+ ALCL, the therapeutic approaches to this lymphoma have not changed significantly. The current treatment is based on doxorubicin-containing combination chemotherapy.33,116 This treatment induces complete remission in up to 95% of the patients, but relapse and resistance occur in more than 40% of the cases.116,117 The exact cause of the high rate of relapse is not known. It has been suggested that a high age-related International Prognostic Index is a reliable indicator for a worse clinical outcome.32,33 It is also possible that ALK+ ALCL patients with poor clinical outcome possess specific biologic factors that affect the outcome of their disease. For example, even though Bcl-2 is infrequently expressed in ALK+ ALCL cells,118 these cells still express high levels of Mcl-1.119 In addition, although ALK+ ALCL cells have a high apoptotic rate,118 some tumor cells may remain dormant and later become the origin of relapses. Furthermore, ALK+ ALCL patients with tumors expressing CD56 or survivin have been shown to have unfavorable clinical outcome.120,121 Similarly, ALK+ ALCL patients with high levels of c-Jun activation binding protein-1 and low levels of p27 have been shown to demonstrate worse clinical outcome.122 In addition, ALK+ ALCL patients with tumors expressing the retinoblastoma protein show a trend, albeit not statistically significant, for a less favorable clinical course.123 One possible explanation is that the tumors that express the retinoblastoma protein demonstrate a lower apoptotic index.123 Recently, Lamant et al124 used gene-expression profiling to show that ALK+ ALCL primary tumors demonstrate significantly up-regulated CEBPB, BCL6, PTPN12, and SERPINA1 genes compared with tumors that lack ALK. The potential significance of CEBPB in NPM-ALK–expressing lymphoma has also been stressed by other investigators.125,126 The relationship between these observations and patient's survival and response to therapy still needs to be investigated.
The fact that NPM-ALK plays a central role in the development and pathogenesis of ALK+ ALCL tumors makes this chimeric protein a legitimate therapeutic target in this disease. Because of its repeatedly documented oncogenic potential and its expression in this type of malignant lymphoma, specific or selective targeting of NPM-ALK will probably be associated with less toxic effects, as compared with targeting other signaling partners that might be involved in maintaining the biologic functions of the nonneoplastic cells. Selective targeting of Bcr-Abl, a chimeric protein with constitutive tyrosine kinase activity and biologic features similar to NPM-ALK, has been used to treat chronic myeloid leukemia and other neoplastic diseases.127 Several recent studies have demonstrated that specific targeting of NPM-ALK may represent a promising therapeutic modality for ALK+ ALCL.128,,,–132 Indirect inhibition of NPM-ALK can also be achieved by inhibitors of Hsp90 such as herbimycin A and 17-AAG.108,133 Nonetheless, similar to Bcr-Abl, resistance to agents selectively targeting NPM-ALK is a real possibility, and therapeutic approaches based on combination or alternative agents will most probably be needed.
With our understanding of the pathobiology of NPM-ALK, blockade of other oncogenic systems interacting with NPM-ALK could be used separately or in conjunction with therapeutic approaches targeting NPM-ALK. Two of the more studied systems in this lymphoma are Jak/Stat and PI3K/Akt. These 2 pathways have recently been proposed to be the focus for the development of new pharmacologic agents to antagonize their effects in malignant diseases including NPM-ALK–expressing lymphoma.47,49,52,75,76,78 Another approach to targeting these systems is by blocking upstream cytokines and growth factors that induce their activation.57
Immunotherapy is another possible approach to treating ALK+ ALCL. In vitro and in vivo studies showed that anti-CD30 antibodies induce ALK+ ALCL apoptotic cell death and tumor regression.134,135 Other studies have suggested that CD26 might represent a promising immunotherapeutic target.136 Finally, gene therapy also may represent a promising future therapeutic modality for ALK+ ALCL. Adenoviral-mediated transfer of p16, p21, p27, and p53 induces apoptosis and cell-cycle arrest in ALK+ ALCL cell lines and tumors implanted in nude mice.137,138 In addition, the transfer of Shp1 suppresses NPM-ALK, Jak3, and Stat3 in ALK+ ALCL cells.66
Summary and conclusions
We have briefly discussed the pathobiology of ALK+ ALCL and emphasized the role of NPM-ALK in this disease. Despite being relatively uncommon, ALK+ ALCL has become an excellent study model for cancer. Over the past 13 years, accumulated data have illustrated and highlighted the collective role of oncoproteins, tumor suppressors, adaptor proteins, and other molecules in the development and progression of ALK+ ALCL. Despite the central role of NPM-ALK in the pathogenesis of this lymphoma, the model emerging from the studies on ALK+ ALCL stresses the collaboration of several members of a molecular network rather than individual culprits. This molecular network transforms ALK+ ALCL into a well-defined clinicopathologic entity. The configuration of this molecular network is most probably a multistep process, which is a well-known characteristic of oncogenesis. The current model provides a rationale for designing therapeutic approaches that can specifically disrupt multiple targets to eliminate the synergistic effects of the various members of the molecular network. As our understanding of the biology of ALK+ ALCL continues to evolve, it is highly anticipated that the knowledge gained from the studies of this lymphoma will be applicable to other neoplastic diseases.
Acknowledgments
The authors are grateful to Elizabeth Hess and Kim-Anh Vu for their valuable help with the preparation of this manuscript. The authors apologize to the laboratories whose contributions to this field could not be discussed or cited because of space limitation.
This work was supported in part by CA114395 grant from the National Institutes of Health (NIH) and by the Physician Scientist Program Award and an Institutional Research Grant from M. D. Anderson Cancer Center to H.M.A.; and by grants from the National Cancer Institute of Canada, Alberta Cancer Foundation, and the Canadian Institute for Health Research awarded to R.L.
National Institutes of Health
Authorship
Contribution: H.M.A. and R.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hesham M. Amin, Department of Hematopathology, Unit 72, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail:hamin@mdanderson.org; or Raymond Lai, Cross Cancer Institute, Room 2342, 11560 University Avenue, Edmonton, Alberta T6G 1Z2, Canada; e-mail: raymondl@cancerboard.ab.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal