Abstract
No consensus exists on whether acyclovir prophylaxis should be given for varicella-zoster virus (VZV) prophylaxis after hematopoietic cell transplantation because of the concern of “rebound” VZV disease after discontinuation of prophylaxis. To determine whether rebound VZV disease is an important clinical problem and whether prolonging prophylaxis beyond 1 year is beneficial, we examined 3 sequential cohorts receiving acyclovir from day of transplantation until engraftment for prevention of herpes simplex virus reactivation (n = 932); acyclovir or valacyclovir 1 year (n = 1117); or acyclovir/valacyclovir for at least 1 year or longer if patients remained on immunosuppressive drugs (n = 586). In multivariable statistical models, prophylaxis given for 1 year significantly reduced VZV disease (P < .001) without evidence of rebound VZV disease. Continuation of prophylaxis beyond 1 year in allogeneic recipients who remained on immunosuppressive drugs led to a further reduction in VZV disease (P = .01) but VZV disease developed in 6.1% during the second year while receiving this strategy. In conclusion, acyclovir/valacyclovir prophylaxis given for 1 year led to a persistent benefit after drug discontinuation and no evidence of a rebound effect. To effectively prevent VZV disease in long-term hematopoietic cell transplantation survivors, additional approaches such as vaccination will probably be required.
Introduction
Varicella-zoster virus (VZV) disease can lead to serious complications, including dissemination, hepatic disease, postherpetic neuralgia, bacterial superinfection, and death after hematopoietic cell transplantation (HCT).1-4 Although acyclovir given at different doses for different time periods has been shown to be effective against VZV reactivation disease after HCT,5-7 no consensus exists on the dose, the duration of treatment, or on which group of HCT recipients may benefit most from acyclovir prophylaxis. The most recent Centers for Disease Control and Prevention/American Society for Blood and Marrow Transplantation/Infectious Diseases Society of America (CDC/ASBMT/IDSA) guidelines do not recommend universal prolonged acyclovir prophylaxis for prevention of VZV disease.8 One major reason for the reluctance to administer long-term prophylaxis comes from several smaller studies that showed that there appeared to be disproportionate increase of VZV disease after discontinuation of prophylaxis, which is often termed “rebound” disease. This led to no detectable differences in VZV disease rate at later time points in these studies.5-7 This observation has been explained by reduced VZV-specific T-cell immunity in recipients of acyclovir prophylaxis.7 A more recent study showed increases of VZV disease after drug discontinuation, although VZV-specific T-cell immune reconstitution was not impaired.9 The same study observed that the “rebound” occurred predominantly in patients with continued need for systemic immunosuppression, suggesting that even longer prophylaxis would be required in some patients.9 However, how to identify persistent immunosuppression clinically is an unresolved question.10 Thus, the issue of acyclovir prophylaxis for VZV remains controversial and many transplantation centers do not routinely use long-term acyclovir prophylaxis for VZV reactivation.
The purpose of this study was to assess in a large cohort of patients undergoing HCT if a 1-year acyclovir prophylaxis strategy leads to significant rebound VZV disease after drug discontinuation, and whether the extension of acyclovir prophylaxis beyond 1 year in patients at high risk for late VZV disease confers additional benefit.
Patients and methods
All VZV-seropositive (enzyme immunoassay; Gull Laboratories, Salt Lake City, UT, or immunofluorescence assay; Hemagen, Columbia, MD) T cell replete HCT recipients who underwent their first transplantation at the Fred Hutchinson Cancer Research Center (FHCRC) between January 1996 and December 2003 were included in the study. Informed consent was obtained in accordance with the Declaration of Helsinki. During each of the 3 time periods, management of VZV infections after transplantation was handled by uniform practice guidelines and patients were grouped by the type of management used at the time of the transplant. Cohort 1 (n = 932, transplantations between January 1996 and November 1998) did not receive acyclovir for VZV prevention; however, herpes simplex virus (HSV)–positive recipients were given acyclovir, 250 mg/m2 twice per day, from day −7 until engraftment and resolution of mucositis. From November 1998 until May 2002 (cohort 2, n = 1117), VZV-seropositive HCT recipients received prophylaxis against VZV (acyclovir 250 mg/m2 intravenously followed by 800 mg orally or valacyclovir 500 mg orally, all drugs given twice per day; valacyclovir was preferred for patients who received > 0.5 mg/kg per day of steroids) for 1 year after transplantation. HCT patients undergoing transplantation from May 2002 to December 2003 (cohort 3, n = 586) received the same regimen prescribed for cohort 2 until 1 year after transplantation or 6 months after cessation of all immunosuppression, whichever occurred later. This strategy was intended for allogeneic HCT recipients who were given prolonged immunosuppression for chronic graft-versus-host disease. Standard care for prevention of candidiasis, cytomegalovirus, and pneumocystis jeroveci infection were given as described elsewhere.11-13 Treatment of VZV disease was as per local standard practice, typically with high-dose acyclovir.14 We reviewed medical and laboratory records to identify posttransplantation VZV reactivation cases with a closing date of December 31, 2005. Recipient and transplant characteristics were retrieved from prospectively collected data. Long-term follow-up data consist of medical records, containing information from medical evaluations performed at the center or by the primary physician, and health questionnaires (asking specifically for VZV reactivation disease) sent annually to the physician and the patient. VZV disease was defined clinically and was classified as either localized VZV disease, defined as the presence of lesions distributed in one or 2 contiguous dermatomes or as disseminated VZV disease, with lesions involving more than 2 dermatomes, or in case of visceral localization. Patients with suspicious skin lesions were tested by viral culture, direct fluorescent antibodies, or polymerase chain reaction at the University of Washington clinical and molecular laboratories while patient were still in Seattle. For patients whose diagnoses were received outside Seattle, virologic confirmation was recommended but test results were not available for review. The occurrence of a new episode of VZV reactivation disease 1 week after discontinuation of adequate antiviral treatment and complete resolution of a preceding episode was defined as recurrent VZV reactivation disease.
The study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center, Seattle, WA. Patients included in the study signed an informed consent allowing the analysis of their clinical information in accordance with the Declaration of Helsinki.
Statistical analysis
All analyses were performed separately for allogeneic (including tandem autologous–allogeneic transplant) and autologous (including tandem autologous–autologous transplant) HCT recipients. The analysis was restricted to the first 2 years after HCT. Because the regimen in cohort 3 was intended for allogeneic HCT recipients on prolonged immunosuppression, cohorts 2 and 3 were combined for autologous HCT recipients.
Cumulative incidence curves were used to estimate the probability of acquiring VZV reactivation disease, with death, morphologic relapse, and nontandem second transplantation treated as competing risks.15 The log-rank test was used to compare the underlying hazards of VZV disease between cohorts. The impact of the acyclovir strategy on first VZV disease was estimated by multivariable Cox regression models, which evaluated the following candidate risk factors: recipient age at transplantation, sex, race, transplant type, human leukocyte antigen matching status, conditioning regimen, cell source, disease prognosis, underlying disease, recipient HSV serostatus, recipient/donor cytomegalovirus serostatus, and acute and chronic graft-versus-host disease as time-dependent covariates. We also estimated the probability of first VZV disease 1 year after transplantation for the allogeneic transplant recipients who survived without relapse, second transplantation, or VZV infection to 1 year after HCT.
Acyclovir prophylaxis regimen and first VZV disease were assessed also as risk factors for nonrelapse and overall mortality, with VZV disease entered as a time-dependent covariate in a Cox regression model. Nonrelapse mortality was defined as death without previous morphologic relapse or nontandem second transplantation. Other candidate risk factors for mortality included age at transplantation, human leukocyte antigen matching status, nonmyeloablative conditioning regimen, cell source, disease prognosis, recipient HSV serostatus, recipient/donor cytomegalovirus serostatus, and acute and chronic graft-versus-host disease as time-dependent covariates.
Crude incidence rates of all episodes of VZV reactivation per 100 person-years were calculated for each year of transplantation. Person-time was censored at loss to follow-up, death, morphologic relapse, second transplantation (except for tandem transplant), or 2 years after HCT, whichever occurred first.
A 2-sided P of less than .05 was considered statistically significant. Analysis was performed using STATA Intercooled 9 statistical software (StataCorp LP, College Station, TX) and SAS (version 8.1; SAS, Cary, NC).
Results
Patients and cohort characteristics
The clinical characteristics of patients in each cohort are displayed in Table 1. Of 1522 patients who survived to 2 years after transplantation, more than 90% had follow-up data up to 2 years or later. By acyclovir prophylaxis cohort, 4% (20/510) of the first cohort, 7% (48/656) of the second cohort, and 7% (25/356) of the third cohort had incomplete data (last day of follow-up before 2 years).
Clinical characteristics of the study population
| Complete follow-up data by 2 years after transplantation . | Cohort 1: 1 mo of ACV . | Cohort 2: 1 yr of ACV . | Cohort 3: over 1 yr of ACV . |
|---|---|---|---|
| Number | 932 | 1117 | 586 |
| Age, y, median (range) | 42 (1-68) | 45 (1-74) | 47 (0-73) |
| Male | 487 (52.3) | 576 (51.6) | 358 (61.1) |
| Ethnicity | |||
| White | 801 (85.9) | 898 (80.4) | 476 (81.2) |
| Not white | 131 (14.1) | 219 (19.6) | 101 (17.2) |
| Missing | 0 (0) | 0 (0) | 9 (1.5) |
| Type of transplant procedure | |||
| Tandem transplant* | 17 (1.8) | 27 (2.4) | 15 (2.6) |
| Single autologous transplantation | 196 (21.0) | 262 (23.5) | 184 (31.4) |
| Single allogeneic transplantation | 719 (77.1) | 828 (74.1) | 387 (66.0) |
| Tandem strategy | |||
| Autologous–allogeneic | 1 (0.1) | 23 (2.1) | 15 (2.6) |
| Autologous–autologous | 16 (1.7) | 4 (0.4) | 0 (0) |
| Conditioning regimen | |||
| Nonmyeloablative† | 6 (0.6) | 126 (11.3) | 82 (14.0) |
| Myeloablative with TBI | 383 (41.1) | 513 (45.9) | 335 (57.2) |
| Myeloablative with combination chemotherapy | 526 (56.4) | 451 (40.4) | 154 (26.3) |
| Tandem | |||
| Myeloablative–myeloablative | 1 (0.1) | 23 (2.1) | 15 (2.6) |
| Myeloablative–nonmyeloablative | 16 (1.7) | 4 (0.4) | 0 (0) |
| Donor status | |||
| Autologous transplantation | 212 (22.7) | 266 (23.8) | 184 (31.4) |
| HLA-matched | 319 (34.2) | 424 (38.0) | 165 (28.2) |
| HLA-mismatched | 73 (7.8) | 33 (3.0) | 23 (3.9) |
| Unrelated | 328 (35.2) | 394 (35.3) | 214 (36.5) |
| Type of transplanted cells | |||
| Bone marrow | 597 (64.1) | 391 (35.0) | 69 (11.8) |
| Cord blood | 8 (0.9) | 12 (1.1) | 8 (1.4) |
| PBSC | 327 (35.1) | 714 (63.9) | 509 (86.9) |
| Underlying disease prognosis‡ | |||
| Good prognosis | 336 (36.1) | 396 (35.5) | 149 (25.4) |
| Poor prognosis | 596 (63.9) | 712 (63.7) | 425 (72.5) |
| Unknown | 0 (0) | 9 (0.8) | 12 (2.0) |
| Recipient HSV serostatus | |||
| Negative | 250 (26.8) | 213 (19.1) | 109 (18.6) |
| Positive | 677 (72.6) | 898 (80.4) | 474 (80.9) |
| Unknown | 5 (0.5) | 6 (0.5) | 3 (0.5) |
| Recipient/donor CMV serostatus in allogeneic recipients | |||
| −/− | 272 (37.8) | 280 (32.9) | 129 (32.1) |
| −/+ | 115 (16.0) | 120 (14.1) | 47 (11.7) |
| +/− | 152 (21.1) | 213 (25.0) | 117 (29.1) |
| +/+ | 180 (25.0) | 230 (27.0) | 108 (26.9) |
| Donor CMV serostatus unknown (recipient negative) | 1 (0.1) | 8 (1.0) | 1 (0.2) |
| Donor CMV serostatus autologous patients | |||
| Negative | 100 (47.2) | 119 (44.7) | 81 (44.0) |
| Positive | 112 (52.8) | 147 (55.3) | 103 (56.0) |
| Complete follow-up data by 2 years after transplantation . | Cohort 1: 1 mo of ACV . | Cohort 2: 1 yr of ACV . | Cohort 3: over 1 yr of ACV . |
|---|---|---|---|
| Number | 932 | 1117 | 586 |
| Age, y, median (range) | 42 (1-68) | 45 (1-74) | 47 (0-73) |
| Male | 487 (52.3) | 576 (51.6) | 358 (61.1) |
| Ethnicity | |||
| White | 801 (85.9) | 898 (80.4) | 476 (81.2) |
| Not white | 131 (14.1) | 219 (19.6) | 101 (17.2) |
| Missing | 0 (0) | 0 (0) | 9 (1.5) |
| Type of transplant procedure | |||
| Tandem transplant* | 17 (1.8) | 27 (2.4) | 15 (2.6) |
| Single autologous transplantation | 196 (21.0) | 262 (23.5) | 184 (31.4) |
| Single allogeneic transplantation | 719 (77.1) | 828 (74.1) | 387 (66.0) |
| Tandem strategy | |||
| Autologous–allogeneic | 1 (0.1) | 23 (2.1) | 15 (2.6) |
| Autologous–autologous | 16 (1.7) | 4 (0.4) | 0 (0) |
| Conditioning regimen | |||
| Nonmyeloablative† | 6 (0.6) | 126 (11.3) | 82 (14.0) |
| Myeloablative with TBI | 383 (41.1) | 513 (45.9) | 335 (57.2) |
| Myeloablative with combination chemotherapy | 526 (56.4) | 451 (40.4) | 154 (26.3) |
| Tandem | |||
| Myeloablative–myeloablative | 1 (0.1) | 23 (2.1) | 15 (2.6) |
| Myeloablative–nonmyeloablative | 16 (1.7) | 4 (0.4) | 0 (0) |
| Donor status | |||
| Autologous transplantation | 212 (22.7) | 266 (23.8) | 184 (31.4) |
| HLA-matched | 319 (34.2) | 424 (38.0) | 165 (28.2) |
| HLA-mismatched | 73 (7.8) | 33 (3.0) | 23 (3.9) |
| Unrelated | 328 (35.2) | 394 (35.3) | 214 (36.5) |
| Type of transplanted cells | |||
| Bone marrow | 597 (64.1) | 391 (35.0) | 69 (11.8) |
| Cord blood | 8 (0.9) | 12 (1.1) | 8 (1.4) |
| PBSC | 327 (35.1) | 714 (63.9) | 509 (86.9) |
| Underlying disease prognosis‡ | |||
| Good prognosis | 336 (36.1) | 396 (35.5) | 149 (25.4) |
| Poor prognosis | 596 (63.9) | 712 (63.7) | 425 (72.5) |
| Unknown | 0 (0) | 9 (0.8) | 12 (2.0) |
| Recipient HSV serostatus | |||
| Negative | 250 (26.8) | 213 (19.1) | 109 (18.6) |
| Positive | 677 (72.6) | 898 (80.4) | 474 (80.9) |
| Unknown | 5 (0.5) | 6 (0.5) | 3 (0.5) |
| Recipient/donor CMV serostatus in allogeneic recipients | |||
| −/− | 272 (37.8) | 280 (32.9) | 129 (32.1) |
| −/+ | 115 (16.0) | 120 (14.1) | 47 (11.7) |
| +/− | 152 (21.1) | 213 (25.0) | 117 (29.1) |
| +/+ | 180 (25.0) | 230 (27.0) | 108 (26.9) |
| Donor CMV serostatus unknown (recipient negative) | 1 (0.1) | 8 (1.0) | 1 (0.2) |
| Donor CMV serostatus autologous patients | |||
| Negative | 100 (47.2) | 119 (44.7) | 81 (44.0) |
| Positive | 112 (52.8) | 147 (55.3) | 103 (56.0) |
Data are numbers (%) except for rows 1 and 2.
