Abstract
Reticulocytes release small membrane vesicles termed exosomes during their maturation into erythrocytes. It has been suggested that reticulocytes remodel the plasma membrane of the immature red cell during erythropoiesis by specifically eliminating various proteins. We report here that exosome release is associated with a physiologic cascade induced by the expression of a 15-lipoxygenase at the reticulocyte stage. We found that the phospholipase iPLA2 specifically associated with the endosomal and exosomal membranes could be activated by reactive oxygen species (ROSs) produced during mitochondria degeneration induced by 15-lipoxygenase. Since iPLA2 has recently been demonstrated to participate in the clearance of apoptotic cells, we investigated its role in vesicle removal. We found that exosomes isolated directly from the blood of an anemic rat or released during in vitro maturation of rat reticulocytes bind IgM antibodies on their surface, in contrast to immature and mature red cells. These natural IgM antibodies recognize lysophosphatidylcholine and are able to specifically bind to apoptotic cells. Finally, evidence of C3 deposition on the exosome surface leads us to hypothesize that this cascade may favor the clearance of exosomes by cells once released into the bloodstream, via a mechanism similar to that involved in the elimination of apoptotic cells.
Introduction
Exosomes are membrane vesicles secreted by various cells, especially hematopoietic and epithelial cells. They correspond to intralumenal vesicles of multivesicular endosomes (MVEs) released in the extracellular medium by fusion of the MVE with the plasma membrane. The physiologic function of exosomes is not completely known. For lymphocytes and epithelial cells, secreted exosomes have been described as devices allowing antigenic information transfer and spreading of the immunologic response. Exosomes either stimulate an immunologic response or induce tolerance to the antigen, depending on the context of peptide presentation by major histocompatibility complexes (MHCs) on the exosomal surface.1,2 The response appears to require the transfer of exosomes to specialized immunologic cells such as dendritic cells and might thus be involved in a relatively local transfer.3-5 In the case of reticulocytes, cells that release high amounts of vesicles into the bloodstream,6 exosome secretion may be viewed differently since these cells do not have any immunologic ability. The function commonly attributed to these particles is the removal of obsolete or unwanted molecules from the surface of mature red cells. Transferrin receptor (TfR) and integrin α4β1 are 2 examples of proteins cleared from the red cell plasma membrane during reticulocyte maturation.7,8 Exosome secretion would therefore be a crucial event leading to membrane remodeling during this last stage of erythropoiesis, since the lysosomal degradation capacity of the reticulocyte is trivial. One can imagine that the released vesicles are then digested by specialized cells, but contrary to exosomes secreted by antigen-presenting cells,5 elimination of reticulocyte exosomes must always occur in a context of tolerance, preventing inflammatory reactions and unwanted immune response to self-antigens
This is reminiscent of the process achieved during apoptotic cell removal, where nonviable “self” cells are specifically recognized and ingested by phagocytes.9 This is particularly important, as recognition of apoptotic cells involves the appearance of altered self-markers (“eat-me” signals) on the cell surface. Among the eat-me signals, the best characterized is the exposure of phosphatidylserine (PS) on the outer leaflet of the lipid bilayer, which is recognized directly by diverse phagocyte receptors or after recruitment of bridging molecules such as milk-fat-globule–EGF-factor 8 (MFG-E8).10 More recently, the phospholipid lysophosphatidylcholine (lysoPC) was identified as another eat-me signal on the surface of apoptotic cells. It has been demonstrated that during apoptosis hydrolysis of plasma membrane phosphatidylcholine by a calcium-independent phospholipase A2 (iPLA2) leads to the exposure of an epitope recognized by natural IgM antibodies.11 Here we show that an 85-kDa iPLA2 is specifically localized in endosomes and exosomes secreted by rat reticulocytes and that its enzymatic activity is increased by reactive oxygen species (ROSs) produced during reticulocyte maturation. Moreover, we demonstrate that IgM antibodies are associated with the vesicles secreted by reticulocytes and that exosome-stripped IgM antibodies specifically bind to lysoPC-containing liposomes and apoptotic cells. We propose that upon exosomal iPLA2 hydrolysis of phosphatidylcholine, IgM binding to lysoPC results in reticulocyte exosome recognition and clearance. In support of this hypothesis, we have also found attachment of C3 on the surface of exosomes, a component of the complement system that could promote their uptake by phagocytes or bystander cells.
Materials and methods
Cells
Reticulocyte production in Sprague-Dawley white rats was induced by phenylhydrazine12 or phlebotomy.13 Erythrocytes were obtained from the blood of untreated rats.
The lymphoid T-cell line Jurkat was maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mM L-glutamine, 50 μg/mL streptomycin, and 50 U/mL penicillin.
Antibodies
Mouse monoclonal antihuman transferrin receptor, raised against the cytoplasmic tail of the receptor, was from Zymed Laboratories (South San Francisco, CA). 15-lipoxygenase (15-LOX) isolated from rabbit reticulocytes and rabbit polyclonal antiserum against the C-terminal peptide (YLRPSIVENSVAI) of rabbit reticulocyte 15-LOX were generous gifts from Klaus van Leyen (Massachusetts General Hospital, Charlestown, MA). Goat polyclonal antibody against rat IgM was purchased from Serotec (Oxford, United Kingdom). Rabbit polyclonal antisera against iPLA2 (Type VI) and C3 component were purchased from Cayman Chemical (Ann Arbor, MI) and Bethyl Laboratories (Montgomery, TX), respectively. Rat monoclonal antibody raised against hsc70 (SPA-815) was from StressGen (Victoria, BC, Canada). Peroxidase-conjugated goat anti–rat IgG, peroxidase-conjugated donkey anti–mouse IgG, and donkey anti–goat IgG were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Peroxidase-conjugated donkey anti–rabbit IgG was from Rockland (Gilbertsville, PA). Cy5-conjugated rabbit anti–goat IgG was from Molecular Probes (Eugene, OR).
Exosome isolation
After removing the buffy coat, red blood cells from anemic rats were washed 3 times with Ringer buffer and cultured for 48 hours at 37°C in RPMI 1640 supplemented with 5 mM glutamine, 5 mM adenosine, 10 mM inosine, 3% FCS, 50 U/mL penicillin, and 50 μg/mL streptomycin. After pelleting the cells, the culture supernatant was centrifuged (20 000g for 20 minutes) to remove cellular debris. Exosomes were separated from the supernatant by ultracentrifugation (100 000g for 2 h) and resuspended in phosphate-buffered saline (PBS) or sucrose, depending on the experiments. Similarly, exosomes contained in the plasma of anemic rats were isolated by the same differential centrifugation method.
Western-blot analysis
Samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using 10%, 12%, or 15% polyacrylamide gels, and the proteins were electrophoretically transferred to polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA). Membranes were blocked for 1 hour in TBST (10 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.05% Tween 20) containing 5% skim milk, followed by 1-hour incubation with the indicated primary antibodies. Blots were washed and incubated for 1 hour at room temperature with the secondary antibody (horseradish-peroxidase [HRP] conjugated). Immunoreactive bands were visualized by the enhanced chemiluminescence (ECL) method (Amersham Bioscience, Sunnyvale, CA) according to standard procedures.
Mitochondria membrane potential measurement
Different populations of age-synchronized reticulocytes were obtained by layering immature red cells from the blood of anemic rats on top of a Percoll/NaCl density gradient (1.100, 1.105, 1.110, 1.123 g/mL) and centrifuged at 15 000g for 10 minutes. Four fractions were collected, corresponding to different reticulocyte maturation stages, from lower to higher density (ie, from younger to older reticulocyte stages).14 As a control of maturation, RNA levels in each fraction were determined by using Thiazole orange.13
The different fractions (2‰ cell suspension in Ringer solution) were then incubated for 30 minutes at 37°C with 100 nM MitoTracker Red CMXRos (Molecular Probes) made from a 100-μM stock solution in dimethyl sulfoxide to monitor the mitochondrial membrane potential (ΔΨm). Red cells were also assessed for ROS liberation occurring during differentiation by incubation (30 minutes at 37°C) with a 1-μM solution of the ROS-sensitive dye hydroethidine (HE; Molecular Probes). Cells were then washed once with Ringer solution and analyzed by flow cytometry on a FACSCanto instrument (BD Biosciences, San Jose, CA) operating with FlowJo 7.2 software (Tree Star, Ashland, OR). In some experiments, inhibitors of the 15-LOX, eicosatetraynoic acid (ETYA; 30 μM), or Nor-dihydroguaiaretic acid (NDGA; 100 μM) were added during maturation of the youngest reticulocyte population isolated on Percoll gradient, and cells were then analyzed for ΔΨm as described earlier in this paragraph.
Reticulocyte subcellular fractionation
Reticulocytes were lysed by freezing/thawing as previously described.12 The lysed cells were pelleted at 1500g for 5 minutes and the supernatant was centrifuged at 10 000g for 10 minutes to pellet mitochondria. An ultracentrifugation (60 000g for 30 minutes in a Beckman TLA110 rotor; Beckman Coulter, Fullerton, CA) finally allowed separation of endosomes (pellet) from cytosol (supernatant). Mitochondria and endosomes were resuspended and washed once in PBS. Reticulocyte plasma membranes were prepared according to Steck and Kant15 with blood from anemic rats.
Sucrose gradient analysis
We assessed the purity of endocytic vesicles by sucrose gradient centrifugation. For that, endosomes were layered on top of a linear sucrose gradient (0.3-1.5 M sucrose) in a Beckman SW 55 tube (Beckman Coulter). Gradients were centrifuged for 1 hour at 120 000g, after which 450 μL was collected from the top of the tube.
Exosomes were layered on top of a linear sucrose gradient (0.5-2.5 M sucrose) in a Beckman SW41 tube. Gradients were centrifuged for 16 hours at 190 000g, after which 700-μL fractions were collected from the top of the tube.
In each case (ie, endosome or exosome isolation), collected fractions were precipitated by trichloracetic acid (TCA), and samples were analyzed by SDS-PAGE and Western blot.
Measurement of iPLA2 activity in endosomes
Freshly obtained endosomes (2.5 mg protein/mL) were maintained in PBS. To assess iPLA2 activity in these vesicles, the bis-BODIPY-FL C11-phosphatidylcholine (BODIPY FL11-PC; Molecular Probes) was used as the fluorescent probe. The proximity of the BODIPY FL fluorophores on adjacent phospholipid acyl chains results in self-quenching of fluorescence, which is alleviated by phospholipase A2–mediated release of a BODIPY FL–labeled fatty acid (BODIPY FL C11). iPLA2 activity was then evaluated by fluorescence (λexcitation: 488 nm, λemission: 510 nm) on an LS 55 spectrofluorometer (Perkin Elmer, Shelton, CT). Results are representative of 3 experiments and presented as mean plus or minus SD.
For inhibition of the iPLA2 activity, 50 μg of endosomes was incubated with BEL ((E)-6-(Bromomethylene)-tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one) (100 μM) for 2 hours at room temperature, then the probe was added and the fluorescence measured.
To test the effect of ROSs on endosomal iPLA2 activity, 2 concentrations of H2O2 (15% and 3%) were added to 50 μg of vesicles, 30 minutes prior to labeling with the BODIPY.
Pull-down assay
Exosomes (100 μg protein) were treated by 0.5 M sodium carbonate (pH 11) for 1 hour at room temperature and ultracentrifuged (100 000g for 30 minutes in a Beckman TLA110 rotor). The pellet containing carbonate-stripped exosomes was then resuspended in PBS.
Supernatant from carbonate-treated exosomes was dialyzed overnight against PBS and reincubated with stripped exosomes for 1 hour at room temperature. After ultracentrifugation (200 000g for 10 minutes in a Beckman TLA110 rotor), pellets and supernatants were loaded on SDS-PAGE 10% and analyzed for the presence of IgM by Western blot. Carbonate-washed proteins and stripped vesicles were submitted separately to the same conditions. To assess exosome integrity, immunoblot was then reprobed for hsc70, a cytosolic exosomal marker.
IgM binding on apoptotic cells
Apoptosis of Jurkat T cells (106/mL) was induced by incubation with 100 ng/mL CD95 antibody (R&D Systems, Minneapolis, MN) for 16 hours and washing twice in RPMI. The percentage of apoptotic cells was then quantified by flow cytometry analysis using annexin V (Sigma, St Louis, MO) according to the manufacturer's instructions.
IgM binding was performed by incubation of supernatant from carbonate-treated exosomes, dialyzed overnight against PBS with Jurkat T cells, induced or not in TC buffer (10 mM Tris, pH 7.4; 140 mM NaCl; 2 mM CaCl2; 1 mM MgCl2; and 1% bovine serum albumin)11 for 30 minutes at 37°C. After 3 washes in PBS, cells were incubated with antibody against rat IgM followed by staining with Cy5-conjugated rabbit anti–goat IgG and analyzed by flow cytometry. Two regions were defined according to annexin V binding.
IgM binding competition with phospholipids
The synthetic phospholipids 1,2-dioleyl-sn-glycero-3-phosphatidylcholine (DOPC) and 1-dodecanoyl-sn-glycero-3-phosphatidylcholine (lysoPC) were purchased from Sigma-Aldrich. Phospholipids (100 μg) were dissolved in methanol and coated on a fluoronunc 96-well plate for 18 hours at 4°C. The following day, dialyzed supernatant from carbonate-treated exosomes was applied on the plates for 2.5 hours at room temperature. Supernatant was then removed and incubated for 1 hour at 37°C with Jurkat T cells induced or not in apoptosis. Finally, IgM binding was assessed by flow cytometry with the anti-rat IgM.
Fluorescence-activated cell sorter analysis of exosomes
Exosome-coated latex beads prepared by incubating exosomes with 4-μm–diameter aldehyde/sulfate latex beads (Interfacial Dynamics, Portland, OR), as previously described,16 were incubated for 45 minutes with a goat anti–rat IgM or a rabbit anti–rat C3 component, followed by incubation with Cy5- or FITC-conjugated antibody and analysis by flow cytometry.
IgM binding to red cells treated with PLA2
Erythrocytes (20 μL packed cell volume) were treated for 18 hours at 4°C with an exogenous PLA2 coming from crotalus venom (65 and 130 U; Sigma). Cells were then washed, incubated with either rat plasma or carbonate wash as described earlier, and analyzed by flow cytometry for the presence of IgM. For BODIPY FL11-PC labeling of red cells, fluorescent phospholipid analog was inserted into the plasma membrane, as previously described.17 Briefly, appropriate amounts of lipid were dried under nitrogen and subsequently solubilized in absolute ethanol. This ethanolic solution was injected with a Hamilton syringe into Hanks buffer (pH 7.4; < 1% vol/vol) while vigorously vortexing. The mixture was then added to the cells and incubation was carried out for 60 minutes at 4°C, after which the medium was removed followed by extensive washing of the cells with cold Hanks buffer. Cells were then incubated with PLA2 as described earlier in this paragraph. Fluorescence (bodipy) dequenching of the probe due to PLA2 hydrolysis was measured by flow-cytometry analysis of the cells (channel FL1).
Results
We have previously shown that phospholipase A2 activity was membrane-associated, enriched in endocytic vesicles and exosomes as compared with cytosol and plasma membrane of rat reticulocyte.18 Moreover, neither the hydrolytic activity nor the membrane association was found to be Ca2+ dependent, and BEL, a specific inhibitor of calcium-independent phospholipase A2, was found to abolish the enzymatic activity.18 We now assessed the subcellular localization of the enzyme after cell fractionation, using an antibody specific for the iPLA2 form. As shown by Coomassie blue staining, the 5 subcellular fractions obtained from reticulocytes clearly have different protein content (Figure 1A). A band of about 85 kDa corresponding to the expected iPLA2 molecular weight (MW)19 was detected by Western blot in endosomal and exosomal fractions (Figure 1B). Moreover, comparable amounts of iPLA2 and TfR were detected in endosomes and exosomes, emphasizing the specificity of iPLA2 association with endosomal-derived membranes. The specificity of this association was confirmed by sucrose gradient analysis. As shown in Figure 1C, iPLA2 specifically cosedimented with endosome fractions as assessed by Western blotting of endosomal markers. While the 85-kDa putative iPLA2 band was detected at only very low levels in other reticulocyte subfractions (cytosol, plasma membrane, and mitochondria; Figure 1B), a 70-kDa band was readily observed in the mitochondrial fraction and at slightly lower levels in the plasma membrane fraction. As the 70-kDa peptide is also detected in endosomes, it might represent a degradation product of the native 85-kDa iPLA2. Moreover, it has been shown that iPLA2 is cleaved by caspase-3 during cell apoptosis, releasing a 30-kDa peptide that possesses the catalytic site and an epitope recognized by the antibody used here.20 Indeed, the 70-kDa and 30-kDa bands detected in the iPLA2 immunoblots of the plasma membrane and mitochondria subfractions may correspond to caspase-derived fragments.20,21 Furthermore, as shown in Figure 1D, when exosomes obtained after ex vivo reticulocyte maturation were incubated for 48 hours at 37°C, an increase in this 30-kDa iPLA2-specific band was detected, in agreement with its potential cleavage by a caspase-3 activity as previously demonstrated in rat reticulocyte exosomes.16 Identical treatment and blotting for TfR revealed no turnover for this protein, suggesting a specific cleavage of iPLA2.
Subcellular localization of reticulocyte iPLA2. Rat reticulocytes were fractionated as described in “Reticuloate subcellular fractionation.” The different fractions obtained (plasma membrane [PM], cytosol, endosomes, mitochondria, and exosomes; 100 μg protein) were loaded on a 12% SDS-PAGE and (A) stained by Coomassie blue or (B) immunoblotted for the proteins indicated on the right. (C) Endosomal vesicles were prepared and loaded on a linear sucrose gradient as described in “Sucrose gradient analysis.” Fractions were collected and after TCA precipitation, proteins were separated by SDS-PAGE and analyzed by Western blot for the presence of the proteins indicated on the right. (D) Exosomes aseptically collected from in vitro maturation of rat reticulocytes were loaded on SDS-PAGE and analyzed for the presence of a 30-kDa cleavage product of iPLA2 by Western blot, before (t0) and after incubation for 48 hours at 37°C. In panels B and D, unfilled arrows indicate the native form of iPLA2 (MW 85 kDa); black arrows indicate the putative 30-kDa cleavage product of iPLA2. The molecular mass (kDa) standards are indicated on the left.
Subcellular localization of reticulocyte iPLA2. Rat reticulocytes were fractionated as described in “Reticuloate subcellular fractionation.” The different fractions obtained (plasma membrane [PM], cytosol, endosomes, mitochondria, and exosomes; 100 μg protein) were loaded on a 12% SDS-PAGE and (A) stained by Coomassie blue or (B) immunoblotted for the proteins indicated on the right. (C) Endosomal vesicles were prepared and loaded on a linear sucrose gradient as described in “Sucrose gradient analysis.” Fractions were collected and after TCA precipitation, proteins were separated by SDS-PAGE and analyzed by Western blot for the presence of the proteins indicated on the right. (D) Exosomes aseptically collected from in vitro maturation of rat reticulocytes were loaded on SDS-PAGE and analyzed for the presence of a 30-kDa cleavage product of iPLA2 by Western blot, before (t0) and after incubation for 48 hours at 37°C. In panels B and D, unfilled arrows indicate the native form of iPLA2 (MW 85 kDa); black arrows indicate the putative 30-kDa cleavage product of iPLA2. The molecular mass (kDa) standards are indicated on the left.
During reticulocyte maturation, mitochondria are known to disappear at least partially by an intracellular degradation pathway involving the proteasome system. This programmed death of mitochondria, called mitoptosis,22 is finely tuned by expression of a 15-lipoxygenase (15-LOX) at the reticulocyte stage that specifically associates with the mitochondrial membrane, creating pores and releasing lumenal proteins.23 This is illustrated in the inset of Figure 2A, where 15-LOX is abundantly present in reticulocytes within in the mitochondria fraction but is not detected in erythrocytes. Mitochondria degeneration occurring during in vitro maturation of reticulocytes is associated with a decrease of the mitochondrial membrane potential (ΔΨm; Figure 2A). When total reticulocytes from an anemic rat are matured for 48 hours, the ΔΨm progressively decreases as assessed by Mitotracker Red staining (Figure 2B). Moreover, when ΔΨm is measured on cells separated on a percoll gradient as a function of their maturation state, there is a clear decrease in ΔΨm from young reticulocytes to older red cells (not shown). This decrease in ΔΨm is abolished when the in vitro reticulocyte maturation is carried out in the presence of the 15-LOX inhibitor eicosatetraynoic acid (ETYA; Figure 2C). Concomitant with this loss of ΔΨm, we determined that intracellular ROS production decreased during red cell maturation, as assessed using the ROS-sensitive dye hydroethidine (Figure 2D).
Mitochondrial membrane potential and ROS production during red cell maturation. Mitochondrial membrane potential (ΔΨm) was monitored using MitoTracker CMXRos as described in “Mitochondria membrane potential measurement.” (A) on mature erythrocytes obtained from a healthy rat (dotted line) and immature red cells obtained from an anemic rat (solid line). (Inset) Mature erythrocytes (1 μL packed cell volume), young reticulocytes (1 μL packed cell volume) corresponding to the lower-density fraction of Percoll-purified immature red cells,14 and mitochondria (50-μg protein) isolated from reticulocytes were loaded on 10% SDS-PAGE and analyzed by Western blotting for 15-lipoxygenase (15-LOX). The 15-LOX (1.4-μg protein) isolated from rabbit reticulocytes was loaded as a control. The molecular mass (kDa) standards are indicated on the left. (B) The ΔΨm was monitored after 6, 20, 30, and 45 hours of in vitro maturation of Percoll-purified young reticulocytes (as indicated). (C) The ΔΨm was monitored on freshly isolated young reticulocytes (tinted pattern) and after 48 hours of cell maturation in the absence (dotted line) or presence (solid line) of the lipoxygenase inhibitor ETYA (30 μM). (Inset) Reticulocytes were matured in vitro for 24 hours in the absence (CTRL) or presence of the lipoxygenase inhibitors (NDGA, ETYA) at the indicated concentrations. Exosomes were then collected from the culture medium, loaded on 10% SDS-PAGE, and analyzed for the presence of TfR by Western blot. (D) Intracellular ROS was assayed using the dye hydroethydine (HE) on mature (–) and immature red cells (—).
Mitochondrial membrane potential and ROS production during red cell maturation. Mitochondrial membrane potential (ΔΨm) was monitored using MitoTracker CMXRos as described in “Mitochondria membrane potential measurement.” (A) on mature erythrocytes obtained from a healthy rat (dotted line) and immature red cells obtained from an anemic rat (solid line). (Inset) Mature erythrocytes (1 μL packed cell volume), young reticulocytes (1 μL packed cell volume) corresponding to the lower-density fraction of Percoll-purified immature red cells,14 and mitochondria (50-μg protein) isolated from reticulocytes were loaded on 10% SDS-PAGE and analyzed by Western blotting for 15-lipoxygenase (15-LOX). The 15-LOX (1.4-μg protein) isolated from rabbit reticulocytes was loaded as a control. The molecular mass (kDa) standards are indicated on the left. (B) The ΔΨm was monitored after 6, 20, 30, and 45 hours of in vitro maturation of Percoll-purified young reticulocytes (as indicated). (C) The ΔΨm was monitored on freshly isolated young reticulocytes (tinted pattern) and after 48 hours of cell maturation in the absence (dotted line) or presence (solid line) of the lipoxygenase inhibitor ETYA (30 μM). (Inset) Reticulocytes were matured in vitro for 24 hours in the absence (CTRL) or presence of the lipoxygenase inhibitors (NDGA, ETYA) at the indicated concentrations. Exosomes were then collected from the culture medium, loaded on 10% SDS-PAGE, and analyzed for the presence of TfR by Western blot. (D) Intracellular ROS was assayed using the dye hydroethydine (HE) on mature (–) and immature red cells (—).
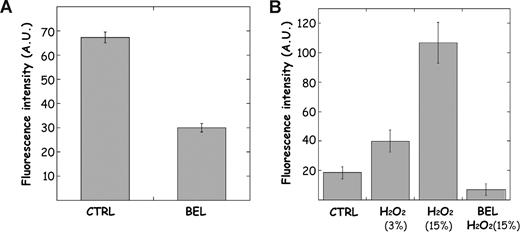
As ROSs have been reported to activate iPLA2 activity in macrophages,24 we assessed the effect of H2O2 on the PLA2 activity present in the reticulocyte endosomal fraction. As we previously demonstrated using radiolabeled phospholipids,18 PLA2 activity, measured by fluorescence dequenching of bis-BODIPY–labeled phospholipid (bis-BODIPY-FL C11-phosphatidylcholine), was inhibited by BEL (Figure 3A). However, when endosomes were incubated in the presence of H2O2, the phospholipase A2 activity was substantially increased (Figure 3B). It is thus possible that during reticulocyte maturation, mitoptosis acts as an unlocking signal, favoring the disappearance of the endosomal compartment through exosome secretion. To test this hypothesis, reticulocytes were matured in the presence or absence of the lipoxygenase inhibitors ETYA or NDGA, and exosomes were collected and analyzed for the presence of TfR. As shown in the inset of Figure 2C, the addition of the lipoxygenase inhibitors decreased the amount of exosome released. One mechanism by which mitoptosis might influence exosome secretion is via ROS-mediated iPLA2 activation, resulting in compartment remodeling by releasing fatty acids from phospholipids. Indeed, arachidonic acid, a cis-unsaturated fatty acid, was demonstrated to promote membrane fusion in different systems.25,26 In addition, iPLA2 activation during mitoptosis could favor the clearance of exosomes once released in the blood circulation, in a manner similar to that occurring in apoptotic cells.27
Activation of endosomal iPLA2 activity by ROSs. Endosomal vesicles were purified from reticulocytes and used to measure iPLA2 activity with the bodipy FL11 PC probe as described in “Measurement of iPLA2 activity in endosomes.” (A) The enzymatic activity was measured in the absence (CTRL) or presence of the iPLA2-specific inhibitor bromoenolactone (BEL; 100μM). (B) Endosomal vesicles were incubated with H2O2 (3% and 15%) for 30 minutes before measuring iPLA2 activity. In some cases, vesicles were treated with BEL, as described in panel A, prior to H2O2 activation. Data are presented as means (± SD) of triplicate samples.
Activation of endosomal iPLA2 activity by ROSs. Endosomal vesicles were purified from reticulocytes and used to measure iPLA2 activity with the bodipy FL11 PC probe as described in “Measurement of iPLA2 activity in endosomes.” (A) The enzymatic activity was measured in the absence (CTRL) or presence of the iPLA2-specific inhibitor bromoenolactone (BEL; 100μM). (B) Endosomal vesicles were incubated with H2O2 (3% and 15%) for 30 minutes before measuring iPLA2 activity. In some cases, vesicles were treated with BEL, as described in panel A, prior to H2O2 activation. Data are presented as means (± SD) of triplicate samples.
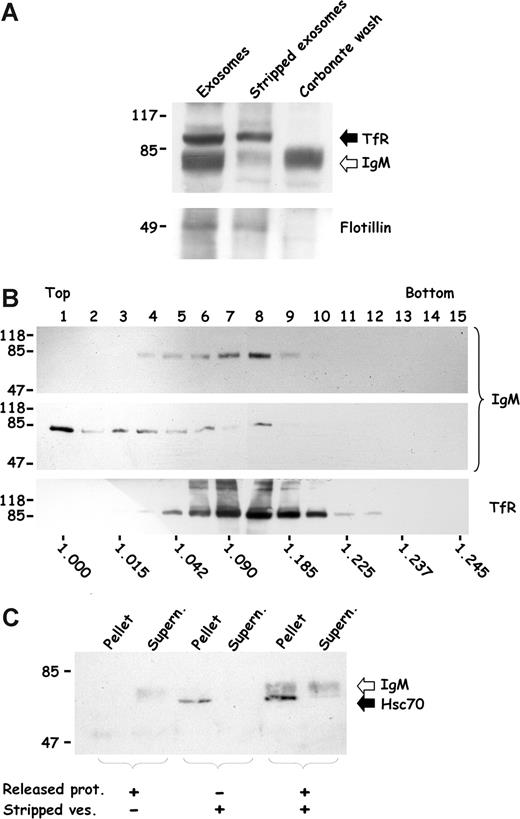
To examine this possibility, exosomes collected from the plasma of phenylhydrazine-treated rats were stripped and analyzed by Western blotting for the presence of IgMs. As shown in Figure 4A, IgM antibodies associated with exosomes were released by a carbonate wash, whereas the stripped vesicles, visualized by the presence of the TfR and flotillin, were almost completely devoid of IgMs. To confirm IgM binding to the vesicles, exosomes collected after reticulocyte maturation were fractionated by flotation on sucrose gradient and analyzed for the presence of TfR and IgM antibodies by Western blot (Figure 4B). Exosomes, as revealed by TfR detection (bottom panel), were mainly distributed in fractions corresponding to densities between 1.08 and 1.18 g/mL, as previously described.16 IgM antibodies revealed an overlapping distribution with exosomal fractions (top panel). As a control, when the proteins stripped from exosomes were loaded on the gradient (middle panel), IgMs were principally recovered on the top of the gradient. To further characterize the exosome-IgM association, we developed a pull-down assay using IgMs collected from a carbonate wash and analyzed their ability to reassociate with stripped vesicles (Figure 4C). As shown, IgMs did not pellet when incubated separately from the vesicles, but a significant fraction was recovered with vesicles following a coincubation. Notably, the stripping conditions used were gentle enough that they did not result in the leakage of the cytosolic heat-shock protein hsc70 from vesicles, as assessed by its detection within the vesicle pellet.
IgM antibodies bind to exosomes. (A) Exosomes were obtained by differential centrifugation and surface-associated proteins were released by a carbonate wash. Untreated exosomes, stripped vesicles, and the released proteins were loaded on SDS-PAGE and analyzed by Western blot for IgM and then for TfR. The membrane was then reprobed for flotillin. (B) Exosomes were deposited on a linear sucrose gradient. Fractions were collected and analyzed for the presence of IgM (top panel) and TfR (bottom panel) using specific antibodies. Proteins stripped from exosomes by carbonate wash were deposited on a sucrose gradient and analyzed for IgM (middle panel). Densities (g/mL) were obtained for each fraction by refractometry and are indicated under each lane. (C) Proteins released from exosomes following a carbonate wash (released proteins) were added back to stripped vesicles (1 h at room temperature) and submitted to ultracentrifugation (200 000g for 10 minutes). Resulting pellets and supernatants were then analyzed for the presence of IgM and hsc70. As controls, stripped vesicles and carbonate wash were submitted to the same centrifugation conditions separately, as indicated at the bottom, and analyzed by Western blot. The molecular mass (kDa) standards are indicated to the left. Arrows point to the bands corresponding to Western blot detection of specified proteins.
IgM antibodies bind to exosomes. (A) Exosomes were obtained by differential centrifugation and surface-associated proteins were released by a carbonate wash. Untreated exosomes, stripped vesicles, and the released proteins were loaded on SDS-PAGE and analyzed by Western blot for IgM and then for TfR. The membrane was then reprobed for flotillin. (B) Exosomes were deposited on a linear sucrose gradient. Fractions were collected and analyzed for the presence of IgM (top panel) and TfR (bottom panel) using specific antibodies. Proteins stripped from exosomes by carbonate wash were deposited on a sucrose gradient and analyzed for IgM (middle panel). Densities (g/mL) were obtained for each fraction by refractometry and are indicated under each lane. (C) Proteins released from exosomes following a carbonate wash (released proteins) were added back to stripped vesicles (1 h at room temperature) and submitted to ultracentrifugation (200 000g for 10 minutes). Resulting pellets and supernatants were then analyzed for the presence of IgM and hsc70. As controls, stripped vesicles and carbonate wash were submitted to the same centrifugation conditions separately, as indicated at the bottom, and analyzed by Western blot. The molecular mass (kDa) standards are indicated to the left. Arrows point to the bands corresponding to Western blot detection of specified proteins.
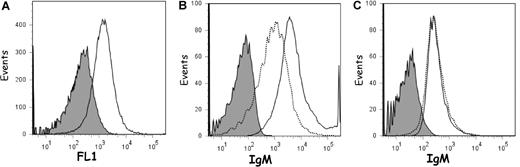
The ability of the carbonate-released IgM antibodies to associate with stripped vesicles strongly suggested the presence of a “receptor” on the exosome surface. To test the hypothesis that the “receptor” was actually lysophosphatidylcholine, we investigated the ability of IgM antibodies released from reticulocyte exosomes to bind to apoptotic cells. To this end, apoptosis was induced in Jurkat cells with an anti-FAS antibody (CD95) for 16 hours. At this time, a subset of cells presented a typical apoptotic FSC (forward scatter; cell volume)/SSC (side scatter; granularity) dot-plot pattern (Figure 5, top panel), allowing 2 regions corresponding to apoptotic and nonapoptotic cells to be distinguished. This characteristic was confirmed by annexin V binding. The cells were then incubated with carbonate-released IgM from exosomes and the 2 regions were monitored for binding of annexin V and IgM antibodies (Figure 5). As shown, nonapoptotic cells did not bind annexin V or carbonate-released IgM (R2 panels), whereas apoptotic cells efficiently bound both annexin V and IgMs (R1 panels). When cells were assessed for IgM binding in the absence of carbonate-released IgM, low level of IgM binding could be detected on both groups of cells (not shown). We tested various phospholipids (eg, phosphatidylethanolamine, lysophosphatidylethanolamine, phosphatidylserine) and found that only liposomes containing lysophosphatidylcholine (ie, the putative epitope for IgM antibodies) were able to pull down exosomal-stripped IgMs (data not shown). As such, we further explored the characteristics of the carbonate-released exosomal IgM by carrying out competition experiments for apoptotic cell binding, using phospholipid-coated plates. As shown in Figure 5 (bottom panels), preincubation of the stripped IgM exosomal antibodies on phosphatidylcholine-coated plates did not modify their binding to annexin V–positive apoptotic cells, whereas preincubation of IgMs on lysoPC-coated plates almost completely abolished binding to apoptotic cells (50% to 7%; Figure 5). Nonapoptotic cells did not bind to carbonate-released exosomal IgMs, irrespective of the preincubation on phospholipid-coated plates. Natural IgM antibodies present in the serum are known to specifically bind to lysophosphatidylcholine present on the surface of apoptotic cells and accelerate phagocyte clearance by mediating their opsonization with complement.11 We therefore assessed whether IgM antibodies present in fetal calf serum would bind to apoptotic Jurkat cells and whether this association would be competed by phosphatidylcholine or its lyso-derivative. Only apoptotic cells could be labeled with serum IgM and, in agreement with our data using exosomal IgMs, lysoPC but not phosphatidylcholine inhibited binding of FCS IgM antibodies (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Moreover, as expected, neither reticulocytes nor erythrocytes, which do not express lysophosphatidylcholine, bound serum IgM (Figure S1). Thus, serum IgM antibodies are likely recruited to the exosomal surface once the vesicles are released into the extracellular medium, rather than membrane associated before secretion.
Binding of carbonate-released exosomal IgM to apoptotic cells. Apoptosis was induced in Jurkat cells by treatment with an anti-FAS antibody (CD95) for 16 hours. Cells were then incubated with dialyzed carbonate wash from exosomes. After washing, cells were analyzed by flow cytometry for the presence of IgM. (Top panel) Dot plot (FCS vs SSC) defining 2 regions, R1 and R2, corresponding to apoptotic and nonapoptotic cells, respectively. (R1 and R2 panels) The 2 regions were analyzed for annexin V and IgM binding. (Bottom panels) IgM binding to apoptotic cells was carried out after preincubation of the carbonate wash to microplates coated with phosphatidylcholine (+PC; tinted pattern) or lysophosphatidylcholine (+LPC; tinted pattern) to deplete specific natural IgM antibodies. CTRL (–) carbonate wash was directly incubated with cells.
Binding of carbonate-released exosomal IgM to apoptotic cells. Apoptosis was induced in Jurkat cells by treatment with an anti-FAS antibody (CD95) for 16 hours. Cells were then incubated with dialyzed carbonate wash from exosomes. After washing, cells were analyzed by flow cytometry for the presence of IgM. (Top panel) Dot plot (FCS vs SSC) defining 2 regions, R1 and R2, corresponding to apoptotic and nonapoptotic cells, respectively. (R1 and R2 panels) The 2 regions were analyzed for annexin V and IgM binding. (Bottom panels) IgM binding to apoptotic cells was carried out after preincubation of the carbonate wash to microplates coated with phosphatidylcholine (+PC; tinted pattern) or lysophosphatidylcholine (+LPC; tinted pattern) to deplete specific natural IgM antibodies. CTRL (–) carbonate wash was directly incubated with cells.
IgM antibodies are potent activators of the classical complement pathway and binding of these molecules to apoptotic cells results in their coating with C3b/bi, which serves as an eat-me signal for phagocytes.9 We thus investigated the C3 deposition on the exosome surface. Indeed, an anti-C3 antibody reacted with several bands from exosomes purified from the plasma of anemic rats, as well as from rat reticulocytes matured in vitro (not shown). Moreover, deposition of C3 component was easily detected on the surface of apoptotic cells by flow cytometry (Figure 6A), as previously demonstrated.28 C3 deposition on the exosome surface was thus monitored by the same technique, after vesicle adsorption on beads. For this, exosomes were isolated either directly from the plasma of an anemic rat or collected from in vitro maturation of rat reticulocytes in FCS-containing medium, adsorbed on the surface of latex beads as previously described,3,16 and then analyzed for the presence of IgM and C3 component. As shown in Figure 6B, IgM and C3 component were detected on the surface of vesicles isolated from both types of exosome complexes, demonstrating that this opsonization process occurs in vitro as well as in vivo. Notably though, lower levels of both IgM and C3 binding were detected when reticulocytes were matured in medium containing heat-inactivated rat plasma (Figure 6C). IgM binding and C3 component deposition on the surface of released exosomes were also found in reticulocytes directly harvested from rats rendered anemic by repeated bleeding, ruling out phenylhydrazine-induced events, even though ROS production was lower when rats were phlebotomized (Figures S2,S3).
C3 component deposition on exosomes. (A) Jurkat cells wherein apoptosis was induced were incubated with FCS and assessed for C3 deposition by flow cytometry. Tinted pattern indicates absence of primary antibodies. (B) Latex beads were coated with exosomes isolated after in vitro maturation or directly from the plasma of an anemic animal. The complexes were then analyzed by flow cytometry for the presence of IgM and C3 using the appropriate antibodies. Tinted patterns indicate absence of primary antibodies. (C) Plasma was collected from an anemic animal and ultracentrifuged to remove exosomes. Reticulocytes were matured in vitro for 48 hours in medium without (tinted pattern) or with plasma, heated-inactivated (–) or not (—). Exosomes were then isolated, coated on latex beads, and analyzed by flow cytometry for IgM or C3 component.
C3 component deposition on exosomes. (A) Jurkat cells wherein apoptosis was induced were incubated with FCS and assessed for C3 deposition by flow cytometry. Tinted pattern indicates absence of primary antibodies. (B) Latex beads were coated with exosomes isolated after in vitro maturation or directly from the plasma of an anemic animal. The complexes were then analyzed by flow cytometry for the presence of IgM and C3 using the appropriate antibodies. Tinted patterns indicate absence of primary antibodies. (C) Plasma was collected from an anemic animal and ultracentrifuged to remove exosomes. Reticulocytes were matured in vitro for 48 hours in medium without (tinted pattern) or with plasma, heated-inactivated (–) or not (—). Exosomes were then isolated, coated on latex beads, and analyzed by flow cytometry for IgM or C3 component.
Our finding that healthy immature and mature red cells do not recruit IgM, in contrast to released exosomes, led us to hypothesize that this was due to a lack of iPLA2 activity in the plasma membrane of former cells. To test this hypothesis, we incubated freshly isolated red cells with exogenous active PLA2 to hydrolyze phospholipids on the cell surface (Figure 7A) and then incubated the cells with serum to provide IgM for potential binding. As presented in Figure 7B, production of lysophospholipids on the red cell surface induced IgM binding, and binding increased further in the presence of higher levels of ectopic PLA2. Finally, upon incubation of PLA2-treated red cells with IgM antibodies, in the form of proteins stripped from exosomes, binding of antibodies to the cell surface was significantly increased (Figure 7C).
IgM binding to red cells treated with exogenous PLA2. (A) Rat red cells were prelabeled with the bodipy FL11 PC probe (1 h at 4°C) by ethanol injection17 before overnight incubation with PLA2 at 4°C. Red cells were then analyzed by flow cytometry to assess probe hydrolysis and fluorescence (FL1) intensity increase. After cell treatment with PLA2, IgM was provided in the form of rat plasma (B) or carbonate wash (C), and IgM association to the red blood cells was monitored. Tinted patterns indicate untreated cells; –, cells treated with 65 U of PLA2; and —, cells treated with 130 U of PLA2.
IgM binding to red cells treated with exogenous PLA2. (A) Rat red cells were prelabeled with the bodipy FL11 PC probe (1 h at 4°C) by ethanol injection17 before overnight incubation with PLA2 at 4°C. Red cells were then analyzed by flow cytometry to assess probe hydrolysis and fluorescence (FL1) intensity increase. After cell treatment with PLA2, IgM was provided in the form of rat plasma (B) or carbonate wash (C), and IgM association to the red blood cells was monitored. Tinted patterns indicate untreated cells; –, cells treated with 65 U of PLA2; and —, cells treated with 130 U of PLA2.
Discussion
Exosomes have been the subject of numerous studies but their biogenesis, function, and fate are still not completely understood. The function of reticulocyte-secreted exosomes appears more obvious, since various membrane proteins are specifically cleared by the exosomal pathway, leading to red cell surface remodeling. Removal of some of these proteins (eg, integrin α4β1) from the erythrocyte surface is essential to avoid blood circulation complications.8,29 Moreover, the cellular strategy employed to eliminate these proteins (ie, sorting to the multivesicular compartment) is in keeping with the use of the ESCRT (endosomal sorting complex required for transport) machinery in charge of protein sorting to lysosomal degradation.30,31 However, because the secreting red cell does not itself degrade the vesicles, the question arises as to how these exosomes are cleared once they are released into the bloodstream. Aged erythrocytes, after a lifetime of about 4 months in the blood circulation, are known to be phagocytosed principally in the spleen upon exposure of phosphatidylserine. Thus, appearance of “new” molecules on the surface of these red blood cells allows discrimination between normal and aged erythrocytes, resulting in a selective removal of the latter cells by the reticuloendothelial system. Our hypothesis is that a similar mechanism might be involved in the removal of reticulocyte exosomes from the bloodstream. Studies in recent years have disclosed that cells undergoing apoptosis express what is called eat-me signals allowing macrophages to recognize and engulf the dying cells.27 These eat-me signals can be of a diverse nature, including lipids (phosphatidylserine), proteins (annexin I), and modified sugar moieties.32 Phosphatidylserine exposure is known to be an early and quite specific sign of cell commitment to apoptosis. Under normal circumstances, the phospholipid distribution of the 2 leaflets of plasma membrane is asymmetric. This lipid asymmetry is maintained by an ATPase called aminophospholipid translocase, which promotes the inner transport of aminophospholipids such as phosphatidylserine.33 Phosphatidylserine on apoptotic cells can be recognized directly by a phagocyte PS receptor34 but also through PS-binding proteins (eg, MFG-E8, β2-glycoprotein I, Gas6) acting as a bridge between apoptotic cells and phagocytes.10,35,36 We previously reported that reticulocyte exosomes freshly collected following in vitro maturation presented a normal membrane asymmetry.37 However in the same study, we demonstrated the absence of the aminophospholipid translocase in reticulocyte exosomes, suggesting that the phospholipid asymmetry should be progressively lost with time, leading to PS exposure on the membrane outer leaflet. It is worth noting that accordingly, the presence of aminophospholipids on the surface of exosomes has been reported in some cases38,39 and that MFG-E8 has been described as associated with exosomes secreted by various cells.5,39-41
More recently, lysophosphatidylcholine, another lipid exposed on the plasma membrane of cells undergoing apoptosis, was demonstrated to play a role in apoptotic cell recognition. LysoPC is a product of phosphatidylcholine hydrolysis by phospholipase A2, enzymes that cleave the sn-2 ester bond in phospholipids to release free fatty acids and lysophospholipids. Based upon the Ca2+ requirement for their enzymatic activities, PLA2s are divided into 3 categories: secretory PLA2s (sPLA2s), cytosolic PLA2s (cPLA2s), and Ca2+-independent PLA2s (iPLA2s). The PLA2 involved in lysoPC production and recognition during apoptosis has been demonstrated to be a member of the iPLA2 group.11 In agreement with our previous biochemical data (ie, Ca2+ independence, sensitivity to the suicide inhibitor BEL),18 a PLA2 with a molecular weight of about 85 kDa, detected by an antibody specific to the iPLA2 form, is present mainly in endosome and exosome fractions. The bands with lower MW detected in the other cell fractions could be degradation products of the same protein. Indeed, iPLA2 has been cloned and identified in several species and a variety of cells. The most studied iPLA2 (iPLA2-VIA) contains several putative consensus sequences for caspase cleavage (DXXD), and corresponding iPLA2-VIA fragments have been reported to exist in cells.20,42 Interestingly, the fragments detected in our fractions fit with a caspase processing, especially the 30-kDa and 70-kDa fragments present in mitochondria, whose degeneration at the reticulocyte stage should favor caspase activation. Thus, it is possible that activation of caspases and the degeneration of mitochondria are early enough to explain the low-level native form of iPLA2 in mitochondria at the blood-circulating reticulocyte stage. Indeed, a caspase-3 activity was found to be involved in red cell differentiation,43 and we detected a caspase-3 activity in reticulocyte exosomes.16 Accordingly, when exosomes were incubated at 37°C, the 85-kDa band diminished whereas a 30-kDa fragment corresponding to a caspase cleavage product was enhanced. It has been shown that 15-lipoxygenase is involved in mitochondria degeneration by creating pores through which proteins can leak. As we demonstrate here, using inhibitors, the 15-LOX induces a loss of ΔΨm but also modulates exosome release. Although we found that 15-LOX was associated with mitochondria (Figure 2) and to some extent with the endosomal compartment (not shown), the mechanism by which its enzymatic activity contributes to vesicle secretion is not fully understood. However, we found a clear correlation between 15-LOX expression and ROS production during the reticulocyte stage, and neither 15-LOX nor ROSs could be detected in mature red cells (Figure 2A,D).
Recent findings have suggested that ROSs are a factor potentiating iPLA2 activity in various cell types.24,44,45 Thus, the possibility that ROSs transiently generated during reticulocyte maturation enhance endosomal iPLA2 activity was investigated. We indeed found that H2O2 increases hydrolysis of 1, 2-bis-BODIPY–labeled phosphatidylcholine and that iPLA2 enzymatic activity is still inhibited by the suicide inhibitor BEL. The mechanism for this H2O2-induced increase of hydrolysis has been reported to be due to an increased susceptibility of the membrane to PLA2 rather than to a change in iPLA2-specific activity.46 In any case, the concomitant increase in iPLA2 activity and mitoptosis is highly reminiscent of a role for iPLA2 in apoptosis, recently proposed beyond its housekeeping role in phospholipid metabolism. It is known that in an apoptotic context, iPLA2 does not have a destructive role, since it is not absolutely required for apoptosis, but it may provide signals involved in the clearance of apoptotic bodies.47 We thus investigated if similarly, exposure of iPLA2-produced lysoPC on the surface of released exosomes could induce binding of IgM and complement factors. We demonstrated that exosomes collected either directly from the plasma of anemic rats or from ex vivo–matured reticulocytes released IgM but not IgG antibodies after carbonate treatment. Moreover, these exosomal IgM antibodies specifically bind to lysoPC and bind to apoptotic Jurkat cells and PLA2-treated red cells but not to viable Jurkat cells, reticulocytes, and erythrocytes, suggesting that they are natural IgM antibodies recognizing the lysoPC eat-me signal.11,27 IgM binding to lysophospholipids on the surface of apoptotic cells may be responsible for complement activation and efficient phagocytosis of exosomes, since C3 component deposition was detected on the surface of the vesicles (model proposed in Figure S4).
Thus, we propose that the exosomal release occurring during reticulocyte maturation is part of a cellular program resulting in the remodeling of the plasma membrane by removal of specific proteins. The process of exosome biogenesis is not limited to obsolete protein sorting but is finely interconnected with an accompanying mitoptosis and downstream vesicle clearance.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by research funding from the CNRS, the Ministère de la Recherche, and the Association pour la Re-cherche sur le Cancer (ARC; no. 3444).
We are grateful to Klaus van Leyen for providing purified 15-LOX and anti–15-LOX antibodies. We wish to thank Naomi Taylor for critical reading of the manuscript.
Authorship
Contribution: M.V. designed the research; L.B., C.B., and P.B.-B. performed the research; and L.B. and M.V. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michel Vidal, UMR 5235, Univ. Montpellier II-cc107, Montpellier 34095, France; e-mail: mvidal@univ-montp2.fr.

![Figure 1. Subcellular localization of reticulocyte iPLA2. Rat reticulocytes were fractionated as described in “Reticuloate subcellular fractionation.” The different fractions obtained (plasma membrane [PM], cytosol, endosomes, mitochondria, and exosomes; 100 μg protein) were loaded on a 12% SDS-PAGE and (A) stained by Coomassie blue or (B) immunoblotted for the proteins indicated on the right. (C) Endosomal vesicles were prepared and loaded on a linear sucrose gradient as described in “Sucrose gradient analysis.” Fractions were collected and after TCA precipitation, proteins were separated by SDS-PAGE and analyzed by Western blot for the presence of the proteins indicated on the right. (D) Exosomes aseptically collected from in vitro maturation of rat reticulocytes were loaded on SDS-PAGE and analyzed for the presence of a 30-kDa cleavage product of iPLA2 by Western blot, before (t0) and after incubation for 48 hours at 37°C. In panels B and D, unfilled arrows indicate the native form of iPLA2 (MW 85 kDa); black arrows indicate the putative 30-kDa cleavage product of iPLA2. The molecular mass (kDa) standards are indicated on the left.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/9/10.1182_blood-2007-04-085845/7/m_zh80220709270001.jpeg?Expires=1767792600&Signature=LX5zR0qaw-mKCwku0EnZr2CWeOcN~gWDk0Ey-GePp67cwNAGtsViwWsFuuHAlPf8DH4sJOQSu6bl-Tv9Kuf02V2OutgngZ44cZvgbyhkEAhcMZmnm6JNcf~PqHk5dG30UBhHjKid2oRFfyYonSZpxvOEyh6gI2VxHQcCJvnRjV0s-mlKnXDMEMtGiEb4EVBuyqkN8sk-hwAAnQh3IYotqTwlWxM6PtLV8nxTBbgKdTJHW-7s~jnWHXFqTjKyuhEAlcjUAOIm4hTBNSalTlyyiQxCiAz62LekpJlseZdalQ0Su18d8d9DsW0V~auYxx7D2gkcSHo60mwDHTmgBXLd5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal