Statins are a class of drugs that inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMGcoA) reductase, a critical enzyme in the mevalonate pathway. Several reports document that statins may prevent different human cancers. However, whether or not statins can prevent cancer is controversial due to discordant results. One possible explanation for these conflicting conclusions is that only some tumors or specific statins may be effective. Here, we demonstrate in an in vivo transgenic model in which atorvastatin reverses and prevents the onset of MYC-induced lymphomagenesis, but fails to reverse or prevent tumorigenesis in the presence of constitutively activated K-Ras (G12D). Using phosphoprotein fluorescence-activated cell sorter (FACS) analysis, atorvastatin treatment was found to result in the inactivation of the Ras and ERK1/2 signaling pathways associated with the dephosphorylation and inactivation of MYC. Correspondingly, tumors with a constitutively activated K-Ras (G12D) did not exhibit dephosphorylation of ERK1/2 and MYC. Atorvastatin's effects on MYC were specific to the inhibition of HMGcoA reductase, as treatment with mevalonate, the product of HMG-CoA reductase activity, abrogated these effects and inhibited the ability of atorvastatin to reverse or suppress tumorigenesis. Also, RNAi directed at HMGcoA reductase was sufficient to abrogate the neoplastic properties of MYC-induced tumors. Thus, atorvastatin, by inhibiting HMGcoA reductase, induces changes in phosphoprotein signaling that in turn prevent MYC-induced lymphomagenesis.
Introduction
Cancer is largely caused by genomic events which result in the activation of oncogenes or the loss of tumor-suppressor genes.1 The targeted inactivation of oncogenes may be a specific and effective therapy for treating certain malignancies.2,3 Experimental results in animal models validate the notion that the targeted inactivation of a single oncogene can be sufficient to reverse tumorigenesis.4 Drugs that target specific proteins have been identified and shown to be effective in the treatment of certain cancers,5,,,,,,,,,,,,,,,–21 which indicates that some neoplasms are susceptible to therapies that target their molecular causes. However, cancers can escape dependence upon oncogenes, as observed in animal models as well as in human patients.21,–23 Thus, the best targets may not be the suspected oncogenes but rather proteins essential to multiple signaling or metabolic pathways required for tumor maintenance.
MYC is one of the oncogenes most commonly associated with human cancer.24 Experimentally, the inactivation of MYC has been shown to induce sustained tumor regression in transgenic mouse models of many different tumors, suggesting that MYC may be a good target for cancer treatment.25,,–28 However, MYC as a transcription factor is not easy to target directly with small molecules. Thus, rather than directly targeting MYC, it might be possible to inactivate MYC through the disruption of upstream regulatory signaling molecules such as those in pathways regulated by Ras and ERK1/2.29,30
The enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMGcoA) reductase is rate-limiting in the mevalonate pathway31 (Figure S1A, available at the Blood website; see the Supplement Figures link at the top of the online article), and is required for the synthesis of isoprenoids that play fundamental roles in the regulation of cell signaling. Isoprenoid groups are necessary for the posttranslational modification of proteins, including Ras, that regulate cellular proliferation and apoptosis.32,–34 Statins are a class of drugs that inhibit HMGcoA reductase,35 and have been widely used to reduce cholesterol levels for the prevention of atherosclerosis.36 Statins have been reported to have potent antitumor properties both in vitro and in vivo.37,,,,,,,,–46 Several reports document that statins may prevent different cancers. Most notably, 1 study documented a 50% decrease in the incidence of colon cancer.47,48 However, 2 recent large meta-analyses suggest that statins may not prevent malignancy.49,50
We hypothesized that the antineoplastic effects of statins may be mediated by inhibiting the function of discrete oncogenic signaling pathways, which implies that these drugs may only demonstrate efficacy in a subset of molecularly defined tumors. Consistent with this possibility, we found in a transgenic mouse model that atorvastatin can reverse and prevent MYC-induced lymphomagenesis, but failed to do so in tumors induced in the context of constitutively activated K-Ras (G12D). We have examined signaling pathways by phosphoprotein fluorescence-activated cell sorter (FACS) analysis and found that atorvastatin induces a specific phosphoprotein signature by shutting down specific signaling networks required to sustain MYC activation.
Materials and methods
Transgenic mice
The “Tet-system” was used to generate transgenic mice that conditionally express the human MYC cDNA in T-cell lymphocytes, as previously described.19,48 Dr Harold Varmus (Memorial Sloan-Kettering Cancer Center, New York, NY) kindly provided the TRE-Ras (murine K-Ras [G12D]) transgenic line.51 Tumorigenesis experiments were performed as previously described.26,27,52 Mice images were acquired using Nikon Fdx-35 camera (Japan).
Transgenic cell lines
Single-cell suspensions were generated from tumors of MYC-overexpressing mice by mechanical disruption. Cells were grown in RPMI 1640 supplemented with 10% FCS, β-mercaptoethanol, glutamine, and penicillin-streptomycin. Early passages of the cell lines were used for the experiments described.
Histology
Tissues were fixed in 10% buffered formalin and embedded in paraffin. Sections (5 μm) were stained with haematoxylin and eosin.
TUNEL assay
Apoptotic cells were detected by the TUNEL (terminal dUTP nick-end labeling) assay in situ death detection kit (Roche Diagnostic, Indianapolis, IN) as described by supplier. Cells were counterstained with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired using a Nikon ECLIPSE E 800 microscope using SPOT RT-SLIDER camera and accompanying software spot 4.6, Diagnostic Instruments (Sterling Heights, MI).
Proliferation assay
Cells were grown in their respective media requirements, and cultures were pulsed for 18 hours with 0.037 MBq (1 μCi) per well of [3H]thymidine. Cells were harvested, and [3H]thymidine incorporation was measured. Results are shown as the mean of triplicate experiments with standard error of the mean (SEM) given as a percentage.
Cholesterol assay
Cellular cholesterol was detected using the Amplex Red Cholesterol Assay Kit (A12216; Molecular Probes, Eugene, OR) as suggested by the manufacturer.
Flow cytometry
Intracellular probes for active kinases were made by conjugating phosphospecific antibodies to the Alexa Fluor dye series as described, and were used in phosphoprotein-optimized conditions.53,–55 Cells (106 cells/mL) were fixed in tissue-culture plates in 2% PFA for 15 minutes at 37°C, and were permeabilized with methanol and stored at −20°C until stained. Cells were seeded into 96-well plates, washed in PBS, and stained with antibody cocktails in 4% FCS for 30 minutes, washed 3 times, and then processed for flow cytometry. All centrifugation, staining, and flow cytometry processes were performed on ice with ice-cold buffers using pretitered antibodies at optimal antibody concentrations and fluorophore to protein (FTP) ratios.53 Flow cytometry data are representative of 3 independent experiments. Flow cytometry (4-color) was collected on a FACSCalibur machine (BD Biosciences, San Jose, CA). Multicolor flow cytometry was collected on a FACSCAN or FACSARIA (BD Biosciences). Data were analyzed using FlowJo software (TreeStar, Ashland, OR). Phosphorylated kinase clustering was performed using TreeView (http://rana.lbl.gov/Eisensoftware/.htm) (Eisen et al56 ).
Antibodies
Phosphospecific antibodies to STAT5 (Y694), STAT1 (Y701), STAT3 (Y705), STAT3 (S727), ERK1/2 (T202/Y204), p38 (T180/Y182), Plcγ1 (Y783), and Lck (Y505) conjugated to Alexa 488, PE, or Alexa 647 were from BD Biosciences. Phosphospecific antibodies raised against IκB-α (S32/36), Ikkα (S176/180), cRaf (S259), cRaf (S338), cRaf (S621), Elk (S338), JNK (Y183/T185), AKT (S473), AKT (T308), cJun (S73), Bad (S112), Bad (S136), Mek (T394), Mek (S298), NFκB (S259), and Vav1 (Y160) were from Biosource International (Camarillo, CA). Antibodies against HMGcoA reductase and MYC were from Upstate Technologies (Billerica, MA). Cleaved caspase-3–PE, cleaved PARP-FITC, Bad-FITC, Bcl2-Fitc, and Annexin-Cy5 were from BD Biosciences. Alexa Fluor dye series 405, 430, 488, 546, 568, 647, and 700 were from Molecular Probes.
The antibodies used in Western blot analysis were the following: ERK1/2 (9102), p-ERK1/2 (4377), p-Raf (9427) (all from Cell Signaling Technology, Danvers, MA); Akt and p-Akt (S473) were from BD Biosciences. Antibodies against α-tubulin (5280) and Bax were from Upstate Cell Signaling Solutions (Billerica, MA); β-actin (A2668) was from Sigma (St Louis, MO); Ras (610002) was from BD Biosciences, DNA J (MS-225-p) was from Lab Vision Corp (Fremont, CA); caspase 3 and cleaved caspase 3 (9662) were from Cell Signaling Technologies; and RhoA (sc-418), RhoB (sc-1), Rap1 (sc-65), and Rac1 (sc-217) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against HMGcoA reductase (07-572) were from Upstate Technologies, and p-c-MYC (9401) was from Cell Signaling Technology.
Immunoblotting
Cell extracts were prepared by washing 2 × 106 cells (treated as indicated) in ice-cold PBS and harvesting in lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM Na2PO4, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, 1 mM PMSF, and protease inhibitor cocktail tablet (Boehringer Mannheim, Mannheim, Germany). Extracts were centrifuged at 18 000g rpm (5 minutes at 4°C), and 10 to 20 μg (BCA protein assay; Pierce, Rockford, IL) was immunoblotted using standard procedures. Blots were incubated with the indicated antibodies and developed using ECL (enhanced chemiluminescence; Amersham, Piscataway, NJ). Immunoblots were stripped by incubating with stripping buffer (62.5 mM Tris [pH 6.8], 10% SDS, and 1% β-mercaptoethanol for 30 minutes at 55°C) and reprobed.
Atorvastatin treatment
Atorvastatin (prescription formulation; Pfizer Inc, New York, NY) was put into suspension in PBS. Atorvastatin was administered orally in 0.5-mL doses weekly using 20-mm feeding needles (Popper and Sons, New Hyde Park, NY). PBS was administered as control. Purified atorvastatin (Sequoia Research Products, Pangbourne, United Kingdom) was used for in vitro studies.
Viral infections
Ad-MycWT, Ad-MycS62A, and Ad-MycT58A viruses were kindly provided as a gift from Dr Rosalie C. Sears (Oregon Health & Science University, Portland, OR).29 HeLa cells were infected as previously described.57 In brief, Hela cells were infected with virus by incubation in DMEM at 37°C for approximately 60 minutes at a cell-to-volume ratio of 4 × 105 cells/mL. Cells were monitored during infection. Media was replaced after infection and the cells were further incubated at 37°C. Infection was confirmed by GFP expression, and cells were then treated with atorvastatin. Cell images were acquired using a Nikon Eclipse TS100 microscope with attached D-50 camera.
Quantitation of mRNA by real-time PCR
Total RNA from lymphoma cells was extracted and purified using TRIzol reagent (Invitrogen, Carlsbad, CA), and RNA was quantified by spectrophotometer (Beckman Coulter, Fullerton, CA). After DNase I digestion (amplification grade, 18068-0158; Invitrogen) 2 μg of total RNA was reverse-transcribed by Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's protocol using random oligo-d(T) as primers. Real-time polymerase chain reaction (PCR) analysis was carried out on an ABI Prism 7900HT system (Applied Biosystems, Foster City, CA). PCR conditions were 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Each reaction contained cDNA (10 ng), primers (800 nM), and SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA) in a total volume of 6 μL. Primers were generously provided by Dr Bruno Amati (European Institute of Oncology, Milan, Italy). All samples were analyzed in triplicates and were quantified using a standard curve generated by serial dilution of cDNA pooled from nontreated and treated lymphoma cells. The level of each mRNA was normalized to that of ubiquitin. The Student t test was performed to compare the treated and nontreated tumors: (1) MYC ON versus MYC OFF; (2) MYC ON versus MYC ON plus atorvastatin; (3) MYC ON versus MYC ON plus mevalonate; and (4) MYC ON versus MYC ON plus atorvastatin plus mevalonate. Results were visualized using TreeView.56
Cell fractionation and protein isolation
Cell fractionation studies to determine Ras localization were performed as previously described.58 In brief, 200 million cells of atorvastatin-treated versus untreated cells were washed 3 times in PBS and homogenized in lysis buffer (1 mM EDTA, 20 mM Tris-HCl [pH 7.4]). Lysate was centrifuged at 31 200 rpm using a Beckman Coulter ultracentrifuge. The supernatant (soluble fraction) was concentrated using a 10 K molecular limit Nanosep centrifugal device (Pall Life Sciences, East Hills, NY). The membrane pellet was solubilized in 2× immunoprecipitation buffer (0.15 M NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS, 10 mM Tris-HCl [pH 7.5]) and centrifuged at 15 000 rpm to obtain a clarified supernatant. Protease and phosphatase inhibitors were included in all buffers. All homogenization, immunoprecipitation, and centrifugation steps were performed using fresh material, on ice with ice-cold buffers. The concentration of proteins in each sample was determined using a BCA assay kit (Pierce).
Results
Atorvastatin is effective in reversing and preventing MYC-induced lymphomagenesis
Whether or not statins can prevent cancer in humans is controversial.8,43,48,–50,59,60 One possible explanation for the discordant results is that only specific tumors or certain statins may be effective. We speculated that the efficacy of statins in treating cancer may be more easily defined in transgenic models of tumorigenesis. Previously, we have described a conditional transgenic model system for MYC-induced lymphomagenesis.52 The model system has the useful feature that the MYC transgene is conditionally regulated through the “Tet-system” via administration of doxycycline where the Ig heavy chain enhancer and the SR α promoter (EμSR) elements were used to drive expression of the Tet-regulatory elements. Thus, MYC is overexpressed in the hematopoietic cells in the absence of doxycycline and is repressed upon addition of doxycycline to the drinking water. MYC overexpression results in the development of T-cell lymphomas. Treatment of tumor bearing mice with doxycycline results in MYC inactivation and in the regression of the tumors.
To gain more insight into the mechanisms by which statins induce proliferative arrest and apoptosis in tumors, we first evaluated the in vitro efficacy of statins in our MYC-induced T-cell lymphomas. We tested the effect of several commercially available statins on the MYC-induced T-cell lymphomas. Atorvastatin was found to be more potent than simvastatin and lovastatin, whereas pravastatin was ineffective in inducing proliferative arrest (Figure 1A). Pravastatin is known to bind less effectively to HMGcoA reductase, which perhaps accounts for the lack of effect on tumor cells when compared with the other statins tested.61 Atorvastatin was found to induce a dose-dependent growth inhibition in the MYC-induced T-cell lymphomas (Figure 1B). Most of the tumor cells ceased to proliferate when treated with 10 μM atorvastatin. Similar results were seen in 6 independently generated transgenic tumor cell lines. Notably, tumors generated from mice in which p53 was knocked out or BCL2 was overexpressed also exhibited apoptosis upon statin treatment (data not shown). The concentration of atorvastatin used in the experiment is comparable with the plasma levels achieved in treated humans.62 The downstream product of HMGcoA reductase, mevalonate, prevented atorvastatin from inhibiting growth of tumor cells (Figure 1C). The effect of atorvastatin (10 μM) was compared with MYC inactivation in the MYC-induced tumor cells. Atorvastatin treatment of MYC-induced transgenic lymphomas in vitro was associated with proliferative arrest and apoptosis at higher levels than those obtained through MYC inactivation (Figures 1C, S2–S3).
Atorvastatin reversed MYC-induced tumorigenesis in vitro and in vivo. (A) The influence of statins on cell proliferation was analyzed in murine lymphoma cell lines derived from a conditional transgenic model of MYC-induced lymphomagenesis using the Tet-system. Tumor-derived cell lines were treated with 10 μM atorvastatin (AT), 10 μM simvastatin (SM), 10 μM lovastatin (LOV), and 10 μM pravastatin (PRA) and analyzed after 24 and 48 hours for survival. Representative data are shown for 1 of the 6 tumor-derived cell lines from our conditional MYC transgenic model. Growth was measured by [3H]thymidine incorporation. Results are presented as stimulation index (SI), measured as the incorporation of [3H]thymidine in the presence versus the absence of treatment. Results are shown as mean of 2 separate experiments performed in triplicate (± SEM). SEMs were within 10% of the means. (B) Atorvastatin induced a dose-dependent inhibition of proliferation in MYC-induced tumors. Conditional transgenic lymphoma cell lines were generated using the Tet-system by overexpressing MYC. Tumor cells were treated with different doses of atorvastatin as indicated, and growth was measured by [3H]thymidine incorporation. Results are presented as SI, measured as the incorporation of [3H]thymidine in the presence versus the absence of treatment with statins. Experiments were performed in triplicate. Cells were treated with different doses of atorvastatin and analyzed after 24 and 48 hours of treatment. Results are presented as the mean of 3 different experiments performed in triplicate. (C) Atorvastatin inhibited proliferation and induced apoptosis in MYC-expressing tumor cells. MYC lymphoma cells where MYC was expressed (MYC ON) or MYC was inactivated (MYC OFF) after doxycycline treatment (20 ng/mL) or in the presence of 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev) were pulsed with BRDU after 36 hours. Live cells were stained with PI and analyzed by FACS. Results are presented as the mean of 2 different experiments. See also Figures S2,S3. (D) Survival of mice transplanted with a MYC-induced lymphoma cell line. Mice were injected with tumor cells of 1 of the MYC-induced tumor derived cell lines and when they were moribund with tumor, were either not treated (MYC ON; squares) or treated with doxycycline in their drinking water (100 μg/mL) to inactivate MYC (MYC OFF; circles) or treated with atorvastatin after 5 weeks of tumor growth, at doses of 1 mg/kg (triangles) or 10 mg/kg (stars). Each cohort consisted of 5 mice. Significant difference in survival was determined by Chi square test (AT, 1 mg/kg P < .002; AT, 10 mg/kg P < .02). (E) Representative picture of mouse prior to treatment and (F) after treatment with atorvastatin (1 mg/kg) (magnification 20× insert 40×) for 8 days. (G) Histology of a tumor sections stained with hematoxylin and eosin prior to treatment, consisting of tumor cells with high nuclear to cytoplasmic ratio, and (magnification 20× insert 40×) (H) after treatment with atorvastatin (magnification 20× insert 40×), where (magnification 20×) tumor cells appear to be apoptotic. Images were acquired as in “Viral infections.” (I) TUNEL assay was performed to determine the presence of apoptotic cells prior to treatment (magnification 20×) and (J) after treatment with atorvastatin (magnification 20×). Representative data are shown. Similar results were observed for 3 different tumor-derived lymphoma cell lines. Images were acquired as in “TUNEL assay.”
Atorvastatin reversed MYC-induced tumorigenesis in vitro and in vivo. (A) The influence of statins on cell proliferation was analyzed in murine lymphoma cell lines derived from a conditional transgenic model of MYC-induced lymphomagenesis using the Tet-system. Tumor-derived cell lines were treated with 10 μM atorvastatin (AT), 10 μM simvastatin (SM), 10 μM lovastatin (LOV), and 10 μM pravastatin (PRA) and analyzed after 24 and 48 hours for survival. Representative data are shown for 1 of the 6 tumor-derived cell lines from our conditional MYC transgenic model. Growth was measured by [3H]thymidine incorporation. Results are presented as stimulation index (SI), measured as the incorporation of [3H]thymidine in the presence versus the absence of treatment. Results are shown as mean of 2 separate experiments performed in triplicate (± SEM). SEMs were within 10% of the means. (B) Atorvastatin induced a dose-dependent inhibition of proliferation in MYC-induced tumors. Conditional transgenic lymphoma cell lines were generated using the Tet-system by overexpressing MYC. Tumor cells were treated with different doses of atorvastatin as indicated, and growth was measured by [3H]thymidine incorporation. Results are presented as SI, measured as the incorporation of [3H]thymidine in the presence versus the absence of treatment with statins. Experiments were performed in triplicate. Cells were treated with different doses of atorvastatin and analyzed after 24 and 48 hours of treatment. Results are presented as the mean of 3 different experiments performed in triplicate. (C) Atorvastatin inhibited proliferation and induced apoptosis in MYC-expressing tumor cells. MYC lymphoma cells where MYC was expressed (MYC ON) or MYC was inactivated (MYC OFF) after doxycycline treatment (20 ng/mL) or in the presence of 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev) were pulsed with BRDU after 36 hours. Live cells were stained with PI and analyzed by FACS. Results are presented as the mean of 2 different experiments. See also Figures S2,S3. (D) Survival of mice transplanted with a MYC-induced lymphoma cell line. Mice were injected with tumor cells of 1 of the MYC-induced tumor derived cell lines and when they were moribund with tumor, were either not treated (MYC ON; squares) or treated with doxycycline in their drinking water (100 μg/mL) to inactivate MYC (MYC OFF; circles) or treated with atorvastatin after 5 weeks of tumor growth, at doses of 1 mg/kg (triangles) or 10 mg/kg (stars). Each cohort consisted of 5 mice. Significant difference in survival was determined by Chi square test (AT, 1 mg/kg P < .002; AT, 10 mg/kg P < .02). (E) Representative picture of mouse prior to treatment and (F) after treatment with atorvastatin (1 mg/kg) (magnification 20× insert 40×) for 8 days. (G) Histology of a tumor sections stained with hematoxylin and eosin prior to treatment, consisting of tumor cells with high nuclear to cytoplasmic ratio, and (magnification 20× insert 40×) (H) after treatment with atorvastatin (magnification 20× insert 40×), where (magnification 20×) tumor cells appear to be apoptotic. Images were acquired as in “Viral infections.” (I) TUNEL assay was performed to determine the presence of apoptotic cells prior to treatment (magnification 20×) and (J) after treatment with atorvastatin (magnification 20×). Representative data are shown. Similar results were observed for 3 different tumor-derived lymphoma cell lines. Images were acquired as in “TUNEL assay.”
To evaluate the efficacy of atorvastatin for the treatment of cancers arising in vivo, we examined the ability of atorvastatin treatment to induce tumor regression in the transgenic model of MYC-induced lymphomagenesis.52 Mice with tumor burden were either left untreated, treated with doxycycline to inactivate the MYC transgene, or treated daily with various doses of atorvastatin given orally. Untreated mice rapidly succumbed to tumor burden (Figure 1D-E), whereas treatment with atorvastatin, similar to inactivation of MYC, induced the rapid regression of tumors within 1 week of treatment and increased survival (1 mg/kg [P < .002; n = 5] and 10 mg/kg [P < .02; n = 5]) (Figure 1D,F). Mice treated daily with 100 mg/kg were unable to tolerate therapy for more than 2 weeks. Treatment with atorvastatin resulted in the apoptosis of tumor cells in vivo, as observed by examination of histologic specimens and measured by TUNEL assay (Figure 1G-J). Thus, atorvastatin can reverse MYC-induced tumorigenesis even in established tumors in vivo.
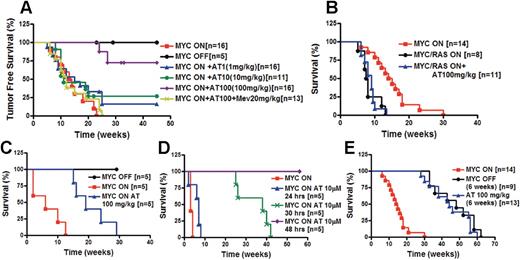
Our results suggested to us that atorvastatin may also be able to prevent MYC-induced tumorigenesis. Cohorts of mice were either treated with doxycycline to suppress MYC expression or not treated with doxycycline and treated with varying doses of atorvastatin, or atorvastatin plus mevalonate (Figure 2A). As expected, transgenic mice not treated with doxycycline developed tumors with a frequency of 100% and a mean latency of tumor onset of 13 weeks.26,52 As we have previously shown, inactivation of MYC with doxycycline (n = 5) completely prevented tumorigenesis.26,27,52 Since mice treated daily with 100 mg/kg of atorvastatin were unable to tolerate therapy for more than 2 weeks, we now treated mice with 100 mg/kg 2 to 3 times per week. Thus, we were able to prevent the acute toxicity observed with the daily treatment. Tumor onset was largely prevented by atorvastatin administered orally 2 to 3 times weekly (100 mg/kg; P < .001 [n = 16]). Importantly, atorvastatin failed to prevent tumorigenesis in mice treated with mevalonate (20 mg/kg; n = 13), suggesting that inhibition of HMGcoA reductase was responsible for preventing tumorigenesis. These mice had a survival similar to mice express-ing MYC that were not treated with statins. When treated with lower doses of atorvastatin (1 mg/kg [n = 16] or 10 mg/kg [n = 10]), 30% of mice remained disease free, suggesting that there may be a dose-dependent ability of atorvastatin to prevent tumorigenesis. Thus, atorvastatin, presumably through inactivation of HMGcoA reductase, induces a dose-dependent inhibition of MYC-induced tumorigenesis.
Atorvastatin prevented the onset of MYC-induced lymphomagenesis. (A) Atorvastatin treatment of mice expressing MYC. Kaplan-Meier survival curves of transgenic mice conditionally overexpressing the MYC transgene in murine T-cell lymphocytes using the Tet-system (MYC ON; n = 16) or treated with doxycycline to suppress MYC expression (MYC OFF; n = 5). Mice overexpressing MYC (MYC ON) were treated with atorvastatin 3 times weekly (MYC ON + AT1, 1 mg/kg; n = 16), (MYC ON + AT10, 10 mg/kg; n = 11), or (MYC ON + AT100, 100 mg/kg; n = 16) as indicated. Mice overexpressing MYC were treated with atorvastatin (100 mg/kg) and mevalonate (20 mg/kg) (MYC ON + AT100 + Mev; n = 13). Significant difference in survival was determined by Chi square test (P < .001; MYC ON + AT100 100 mg/kg). (B) Atorvastatin treatment of mice expressing MYC and constitutively activated K-Ras (G12D). Survival curve of mice overexpressing MYC alone (MYC ON; n = 14) or together with activated murine K-Ras (G12D) (MYC/Ras ON; n = 8) or treated 3 times weekly with 100 mg/kg atorvastatin (MYC/Ras ON + AT100, 100 mg/kg; n = 11). (C) Atorvastatin treatment prevents tumor onset in mice given transplants of bone marrow from MYC transgenic mice. Lethally irradiated mice were given transplants of bone marrow cells (5 × 106) pooled from 3 mice that overexpress MYC. Mice were treated with doxycycline to inactivate MYC (MYC OFF; n = 5), not treated with doxycycline (MYC ON; n = 5), or not treated with doxycycline but treated with atorvastatin (AT100, 100 mg/kg; n = 5) 3 times weekly. Significant difference in survival of atorvastatin-treated mice compared with nontreated mice was determined by Chi square test (P < .001). (D) In vitro purging of bone marrow from MYC transgenic mice with atorvastatin prevents tumor development. Bone marrow of MYC-overexpressing mice with tumor burden was treated in vitro with atorvastatin (10 μM) for 24 hours (n = 5), 30 hours (n = 5), or for 48 hours (n = 5). Treated or nontreated bone marrow cells (5 × 106) were injected into lethally irradiated mice. Significant difference in survival was determined by Chi square test (24 hours, P > .04; 30 hours, P < .002; 48 hours, P < .001). (E) Transient treatment with atorvastatin delays tumor onset. Transgenic mice conditionally overexpressing MYC were either treated with doxycycline for 6 weeks (MYC OFF, 6 weeks; n = 9) or not treated with doxycycline (AT 100 mg/kg, 6 weeks; n = 14) but treated with atorvastatin at 100 mg/kg 3 times weekly for 6 weeks (n = 13) when weaned. The difference in survival of atorvastatin-treated mice was compared with that of nontreated mice using the Chi square test (P < .001).
Atorvastatin prevented the onset of MYC-induced lymphomagenesis. (A) Atorvastatin treatment of mice expressing MYC. Kaplan-Meier survival curves of transgenic mice conditionally overexpressing the MYC transgene in murine T-cell lymphocytes using the Tet-system (MYC ON; n = 16) or treated with doxycycline to suppress MYC expression (MYC OFF; n = 5). Mice overexpressing MYC (MYC ON) were treated with atorvastatin 3 times weekly (MYC ON + AT1, 1 mg/kg; n = 16), (MYC ON + AT10, 10 mg/kg; n = 11), or (MYC ON + AT100, 100 mg/kg; n = 16) as indicated. Mice overexpressing MYC were treated with atorvastatin (100 mg/kg) and mevalonate (20 mg/kg) (MYC ON + AT100 + Mev; n = 13). Significant difference in survival was determined by Chi square test (P < .001; MYC ON + AT100 100 mg/kg). (B) Atorvastatin treatment of mice expressing MYC and constitutively activated K-Ras (G12D). Survival curve of mice overexpressing MYC alone (MYC ON; n = 14) or together with activated murine K-Ras (G12D) (MYC/Ras ON; n = 8) or treated 3 times weekly with 100 mg/kg atorvastatin (MYC/Ras ON + AT100, 100 mg/kg; n = 11). (C) Atorvastatin treatment prevents tumor onset in mice given transplants of bone marrow from MYC transgenic mice. Lethally irradiated mice were given transplants of bone marrow cells (5 × 106) pooled from 3 mice that overexpress MYC. Mice were treated with doxycycline to inactivate MYC (MYC OFF; n = 5), not treated with doxycycline (MYC ON; n = 5), or not treated with doxycycline but treated with atorvastatin (AT100, 100 mg/kg; n = 5) 3 times weekly. Significant difference in survival of atorvastatin-treated mice compared with nontreated mice was determined by Chi square test (P < .001). (D) In vitro purging of bone marrow from MYC transgenic mice with atorvastatin prevents tumor development. Bone marrow of MYC-overexpressing mice with tumor burden was treated in vitro with atorvastatin (10 μM) for 24 hours (n = 5), 30 hours (n = 5), or for 48 hours (n = 5). Treated or nontreated bone marrow cells (5 × 106) were injected into lethally irradiated mice. Significant difference in survival was determined by Chi square test (24 hours, P > .04; 30 hours, P < .002; 48 hours, P < .001). (E) Transient treatment with atorvastatin delays tumor onset. Transgenic mice conditionally overexpressing MYC were either treated with doxycycline for 6 weeks (MYC OFF, 6 weeks; n = 9) or not treated with doxycycline (AT 100 mg/kg, 6 weeks; n = 14) but treated with atorvastatin at 100 mg/kg 3 times weekly for 6 weeks (n = 13) when weaned. The difference in survival of atorvastatin-treated mice was compared with that of nontreated mice using the Chi square test (P < .001).
We observed that mice did not tolerate higher doses (100 mg/kg) 3 times per week for more than 20 weeks due to increased mortality. We could not attribute this effect to changes in liver or kidney function or to rhabdomyolysis, which are all potential toxic effects that are seen in humans (data not shown). Reported doses used in pharmocokinetic studies of mice range from 100 to 800 mg/kg/day.63 In C57Bl6 mice, the toxic dose is 800 mg/kg/day. In humans, the toxic dose is more than 80 mg/day.64
Atorvastatin can be used in vitro or in vivo to purge preneoplastic or neoplastic cells
Next, to evaluate the ability of atorvastatin to specifically target tumor cells, bone marrow cells from MYC transgenic mice either before or after tumor onset were treated with atorvastatin. Lethally irradiated syngeneic, nontransgenic mice were transplanted with bone marrow from newly weaned MYC-overexpressing transgenic mice prior to tumor formation (4-5 weeks old). Atorvastatin treatment of these mice delayed tumor onset by 20 weeks (P < .001; n = 5) (Figure 2C). The in vitro atorvastatin treatment of bone marrow from mice with tumor burden for 30 or 48 hours, but not 24 hours, was sufficient to delay tumor onset by more than 30 weeks (30 hours, (P < .002; n = 5), or to completely prevent tumor onset (48 hours, P < .001; n = 5), respectively (Figure 2D). Thus, atorvastatin may be effective in delaying tumor onset of preneoplastic cells or in purging bone marrow of neoplastic cells.
We also found that even the transient treatment of transgenic mice with atorvastatin for 6 weeks (n = 13) delays tumor onset by more than 40 weeks compared with untreated mice (P < .001; n = 16) in a manner similar to what is observed during transient MYC inactivation (n = 9) in lymphoid tumors (Figure 2E). To explain these results, we found in vivo that atorvastatin treatment induced apoptosis in MYC-overexpressing thymocytes prior to tumor formation (Figure S5). To interrogate the kinetics of tumor cell elimination, bioluminescence imaging was performed using methods we have described.26 MYC inactivation and atorvastatin treatment induced a similar degree of tumor regression, albeit statins induced tumor regression with delayed kinetics (Figure S6). Hence, atorvastatin appears to prevent MYC from initiating tumorigenesis by inducing apoptosis in MYC-activated preneoplastic cells.
Atorvastatin inactivates multiple signaling pathways
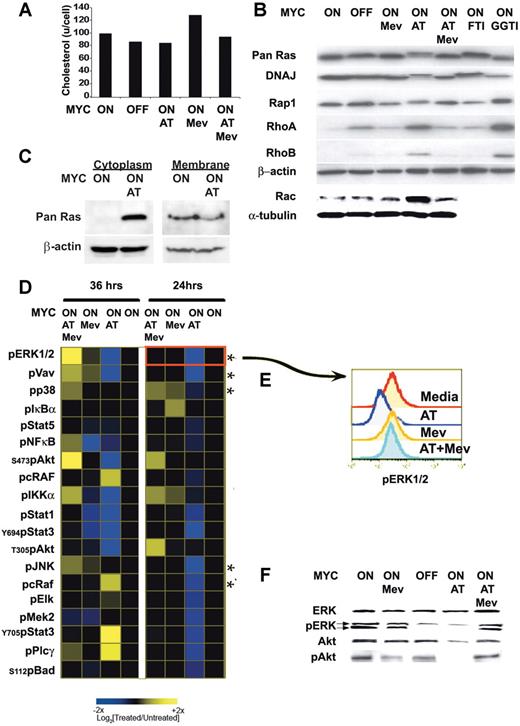
To account for the dramatic affects of atorvastatin on the reversal and prevention of MYC-induced tumorigenesis, we reasoned that atorvastatin may be inhibiting key effectors required to maintain MYC activation and function. Mevalonate is required for cholesterol synthesis and for the prenylation and activation of many proteins.65,–67 The inhibition of HMGcoA reductase inhibits the synthesis of mevalonate (Figure S1). Atorvastatin treatment induced a modest reduction of 10% to 30% in cellular cholesterol levels of MYC-expressing lymphoma cells (Figure 3A). Atorvastatin treatment of the MYC-induced lymphomas was found to influence the prenylation of several proteins (Figure 3B). Within 24 hours of atorvastatin (10 μM) treatment, farnesylation of Ras and DNAJ was inhibited. A shift in the mobility of these proteins was observed by Western blot analysis.
Atorvastatin-mediated disruption of prenylation and phosphorylation of signaling proteins. (A) Cellular cholesterol levels. MYC-expressing tumor cells were analyzed 24 hours after treatment. Equivalent numbers of cells were analyzed when MYC was expressed (MYC ON) or not expressed (MYC OFF) after doxycycline treatment (20 ng/mL) in the absence or presence of 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev). (B) Prenylation of several proteins was assessed by immunoblot when treated as described. Treatment with FTI-277 (5 μM) or GGTI-298 (5 μM) were used as controls to visualize changes observed in farnesylation and geranylation, respectively. (C) Ras protein accumulates in cytoplasm following atorvastatin treatment. MYC-induced lymphoma cell lines were treated for 6 hours with atorvastatin (AT; 10 mM). The cytoplasmic fraction was isolated and analyzed by Western blot for Ras protein. Representative data from 1 of 3 experiments is shown. (D) Kinetic analysis of changes in phosphoprotein expression analyzed by FACS analysis. MYC-induced lymphoma cell lines were treated for 24 and 36 hours, as indicated. The response to AT, Mev, and AT + Mev were determined and compared with the basal state, shown in the profile in black. Cells were analyzed for changes in phosphorylation of 56 different phosphoprotein epitopes. Changes observed for 19 of the epitopes are presented. (E) FACS plot analysis for changes in ERK1/2 phosphorylation. (F) Analysis of levels of protein expression and phosphorylation. Levels of ERK1/2, phosphorylated ERK1/2 (pERK1/2), Akt, and phosphorylated Akt (pS473) (pAkt) are measured by Western analysis.
Atorvastatin-mediated disruption of prenylation and phosphorylation of signaling proteins. (A) Cellular cholesterol levels. MYC-expressing tumor cells were analyzed 24 hours after treatment. Equivalent numbers of cells were analyzed when MYC was expressed (MYC ON) or not expressed (MYC OFF) after doxycycline treatment (20 ng/mL) in the absence or presence of 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev). (B) Prenylation of several proteins was assessed by immunoblot when treated as described. Treatment with FTI-277 (5 μM) or GGTI-298 (5 μM) were used as controls to visualize changes observed in farnesylation and geranylation, respectively. (C) Ras protein accumulates in cytoplasm following atorvastatin treatment. MYC-induced lymphoma cell lines were treated for 6 hours with atorvastatin (AT; 10 mM). The cytoplasmic fraction was isolated and analyzed by Western blot for Ras protein. Representative data from 1 of 3 experiments is shown. (D) Kinetic analysis of changes in phosphoprotein expression analyzed by FACS analysis. MYC-induced lymphoma cell lines were treated for 24 and 36 hours, as indicated. The response to AT, Mev, and AT + Mev were determined and compared with the basal state, shown in the profile in black. Cells were analyzed for changes in phosphorylation of 56 different phosphoprotein epitopes. Changes observed for 19 of the epitopes are presented. (E) FACS plot analysis for changes in ERK1/2 phosphorylation. (F) Analysis of levels of protein expression and phosphorylation. Levels of ERK1/2, phosphorylated ERK1/2 (pERK1/2), Akt, and phosphorylated Akt (pS473) (pAkt) are measured by Western analysis.
To evaluate if this shift was similar to that resulting from defarnesylation mediated by farnesyltransferase inhibitors (FTIs), the specific farnesyltransferase inhibitor FTI-277 was administered and shown to induce the same change in protein migration (Figure 3B). In addition, atorvastatin affected the geranylation process of several proteins, such as Rap1, RhoA, and RhoB, which was confirmed by demonstrating similar changes in protein mobility after treatment with the specific geranylgeranyltransferase inhibitor GGTI-298 (Figure 3B). A shift in the mobility was observed for Ras, DNAJ, and Rap1. However, RhoA and RhoB showed increased levels of expression after atorvastatin treatment, an effect previously described as translational and posttranslational due to the increase of de novo protein synthesis and decrease in the Rho protein degradation following treatment with statins.68 We confirmed that atorvastatin treatment decreased membrane-bound Ras and increased the accumulation of Ras in the cytoplasm of the cell as early as 6 hours after treatment, as would be expected with decreased Ras prenylation (Figure 3C). Mevalonate reversed the effects of atorvastatin, suggesting that the changes may be mediated through the inhibition of HMGcoA reductase function. Indeed, it has recently been shown that atorvastatin mediates its affects on lymphocyte biology by perturbing prenylation.58 As a negative control, we demonstrated that the suppression of MYC expression did not affect the posttranslational modification of these proteins. Thus, atorvastatin influenced the prenylation of several signaling molecules, as has been described previously.65,–67
Atorvastatin fails to prevent Ras-associated lymphomagenesis
To evaluate whether atorvastatin specifically reverses and prevents MYC-induced tumorigenesis by perturbing protein pathways regulated by posttranslational modification,32,–34 we examined how atorvastatin affects tumorigenesis induced by the overexpression of MYC together with a potent oncogene that may regulate MYC activation. Ras mutations that often occur in human tumors did not occur spontaneously in the MYC-overexpressing mice tumors (data not shown). The G12D mutation in K-Ras causes the protein to be constitutively activated, which then activates the MAP kinase pathway and subsequently ERK1/2, which in turn phosphorylates and activates MYC.69 Although Ras requires prenylation to localize to the plasma membrane and become activated, several recent papers have described that constitutively activated Ras can mediate oncogenic effects without localization to the plasma membrane.70,71 We therefore hypothesized that the oncogenic activity of MYC would not be affected by HMGcoA reductase inhibition in the presence of constitutively activated, mutated K-RAS (G12D).
Cohorts of transgenic mice were generated that overexpressed both MYC and mutated K-Ras (G12D)51 using the Tet-system. In contrast to tumors induced by MYC alone, tumorigenesis was not prevented by atorvastatin when MYC activation was combined with mutated K-Ras (G12D) (Figure 2B). Moreover, tumors caused by MYC and mutated K-Ras (G12D) did not show any survival advantage with atorvastatin treatment compared with untreated mice (Figure S4). Therefore, atorvastatin reverses and prevents lymphomagenesis associated with MYC, but not in the presence of mutated K-Ras (G12D).
Phosphoprotein FACS analysis of the effects of atorvastatin on cell signaling
To broadly evaluate the effects of atorvastatin on changes in cell signaling as a result of alterations in protein prenylation and cellular cholesterol content, several signal transduction pathways were surveyed using a multiparameter phosphoproteomic approach53,–55 to assess changes in the phosphorylation status of multiple signaling proteins after treatment. Single-cell analysis of phosphorylation changes was accomplished by intracellular staining of 56 different phosphorylation epitopes using directly conjugated multicolor phosphospecific antibodies at 11 time points following treatment. The geometric means of fluorescent intensity values were computed relative to the nontreated media control and analyzed using heat-map visualization tools to identify patterns of phosphorylation.
In the presence of atorvastatin, significant changes for 19 epitopes were observed after 24 and 36 hours of treatment. A dephosphorylation of ERK1/2, Vav, p38, JNK, and cRaf was observed within 24 hours (Figure 3D-E; see asterisk), suggesting that MAPK signaling proteins and their transcriptional targets such as Elk-1 were being deactivated. Critically, phosphorylation of Akt at serine 473 and threonine 308, known to be required for cell survival,72 dropped significantly by 36 hours. Interestingly, phosphorylated proteins (JNK, Bad, Raf, PLCγ, and Stat5) that had decreased phosphorylation at 24 hours of treatment regained phosphorylation at 36 hours, suggesting a compensatory mechanism or program had been initiated. By 36 hours, atorvastatin-treated cells became Annexin-V positive (Figure S3). By contrast, in the presence of mevalonate, the dephosphorylation of ERK1/2, Vav1, AKT, and IKKα that resulted from atorvastatin treatment was reversed, indicating that the effects observed were due to the inhibition of HMGcoA reductase. In addition, mevalonate also inhibited cell death induced by atorvastatin (Figure S3). Thus, the inhibition of mevalonate biosynthesis via atorvastatin inactivation of HMGcoA reductase results in the rapid dephosphorylation of many integral signaling components of the ERK1/2 MAPK pathway (Raf, Mek, ERK1/2, Elk, p38, and JNK) and cell survival pathways (Akt), as well as T-cell signaling molecules (Vav, IKKα, and NF-κB) and several transcription factors (STATs). Erk1/2 and Akt changes were confirmed by Western blot (Figure 3D-F). These changes could reflect the effects of atorvastatin on prenylation and/or lipid rafts.
Atorvastatin treatment results in the dephosphorylation and inactivation of MYC in murine and human cell lines
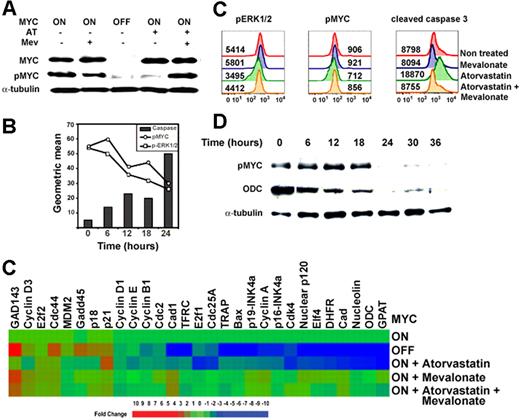
Many of the signaling pathways affected by atorvastatin treatment are involved in the regulation of MYC (Figure S7). In particular, Ras and ERK1/2 are known to be required for MYC phosphorylation.73,74 Atorvastatin treatment did not affect MYC protein levels, but did decrease MYC phosphorylation (Figure 4A). A temporal analysis of ERK1/2 and MYC phosphorylation upon atorvastatin treatment indicated that atorvastatin treatment first inhibits ERK1/2 phosphorylation, followed by the loss of phosphorylation of MYC at S62, T58. Phosphorylation of S62 is required for MYC activation. Differences in phosphorylation between nontreated and atorvastatin-treated cells was also observed by mass spectrometry (data not shown). At 24 hours, cells exhibit increased levels of cleaved caspase 3, an indicator of the initiation of apoptosis (Figure 4B). Importantly, atorvastatin treatment of a human Burkitt lymphoma cell line, ST486, induced the loss of phosphorylated ERK1/2 and MYC and subsequently induced the cleavage of caspase 3 and cell death (Figures 4C, S8). Hence, atorvastatin treatment of murine and human MYC-induced tumors resulted in the dephosphorylation of MYC followed by an increase in levels of cleaved caspase 3.
Atorvastatin treatment results in the dephosphorylation and inactivation of MYC. (A) Analysis of levels of MYC protein expression and phosphorylation. Levels of MYC and MYC phosphorylated at Ser62 and Thr58 (p-MYC) measured by Western analysis. (B) Temporal analysis of MYC phosphorylation. MYC-induced lymphoma cell lines were treated with atorvastatin and analyzed at indicated times for phosphorylation of ERK1/2, phosphorylation of MYC, and cleavage of caspase 3 by FACS. (C) Treatment of human Burkitt lymphoma cell line ST486 with atorvastatin. Levels of pERK1/2, pMYC, and cleaved caspase 3 were analyzed by FACS following treatment with 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev). (D) Temporal analysis of MYC phosphorylation. MYC-expressing tumor cells (0.3 × 106) were treated with atorvastatin (10 μM) for different lengths of time and analyzed for changes in MYC phosphorylation and expression of ODC, a known MYC transcriptional target. This image was cropped so that the 0- and 6-hour time points were next to each other. (E) Quantitative real-time PCR analysis of cDNA from MYC lymphoma cells where MYC was expressed (MYC ON) or MYC was inactivated (MYC OFF) after doxycycline treatment (20 ng/mL) or in the presence of 10 μM AT, 100 μM Mev, or 10 μM AT plus 100 μM Mev. Expression of each gene was normalized to the expression of ubiquitin. Changes in gene expression were measured for each treatment and compared with MYC-expressing nontreated tumor cells. Changes greater than 2-fold are statistically significant, as indicated in blue and orange/red (t test: P < .05-.001).
Atorvastatin treatment results in the dephosphorylation and inactivation of MYC. (A) Analysis of levels of MYC protein expression and phosphorylation. Levels of MYC and MYC phosphorylated at Ser62 and Thr58 (p-MYC) measured by Western analysis. (B) Temporal analysis of MYC phosphorylation. MYC-induced lymphoma cell lines were treated with atorvastatin and analyzed at indicated times for phosphorylation of ERK1/2, phosphorylation of MYC, and cleavage of caspase 3 by FACS. (C) Treatment of human Burkitt lymphoma cell line ST486 with atorvastatin. Levels of pERK1/2, pMYC, and cleaved caspase 3 were analyzed by FACS following treatment with 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev). (D) Temporal analysis of MYC phosphorylation. MYC-expressing tumor cells (0.3 × 106) were treated with atorvastatin (10 μM) for different lengths of time and analyzed for changes in MYC phosphorylation and expression of ODC, a known MYC transcriptional target. This image was cropped so that the 0- and 6-hour time points were next to each other. (E) Quantitative real-time PCR analysis of cDNA from MYC lymphoma cells where MYC was expressed (MYC ON) or MYC was inactivated (MYC OFF) after doxycycline treatment (20 ng/mL) or in the presence of 10 μM AT, 100 μM Mev, or 10 μM AT plus 100 μM Mev. Expression of each gene was normalized to the expression of ubiquitin. Changes in gene expression were measured for each treatment and compared with MYC-expressing nontreated tumor cells. Changes greater than 2-fold are statistically significant, as indicated in blue and orange/red (t test: P < .05-.001).
To evaluate whether the dephosphorylation of MYC was associated with decreased transcriptional function, a temporal analysis of the expression of a known MYC target gene, ODC, was performed. Notably, the loss of MYC phosphorylation occurred after 18 hours of atorvastatin treatment and was found to also be associated with the loss of expression of ODC as measured by Western blot analysis (Figure 4D). Furthermore, we compared the ability of atorvastatin to affect MYC-related gene transcription by quantitative real-time (QT)–PCR. A series of target genes strongly associated with MYC's function were examined, including cell-cycle–regulatory genes (cyclin A, cdc4, cdc25a, nuclear protein p120, nucleolin, and p21) and genes associated with cell metabolism (GPAT, DHFR, and CAD). We found that after atorvastatin treatment, 18 of these 29 MYC target genes exhibited a down-regulation in gene expression similar to those following the inactivation of MYC expression by doxycycline (Figure 4E). The cotreatment with mevalonate and atorvastatin was shown to reverse the decrease in the gene expression induced by atorvastatin treatment. On the other hand, while MYC inactivation induced an up-regulation in 6 of the 29 genes analyzed (p21, p18, Gadd45, cdc44, cyclin D3, and GAD143), the expression of the remaining 5 genes was not altered by atorvastatin treatment. This difference in the effect of atorvastatin on gene transactivation and transrepression suggests that phosphorylation may be essential for MYC's transactivational function but not sufficient for MYC's transrepressional function. This suggests that the atorvastatin treatment compromises MYC's transactivation function but may not affect MYC's transrepressional function. Therefore, atorvastatin treatment of MYC-induced tumor cells affects MYC-regulated transcriptional activity.
Atorvastatin fails to inactivate MYC in the presence of constitutively activated K-Ras (G12D)
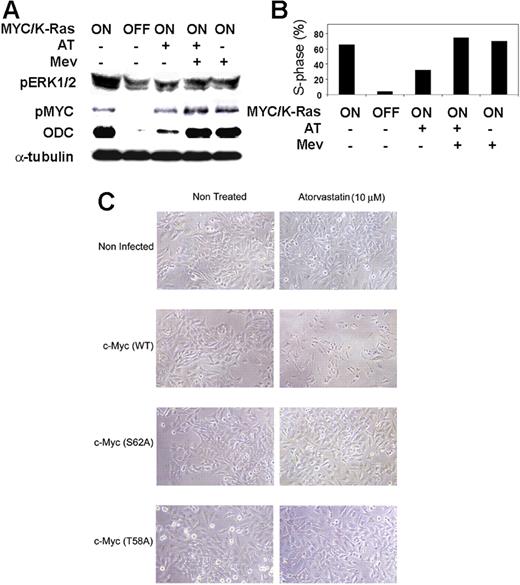
Since Ras regulates signaling pathways that are required to activate MYC through phosphorylation, we considered the possibility that constitutively activated K-Ras (G12D) was preventing atorvastatin from inactivating MYC signaling pathways. As predicted, atorvastatin treatment of MYC-induced tumors that had constitutively activated K-Ras (G12D) failed to exhibit decreased phosphorylation of ERK1/2 or MYC (Figure 5A), continued to express the MYC target gene ODC (Figure 5A), and failed to undergo complete cell-cycle arrest (Figure 5B). Thus, the presence of a constitutively activated K-Ras (G12D) prevented atorvastatin from inducing MYC dephosphorylation and regression of tumors overexpressing MYC.
Atorvastatin fails to decrease MYC phosphorylation or induce proliferative arrest in K-Ras (G12D) tumors. (A) Analysis of pERK1/2 and pMYC expression in tumor cells overexpressing MYC together with activated K-Ras (G12D). Tumor cells were treated as described and analyzed by Western blot analysis to assess the state of phosphorylation of ERK1/2 and MYC. (B) Analysis of proliferation of tumor cells overexpressing MYC together with activated K-Ras (G12D). Tumor cells were treated with doxycycline to inactivate MYC and K-Ras (G12D) expression or treated with 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev). (C) Effect of atorvastatin on cells expressing WT c-Myc or mutant c-Myc (S62A) or c-Myc (T58A). HeLa cells were not infected or infected with adenovirus containing murine Myc (Ad-MycWT) or Myc mutated at S62 (Ad-MycS62A), which prevents Myc phosphorylation and activation, or Myc mutated at T58 (Ad-MycT58A), which prevents dephosphorylation of Myc's activation site. Cells were treated or not treated with atorvastatin (10 μM). Images were acquired as in “Viral infections” (magnification 20×).
Atorvastatin fails to decrease MYC phosphorylation or induce proliferative arrest in K-Ras (G12D) tumors. (A) Analysis of pERK1/2 and pMYC expression in tumor cells overexpressing MYC together with activated K-Ras (G12D). Tumor cells were treated as described and analyzed by Western blot analysis to assess the state of phosphorylation of ERK1/2 and MYC. (B) Analysis of proliferation of tumor cells overexpressing MYC together with activated K-Ras (G12D). Tumor cells were treated with doxycycline to inactivate MYC and K-Ras (G12D) expression or treated with 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev). (C) Effect of atorvastatin on cells expressing WT c-Myc or mutant c-Myc (S62A) or c-Myc (T58A). HeLa cells were not infected or infected with adenovirus containing murine Myc (Ad-MycWT) or Myc mutated at S62 (Ad-MycS62A), which prevents Myc phosphorylation and activation, or Myc mutated at T58 (Ad-MycT58A), which prevents dephosphorylation of Myc's activation site. Cells were treated or not treated with atorvastatin (10 μM). Images were acquired as in “Viral infections” (magnification 20×).
Increased MYC expression potentiates sensitivity of HeLa cells to atorvastatin
Our results suggested the possibility that MYC overexpression and phosphorylation generally sensitizes cells to statins. Since the MYC-expressing lymphoma cells are extremely hard to infect, we conducted the following experiment in HeLa cells infected with 3 adenoviral constructs.57 The first construct contained wild-type murine Myc (Ad-MycWT). The second contained a mutation in Myc at the S62 phosphorylation site (Ad-MycS62A), which prevents Myc activation. The third contained a mutation at the T58 phosphorylation site (Ad-MycT58A), which prevents dephosphorylation of Myc at S62 and mediates Myc degradation. Introduction of wild-type Myc, but not mutant Myc, enhanced the sensitivity of HeLa cells to apoptosis mediated by atorvastatin treatment (Figure 5C[b]). Thus, atorvastatin treatment induces cell death in HeLa cells expressing wild-type Myc, but not in cells where crucial sites of Myc phosphorylation were disrupted.
HMGcoA reductase is required for tumor cell survival
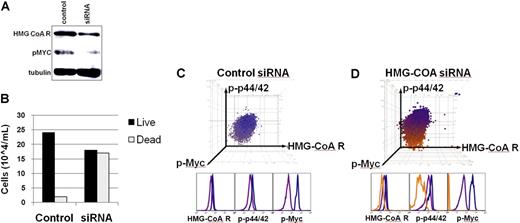
Our results suggested that HMGcoA reductase was required for MYC-induced tumor maintenance. We validated that atorvastatin treatment as well as MYC inactivation resulted in decreased levels of HMGcoA reductase protein levels but not in gene expression (Figure S9). We targeted HMGcoA reductase with specific RNAi oligomers (Dharmacon RNA Technologies, Lafayette, CO). We confirmed that transfection with HMGcoA reductase–specific RNAi decreased HMGcoA reductase protein levels, as measured by Western blot analysis and by FACS analysis after 18 hours of transfection (Figure 6A). At 30 hours following transfection, a decreased viability of tumor cells was observed as measured by trypan blue staining (Figure 6B). Following transfection with RNAi oligomers, we monitored individual tumor cells by FACS to assess HMGcoA reductase expression as well as phosphorylation of ERK1/2 and MYC in the cells. The targeted inactivation of HMGcoA reductase using siRNA-specific oligomers was sufficient to inhibit phosphorylation of ERK1/2 and MYC (siRNA/HMGcoA reductase; Figure 6D; orange), compared with control mock-transfected cells, which had unaltered levels of HMGcoA reductase and phosphorylated ERK1/2 and MYC (Control; Figure 6C; blue/purple). Thus, the direct targeting of HMGcoA reductase by RNAi inhibits ERK1/2 and MYC phosphorylation in a manner that is sufficient to induce tumor cell death.
Inactivation of HMGcoA reductase by RNAi was sufficient to induce loss of a neoplastic phenotype. (A) HMGcoA reductase and pMYC protein expression in cells treated with siRNA. An MYC-induced lymphoma cell line was transfected with smart-pool siRNA (Dharmacon RNA Technologies) directed against HMGcoA reductase and compared with mock-transfected cells. Protein expression was analyzed 18 hours after treatment by Western analysis. (B) Cell survival upon siRNA knockdown of HMGcoA reductase. Cells were stained with trypan blue and analyzed for survival 30 hours after transfection with siRNA. (C-D) Single-cell FACS analysis of cells transfected with siRNA. Levels of expression of HMGcoA reductase, phosphorylated ERK1/2, and phosphorylated MYC after (C) control/mock transfection or (D) transfection with siRNA against HMGcoA reductase, as measured by FACS analysis. A 3-color clustering of cellular populations was performed in FlowJo, and unique populations were color coded. Cells that do not incorporate the siRNA are blue/purple. Cells that incorporate the siRNA are orange.
Inactivation of HMGcoA reductase by RNAi was sufficient to induce loss of a neoplastic phenotype. (A) HMGcoA reductase and pMYC protein expression in cells treated with siRNA. An MYC-induced lymphoma cell line was transfected with smart-pool siRNA (Dharmacon RNA Technologies) directed against HMGcoA reductase and compared with mock-transfected cells. Protein expression was analyzed 18 hours after treatment by Western analysis. (B) Cell survival upon siRNA knockdown of HMGcoA reductase. Cells were stained with trypan blue and analyzed for survival 30 hours after transfection with siRNA. (C-D) Single-cell FACS analysis of cells transfected with siRNA. Levels of expression of HMGcoA reductase, phosphorylated ERK1/2, and phosphorylated MYC after (C) control/mock transfection or (D) transfection with siRNA against HMGcoA reductase, as measured by FACS analysis. A 3-color clustering of cellular populations was performed in FlowJo, and unique populations were color coded. Cells that do not incorporate the siRNA are blue/purple. Cells that incorporate the siRNA are orange.
Discussion
Statins are some of the most commonly prescribed medications in humans. They have been in use now for almost 2 decades for the treatment of hypercholesterolemia and atherosclerosis.36 Statins have been shown by many investigators to have potential antineoplastic properties both in vitro and in vivo.8,37,,,,,,,,–46 Many epidemiologic studies have noted a significant reduction in the incidence of colon, breast, prostate, and lung cancers in patients taking statins.8,43,48,59,60 Most notably, 1 report documented a 50% decrease in the incidence of colon cancer.47 However, 2 recent large meta-analyses concluded that statins do not prevent cancer.49,50 To date, no explanation has been offered for these conflicting conclusions. Our results suggest a possible explanation: perhaps only specific molecular signaling pathways associated with certain types of cancer may be sensitive to particular statins.
We provide evidence suggesting that atorvastatin can reverse and prevent tumors induced by MYC overexpression, but not tumors also carrying constitutively activated K-Ras (G12D). The mechanism by which atorvastatin inhibits MYC is not clear. One possible explanation raised from our results is that atorvastatin induces changes in protein prenylation and in the phosphoprotein signature associated with the inhibition of several signaling pathways, such as Ras and ERK1/2, that are thought to be required for MYC phosphorylation and activation (Figure S7).73,–75 Atorvastatin caused the dephosphorylation and inactivation of MYC, which in turn was assossciated with the suppression of the expression of MYC target genes. Atorvastatin's effects on the phosphorylation state of MYC were specific to the inhibition of HMGcoA reductase, as treatment with mevalonate abrogated these effects and the ability of atorvastatin to reverse or suppress tumorigenesis. When treated with atorvastatin, tumors expressing constitutively activated K-Ras (G12D) did not exhibit MYC dephosphorylation or inactivation. Hence, from these results, we predicted and indeed showed that in the presence of constitutively activated K-Ras (G12D), atorvastatin failed to prevent tumorigenesis in MYC-induced tumors.
Our results are consistent with recent reports that constitutively activated Ras does not necessarily require prenylation to activate cellular signaling or to function as an oncogene.70,71 Hence, given their putative mechanism of action, statins would not necessarily be expected to be fully capable of inactivating tumors with constitutively activated Ras that obviates the requirement for prenylation. Our results suggest that MYC-induced lymphomas may be particularly sensitive to statins. Moreover, we show that atorvastatin resulted in ERK1/2 and MYC dephosphorylation in a Burkitt lymphoma cell line, a tumor type causally associated with MYC activation. We recognize that these results in our transgenic model and in vitro in a human cell line will have to be validated in vivo in humans.
Our results raise the possibility that atorvastatin may be effective in preventing specific types of cancer. Our findings also suggest the possibility that atorvastatin may be effective in purging these types of cancer cells from bone marrow before bone marrow transplantation. As also noted in previous studies, the dose of atorvastatin required to induce tumor regression of established tumors was different from the dose required to prevent tumorigenesis.76 Daily treatment with atorvastatin induced the regression of established tumors at doses of 1 to 10 mg/kg, which appears to be comparable to doses used to treat hypercholesterolemia in humans.77 By contrast, for prevention of MYC-induced tumorigenesis, we treated mice 3 times weekly with 100 mg/kg. HMGcoA reductase activity is inhibited in specific mouse strains only at daily doses of 100 mg/kg and higher.78 At a dose of 100 mg/kg/day in male mice, the area under the curve (AUC) is 10 times higher than that achieved in humans treated with 40 mg/day. However, when mice were given just 1 dose, the AUC is equivalent to that of humans, implying that mice are unable to clear the drug as quickly as humans. Perhaps these results would explain why continuous atorvastatin treatment is toxic to mice.63 Our results suggest that toxicity may also depend upon the strain of mouse. In addition, mevalonate-derived intermediates have a higher affinity for enzymes involved in nonsterol biosynthesis than cholesterol biosynthesis. Lower doses of statins have a far greater impact on cholesterol levels than on protein prenylation, but at higher doses affect protein prenylation.76 Our results suggest that atorvastatin prevents MYC-induced tumorigenesis by blocking HMGcoA reductase activity, because treatment with mevalonate completely abrogated tumor prevention.
Epidemiologic studies have yet to describe differences among various statins or a dose response in their prevention of hematopoietic tumorigenesis.8,43,48,59,60 However, in these studies, patients usually did not receive atorvastatin but instead were treated with pravastatin or simvastatin. Also, most studies did not specifically look at the affect of statins on hematopoietic tumorigenesis.
Statins appear to mediate their antineoplastic effects by inhibiting pathways downstream of HMGcoA reductase that are essential for MYC-associated oncogenic signaling pathways (Figure S7). Two observations confirm that the antineoplastic effects of statins are mediated through the inhibition of HMGcoA reductase. First, mevalonate rescued tumors treated with atorvastatin. Second, RNAi directed against HMGcoA reductase was sufficient to induce the loss of ERK1/2 and MYC phosphorylation in tumor cells in vitro. Specific pharmacologic inhibitors for geranylgeranylation (GGTIs) and farnesylation (FTIs) as well as inhibitors for ERK1/2 and Akt are known to contribute to the loss of a neoplastic phenotype in tumors expressing wild-type Ras.79,80 In combination, these results suggest that statins may induce the loss of neoplastic properties by inhibiting multiple signaling processes that appear to be required for MYC-induced tumorigenesis.
Statins have yet to be described to have clinical efficacy in the treatment of cancer. Our results suggest that statins may be more capable of targeting early neoplastic cells, which tend to be less genetically complex, thereby preventing tumorigenesis. Specifically, our results suggest that MYC-associated lymphomagenesis may be prevented by treatment with atorvastatin. However, cancers appear to be capable of escaping dependence upon individual oncogenic signaling proteins, so we recognize that atorvastatin is likely to be more effective when combined with other therapies for the treatment of tumors.22,81,82
Finally, we have shown that by using phosphoprotein FACS profiling of multiple proteins in defined conditional transgenic models, we were able to demonstrate that MYC-induced tumorigenesis is sensitive to atorvastatin-mediated inhibition of HMGcoA reductase. The identification of the phosphoprotein signature of signaling molecules may be useful in identifying drugs optimized for their antineoplastic properties in specific oncogenic signaling states.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to dedicate this work to the memory of Cleto Fernandes and Arthur Lantz. We would like to thank Dr Rosalie C. Sears (Oregon Health & Science University, Portland, OR) for kindly providing us with Myc viruses, and Dr Bruno Amati (European Institute of Oncology, Milan, Italy) for providing us with primers for the quantitative PCR. We thank the members of the Felsher, Steinman, and Nolan laboratories for their helpful comments. This work was supported, in part, by the National Cancer Institute grants 1R01 CA89305–01A1, 3RO1 CA89305–0351, and 1RO1 CA105102; the Leukemia and Lymphoma Society, the Damon Runyon Foundation, and the Burroughs Wellcome Fund (D.W.F.); the Weiland Family Fellowship and Flight Attendant Research Institute (FAMRI)–Young Clinical Scientist Award (C.M.S.); Bristol-Meyer Squibb Irvington Institute Fellow, the Dana Foundation, the National Heart, Lung and Blood Institute (N01-HV-28183I), and BD Biosciences (O.D.P.); and the Leukemia Society of America, National Institutes of Health grants P01-AI39646, AR44565, AI35304, N01-AR-6–2227, and A1/GF41520–01, and the Juvenile Diabetes Foundation (G.P.N.), and the Ludwig Foundation (C.N.S., D.W.F., O.D.P., G.P.N.).
National Institutes of Health
Authorship
Contribution: C.M.S., O.D.P., and S.Y. designed and performed research and wrote the paper; A.C.F., M.J.G., S.E., A.E.S., J.C., O.S., D.J.M., and M.C. performed research; G.P.N., L.S., and D.W.F. designed the research and wrote the paper. C.M.S., O.D.P., and S.Y. contributed equally to the manuscript. G.P.N., L.S., and D.W.F. contributed equally to the manuscript as senior authors.
Conflict-of-interest disclosure: O.D.P. and G.P.N. hold patents through Stanford University related to technology employed in the study. All other authors declare no competing financial interests.
Correspondence: Dean W. Felsher, 269 Campus Dr, CCSR 1105b, Stanford, CA 94305-5151; e-mail:dfelsher@stanford.edu.

![Figure 1. Atorvastatin reversed MYC-induced tumorigenesis in vitro and in vivo. (A) The influence of statins on cell proliferation was analyzed in murine lymphoma cell lines derived from a conditional transgenic model of MYC-induced lymphomagenesis using the Tet-system. Tumor-derived cell lines were treated with 10 μM atorvastatin (AT), 10 μM simvastatin (SM), 10 μM lovastatin (LOV), and 10 μM pravastatin (PRA) and analyzed after 24 and 48 hours for survival. Representative data are shown for 1 of the 6 tumor-derived cell lines from our conditional MYC transgenic model. Growth was measured by [3H]thymidine incorporation. Results are presented as stimulation index (SI), measured as the incorporation of [3H]thymidine in the presence versus the absence of treatment. Results are shown as mean of 2 separate experiments performed in triplicate (± SEM). SEMs were within 10% of the means. (B) Atorvastatin induced a dose-dependent inhibition of proliferation in MYC-induced tumors. Conditional transgenic lymphoma cell lines were generated using the Tet-system by overexpressing MYC. Tumor cells were treated with different doses of atorvastatin as indicated, and growth was measured by [3H]thymidine incorporation. Results are presented as SI, measured as the incorporation of [3H]thymidine in the presence versus the absence of treatment with statins. Experiments were performed in triplicate. Cells were treated with different doses of atorvastatin and analyzed after 24 and 48 hours of treatment. Results are presented as the mean of 3 different experiments performed in triplicate. (C) Atorvastatin inhibited proliferation and induced apoptosis in MYC-expressing tumor cells. MYC lymphoma cells where MYC was expressed (MYC ON) or MYC was inactivated (MYC OFF) after doxycycline treatment (20 ng/mL) or in the presence of 10 μM atorvastatin (AT), 100 μM mevalonate (Mev), or 10 μM atorvastatin plus 100 μM mevalonate (AT + Mev) were pulsed with BRDU after 36 hours. Live cells were stained with PI and analyzed by FACS. Results are presented as the mean of 2 different experiments. See also Figures S2,S3. (D) Survival of mice transplanted with a MYC-induced lymphoma cell line. Mice were injected with tumor cells of 1 of the MYC-induced tumor derived cell lines and when they were moribund with tumor, were either not treated (MYC ON; squares) or treated with doxycycline in their drinking water (100 μg/mL) to inactivate MYC (MYC OFF; circles) or treated with atorvastatin after 5 weeks of tumor growth, at doses of 1 mg/kg (triangles) or 10 mg/kg (stars). Each cohort consisted of 5 mice. Significant difference in survival was determined by Chi square test (AT, 1 mg/kg P < .002; AT, 10 mg/kg P < .02). (E) Representative picture of mouse prior to treatment and (F) after treatment with atorvastatin (1 mg/kg) (magnification 20× insert 40×) for 8 days. (G) Histology of a tumor sections stained with hematoxylin and eosin prior to treatment, consisting of tumor cells with high nuclear to cytoplasmic ratio, and (magnification 20× insert 40×) (H) after treatment with atorvastatin (magnification 20× insert 40×), where (magnification 20×) tumor cells appear to be apoptotic. Images were acquired as in “Viral infections.” (I) TUNEL assay was performed to determine the presence of apoptotic cells prior to treatment (magnification 20×) and (J) after treatment with atorvastatin (magnification 20×). Representative data are shown. Similar results were observed for 3 different tumor-derived lymphoma cell lines. Images were acquired as in “TUNEL assay.”](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2006-09-048033/2/m_zh80210708420001.jpeg?Expires=1767707757&Signature=ggc0HaWUBHXHdJWuL5LoFjSQSS3UNm44ie4T3EkjaObxspyRqa-b6ZWnVe~ivmtHF~Mk43L9PJDJtmde9~haRK4gk0sf-azpekgJ-S4rCxsopv-o8OANExe5cBuJz0GPZHV349BSK6cHsaUwltnsct3sKuBz5bWRCKkB~~P5b0jr-3AtDtiRO9jEHpZDKump32COMvDAy54gL78MVYgWIj5n8dfbhf4UzDbGZwW9Ncmp60C7N5UWwqbexGQO15f0dWuZMAgeZyEU4KA1PIHwhN8RrvKYseD3TruqHkrp1qnWS3r~qDEtDc3HIDQcqYOX3c4OB7unNgn81jkpc033Gw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)