Abstract
Rituximab, an anti-CD20 monoclonal antibody, has been used to treat autoimmune disorders such as idiopathic thrombocytopenic purpura (ITP). However, its mechanisms of action as well as the effects on cellular immunity remain poorly defined. We investigated the changes of different peripheral blood T-cell subsets, the apoptosis profile, as well as the changes of T-cell receptor (TCR) β-variable (VB) region gene usage of CD4+ and CD8+ T-cell subpopulations following rituximab therapy. The study involved 30 patients with chronic ITP who received rituximab, of whom 14 achieved a durable (> 6 months) response. Compared with the control group, pretreatment abnormalities of T cells in ITP patients included an increase of the Th1/Th2 ratio and of the Tc1/Tc2 ratios (P < .001), increased expression of Fas ligand on Th1 and Th2 cells (P < .001), increased expression of Bcl-2 mRNA (P = .003) and decreased expression of bax mRNA (P = .025) in Th cells, and expansion of oligoclonal T cells with no preferential use of any TCR VB subfamily. These abnormalities were reverted in responders at 3 and 6 months after treatment, whereas they remained unchanged in nonresponders. Our findings indicate that in patients with ITP, response to B-cell depletion induced by rituximab is associated with significant changes of the T-cell compartment.
Introduction
Rituximab, a genetically engineered chimeric murine/human anti-CD20 monoclonal antibody originally developed for the treatment of non-Hodgkin lymphoma, has been used successfully in a number of autoimmune disorders.1 The use of this drug in patients with chronic idiopathic thrombocytopenic purpura (ITP) produces some long-term responses, which are usually associated with a sustained B-cell depletion and a decrease or disappearance of antiplatelet antibodies.2 It is noteworthy that responders have not shown significant changes in the CD4/CD8 ratio or in the number of NK cells as evaluated by conventional immunophenotyping.2 Nevertheless, the production of antiplatelet antibodies by autoreactive B lymphocytes is known to be driven and regulated by cellular mechanisms, primarily involving CD4+ T (Th) lymphocytes and to a lesser degree CD8+ T (Tc) lymphocytes.3 Both Th and Tc lymphocytes can be functionally divided into type 1 and type 2 subsets based on the secretion of either IFN-γ (T1) or IL-4 (T2), since the synthesis of cytokines such as IL-2, IL-6, and IL-10 is not stringently restricted to a single subset.4 Th0 cells are considered precursors of other Th cells and may produce both IFN-γ and IL-4. Interestingly, the cytokine pattern of lymphocytes seen in patients with chronic ITP suggests an early CD4+ Th0 and Th1 cell activation.5-7
Changes in both the intrinsic and extrinsic apoptotic pathways have also been suggested to be causative of autoimmune diseases, a good example being the autoimmune lymphoproliferative syndrome where mutations in Fas or caspase results in loss of appropriate T-cell apoptosis. Alterations in the Fas/FasL pathway have been found in patients with ITP. For example, an enhanced expression of FasL on Th1 and Tc1 and of Fas on Th2 and Tc2 have been described.7 Furthermore, when soluble Fas was present in patients with ITP it correlated to an increase in IL-2 and sIL-2R levels.8 More recent data describe the role of the bcl-2 family members, which control the mitochondrial membrane integrity, in the intrinsic cell death pathway. This family includes antiapoptotic proteins such as bcl-2 and proapoptotic proteins such as bax. This family of proteins is critical for T-cell homeostasis and interference or abnormalities in this pathway result in either immunodeficiency or autoimmunity. One study has shown changes in bcl-2 and bax mRNA in 50 children with ITP. Compared with healthy children, expression of bcl-2 mRNA was increased in children with ITP and expression of bax mRNA was decreased. Additionally, a positive correlation was found with the bcl-2/bax mRNA ratio and the Th1/Th2 ratio.6
Finally, another well-known abnormality is the oligoclonal accumulation of CD4+ Th cells in the peripheral blood of patients with ITP.9 Size analysis of cDNAs for the complementarity-determining region 3 (CDR3) of the T-cell receptor (TCR) β-variable (VB) region genes has enabled to demonstrate the frequent use of VB3, 6, 10, and 13.1 to 14 genes.10
To further evaluate the effects of rituximab treatment on T lymphocytes, we investigated the changes in peripheral blood Th and Tc subsets by analysis of intracellular cytokines, the apoptosis profile of different T-cell subsets, as well as the changes of TCR VB gene patterns.
Patients, materials, and methods
Patients
The study included 30 adults with chronic ITP who received rituximab therapy, some of whom as part of a clinical trial between February 2004 and December 2005 (trial ML18542 registered at http://www.roche-trials.com/patient/trials/trial100026.html). It was approved by the institutional review boards of participating centers (S. Eugenio Hospital and Tor Vergata University Hospital in Rome, Regina Apostolorum Hospital in Albano Laziale, Italy), and informed consent was obtained from each patient in accordance with the Declaration of Helsinki. The diagnosis of ITP was based on the criteria reported previously in the guidelines of the American Society of Hematology.11 Rituximab (Mabthera; Roche, Milan, Italy) was administered intravenously at the dose of 375 mg/m2 once weekly for a total of 4 infusions (days 1, 8, 15, and 22). Patients were not allowed to take immunosuppressive or immunomodulating therapies other than corticosteroids during and up to 3 months after rituximab.
Laboratory investigations were performed centrally at S. Eugenio Hospital. Peripheral blood samples were obtained from all patients before and 3 months after rituximab therapy. We chose 3 months as the main evaluation point because patients were not supposed to respond after that time, and nonresponders had not yet received other treatments. Additional samples were obtained from patients with sustained response at 6 months (14 cases) and at 12 months (9 cases). The control group consisted of 30 adult healthy volunteers matched for sex and age (± 3 years) with the study population. Patients' and controls' characteristics are detailed in Table 1.
Summary of patients' and controls' characteristics
| Characteristics . | Patients . | Controls . |
|---|---|---|
| Total no. | 30 | 30 |
| Men | 8 | 8 |
| Women | 22 | 22 |
| Age, y | ||
| Median | 46 | 46.5 |
| Range | 24-68 | 23-68 |
| Baseline platelet count, × 109/L | ||
| Median | 12 | 242 |
| Range | 3-22 | 161-285 |
| Previous therapies | NA | |
| Prednisone | 30 | — |
| Intravenous immunoglobulin | 30 | — |
| High-dose dexamethasone | 10 | — |
| Splenectomy | 9 | — |
| Azathioprine | 8 | — |
| Vincristine | 5 | — |
| Pulse cyclophosphamide | 4 | — |
| ITP duration before rituximab treatment, mo | NA | |
| Median | 37 | — |
| Range | 9-87 | — |
| Elevated PAIgG | 26/26 | 5/26 |
| Elevated ACA/LLA | 12/26 | 0/26 |
| ANA positive | 0/30 | 0/26 |
| Coombs tests positive | 0/24 | 0/26 |
| Response to rituximab* | 14/30 | NA |
| Characteristics . | Patients . | Controls . |
|---|---|---|
| Total no. | 30 | 30 |
| Men | 8 | 8 |
| Women | 22 | 22 |
| Age, y | ||
| Median | 46 | 46.5 |
| Range | 24-68 | 23-68 |
| Baseline platelet count, × 109/L | ||
| Median | 12 | 242 |
| Range | 3-22 | 161-285 |
| Previous therapies | NA | |
| Prednisone | 30 | — |
| Intravenous immunoglobulin | 30 | — |
| High-dose dexamethasone | 10 | — |
| Splenectomy | 9 | — |
| Azathioprine | 8 | — |
| Vincristine | 5 | — |
| Pulse cyclophosphamide | 4 | — |
| ITP duration before rituximab treatment, mo | NA | |
| Median | 37 | — |
| Range | 9-87 | — |
| Elevated PAIgG | 26/26 | 5/26 |
| Elevated ACA/LLA | 12/26 | 0/26 |
| ANA positive | 0/30 | 0/26 |
| Coombs tests positive | 0/24 | 0/26 |
| Response to rituximab* | 14/30 | NA |
ITP indicates idiopathic thrombocytopenic purpura IgG; PAIgG, platelet-associated immunoglobulin; ACA/LLA, anticardiolipin antibodies/lupus-like anticoagulant; ANA, antinuclear antibodies; NA, not applicable; and —, not available.
Platelet count higher than 150 × 109/L of at least 6-month duration.
Antibodies and reagents
We used the following monoclonal antibodies (all from BD Biosciences, Milan, Italy): anti-IFNγ conjugated to phycoerythrin (PE); anti–IL-4 PE; anti-CD3 (T cells) conjugated to peridin chlorophyll protein (PerCP); anti-CD4 (T-helper inducer) conjugated to fluorescein isothiocyanate (FITC), PE, and PerCP; anti-CD8 (T-suppressor cytotoxic) FITC, PE, and PerCP; anti-CD25 (p55 IL-2 receptor) PE; anti–HLA-DR (class II MHC) PE; anti-Fas and anti-FasL FITC (clone NOK-1); isotype-matched, directly conjugated (FITC, PE, and PerCP) control antibodies. FACS Lysing Solution and FACS Permeabilizing Solution were obtained from BD Biosciences. Phorbol myristate acetate (PMA), ionomycin, brefeldin A, paraformaldehyde, l-glutamine, RPMI 1640, and AB serum were obtained from Sigma Chemical (St Louis, MO).
T-cell separation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by centrifugation over Ficoll-Hypaque gradients (Pharmacia, Uppsala, Sweden). Cells were resuspended at a concentration of 2 × 106 cells/mL in RPMI medium 1640 supplemented with 10% fetal bovine serum, 4 mM l-glutamine, 25 mM Hepes buffer, 50 units/mL penicillin, and 50 μg/mL streptomycin. T cells were separated from PBMCs by negative depletion of non-T cells with a human T-cell enrichment column (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions. This isolation procedure yielded greater than 90% CD3+ T cells. T cells were tested by trypan blue exclusion for viability, which was generally more than 95%. To obtain highly purified CD4+ and CD8+ T cells for analysis of the T-cell receptor repertoire, as well as of bcl-2 and bax gene expression, T cells collected after the enrichment column procedure were stained with CD4 and CD8 monoclonal antibodies and sorted on a FAC-Star flow cytometer (BD Biosciences).
Intracellular cytokine analysis
Intracellular cytokines were studied by flow cytometry. Briefly, 100 μL of T-cell suspensions were incubated in tubes (Falcon; BD Biosciences) for 4 hours at 37°C, 5% CO2 in the presence of 25 ng/mL phorbol 12-myristate 13-acetate (PMA), 1 mg/mL ionomycin, and 10 μg/mL brefeldin A (Sigma Chemical). PMA and ionomycin are pharmacological T-cell–activating agents that mimic signals generated by the TCR complex and have the advantage of stimulating T cells of any antigen specificity. Brefeldin-A was used to block intracellular transport mechanisms, thereby leading to an accumulation of cytokines in the cells.
After 2 washes with buffered solution (phosphate-buffered saline containing 0.5% bovine serum albumin and 0.1% sodium azide), cells were stained with PerCP-conjugated anti-CD4 or anti-CD8 monoclonal antibodies at room temperature in the dark for 15 minutes. Cells were then washed with PBS containing 0.5% albumin bovine serum and 0.1% sodium azide and subsequently resuspended in 500 μL FACS permeabilizing solution. After another wash with wash buffer, cells were stained with FITC-conjugated anti–IFN-γ and PE-conjugated anti–IL-4 monoclonal antibodies at room temperature for 30 minutes. As the last step, the cells were washed with wash buffer and resuspended in 1% paraformaldehyde for analysis by 3-color flow cytometry. A FACScan flow cytometer (BD Biosciences) equipped with a 15-MW argon ion laser and appropriate filters for FITC (530 nm), PE (585 nm), and PerCP (> 650 nm) was used. For surface marker analysis of peripheral blood lymphocytes, 10 000 cells were computed in list mode and analyzed using the Lysis software (BD Biosciences). For intracellular cytokine detection, 10 000 cells were computed and analyzed with the same software. An electronic gate was set on the lymphocytes on the forward and side scatter plot, and CD4+ or CD8+ (PerCP+) cells falling within the gated area were then identified and selected in a second gate. Cytokine production was analyzed by detection of FITC and PE staining, and the number of cells staining for each cytokine was expressed as a percentage of CD4+ and CD8+ T cells. Specific binding of mAb was controlled by subtraction of isotype-matched mouse immunoglobulins.
Analysis of Fas and FasL expression on T-cell subsets
The expression of Fas and FasL on T-cell subsets was detected by immunofluorescent staining and tricolor flow cytometry. At least 10 000 events per sample were analyzed. Analysis of Fas or FasL expression with FITC-conjugated antibodies was performed on T-cell subsets that had been gated according to the double expression of IFN-γ or IL-4 (PE-conjugated antibodies) and CD4 or CD8 (PerCP-conjugated antibodies). The threshold level for positive cells was set for each sample at the intersection of the histogram curves obtained for cells stained with the isotype control and the specific mAb.
Analysis of bcl-2 and bax gene expression in T cells
Total RNA of purified CD4+ and CD8+ T cells was isolated by Trizol reagent according to manufacturer's protocol (Life Technologies, Bethesda, MD). Bcl-2 and bax cDNA fragments were amplified by one-step reverse-transcriptase–polymerase chain reaction (RT-PCR) Kit (Applied Biosystems, Foster City, CA) using 2 μg of total RNA. The primers were as follows: for bcl-2, 5′-CAG CTG CAC CTG ACG CCC TT-3′ and 5′-GCC TCC GTT ATC CTG GAT CC-3′; for bax, 5′-TTT GCT TCA GGG TTT CAT CC-3′ and 5′-CGT CCC AAA GTA GGA GAG GGA-3′; for β-actin, 5′-AGC GGG AAA ACG TGC GTG AC-3′ and 5′-ACT CCT GCT TGC TGA TCC ACA TC-3′. β-Actin was used as positive control. The PCR products were analyzed by electrophoresis in 1.5% agarose gel containing ethidium bromide. The image of amplified strips was analyzed by two-dimension laser scanner and corrected by β-actin, indicated with the optical absorption ratio.
CDR3 size analysis of TCR VB transcripts
Total RNA was extracted from purified T cells using Trizol reagent and then subjected to reverse transcription. The cDNA was amplified through 35 cycles (94°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds) with primers specific to each of the 22 VB subfamilies (excluding VB10 and VB19, which are pseudogenes), and a fluorescently labeled constant region primer. PCR products were denatured for 3 minutes at 90°C and size separated on a high-resolution polyacrylamide gel and analyzed using the GeneMapper software (Applied Biosystems). Products from clonal TCRBV cell populations were visualized as 1 or 2 sharp peaks of fluorescence corresponding to PCR-amplified clonal rearranged TCR alleles, while cDNA from polyclonal TCR VB cell populations was visualized as a fluorescence spectrum composed of polyclonal PCR fragments of different sizes in a normal Gaussian distribution with peaks spaced by 3 bp corresponding to selected in-frame TCR VB rearrangements. CDR3 size patterns were judged to be abnormal if a Gaussian distribution was absent, due to either a reduced (< 5) peak number or the appearance of prominent peaks.12 Three independent investigators experienced in the technique made this determination in a blinded fashion; CDR3 size patterns were reported as normal or abnormal only after consensus to the designation had been reached. The number of VB subfamilies displaying an abnormal CDR3 size pattern was determined for each subject.
Statistical analysis
Statistics was carried out using NCSS for Windows (NCSS, Kaysville, UT). Continuous variables were summarized by descriptive statistics. Categoric variables were summarized by frequency statistics. Student t test for independent samples was used for comparisons between different groups and the paired sample t test for within-group comparisons. Linear regression was used to determine the correlation between different study parameters. Values of P less than .05 were accepted as statistically significant. All P values are 2 tailed.
Results
Expression of intracellular cytokines
The results are summarized as both absolute concentrations and as percentage of CD3+ cells in Table 2, and representative dot-plots are shown in Figure 1.
Results of intracellular cytokine analysis
| Variable . | Baseline . | 3 mo . | 6 mo . | 12 mo . | Controls, N = 30 . | ||
|---|---|---|---|---|---|---|---|
| ITP all pts, N = 30 . | ITP all pts, N = 30 . | Responders, N = 14 . | Nonresponders, N = 16 . | Responders, N = 14 . | Responders, N = 9 . | ||
| Th1, × 109/L | 0.23 ± 0.10 | 0.24 ± 0.13 | 0.24 ± 0.14 | 0.24 ± 0.13 | 0.24 ± 0.14 | 0.22 ± 0.12 | 0.25 ± 0.08 |
| Th1, % | 16.3 ± 5.3 | 15.2 ± 6.1 | 14.6 ± 5.3 | 15.8 ± 6.9 | 15.7 ± 6.8 | 15.3 ± 6.4 | 15.9 ± 2.6 |
| Th2, × 109/L | 0.018 ± 0.008 | 0.29 ± 0.017* | 0.038 ± 0.017* | 0.021 ± 0.011 | 0.039 ± 0.018* | 0.032 ± 0.015* | 0.035 ± 0.013* |
| Th2, % | 1.4 ± 0.6 | 1.9 ± 0.9* | 2.5 ± 0.9* | 1.3 ± 0.6 | 2.6 ± 1.0* | 2.4 ± 0.8* | 2.3 ± 0.5* |
| Th1/Th2 | 19.1 ± 13.5 | 13.3 ± 6.4 | 10.9 ± 9.8* | 15.9 ± 13.6 | 7.1 ± 5.8* | 8.2 ± 3.6* | 7.5 ± 2.1* |
| Tc1, × 109/L | 0.38 ± 0.17 | 0.33 ± 0.11 | 0.32 ± 0.11 | 0.33 ± 0.11 | 0.29 ± 0.10 | 0.32 ± 0.12 | 0.36 ± 0.11 |
| Tc1, % | 26.2 ± 7.4 | 21.2 ± 3.8 | 20.4 ± 3.9 | 21.8 ± 3.7 | 19.1 ± 4.1 | 22.4 ± 3.3 | 23.4 ± 3.0 |
| Tc2, × 109/L | 0.011 ± 0.004 | 0.021 ± 0.011* | 0.025 ± 0.012* | 0.017 ± 0.007 | 0.030 ± 0.12* | 0.028 ± 0.010* | 0.034 ± 0.013* |
| Tc2, % | 0.8 ± 0.3 | 1.4 ± 0.6* | 1.6 ± 0.6* | 1.1 ± 0.4 | 2.0 ± 0.4* | 1.9 ± 0.5* | 2.2 ± 0.6* |
| Tc1/Tc2 | 34.0 ± 14.2 | 18.4 ± 8.4* | 15.1 ± 8.1* | 21.3 ± 7.8* | 10.0 ± 2.8* | 11.8 ± 0.7* | 11.0 ± 2.7* |
| Variable . | Baseline . | 3 mo . | 6 mo . | 12 mo . | Controls, N = 30 . | ||
|---|---|---|---|---|---|---|---|
| ITP all pts, N = 30 . | ITP all pts, N = 30 . | Responders, N = 14 . | Nonresponders, N = 16 . | Responders, N = 14 . | Responders, N = 9 . | ||
| Th1, × 109/L | 0.23 ± 0.10 | 0.24 ± 0.13 | 0.24 ± 0.14 | 0.24 ± 0.13 | 0.24 ± 0.14 | 0.22 ± 0.12 | 0.25 ± 0.08 |
| Th1, % | 16.3 ± 5.3 | 15.2 ± 6.1 | 14.6 ± 5.3 | 15.8 ± 6.9 | 15.7 ± 6.8 | 15.3 ± 6.4 | 15.9 ± 2.6 |
| Th2, × 109/L | 0.018 ± 0.008 | 0.29 ± 0.017* | 0.038 ± 0.017* | 0.021 ± 0.011 | 0.039 ± 0.018* | 0.032 ± 0.015* | 0.035 ± 0.013* |
| Th2, % | 1.4 ± 0.6 | 1.9 ± 0.9* | 2.5 ± 0.9* | 1.3 ± 0.6 | 2.6 ± 1.0* | 2.4 ± 0.8* | 2.3 ± 0.5* |
| Th1/Th2 | 19.1 ± 13.5 | 13.3 ± 6.4 | 10.9 ± 9.8* | 15.9 ± 13.6 | 7.1 ± 5.8* | 8.2 ± 3.6* | 7.5 ± 2.1* |
| Tc1, × 109/L | 0.38 ± 0.17 | 0.33 ± 0.11 | 0.32 ± 0.11 | 0.33 ± 0.11 | 0.29 ± 0.10 | 0.32 ± 0.12 | 0.36 ± 0.11 |
| Tc1, % | 26.2 ± 7.4 | 21.2 ± 3.8 | 20.4 ± 3.9 | 21.8 ± 3.7 | 19.1 ± 4.1 | 22.4 ± 3.3 | 23.4 ± 3.0 |
| Tc2, × 109/L | 0.011 ± 0.004 | 0.021 ± 0.011* | 0.025 ± 0.012* | 0.017 ± 0.007 | 0.030 ± 0.12* | 0.028 ± 0.010* | 0.034 ± 0.013* |
| Tc2, % | 0.8 ± 0.3 | 1.4 ± 0.6* | 1.6 ± 0.6* | 1.1 ± 0.4 | 2.0 ± 0.4* | 1.9 ± 0.5* | 2.2 ± 0.6* |
| Tc1/Tc2 | 34.0 ± 14.2 | 18.4 ± 8.4* | 15.1 ± 8.1* | 21.3 ± 7.8* | 10.0 ± 2.8* | 11.8 ± 0.7* | 11.0 ± 2.7* |
T-cell subset values are expressed as both absolute concentrations (× 109/L) and as percentage of CD3+ cells (%). Data are shown as mean plus or minus a standard deviation.
Statistically significant (P < .05) difference compared with baseline.
Representative dot-plots of intracellular cytokine expression as evaluated by flow cytometry. Samples from the same patient, in this case a responder, were analyzed before rituximab therapy and 3 months after the first rituximab infusion. The sample of a control subject has been reported for comparison. In the upper plots, CD4+ cells were sorted into Th1 and Th2 subsets according to the expression of either IFN-γ or IL-4. In the lower plots, CD8+ cells were sorted into Tc1 and Tc2 subsets according to the expression of either IFN-γ or IL-4. At 3 months, the patient sample showed an increase of the percentage of Th2 and Tc2 cells compared with baseline, with values very similar to the control sample.
Representative dot-plots of intracellular cytokine expression as evaluated by flow cytometry. Samples from the same patient, in this case a responder, were analyzed before rituximab therapy and 3 months after the first rituximab infusion. The sample of a control subject has been reported for comparison. In the upper plots, CD4+ cells were sorted into Th1 and Th2 subsets according to the expression of either IFN-γ or IL-4. In the lower plots, CD8+ cells were sorted into Tc1 and Tc2 subsets according to the expression of either IFN-γ or IL-4. At 3 months, the patient sample showed an increase of the percentage of Th2 and Tc2 cells compared with baseline, with values very similar to the control sample.
At baseline the number of Th1 cells (IFN-γ single-positive CD4 cells) in ITP patients was not significantly different from controls and did not change significantly during follow-up. The number of Th2 cells (IL-4 single-positive CD4 cells) in patients with active ITP was lower than in the control group (P < .001), but increased significantly (P < .001) in responders at all subsequent controls (3 months, 6 months, and 12 months). The number of Th2 cells was not significantly different between patients in remission and controls.
The number of Tc1 cells (IFN-γ single-positive CD8 cells) was similar between patients and the control group at all study points. The number of Tc2 cells (IL-4 single-positive CD8 cells) was significantly lower in patients with active ITP than in the control group (P < .001), but increased significantly (P < .001) in responders at subsequent study points. Again, the number of Tc2 cells was not significantly different between patients in remission and controls.
The Th1/Th2 and Tc1/Tc2 ratios were significantly higher (P < .001) in patients with active ITP than in the control group, whereas these ratios were not significantly different between patients in remission and controls.
Expression of Fas and FasL on T-cell subsets
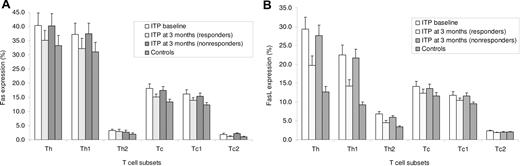
Fas expression on all T-cell subsets of ITP patients was not significantly different from that of healthy individuals both before and after rituximab treatment (Figure 2A). Patients' pretreatment expression of FasL was significantly increased (P < .01) on Th1 (22.5%; 95% CI, 17.4%-27.6%) and Th2 (6.8%; 95% CI, 5.5%-8.1%) cells compared with both controls (Th1, 9.3%; 95% CI, 8.1%-10.6%; Th2, 3.4%; 95% CI, 2.8%-3.9%) and responders to rituximab (Th1, 14.2%; 95% CI, 10.8%-17.9%; Th2, 4.5%; 95% CI, 4.1%-5.0%), but did not change significantly in nonresponders (Th1, 21.7%; 95% CI, 17.2%-26.1%; Th2, 5.9%; 95% CI, 5.3%-6.4%) (Figure 2B). The changes observed at 3 months persisted in responders at 6 and 12 months. No significant differences in FasL expression were observed on Tc subsets.
Flow cytometry analysis of Fas or FasL expression with FITC-conjugated antibodies was performed on T-cell subsets that had been gated according to the double expression of IFN-γ or IL-4 (PE-conjugated antibodies) and CD4 or CD8 (PerCP-conjugated antibodies). (A) Fas expression on all T-cell subsets of ITP patients was not different from that of controls at any study point. (B) Patients' pretreatment expression of FasL was significantly increased (P < .01) on Th1 and Th2 cells compared with both controls and responders to rituximab, but comparable with that of nonresponders. No significant differences in FasL expression were observed on Tc subsets.
Flow cytometry analysis of Fas or FasL expression with FITC-conjugated antibodies was performed on T-cell subsets that had been gated according to the double expression of IFN-γ or IL-4 (PE-conjugated antibodies) and CD4 or CD8 (PerCP-conjugated antibodies). (A) Fas expression on all T-cell subsets of ITP patients was not different from that of controls at any study point. (B) Patients' pretreatment expression of FasL was significantly increased (P < .01) on Th1 and Th2 cells compared with both controls and responders to rituximab, but comparable with that of nonresponders. No significant differences in FasL expression were observed on Tc subsets.
Expression of bcl-2 and bax genes
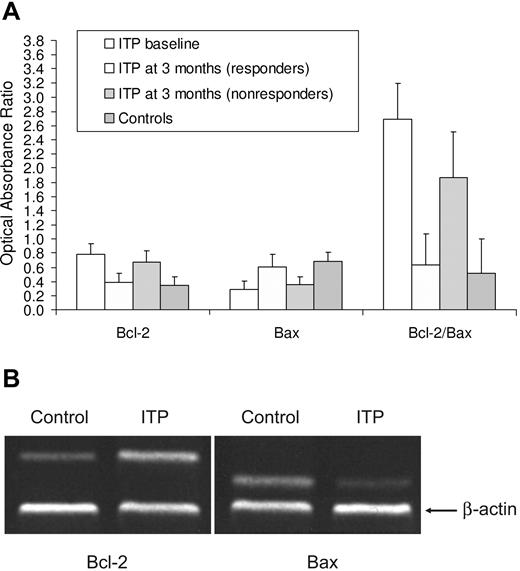
In ITP patients at baseline the expression of Bcl-2 mRNA in CD4+ T cells was higher (P = .003) and that of Bax mRNA was lower (P = .025) than in controls (Figure 3). The Bcl-2/Bax ratio was also significantly higher than in normal samples (P < .001). The expression of Bcl-2 and Bax mRNAs in CD8+ T cells, indicated with the optical absorption ratio, was not different between patients (Bcl-2 = 0.53 ±0.12; Bax = 0.17 ± 0.11) and controls (Bcl-2 = 0.61 ± 0.14; Bax = 0.15 ± 0.09). Bcl-2 and Bax expressions were not significantly different between responders to rituximab therapy and controls, whereas they were not substantially different from baseline in nonresponders.
bcl-2 and bax gene expression in T cells. (A) Relative expressions of Bcl-2 and Bax mRNA in peripheral blood CD4+ T cells. In ITP patients at baseline, the expression of Bcl-2 mRNA in CD4+ T cells was higher (P = .003) and that of Bax mRNA was lower (P = .025) than in controls. The Bcl-2/Bax ratio was also significantly higher than in normal samples (P < .001). Patients and controls had similar expressions of Bcl-2 and Bax mRNAs in CD8+ T cells, indicated with the optical absorption ratio. Bcl-2 and Bax expressions were also not significantly different between responders and controls, whereas they were not substantially different from baseline in nonresponders. (B) Representative RT-PCR analysis for Bcl-2 and Bax on peripheral blood CD4+ T cells. Expression of Bcl-2 was on average increased and that of Bax reduced in ITP patients compared with controls. β-Actin was used as an internal control.
bcl-2 and bax gene expression in T cells. (A) Relative expressions of Bcl-2 and Bax mRNA in peripheral blood CD4+ T cells. In ITP patients at baseline, the expression of Bcl-2 mRNA in CD4+ T cells was higher (P = .003) and that of Bax mRNA was lower (P = .025) than in controls. The Bcl-2/Bax ratio was also significantly higher than in normal samples (P < .001). Patients and controls had similar expressions of Bcl-2 and Bax mRNAs in CD8+ T cells, indicated with the optical absorption ratio. Bcl-2 and Bax expressions were also not significantly different between responders and controls, whereas they were not substantially different from baseline in nonresponders. (B) Representative RT-PCR analysis for Bcl-2 and Bax on peripheral blood CD4+ T cells. Expression of Bcl-2 was on average increased and that of Bax reduced in ITP patients compared with controls. β-Actin was used as an internal control.
Clonality analysis
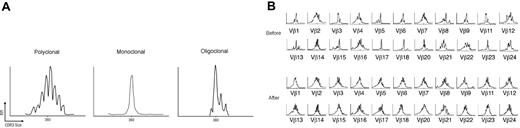
Spectratype analysis in healthy control subjects revealed a polyclonal pattern in TCR VB families (Figure 4A). The pretreatment number of abnormal CDR3 size patterns in responders was 3.1 ± 1.4, which was higher than in controls (1.9 ± 1.3; P = .012) and lower than in nonresponders (9.8 ± 3.6; P < .001). The number of abnormal CDR3 size patterns at baseline tended to correlate with disease duration (r = 0.376; P = .041; Figure 5A).
(A) The normal Vβ spectratype pattern consists of 6 to 8 bands and shows a Gaussian distribution in which the density of bands is generally higher in the middle part of the spectratype. Contracted spectratypes consisting of 1 to 4 peaks suggest the presence of a monoclonal (1 dominant peak) or oligoclonal (2-4 peaks) T-cell population. (B) Reversion of the T-cell repertoire to “normal” in an ITP patient after rituximab therapy. Several monoclonal (eg, VB 17, VB 18) or oligoclonal baseline patterns (eg, VB 1, VB 3, VB 5, VB 6, VB 9) turn to polyclonal after the B-cell–depleting therapy. Shown are the CDR3 size length (40 amino acids) in x-axis versus the relative fluorescence intensity in y-axis.
(A) The normal Vβ spectratype pattern consists of 6 to 8 bands and shows a Gaussian distribution in which the density of bands is generally higher in the middle part of the spectratype. Contracted spectratypes consisting of 1 to 4 peaks suggest the presence of a monoclonal (1 dominant peak) or oligoclonal (2-4 peaks) T-cell population. (B) Reversion of the T-cell repertoire to “normal” in an ITP patient after rituximab therapy. Several monoclonal (eg, VB 17, VB 18) or oligoclonal baseline patterns (eg, VB 1, VB 3, VB 5, VB 6, VB 9) turn to polyclonal after the B-cell–depleting therapy. Shown are the CDR3 size length (40 amino acids) in x-axis versus the relative fluorescence intensity in y-axis.
The number of abnormal CD3 size patterns correlated: (A) positively with ITP duration (r = 0.376; P = .041); and (B) negatively with B-cell depletion duration (r = −0.587; P = .006).
The number of abnormal CD3 size patterns correlated: (A) positively with ITP duration (r = 0.376; P = .041); and (B) negatively with B-cell depletion duration (r = −0.587; P = .006).
After rituximab, responders showed a mean 2.2 ± 1.2 abnormal CDR3 size patterns per patient, similar to that of healthy volunteers. In contrast, patients who had failed rituximab demonstrated 9.2 ± 3.8 abnormal CDR3 size patterns per patient, comparable with their baseline values. No preferential use of any the 22 VB subfamilies was observed. Figure 4B shows the reversion of the T-cell repertoire to a normal Vβ spectratype pattern in a representative ITP patient who responded to rituximab therapy.
B-cell counts and T-cell parameter changes
All patients experienced a quick and sharp decrease of the B-cell counts following rituximab administration. The duration of B-cell depletion, defined as a B-cell count less than 0.02 × 109/L, was 26.9 ± 9.4 weeks in responders and 13.0 ± 6.9 weeks in nonresponders (P < .001). There was no correlation between the duration of B-cell depletion and T-cell parameters, excepting the number of abnormal CDR3 size patterns at baseline (r = −0.587; P = .006; Figure 5B).
Discussion
The pathogenesis of ITP has traditionally been considered to be antibody mediated. However, a number of T-cell abnormalities have been described, including a polarization toward T1 subsets and also direct antiplatelet T-cell lysis.3
More recently B-cell depletion has been used in the treatment of patients with a number of different autoimmune diseases with varying degrees of success. There is a response rate in patients with ITP in approximately 60% of patients,13 with 15% to 20% of cases achieving long-term responses.14 Given the described T-cell abnormalities in patients with ITP it has not been clear how B-cell depletion works in patients with ITP. Although tempting to suggest the response is related to depletion of the antibody producing B cells, a number of points suggest it may be more complicated, such as the immediate platelet response seen in one third of patients with ITP and the fact that response often occurs while autoantibodies can still be measured.
Although there has been no increase in infections following rituximab, limited evidence from the literature supports the view that rituximab affects both the cellular and the humoral arm of the immune system. Vallerskog et al studied 11 patients with systemic lupus erythematosus.15 They observed that upon B-cell repopulation there was a significant increase in activated CD4+ and CD8+ T cells, as well as CD25brightFOXP3+ regulatory T cells. Cross et al demonstrated that rituximab reduces both B cells and T cells in the cerebrospinal fluid of multiple sclerosis patients.16 Given the unclear nature of response to B-cell depletion, the multiple action of B-cell regulation of T cells, and the unclear mechanism of autoimmunity in ITP, we decided to explore the changes in T-cell subsets in patients who received B-cell depletion with rituximab.
Our results indicate that many T-cell abnormalities that are found in patients with chronic ITP can actually be reverted after treatment with rituximab, but only in responders. In fact, patients with active ITP showed significantly higher Th1/Th2 and Tc1/Tc2 ratios, increased expression of FasL, and higher Bcl-2/Bax ratio, as well as oligoclonal expansion of T cells compared with both healthy controls and patients in remission. These findings support the apparent notion that only when T-cell subsets can be modulated, is rituximab therapy effective. They also stimulate several considerations. First of all, our results are in agreement with previous studies conducted in ITP patients treated with other immunosuppressive agents or splenectomy. Most of the evidence in the literature points to a Th1 polarization of the immune response in ITP in vitro,5,6,17,18 with only a few studies yielding inconsistent results.19,20 The Th1/Th2 balance is well known to regulate the immune system under normal conditions, and is known to be impaired in many autoimmune diseases. The role of Tc subsets has been less well defined. In one study, it was shown that Tc1 clones can favor the development of CD4 effectors that are Th1 biased, whereas Tc2 clones have the opposite effect.4 Tc2 clones not only can promote Th2 effectors but also can efficiently suppress the development of Th1 cells. These findings suggest that Tc1 and Tc2 cells develop and function under the influence of a particular microenvironment, probably alongside Th1 and Th2 responses. In this regard, it is important to emphasize that several factors can determine the fate of activated T cells, including type of antigen presenting cells, costimulatory molecules, chromatin structure, and cytokines present in the local environment of the cell at the time of stimulation.21 Interestingly the data presented here suggest that the polarization occurs due to a decrease in Th2 and Tc2 cells rather than increase in Th1 and Tc1 cells.
Recent investigations have shown that while the Th1/Th2 imbalance plays a central pathogenetic role in ITP, cytotoxic T-cell–mediated lysis of platelets may also contribute to the thrombocytopenia. Applying gene expression and fluorescence-activated cell sorting analysis of T cells, Olsson et al have demonstrated that samples from ITP patients have an increased expression of several cytotoxic genes, such as perforin 1, granzyme A, granzyme B, and Apo-1/Fas, together with genes involved in the Th1 cell response, such as interferon-γ and interleukin-2 receptor-β, compared with controls.22 This study emphasizes the heterogeneity of the mechanisms involved in platelet destruction in ITP, which, in addition to T-cell–mediated destruction, includes phagocytosis by macrophages, and antibody-dependent cell-mediated cytotoxicity.
Several assays have been developed to detect apoptosis. The apoptotic cascade can be initiated via 2 major pathways, involving either the release of cytochrome c from the mitochondria (mitochondrial or intrinsic pathway) or activation of death receptors in response to ligand binding (death receptor or extrinsic pathway). Upon triggering of either pathway, caspases, a family of cysteine proteases that enact the final, irreversible commitment to cell death, are activated. Both pathways are differentially involved in the cellular response to diverse apoptotic stimuli. We have therefore used methods that study both the intrinsic (Bcl-2 gene expression and Bcl-2/Bax ratio) and the extrinsic (Fas/FasL) pathways of apoptosis to integrate these data with the changes of the T-cell subsets. Our findings corroborate previous reports,8,23 demonstrating an altered apoptosis profile of T cells in patients with active ITP. In fact, the pretreatment expression of FasL and the Bcl-2/Bax ratio were significantly higher than in controls and responders to rituximab.
Shenoy et al proposed that altered Fas-pathway signaling may be involved in the etiology of autoimmunity in at least some patients with hematologic disorders; they found T cells resistant to Fas-mediated cell death (although only in 5 of 20 patients), which could support the expansion of self-reactive clones.24 Olsson et al speculated that apoptotic resistance of activated T lymphocytes in patients with active ITP may lead to defective clearance of autoreactive T lymphocytes through activation-induced cell death, which might cause a continued immune destruction of platelets.23 Conversely, a loss of resistance to induced apoptosis might be an important mechanism for the achievement of remission in ITP. It was unclear from the results of this study whether disturbed apoptosis is a primary pathogenetic event or rather the consequence of other deranged immunologic processes. It is interesting to note that the apparent decreased susceptibility to apoptosis is actually combined with a decreased number of Th2 and Tc2 cells. Further work is clearly warranted in this area. Nevertheless, this study provides a possible explanation for the failure of rituximab therapy in those patients whose T-cell subsets are not modulated (ie, T-cell resistance to therapy due to abnormal apoptotic mechanisms).
The changes we have seen include an increase in FasL expression on Th1 and Th2 cells and an increase in the bcl-2/bax mRNA ratio. Unlike Liu et al data,7 there was no change in expression in Fas in these adult patients. Although the relevance of increased FasL on Th cells is not clear, the fact that this and other T-cell abnormalities are corrected by B-cell depletion suggests these findings may be in response to T-cell stimulation. Interestingly, patients with ITP who respond to rituximab do not tend to relapse when B cells return, suggesting a complex B/T-cell interaction in patients with ITP.
Aberrant T-cell repertoires assessed by the CDR3 size determination method have been demonstrated in ITP. Fogarty et al have suggested an association between response to splenectomy and T-cell repertoire characteristics as assessed by the CDR3 size determination method.25 However, the small number of patients and the lack of presplenectomy data strongly limited the interpretation of their findings. On the other hand, our data demonstrate a strong association between failure to rituximab therapy and oligoclonal expansion of T cells. An attractive hypothesis to explain this finding is that rituximab may be sufficiently immunomodulatory to produce a clinical remission at an early stage of ITP, when expansion of pathogenic T cells has not reached a “critical” level and is still dependent upon B-cell costimulation. In contrast, when T lymphocyte clones are more expanded they continue to drive antibody production irrespective of the cytokine microenvironment produced by B cells. The results of a large clinical trial with rituximab are consistent with such speculation.26 In that study, patients with a short disease history, in whom presumably T-cell expansion was not yet critical, were much more likely to respond to treatment than those with an ITP duration of many years. Therefore, nonresponsiveness to anti-CD20 therapy in ITP may be related to an inability to modify the oligoclonal nature of the T-cell subsets.
In summary, our study shows for the first time that B-cell depletion induced by rituximab can revert the abnormalities of the T-cell compartment in patients with ITP who respond to treatment. Further exploration of these changes will be needed in order to fully understand the effects of rituximab on the immune system both in ITP and in a broader range of autoimmune disorders.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We wish to thank Dr Howard Liebman, Division of Hematology, and Dr David Horwitz, Division of Rheumatology and Immunology, Keck School of Medicine, University of Southern California, Los Angeles, for helpful suggestions and a critical review of the paper.
Authorship
Contribution: R.S. designed research, analyzed the data, performed statistical analysis, and drafted the paper; G.D.P., E.S., M.L.E., and M.M.T. performed research; N.C. analyzed and interpreted the data and contributed to writing the paper; and S.A. designed research and analyzed the data. All authors took part in the revision of the paper and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roberto Stasi, Department of Medical Sciences, Regina Apostolorum Hospital, Via S. Francesco, 50, 00041 Albano Laziale, Italy; e-mail: roberto.stasi@uniroma2.it.