CMV indicates cytomegalovirus; PBSC, peripheral blood stem cells; and TBI, total body irradiation.
Tandem regimens combine two transplantation procedures, autologous transplantation with subsequent autologous or nonmyeloablative allogeneic transplantation (auto–auto or auto–allo).
Nonmyeloablative regimen includes TBI 200 cGy alone or fludarabine (90 mg/m2) plus TBI (200 cGy).
Low-risk disease included chronic myeloid leukemia in chronic phase and hematomalignancy in first remission aplastic anemia. High-risk disease included refractory anemia, chronic lymphoid leukemia, paroxysmal nocturnal hemoglobinuria, chronic leukemia in blast phase, juvenile chronic leukemia, hematologic malignancy in relapse, multiple myeloma, myeloid metaplasia, and solid organ tumor.
Incidence and clinical features of VZV reactivation disease
Allogeneic patients.
Posttransplantation VZV disease was documented in 269 patients within 2 years after transplantation (including tandem autologous–allogeneic transplant recipients: no patients presented VZV between the first and the second transplantation), including 178 patients (25%) in cohort 1, 73 (9%) in cohort 2, and 18 (4%) in cohort 3. VZV disease occurred at a median of 6 months (range, 0-24) in cohort 1, 13 months (range,0-23 months) in cohort 2, and 16 months (range, 2-23 months) in cohort 3 (Kruskall-Wallis, χ2 = 57.1; P < .001). Recurrent VZV disease occurred in 8 (1%) and 3 ( < 1%) HCT recipients in cohort 1 and cohort 2, respectively.
A similar distribution of clinical presentation of VZV reactivation disease was observed across cohorts, with a majority of localized disease manifestations (Table 2). Disseminated VZV disease occurred at a median of 98 (range, 52-172) days after HCT and was the cause of death in 6 patients in cohort 1. Two of the 6 patients presented with abdominal pain and liver failure: the first had VZV documented in the liver and subsequently also in skin lesion; the second patient had VZV detected in the liver and gut. One patient presented with cutaneous disseminated VZV disease. The remaining 3 patients with disseminated disease had intravascular coagulopathy-associated VZV disease16,17 with VZV in bone marrow, cerebrospinal fluid, and skin specimens; VZV encephalitis and VZV pneumonia, respectively. No fatal cases were identified in cohorts 2 and 3.
Distribution and characteristics of VZV reactivation disease across cohorts
| . | Allogeneic HCT recipients . | Autologous HCT recipients . | |||
|---|---|---|---|---|---|
| Cohort 1 . | Cohort 2 . | Cohort 3 . | Cohort 1 . | Cohorts 2 and 3 . | |
| Number | 720 | 851 | 402 | 212 | 450 |
| First VZV reactivation disease, no. | 178 | 73 | 18 | 45 | 35 |
| Disseminated disease, N (%) | 35 (19.6) | 15 (20.6) | 2 (1.1) | 6 (13.3) | 1 (2.9) |
| Ocular zoster | 1 (0.6) | 2 (2.7) | 0 | 0 | 2 (5.7) |
| Localized zoster | 142 (79.8) | 56 (76.7) | 16 (98.9) | 39 (86.7) | 32 (91.4) |
| Months to event, median (range) | 6 (<1-24) | 13 (<1-24) | 16 (1.5-23) | 5 (<1-22) | 14 (<1-24) |
| Recurrent VZV reactivation disease* | 8 | 3 | 0 | 0 | 2 |
| Disseminated disease | 1 (12.5) | 0 | — | — | 0 |
| Localized zoster | 7 (87.5) | 3 (100) | — | — | 2 (100) |
| Months to event, median (range) | 6 (4-22) | 17 (16-21) | — | — | (12-18) |
| . | Allogeneic HCT recipients . | Autologous HCT recipients . | |||
|---|---|---|---|---|---|
| Cohort 1 . | Cohort 2 . | Cohort 3 . | Cohort 1 . | Cohorts 2 and 3 . | |
| Number | 720 | 851 | 402 | 212 | 450 |
| First VZV reactivation disease, no. | 178 | 73 | 18 | 45 | 35 |
| Disseminated disease, N (%) | 35 (19.6) | 15 (20.6) | 2 (1.1) | 6 (13.3) | 1 (2.9) |
| Ocular zoster | 1 (0.6) | 2 (2.7) | 0 | 0 | 2 (5.7) |
| Localized zoster | 142 (79.8) | 56 (76.7) | 16 (98.9) | 39 (86.7) | 32 (91.4) |
| Months to event, median (range) | 6 (<1-24) | 13 (<1-24) | 16 (1.5-23) | 5 (<1-22) | 14 (<1-24) |
| Recurrent VZV reactivation disease* | 8 | 3 | 0 | 0 | 2 |
| Disseminated disease | 1 (12.5) | 0 | — | — | 0 |
| Localized zoster | 7 (87.5) | 3 (100) | — | — | 2 (100) |
| Months to event, median (range) | 6 (4-22) | 17 (16-21) | — | — | (12-18) |
Data are number (%) of patients, unless otherwise indicated.
— indicates no entry (0 recurrents).
The occurrence of a new episode of VZV reactivation disease 1 week after discontinuation of adequate antiviral treatment and complete resolution of a preceding episode was defined as recurrent VZV reactivation disease.
Of 14 HCT recipients in cohort 3 in whom VZV developed, reactivation disease during the second year after transplantation, 8 presented with VZV reactivation while on immunosuppressive therapy given for chronic graft-versus-host disease. Among those 8 patients, 2 were not on antiviral prophylaxis, 2 had no information on whether antiviral prophylaxis was prescribed, and 4 were reported to be on antiviral medication, although no information was available concerning adherence to the prescribed antiviral regimen. All of the 14 VZV disease cases responded to higher doses of acyclovir.
Autologous patients.
Posttransplantation VZV disease was documented in 80 patients within 2 years after first transplantation (including tandem autologous–autologous transplant recipients; no patients presented VZV reactivation between the first and the second transplant), including 45 cases (21.2%) in cohort 1, and 35 cases (7.1%) in cohorts 2 and 3. VZV disease occurred at a median of 5 months (range, 0-22) in cohort 1, and 14 months (range, 2-24) in cohort 2 and 3 (Kruskall-Wallis, χ2 = 31.5; P < .001). One patient in cohort 2 presented with a recurrent VZV disease. No death was directly attributable to VZV disease. A similar distribution of clinical presentation of VZV reactivation disease was observed across cohorts, with a majority of localized diseases (Table 2).
Impact of acyclovir on VZV disease
Allogeneic patients.
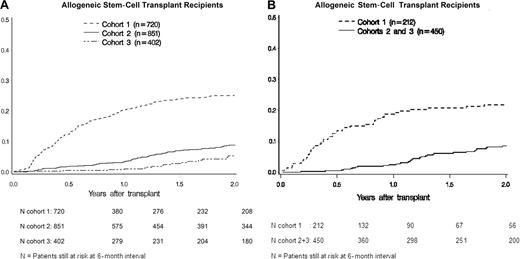
The probability of first VZV reactivation disease at year 2 after transplantation in patients who were not given long-term acyclovir (cohort 1, 24.9%; 95% confidence interval [CI], 21.7%-28.1%) was significantly higher than in those who received either 1 year (cohort 2, 8.8%; 95% CI, 6.9%-10.7%; P < .001) or more than 1 year of acyclovir prophylaxis (cohort 3, 4.5%; 95% CI, 2.5-6.6%; P < .001; Figure 1A). There was no evidence of a disproportionate increase of VZV disease during the second year among patients who received 1 year of acyclovir (cohort 2) compared with patients in cohort 1 (12.5%, cohort 1; 10.0%, cohort 2; P > .05).
Probability of VZV disease for 2 years after transplantation in allogeneic HCT recipients and autologous HCT recipients. (A) Among allogeneic HCT recipients, the probability of VZV reactivation disease was significantly lower in cohort 2 (8.8%; P < .001) and in cohort 3 (4.5%; P < .001) compared with cohort 1 (24.9%), and in cohort 3 compared with cohort 2 (P = .01). (B) Among autologous HCT recipients, the probability of VZV reactivation disease was significantly lower in cohorts 2 and 3 (8.2%; P < .001) compared with cohort 1 (21.7%).
Probability of VZV disease for 2 years after transplantation in allogeneic HCT recipients and autologous HCT recipients. (A) Among allogeneic HCT recipients, the probability of VZV reactivation disease was significantly lower in cohort 2 (8.8%; P < .001) and in cohort 3 (4.5%; P < .001) compared with cohort 1 (24.9%), and in cohort 3 compared with cohort 2 (P = .01). (B) Among autologous HCT recipients, the probability of VZV reactivation disease was significantly lower in cohorts 2 and 3 (8.2%; P < .001) compared with cohort 1 (21.7%).
The extended regimen of cohort 3 provided further protection from VZV disease than the 1-year regimen of cohort 2 (P = .006). The effectiveness of long-term acyclovir in preventing VZV reactivation disease was manifest mainly during the first year after transplantation. Indeed, the probability of a first VZV reactivation disease during the second year after transplantation among patients who had survived to 1 year without relapse, second transplantation, or VZV disease did not vary significantly by cohort (12.5%, 10.0%, and 6.1% in cohorts 1, 2, and 3, respectively; P = .06).
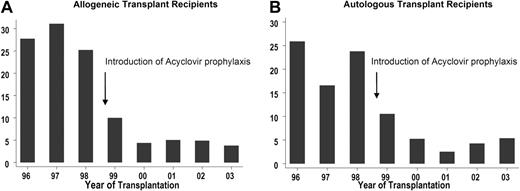
The post-HCT 2-year incidence rate (cases per 100 person-years) of any VZV reactivation disease decreased significantly from 27.7 (95% CI, 23.9-31.9) in cohort 1 to 7.4 (95% CI, 5.9-9.3) in cohort 2 (P < .001), and to 3.6 (95% CI, 2.2-5.7) in cohort 3 (cohort 2 vs cohort 3, P = .003) after allogeneic HCT (Figure 2A). The change over the years in the incidence rate of VZV reactivation disease is displayed in Figure 2A.
The 2-year-incidence rates (per 100 person-years) of VZV reactivation disease after HCT. Allogeneic (A) and autologous (B) HCT recipients. Acyclovir prophylaxis for VZV prevention was first introduced in 1999.
The 2-year-incidence rates (per 100 person-years) of VZV reactivation disease after HCT. Allogeneic (A) and autologous (B) HCT recipients. Acyclovir prophylaxis for VZV prevention was first introduced in 1999.
The acyclovir prophylaxis regimen remained a statistically significant predictor of lower risk of VZV disease after adjustment for other risk factors (Table 3). In a multivariable model that included the acyclovir cohort, HSV seropositivity and age older than 50 years were associated with a lower risk, and receipt of total body irradiation as part of a myeloablative conditioning regimen was associated with a higher risk of VZV reactivation disease.
Factors influencing the development of VZV reactivation disease after allogeneic HCT
| . | Allogeneic HCT recipients* . | 95% CI . | P . |
|---|---|---|---|
| HR . | |||
| Acyclovir prophylaxis† | |||
| No long-term acyclovir (cohort 1) | 3.3 | 2.5-4.4 | <.001 |
| 1 year of acyclovir use (cohort 2) | 1.0 | — | — |
| 1 year or more of acyclovir use (cohort 3) | 0.5 | 0.3-0.9 | .01 |
| Age, y | |||
| 0 to 49 | 1.0 | — | — |
| 50 to 73 | 0.6 | 0.4-0.9 | .01 |
| Conditioning regimen | |||
| Myeloablative with combination therapy | 1.0 | — | — |
| Myeloablative with total body irradiation | 1.5 | 1.2-2.0 | .003 |
| Nonmyeloablative | 1.2 | 0.6-2.2 | .70 |
| Tandem transplantations | 2.1 | 0.8-5.3 | .11 |
| Recipient herpes simplex virus serostatus | |||
| Negative | 1.0 | — | — |
| Positive | 0.6 | 0.5-0.8 | <.001 |
| . | Allogeneic HCT recipients* . | 95% CI . | P . |
|---|---|---|---|
| HR . | |||
| Acyclovir prophylaxis† | |||
| No long-term acyclovir (cohort 1) | 3.3 | 2.5-4.4 | <.001 |
| 1 year of acyclovir use (cohort 2) | 1.0 | — | — |
| 1 year or more of acyclovir use (cohort 3) | 0.5 | 0.3-0.9 | .01 |
| Age, y | |||
| 0 to 49 | 1.0 | — | — |
| 50 to 73 | 0.6 | 0.4-0.9 | .01 |
| Conditioning regimen | |||
| Myeloablative with combination therapy | 1.0 | — | — |
| Myeloablative with total body irradiation | 1.5 | 1.2-2.0 | .003 |
| Nonmyeloablative | 1.2 | 0.6-2.2 | .70 |
| Tandem transplantations | 2.1 | 0.8-5.3 | .11 |
| Recipient herpes simplex virus serostatus | |||
| Negative | 1.0 | — | — |
| Positive | 0.6 | 0.5-0.8 | <.001 |
Allogeneic HCT recipients: gender, race, type of cells (bone marrow or cord blood vs. peripheral blood stem cells), underlying disease (good vs bad prognosis for relapse), CMV serostatus (donor/recipient), donor HLA serostatus, chronic and acute graft-versus host disease (time-dependent covariates) were not independently associated with VZV reactivation disease.
Cohort 1: acyclovir 5 mg/kg intravenous every 8 hours or 400 mg twice daily orally until engraftment for patients who were HSV-seropositive HCT recipients. Cohort 2 and cohort 3: acyclovir 250 mg/m2 intravenous every12 hours followed by acyclovir 800 mg orally twice daily or valacyclovir 500 mg orally twice daily (valacyclovir is preferred for patients who were receiving 0.5 mg/kg or more per day of corticosteroids).
Autologous patients.
The probability of first VZV reactivation disease at year 2 after transplantation for patients who were not given long-term acyclovir (cohort 1, 21.7%; 95% CI, 16.1-27.3%) was significantly higher than for those who received at least 1 year of acyclovir prophylaxis (cohorts 2 and 3, 8.2%; 95% CI, 5.6-10.9%; P < .001) (Figure 1B).
The post-HCT 2-year incidence rate (per 100 person-years) of any VZV reactivation disease decreased significantly from 20.4 (95% CI, 15.2-27.3) among patients who were not given long-term acyclovir prophylaxis (cohort 1) to 5.8 (95% CI, 4.2-8.1) for patients who received 1 year of acyclovir prophylaxis (cohorts 2 and 3, P < .001) after autologous HCT (Figure 2B).
Acyclovir prophylaxis regimen was the only factor associated with VZV reactivation disease in multivariable analysis. The risk of VZV disease was significantly higher among patients who did not receive long-term acyclovir prophylaxis than that among patients who did (cohort 1 vs cohorts 2 and 3, adjusted hazard ratio [HR], 3.8; 95% CI, 2.4-5.9; P < .001).
Impact of acyclovir prophylaxis and first VZV reactivation disease on nonrelapse and overall mortality
Allogeneic HCT recipients.
Among 1973 allogeneic HCT recipients, there were 901 deaths in the first 2 years after HCT. This included 337 in cohort 1, 387 in cohort 2, and 177 in cohort 3; 593 patients died without relapse or second transplantation, including 211 in cohort 1, 278 in cohort 2, and 104 in cohort 3.
The use of 1 year of acyclovir prophylaxis was associated with improved overall survival in allograft (cohort 1 vs. cohort 2, adjusted risk of death [HR], 1.2; 95% CI, 1.0-1.4, p = .03) recipients in a model including age, donor type, the conditioning regimen (myeloablative vs. nonmyeloablative), disease prognosis, recipient HSV, paired cytomegalovirus serostatus, acute and chronic graft-versus-host disease, and disseminated VZV disease. Compared with 1-year acyclovir, prolonged use of acyclovir for more than 1 year did not significantly affect overall mortality (cohort 3 vs. cohort 2, adjusted HR, 0.9; 95% CI, 0.8-1.1; P = .48). Neither the use of 1-year acyclovir (cohort 1 vs cohort 2, adjusted HR, 1.1; 95% CI, 0.9-1.4; P = .17) nor the use of more than 1-year acyclovir (cohort 3 vs cohort 2, adjusted HR, 0.8; 95% CI, 0.6-1.0; P = .09) significantly affected the risk of nonrelapse mortality in the multivariable model.
In these models, disseminated VZV disease was independently associated with a higher risk of overall mortality (HR, 2.5; 95% CI, 1.6-4.0; P < .001) and nonrelapse mortality (HR, 3.8; 95% CI, 2.3-6.4; P < .001); localized VZV disease did not affect these outcomes (data not shown).
Among 20 allogeneic HCT recipients who presented with VZV disease and died without relapse or second transplantation (nonrelapse mortality), VZV infection was the direct cause of death in 6 patients, other infections in 9 patients, and 5 patients died from noninfectious cause.
Autologous HCT recipients.
Among 662 autologous HCT recipients, there were 212 deaths, including 85 in cohort 1, 74 in cohort 2, and 53 in cohort 3. Acyclovir prophylaxis was the only risk factor associated with overall mortality among autologous HCT recipients. The risk of overall mortality in this subgroup of HCT recipients was significantly lower in the long-term acyclovir cohort (cohorts 2 and 3) compared with that in cohort 1 (nonadjusted HR, 0.7; 95% CI, 0.5-0.9; P = .003). No significant association was observed between nonrelapse mortality and acyclovir prophylaxis.
Discussion
This large retrospective population-based study addressed 2 controversial issues related to the use of acyclovir for VZV prophylaxis: (1) whether a 1-year course of acyclovir leads to “rebound” VZV disease after drug discontinuation; and (2) whether continued use of acyclovir in allograft recipients who receive immunosuppressive drugs at 1 year adds additional benefit. Regarding the first question, we confirmed that acyclovir is highly effective in preventing VZV reactivation disease during the time of its administration.6,7,18 The regimen was associated with a significant improvement in all-cause mortality, as well as the elimination of disseminated VZV infection among both allogeneic and autologous HCT transplant recipients. It was also safe and well-tolerated in a previous randomized trial.9 The cases of VZV disease that occurred after discontinuation of acyclovir were localized and responded well to treatment doses of acyclovir. Of interest was our observation that the 1-year course of acyclovir after HCT was associated with a persistently decreased risk of VZV reactivation disease even after drug discontinuation. The rates of VZV disease during the second year were not significantly different between the untreated patients in co-hort 1 and acyclovir recipients in cohort 2, and the slopes of the cumulative incidence curves were virtually identical during the second year (Figure 1A,B). Thus, this study provides evidence that discontinuation of daily antiviral prophylaxis after the first year after transplantation is not associated with a “rebound” effect, ie, there is not an increased VZV disease rate after drug discontinuation when acyclovir is given for 1 year. The results are also consistent with findings from a recent randomized study that also used a 1-year regimen, which showed that VZV-specific T-cells reconstitution was not affected with the acyclovir regimen used in this study.9
We initiated routine VZV prophylaxis for our transplant patients in 1998 because our active surveillance questionnaire in our long-term follow-up unit indicated several cases of disseminated infection and associated death late in the transplantation period. In 2002, we started continuing acyclovir prophylaxis in allograft recipients if they still required systemic immunosuppressive drugs at 1 year after HCT. Thus, the clinical criteria for the continued use of prophylaxis were similar to that recommended for prophylaxis of pneumocystis jeroveci pneumonia.
Whether the use of antiviral prophylaxis after the initial year after HCT transplantation is necessary remains unclear from our analyses. Whereas cohort 3 did exhibit lower rates of VZV over the course of transplantation than cohort 2, this difference was mainly driven by lower rates of VZV disease during the first year after HCT, when both strategies were identical. An analysis of breakthrough cases observed during the second year suggested potential lack of adherence to the prolonged drug regimen. There was also evidence that the clinical criteria used in this study to administer prophylaxis for more than 1 year (cohort 3) did not identify all patients at risk. This suggests that there is a continued VZV-specific immunodeficiency that persists beyond 6 months after discontinuation of all systemic immunosuppression, which was the criterion used for discontinuation of prophylaxis in this study. This finding is consistent with a recent report by Thompson et al.10
The reduction in overall mortality with 1-year acyclovir is an interesting finding that would further support the use of acyclovir for 1 year. The effect could be mediated by a reduction of disseminated VZV disease, which was independently associated with death in this study, as well as through suppression of as yet uncharacterized “indirect effects” of active herpesvirus infections. This increase in survival while on daily acyclovir has been noted in previous studies.19 We were unable to show an effect on overall or nonrelapse survival with the extended use beyond 1 year (cohort 3) compared with the 1-year regimen.
This study used a rather high dose of acyclovir or valacyclovir for VZV prophylaxis.20 We chose this dose based on the previous randomized trial,20 as well as the concern that lower doses would result in a higher frequency of acyclovir-resistant VZV. While we did not routinely test breakthrough cases for acyclovir resistance, clinical resistance was not observed in this cohort and routine use of higher doses of acyclovir was associated with resolution of infection in all cases that we treated.21 The dose used in this study also effectively prevented wild-type and drug-resistant HSV disease among HSV-seropositive transplant recipients.21
This study has strengths and limitations. The large sample size allowed for multivariable analyses, which accounted for changes in transplantation practices over the study period. The “real-world” experience is an additional strength of the study: our data are not derived from a single center experience under a research protocol, but most of the prescriptions were dispensed off-site after the patients' discharge from the cancer center. One of the limitations consists of the comparison of noncontemporaneous cohorts of HCT recipients. It is thus possible that variations in transplant procedures and in the management of posttransplantation complications at different time periods may have influenced the differences seen between the cohorts. However, regarding VZV reactivation disease, the difference remained significant after adjusting for major variables related to transplant procedures, such as the use of peripheral blood stem cells (PBSC) as cell source and nonmyeloablative conditioning. Of note, the risk of VZV disease after PBSC transplantation was similar to that after marrow transplantation in an earlier randomized trial.22 It is possible that regarding their acyclovir prophylaxis, some patients might have been misclassified in the different cohorts; however, if true, this would have led to an attenuation of the estimated size of the true association between acyclovir prophylaxis and VZV reactivation disease. A diagnostic or reporting bias with less testing or insufficient reporting in more recent years is another potential limitation. However, because we were concerned about the possibility of atypical clinical manifestations and the possibility of drug resistance among acyclovir recipients, we aggressively pursued the diagnostic work-up of skin lesions, including polymerase chain reaction testing. We also recommended aggressive work-up to the referring physicians. This retrospective study did not include a formal analysis of safety and tolerability. Acyclovir prophylaxis at the same dose has been shown to be safe when given for 1 year in a randomized, placebo-controlled trial.9 In addition, acyclovir and valacyclovir have been shown to be safe in long-term prevention studies in HCT recipients at doses much higher than those used in this study.19,23 There is presently no controlled safety information beyond 1 year. Finally, the study was not able to address the VZV disease risk after 2 years after transplantation.
In conclusion, this study provides strong support for the use of long-term acyclovir or valacyclovir during the first year after allogeneic and autologous HCT. There was no evidence that the regimen used in this study led to a disproportionate or “rebound” VZV disease during the second year after HCT among acyclovir/valacyclovir recipients, and VZV disease that occurred after prophylaxis responded well to treatment, suggesting that drug resistance was not a major problem. A reconsideration of the current guidelines for VZV prophylaxis, which presently prevents the widespread use of VZV prophylaxis in the HCT setting,8 seems to be justified based on the present study and a recently published randomized placebo-controlled trial.9 This may be especially important for countries and regions where the insurance coverage for acyclovir use is dictated by the current guidelines. With less strong evidence, the present study also suggests an advantage of extending use of acyclovir beyond 1 year among patients with continued need for immunosuppressive therapy, although adherence to the drug regimen and the identification of candidates for long-term use emerged as important issues. Our data show that even with the strategy used in this study, the risk of VZV disease after 1 year is still substantially greater than that observed in the general population.24 Thus, ultimately, control of VZV after 1 year may not be possible with acyclovir alone. A recent study by Thomson et al10 showed a continued risk of VZV disease even after years of acyclovir in patients with chronic immunosuppression. Both the Thomson study and our study suggest that a combined antiviral drug and vaccination approach may be needed to completely address the VZV risk in patients with continued immunosuppression at 1 year. Heat-inactivated vaccine administered in the first 3 months after HCT has been shown to be safe in autologous patients.25,26 Vaccination at 1 year, when partial T cell-immunity is restored, might result in a sufficient increase of virus-specific immunity to completely suppress VZV reactivation disease in long-term allogeneic and autologous HCT survivors. Studies are needed to examine this strategy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Joel Meyers Endowment fund, National Institutes of Health CA 18029, CA 15704, K24 AI071113.
The authors thank Chris Davis for database services, and Debra Mattson, the health care providers, nurses, other professionals, and members of the long-term follow-up team for providing information on long-term follow-up data.
National Institutes of Health
Authorship
V.E. designed the study, collected the data, analyzed the data, and wrote the paper. K.A.G. analyzed the data and critically reviewed the manuscript. C.V. and J.H. contributed to data collection and critically reviewed the manuscript. A.W. contributed to the study design and critically reviewed the manuscript. M.E.D.F. was responsible for chronic graft-versus-host disease grading and critically reviewed the manuscript. L.C. critically reviewed the manuscript. M.B. was responsible for the overall study, contributed to the study design, and carefully reviewed the paper.
Conflict-of-interest disclosure: A.W. has received grant support from GlaxoSmithKline, Antigenics, Roche Laboratories, and Vical. She is a consultant or speaker for Novartis, Powdermed, Medigene, and Merck. L.C. is head of the Virology Division at the University of Washington. The laboratories that he directs receive grant support from a variety of studies from GlaxoSmithKline and Novartis to perform polymerase chain reaction–based assays for clinical tests of HSV. He receives no salary support from these funds. M.B. has received grant support from Roche Laboratories and Novartis. He has served as a consultant and/or speaker for Novartis and Roche Laboratories. All other authors declare no competing financial interests.
Correspondence: Michael Boeckh, Fred Hutchinson Cancer Research Center, Program in Infectious Diseases, 1100 Fairview Avenue North D3-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: mboeckh@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal