Hematopietic stem cells (HSCs) maintain life-long hematopoiesis in the bone marrow via their ability to self-renew and to differentiate into all blood lineages. Although a central role for the canonical wnt signaling pathway has been suggested in HSC self-renewal as well as in the development of B and T cells, conditional deletion of β-catenin (which is considered to be essential for Wnt signaling) has no effect on hematopoiesis or lymphopoiesis. Here, we address whether this discrepancy can be explained by a redundant and compensatory function of γ-catenin, a close homolog of β-catenin. Unexpectedly, we find that combined deficiency of β- and γ-catenin in hematopoietic progenitors does not impair their ability to self-renew and to reconstitute all myeloid, erythroid, and lymphoid lineages, even in competitive mixed chimeras and serial transplantations. These results exclude an essential role for canonical Wnt signaling (as mediated by β- and/or γ-catenin) during hematopoiesis and lymphopoiesis.
Introduction
Long-term hematopoietic stem cells (LT-HSCs) maintain life-long hematopoiesis in the bone marrow (BM) via their ability to self-renew and to differentiate into all blood lineages.1 During differentiation, LT-HSCs transit through short-term (ST)–HSCs and committed progenitor stages (characterized by increasingly restricted lineage potential and reduced self-renewal capacity) before adopting a mature myeloid, erythroid, or lymphoid cell fate. Molecular mechanisms controlling self-renewal and cell-fate decisions within the hematopoietic system remain poorly defined, but recent studies suggest that Wnt signaling might be involved.2 Thus, in vitro experiments show that Wnt proteins have stem cell growth factor activity,3 and retroviral gain-of-function experiments indicate that HSCs expressing a dominant-active form of β-catenin exhibit increased reconstitution potential in vivo.4 Additional gain-of-function studies for β-catenin showed that committed lymphoid and myeloid progenitors can be reprogrammed to uncommitted cells with multilineage potential,5 whereas high levels of constitutive β-catenin–mediated signaling leads to multilineage defects and exhaustion of the LT-HSC pool.6,7 Furthermore, HSCs transduced with Axin, a negative regulator of β-catenin, have reduced growth potential in vitro and a drastically reduced ability to reconstitute the hematopoietic compartment of irradiated mice.4 Although these gain-of-function experiments clearly show that Wnt components can potentially regulate HSC self-renewal, they are not readily compatible with genetic data showing that conditional deletion of β-catenin in BM progenitors does not result in any detectable perturbation of the hematopoietic system.8 One possible explanation for this discrepancy is functional redundancy of β-catenin in hematopoietic cells with γ-catenin, another member of the catenin family
Methods
Generation of β- and/or γ-catenin gene-targeted mice
The generation of β-cateninlox/loxMx-Cre mice8 and conventional gene-targeted mice for γ-catenin9 were previously described. Embryos (embryonic day [E] 12.5-E14.5) from timed pregnancies crossing β-cateninlox/lox γ-catenin+/− Mx-Cre males with β-cateninlox/lox γ-catenin+/− females were isolated. Fetal livers (FLs) were individually isolated, and the genotype of single embryos was determined by polymerase chain reaction (PCR; conditions and primers available upon request).
Generation of FL chimeras
Single-cell suspensions were prepared from FLs with the desired genotypes. A total of 2 to 3 × 106 cells were injected intravenously in a volume of 200 μL phosphate-buffered saline (PBS) into lethally irradiated congenic B6.SJL-Ptprca mice (CD45.1+; Jackson Laboratory, Bar Harbor, ME).
Activation of the Cre-recombinase
Adult FL chimeras received 5 intraperitoneal injections of 2 μg/g body weight polyI-polyC (pI-pC; InvivoGen, Toulouse, France) at 2-day intervals. Mice were killed at indicated times, and genomic DNA was prepared from 10 × 106 BM cells. The deletion efficiency in sorted CD45.2+ BM cells from control and γ-catenin−/−β-cateninlox/loxMx-Cre (β/γ-catenin dko) chimeras was assessed by Southern blot analysis and quantified by PhosphorImager (FUJIFILM FLA3000, Kanagawa, Japan) as previously described.8
Mixed BM chimeras and serial BM transplantation
Reconstituted FL chimeras were used to generate mixed chimeras and serial BM transplants. At 3 to 4 months after reconstitution, a 1:2 mixture of wild-type (wt) CD45.1+ and either control or β/γ dko CD45.2+ BM cells were injected into lethally irradiated CD45.1+ recipients to set up mixed BM chimeras. Animals who underwent serial transplantations were generated by retransplanting BM cells from either control or β/γ dko chimeras into secondary lethally irradiated CD45.1+ recipients.
Immunoblot analysis
Immunoblot analysis to detect β- and γ-catenin protein expression in total BM cells, thymocytes, and splenocytes from a wt or γ-catenin−/− mouse was performed as previously described.8 Monoclonal antibodies specific for the carboxyl terminus of mouse β-catenin (clone 14) or γ-catenin (clone 15; BD Transduction Laboratories, LePont de Claix, France) were used.
Monoclonal antibodies and flow cytometry
Single-cell suspensions from BM and thymus were prepared and stained using standard protocols as previously described.10 (For a detailed record of all antibodies used, refer to Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Fluorescence-activated cell sorter (FACS) analyses (4- and 6-color) were performed using a FACSCanto Flow cytometer (Becton Dickinson, San Jose, CA). Data were analyzed using FlowJo software (Tree Star, Ashland, OR). FACS sorting was performed using a FACS Aria Flow Cytometer (Becton Dickinson).
Results and discussion
Analysis of the expression pattern of β- and γ-catenin proteins is consistent with the hypothesis that γ-catenin can compensate for loss of β-catenin during hematopoiesis, as both proteins can be detected by Western blot analysis in all hematopoietic tissues investigated (Figure S1). To test this hypothesis directly, we analyzed the hematopoietic system of mice that lack γ-catenin, or both β- and γ-catenin simultaneously. Since conventional gene-targeted mice for both β-catenin and γ-catenin die during embryogenesis,9,11 floxed β-catenin mice carrying a Mx-Cre transgene were crossed with conventionally gene-targeted γ-catenin+/− mice, which were subsequently intercrossed. CD45.2+ FL cells from embryos with the genotypes γ-catenin+/+β-cateninlox/lox (wild-type (wt)), γ-catenin−/− β-cateninlox/lox (γ-catenin−/−), γ-catenin+/+β-cateninlox/lox,Mx-Cre (β-catenin−/−), and γ-catenin−/− β-cateninlox/lox,Mx-Cre (β/γ dko) were transferred separately into lethally irradiated CD45.1+ recipient mice (Figure 1A). γ-catenin deficiency of the transplanted FL cells was verified by PCR (data not shown) and Western blot analysis using the remaining embryo (Figure 1B). Inactivation of the β-catenin gene on either wt or γ-catenin−/− background was performed via Mx-Cre induction after FL cells had successfully reconstituted in the hosts that received transplants. Efficient inactivation of β-catenin (deletion efficiency: 98%-100%) was verified by Southern blot analysis of sorted CD45.2+ BM cells from β/γ dko FL chimeras (Figure 1B), which were analyzed 5 to 7 weeks after Mx-Cre induction.
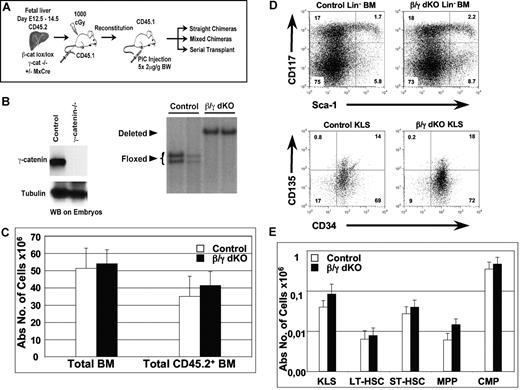
Normal hematopoiesis in the combined absence of β- and γ-catenin. (A) Experimental strategy: CD45.2+ FL cells (E12.5-E14.5) from γ-catenin−/− β-cateninlox/lox (control) and γ-catenin−/− β-cateninlox/lox,Mx-Cre (β/γ dko) embryos were isolated and transplanted into lethally irradiated congenic wt mice (CD45.1+). At 8 weeks after reconstitution, all mice were injected 5 times at 2-day intervals with pI-pC to inactivate the floxed β-catenin alleles via induction of the Mx-Cre transgene. Mice were analyzed 5 to 7 weeks after the last pI-pC injection, and a fraction of T cell–depleted BM cells derived from these FL chimeras were used to set up mixed BM chimeras and serial transplantations, which were both analyzed between 6 weeks and 6 months after transplantation. B. (Left) Western blot analysis performed on embryos from either wt (control) or γ-catenin−/− mice showing the absence of γ-catenin protein in the conventional γ-catenin knock-out embryos. Tubulin was used as a loading control. (Right) Southern blot analysis of EcoRI-digested genomic DNA derived from sorted CD45.2+ BM cells from 2 control and 2 β/γ dko chimeras 7 weeks after inactivation of the floxed β-catenin alleles are shown. Floxed indicates the floxed β-catenin alleles, and deleted indicates the inactivated alleles (98%-100%). (C) Absolute numbers of either total or CD45.2+ BM cells derived from control (n = 7; □) and β/γ dko (n = 8; ■) chimeras. (D) Representative FACS analysis of KLS HSCs defined by CD117 and Sca1 after gating on Lin− BM (top row) and by CD135 and CD34 after gating on KLS (bottom) BM cells derived from control and β/γ dko chimeras. (E) Absolute numbers of KLS HSCs (CD117+Lin−Sca1+), LT-HSCs (CD117+Sca1+CD34−CD135−), ST-HSCs (CD117+Sca1+CD34+CD135−), MPPs (CD117+Sca1+CD34+CD135+), and CMPs (CD117+Sca−) gated on Lin− CD45.2+ BM cells derived from control (n = 7; □) and β/γ dko (n = 8; ■) chimeras. The error bars represent mean (± SD) in panels C and E.
Normal hematopoiesis in the combined absence of β- and γ-catenin. (A) Experimental strategy: CD45.2+ FL cells (E12.5-E14.5) from γ-catenin−/− β-cateninlox/lox (control) and γ-catenin−/− β-cateninlox/lox,Mx-Cre (β/γ dko) embryos were isolated and transplanted into lethally irradiated congenic wt mice (CD45.1+). At 8 weeks after reconstitution, all mice were injected 5 times at 2-day intervals with pI-pC to inactivate the floxed β-catenin alleles via induction of the Mx-Cre transgene. Mice were analyzed 5 to 7 weeks after the last pI-pC injection, and a fraction of T cell–depleted BM cells derived from these FL chimeras were used to set up mixed BM chimeras and serial transplantations, which were both analyzed between 6 weeks and 6 months after transplantation. B. (Left) Western blot analysis performed on embryos from either wt (control) or γ-catenin−/− mice showing the absence of γ-catenin protein in the conventional γ-catenin knock-out embryos. Tubulin was used as a loading control. (Right) Southern blot analysis of EcoRI-digested genomic DNA derived from sorted CD45.2+ BM cells from 2 control and 2 β/γ dko chimeras 7 weeks after inactivation of the floxed β-catenin alleles are shown. Floxed indicates the floxed β-catenin alleles, and deleted indicates the inactivated alleles (98%-100%). (C) Absolute numbers of either total or CD45.2+ BM cells derived from control (n = 7; □) and β/γ dko (n = 8; ■) chimeras. (D) Representative FACS analysis of KLS HSCs defined by CD117 and Sca1 after gating on Lin− BM (top row) and by CD135 and CD34 after gating on KLS (bottom) BM cells derived from control and β/γ dko chimeras. (E) Absolute numbers of KLS HSCs (CD117+Lin−Sca1+), LT-HSCs (CD117+Sca1+CD34−CD135−), ST-HSCs (CD117+Sca1+CD34+CD135−), MPPs (CD117+Sca1+CD34+CD135+), and CMPs (CD117+Sca−) gated on Lin− CD45.2+ BM cells derived from control (n = 7; □) and β/γ dko (n = 8; ■) chimeras. The error bars represent mean (± SD) in panels C and E.
In previous studies,8 we verified that high-efficiency deletion of this β-catenin allele in hematopoietic cells resulted in undetectable levels of β-catenin protein as measured by Western blot.
Mx-Cre induced FL chimeras of wt, γ-catenin−/−, and β-catenin−/− genotypes had comparable hematopoietic compartments (Cobas et al8 and data not shown). Therefore, only the results from γ-catenin−/− (control) and β/γ dko genotypes are shown. Absolute numbers of total and FL donor-derived CD45.2+ BM cells in control and β/γ dko chimeras showed no significant differences 7 weeks after deletion (Figure 1C). To investigate whether β- and γ-catenin play redundant roles for HSC homeostasis under steady-state conditions, we phenotypically investigated the HSC compartment (defined as c-kit+lin−Sca1+ [KLS]) of β/γ dko mice 5 to 7 weeks after Mx-Cre induction. Surprisingly, the proportion of CD45.2+ KLS donor cells in control and β/γ dko chimeras was very similar (Figure 1D top panel). The adult KLS compartment contains a mixture of cells, including LT-HSCs, ST-HSCs and multipotent progenitors (MPPs) that have been defined by additional markers, including CD34, CD135, and CD150.12,13 However, even using these additional criteria, we were unable to find any significant differences between control and β/γ dko chimeras (Figure 1D bottom panel; Figure S2). Indeed, the absolute numbers of LT-HSCs, ST-HSCs, and MPPs as well as common myeloid precursors (CMPs) were not significantly different (Figure 1E), indicating that β- and γ-catenin are dispensable for HSC steady-state homeostasis. Furthermore, absolute numbers of more mature myeloid and erythroid lineages (Figure S3A), as well as immature and mature B cells (Figure S3B), were similar for both control and β/γ dko chimeras.
Since analysis of the hematopoietic system under homeostatic conditions does not address the reconstitution efficiency of HSCs, competitive mixed BM chimeras were set up using BM derived from FL chimeras (Figure 1A schematic) and analyzed between 6 weeks and 6 months after transplantation. The global reconstitution efficiency of CD45.2+ donor-derived BM cells within the 2 groups of mixed BM chimeras was variable but similar, ranging from 10% to 60% (data not shown). The relative percentages of donor-derived progenitor subsets, including KLS HSCs, LT-HSCs, ST-HSCs, and MPPs (Figure 2A left panel) as well as more mature myeloid and erythroid lineages (Figure 2B), were similar in both control and β/γ dko mixed chimeras. Furthermore, all immature and mature subsets of all hematopoietic lineages tested were reconstituted to a similar extent in control and β/γ dko chimeras following a secondary BM transfer (Figure 2A right panel; data not shown). Collectively, these data demonstrate that β- and γ-catenin are dispensable for hematopoiesis even under highly competitive conditions imposed by mixed chimeras and serial transplantation.
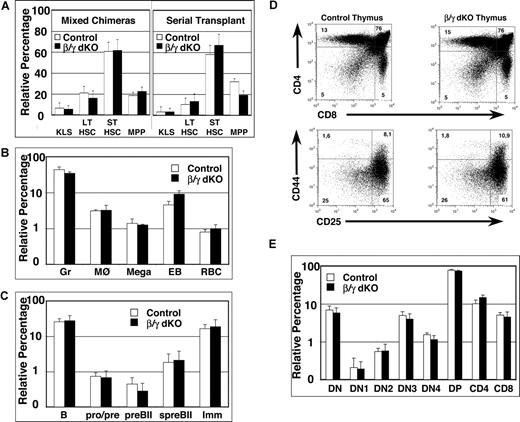
Simultaneous lack of β- and γ-catenin in HSCs of mixed BM chimeras and serial transplants does not affect their repopulation capacity, nor does it influence B- or T-cell development. (A) Mixed BM chimeras were analyzed between 6 and 26 weeks after reconstitution with a 1:2 mixture of CD45.1+ and either control or β/γ dko CD45.2+ BM cells (left panel). BM cells from primary chimeric mice of either control or β/γ dko were serially transplanted into CD45.1+ recipients and analyzed at 12 and 24 weeks after reconstitution (right panel). Percentages of CD45.2+ KLS (CD117+lin−Sca1+), LT-HSCs (CD117+lin−Sca1+CD34−CD135−), ST-HSCs (CD117+lin−Sca1+CD34+CD135−), and MPPs (CD117+lin−Sca1+CD34+CD135+) from either control (□) or β/γ dko (■) mixed BM chimeras (n = 6 for control and n = 7 for β/γ dko) and the serial transplants (n = 5 for control and n = 5 for β/γ dko) are shown. (B) Percentages of myeloid lineages, including granulocytes (Gr; Gr1+Mac1+), macrophages (MØ; Gr1−Mac1+), megakaryocytes (Mega; CD41+), early erythroblasts (EB; Ter119+CD71+), and red blood cells (RBC; Ter119+CD71−) gated on CD45.2+ donor-derived cells derived from either control (□) or β/γ dko (■) mixed BM chimeras (n = 6 for control and n = 7 for β/γ dko). (C) Percentages of BM-derived B-cell subsets, including total BM B cells (B220+), pro-/pre-B cells (B220+CD43+BP1−), large pre-BII cells (preBII; B220+CD43+BP1+), small pre-BII cells (spreBII; B220+CD43−BP1+IgM−), and immature B cells (Imm; B220+CD43−BP1−IgM+), gated on CD45.2+ donor-derived cells derived from either control (□) or β/γ dko (■) mixed BM chimeras (n = 6 for control and n = 7 for β/γ dko). (D) Representative FACS analysis of CD45.2+ total thymocytes stained with anti-CD4 and anti-CD8 antibodies (top row), or with anti-CD44 and anti-CD25 antibodies (bottom row) after gating on Lin− cells (and excluding CD44+CD117− cells) derived from control and β/γ dko mixed chimeras. (E) Bar graphs represent relative percentages of CD45.2+ thymocyte subsets; DN (double negative; CD4−CD8−TCRβ−), DN1 (CD117+CD44+CD25−), DN2 (CD117+CD44+CD25+), DN3 (CD44−CD25+), DN4 (CD44−CD25−), DP (double positive; CD4+CD8+), CD4 (CD4+CD8−), and CD8 (CD4−CD8+) derived from control (□) and β/γ dko (■) mixed chimeras (n = 6 for control and n = 7 for β/γ dko). The error bars represent mean (± SD) in panels A, B, C, and E. No statistically significant differences (P values ranged between .06 and .89) were observed between control and β/γ dko chimeras in all populations analyzed.
Simultaneous lack of β- and γ-catenin in HSCs of mixed BM chimeras and serial transplants does not affect their repopulation capacity, nor does it influence B- or T-cell development. (A) Mixed BM chimeras were analyzed between 6 and 26 weeks after reconstitution with a 1:2 mixture of CD45.1+ and either control or β/γ dko CD45.2+ BM cells (left panel). BM cells from primary chimeric mice of either control or β/γ dko were serially transplanted into CD45.1+ recipients and analyzed at 12 and 24 weeks after reconstitution (right panel). Percentages of CD45.2+ KLS (CD117+lin−Sca1+), LT-HSCs (CD117+lin−Sca1+CD34−CD135−), ST-HSCs (CD117+lin−Sca1+CD34+CD135−), and MPPs (CD117+lin−Sca1+CD34+CD135+) from either control (□) or β/γ dko (■) mixed BM chimeras (n = 6 for control and n = 7 for β/γ dko) and the serial transplants (n = 5 for control and n = 5 for β/γ dko) are shown. (B) Percentages of myeloid lineages, including granulocytes (Gr; Gr1+Mac1+), macrophages (MØ; Gr1−Mac1+), megakaryocytes (Mega; CD41+), early erythroblasts (EB; Ter119+CD71+), and red blood cells (RBC; Ter119+CD71−) gated on CD45.2+ donor-derived cells derived from either control (□) or β/γ dko (■) mixed BM chimeras (n = 6 for control and n = 7 for β/γ dko). (C) Percentages of BM-derived B-cell subsets, including total BM B cells (B220+), pro-/pre-B cells (B220+CD43+BP1−), large pre-BII cells (preBII; B220+CD43+BP1+), small pre-BII cells (spreBII; B220+CD43−BP1+IgM−), and immature B cells (Imm; B220+CD43−BP1−IgM+), gated on CD45.2+ donor-derived cells derived from either control (□) or β/γ dko (■) mixed BM chimeras (n = 6 for control and n = 7 for β/γ dko). (D) Representative FACS analysis of CD45.2+ total thymocytes stained with anti-CD4 and anti-CD8 antibodies (top row), or with anti-CD44 and anti-CD25 antibodies (bottom row) after gating on Lin− cells (and excluding CD44+CD117− cells) derived from control and β/γ dko mixed chimeras. (E) Bar graphs represent relative percentages of CD45.2+ thymocyte subsets; DN (double negative; CD4−CD8−TCRβ−), DN1 (CD117+CD44+CD25−), DN2 (CD117+CD44+CD25+), DN3 (CD44−CD25+), DN4 (CD44−CD25−), DP (double positive; CD4+CD8+), CD4 (CD4+CD8−), and CD8 (CD4−CD8+) derived from control (□) and β/γ dko (■) mixed chimeras (n = 6 for control and n = 7 for β/γ dko). The error bars represent mean (± SD) in panels A, B, C, and E. No statistically significant differences (P values ranged between .06 and .89) were observed between control and β/γ dko chimeras in all populations analyzed.
A role for the canonical wnt pathway in lymphopoiesis has also been suggested, since defects in both B-cell and especially in T-cell development14 have been reported in mice lacking LEF115 and TCF1,16,17 the transcriptional activators of Wnt signaling.2 Therefore, these 2 lymphoid lineages were analyzed in detail in mixed BM chimeras. The relative percentages of cells at various stages of B-cell development within the BM of the CD45.2+ donor population were similar between control and β/γ dko mixed chimeras (Figure 2C). Even more surprisingly, β/γ dko BM cells reconstituted the immature and mature T-cell compartments in the thymus of mixed chimeras to the same extent as control BM (Figure 2D,E), indicating that β- and γ-catenin are both dispensable for T-cell development.
This latter outcome is unexpected, since TCF1−/− mice show multiple developmental blocks during early thymocyte development,16,–18 as well as increased apoptosis at the CD4+CD8+ (double-positive [DP]) stage.19 Moreover, adult TCF1−/− BM cells fail to reconstitute the T-cell compartment of lethally irradiated hosts, whereas the development of all other blood lineages appears to be normal.16,20 The dramatically discrepant T-cell phenotypes of β/γ dko and TCF1−/− mice is intriguing and could theoretically be explained by an unknown protein that activates Wnt signaling in T cells via TCF1 in the absence of both β- and γ-catenin. Alternatively, TCF1 may exert catenin-independent (or even wnt-independent) functions that mostly impinge on T lymphocyte development and/or survival. Whatever the explanation, our results unequivocally demonstrate that β- and γ-catenin are dispensable for both hematopoiesis and lymphopoiesis, although we cannot formally exclude the possibility that other pathways may compensate for their absence in dko mice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Frederic Grosjean and Steven Merlin for cell sorting.
This work was supported in part by the Swiss National Science Foundation (Foerderungsprofessur to F. R.) and the Swiss Cancer League.
Authorship
Contribution: U.K., A.W., and M.C. performed experiments and analyzed the results. R.K. contributed essential reagents. H.R.M. and F.R. wrote the manuscript.
U.K., A.W., and M.C. contributed equally to the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Freddy Radtke, Swiss Institute for Experimental Cancer Research (ISREC), Chemin des Boveresses 155, 1066 Epalinges, Switzerland; e-mail: freddy.radtke@isrec.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal