Abstract
Human embryonic stem cells (hESCs) could potentially represent an alternative source for blood transfusion therapies and a promising tool for studying the ontogeny of hematopoiesis. When we cultured hESCs on either C3H10T1/2 or OP-9 cells to facilitate hematopoiesis, we found that exogenous administration of vascular endothelial growth factor promoted the emergence of sac-like structures, which we named embryonic stem cell–derived sacs (ES-sacs). These ES-sacs consisted of multiple cysts demarcated by cellular monolayers that retained some of the properties of endothelial cells. The spherical cells inside ES-sacs expressed primarily CD34, along with VE-cadherin, CD31, CD41a, and CD45, and were able to form hematopoietic colonies in semisolid culture and to differentiate into mature megakaryocytes by day 24 in the presence of thrombopoietin. Apparently, ES-sacs provide a suitable environment for hematopoietic progenitors. Relatively large numbers of mature megakaryocytes could be induced from the hematopoietic progenitors within ES-sacs, which were then able to release platelets that displayed integrin αIIbβ3 activation and spreading in response to ADP or thrombin. This novel protocol thus provides a means of generating platelets from hESCs, which could serve as the basis for efficient production of platelets for clinical transfusion and studies of thrombopoiesis.
Introduction
Human embryonic stem cells (hESCs) are pluripotent cells that can proliferate almost infinitely in vitro,1 and thus could represent a potent source for cell transplantation therapies. Indeed, studies are currently under way to obtain a variety of differentiated cell types from hESCs, including hematopoietic cells, endothelial cells, cardiomyocytes, hepatocytes, and neuronal cells.2-7 Although, at present, derivation of hESCs from an individual patient is difficult, technologies to establish patient-derived multipotent stem cells that should, in theory, overcome the issue of immunologic rejection are being developed. These include nuclear-transfer ESCs,8 induced pluripotent stem (iPS) cells through gene manipulation,9,10 germ stem cell–derived pluripotent stem cells,11 and establish-ment of hESC banks for various and distinct types of human leukocyte antigen (HLA).12 Another issue that must be overcome is the potential for teratoma formation that can occur if undifferentiated or immature hESCs are contaminated with transplanted mature cells.13
The aforementioned problems must be resolved before hESC-derived cells can be applied in clinical settings. Platelets, which are terminally differentiated and anucleate cells, can be irradiated to eliminate residual white blood cells involved in immediate immune reactions before transfusion, particularly in immunocompromised patients.14 Irradiation of hESC-derived platelets would also kill passenger hESCs and their derivatives that could form teratomas or induce immunologic rejection through expression of polymorphic major histocompatibility complex molecules. Therefore, when an hESC bank containing various types of HLA,12 or the clinical use of iPS cells,9,10 becomes applicable, it might be feasible to use hESC/human iPS cell–derived platelets for transfusion. Such generation of platelets from hESCs could potentially lead to a constant and safe source of platelets that would eliminate the need to obtain platelets through blood donation.
With this in mind, we developed a system in which large numbers of mature, proplatelet-producing megakaryocytes can be derived from murine ESCs cultured on OP-9 stromal cells.15 Using this coculture system, Gaur et al recently succeeded in producing megakaryocytes from H9 hESCs; however, their yield of megakaryocytes was low, and they failed to obtain functional platelets.16 The aim of the present study was to modify this in vitro culture system such that hESCs would be able to differentiate into platelet-forming megakaryocytes. Here we describe a novel protocol that enables hESCs to efficiently develop into hematopoietic progenitors and megakaryocytes capable of releasing functional platelets via the formation of unique sac-like structures.
Methods
Reagents and cell lines
All reagents were from Sigma-Aldrich (St Louis, MO) unless indicated otherwise. Three hESC lines, Kyoto hESC-1 (KhES), KhES-2 and KhES-3, were obtained from the Institute for Frontier Medical Science, Kyoto University (Kyoto, Japan), with approval for hESC use granted by the Minister of Education, Culture, Sports, Science, and Technology of Japan. The Review Board of the Institute of Medical Science, University of Tokyo approved this research. The entire study was conducted in accordance with the Declaration of Helsinki. KhESCs were maintained as described previously17 ; they were cultured on irradiated mouse embryonic fibroblasts in a 1:1 mixture of Dulbecco modified Eagle medium and Ham F-12 medium supplemented with 0.1 mM nonessential amino acids (Invitrogen, Carlsbad, CA), 2 mM l-glutamine (Invitrogen), 20% knockout serum replacement (Invitrogen), 0.1 mM 2-mercaptoethanol, and 5 ng/mL basic fibroblast growth factor (bFGF; Upstate, Lake Placid, NY). The cells were passaged every 3 days to maintain them in an undifferentiated state. The mouse C3H10T1/2 cell line was purchased from the RIKEN BioResource Center (Tsukuba, Ibaraki, Japan) and was cultured in Eagle basal medium (Invitrogen) containing 10% fetal bovine serum (FBS) and 2 mM l-glutamine. The OP-9 cell line was a gift from Dr T. Nakano (Osaka University, Osaka, Japan) and was cultured in α-minimum essential medium (MEM; Invitrogen) containing 20% FBS and 2 mM l-glutamine. ESC differentiation medium was Iscove modified Dulbecco medium supplemented with a cocktail of 10 μg/mL human insulin, 5.5 μg/mL human transferrin, 5 ng/mL sodium selenite, 2 mM l-glutamine, 0.45 mM α-monothioglycerol, 50 μg/mL ascorbic acid, and 15% highly filtered FBS (Cellect Gold; ICN Biomedicals, Aurora, OH) in the absence or presence of the cytokines/mediators listed in Figures 3 and 5. Human vascular endothelial growth factor (VEGF), placental growth factor (PlGF), and human bone morphogenetic protein-4 (BMP-4) were from R&D Systems (Minneapolis, MN). Human thrombopoietin (TPO), human interleukin-6 (IL-6), IL-11, insulin-like growth factor II (IGF-II), and human stem cell factor (SCF) were from Peprotech (Rocky Hill, NJ). The following antibodies were used: phycoerythrin (PE)–conjugated anti-CD31, PE- or fluorescein isothiocyanate (FITC)–conjugated anti-CD34, unconjugated CD41a (integrin αIIb subunit), PE-conjugated CD41a or allophycocyanin (APC)–conjugated anti-CD41a, FITC-conjugated anti-CD42a (GPIX), PE-conjugated anti-CD42b (GPIbα), Alexa 405–conjugated anti-CD45, unconjugated anti–vascular endothelial (VE)–cadherin, and APC-conjugated anti–VEGF-receptor 2 (VEGF-R2). Antihuman VEGF neutralizing antibody (bevacizumab)18 was from Roche (Basel, Switzerland). Antihuman c-Mpl antibody was a kind gift from Kirin (Tokyo, Japan). FITC-conjugated PAC-1 antibody (BD Biosciences, San Jose, CA) was used for integrin activation studies.19 Tirofiban,20 a specific antagonist to human integrin αIIbβ3, was from Merck (Whitehouse Station, NJ).
Cell culture
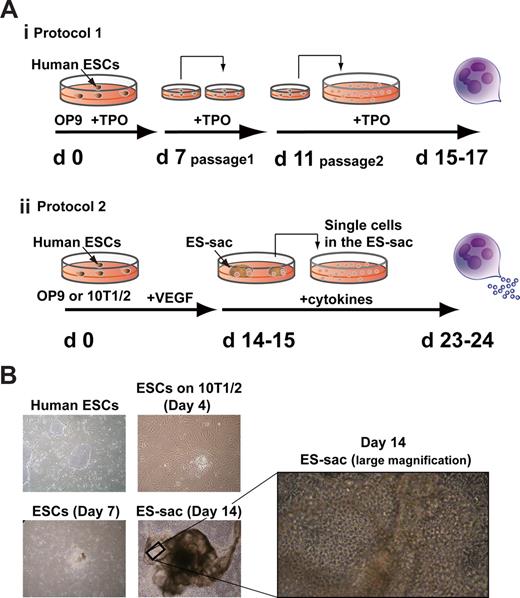
C3H10T1/2 cells or OP-9 cells were irradiated (50 Gy) in 100-mm dishes before use. We compared 2 different protocols, as depicted in Figure 1Ai-ii. Protocol 1: Small clumps (< 100 cells) of hESCs were transferred onto untreated OP9 cells and cultivated in the presence of 100 ng/mL TPO throughout the culture. On days 7 and 11, the cells were passed onto fresh OP-9 cells, leading to the generation of mature megakaryocytes on days 15 to 17, as previously demonstrated.16 Protocol 2: Small clumps of hESCs (suspended in PBS containing 0.25% trypsin, 1 mM CaCl2, and 20% KSR) were transferred onto C3H10T1/2 or OP-9 cells and cultured in hESC differentiation medium, which was refreshed every 3 days. On days 14 to 15 of culture, embryonic stem cell–derived sacs (ES-sacs) were collected into a 50-mL tube, gently crushed with a pipette, and passed through a 70-μm cell strainer to obtain hematopoietic progenitors. These cells were transferred onto fresh, irradiated feeder cells at a density of 2 to 3 × 104 cells per a well in 6-well plates and maintained in differentiation medium supplemented with human TPO or other combinations of cytokines/mediators (human IL-6, IL-11, human SCF, and heparin; Pharmacia & Upjohn, Bridgewater, NJ). The medium was replaced every 3 days; nonadherent cells were collected and analyzed after 20 to 32 days.
Immunohistochemical studies and flow cytometric analyses
Immunohistochemical staining of ES-sacs was carried out on days 14 to 15. Intact ES-sacs were fixed with either 10% methanol or 4% formaldehyde in PBS and then stained with antibodies against CD31, CD34, VEGF-R2, and/or FITC-conjugated Ulex europaeus agglutinin-1 (UEA-1) lectin, an endothelial cell marker (Vector Laboratories, Burlingame, CA), after which they were labeled with secondary antibodies and observed under a fluorescence microscope (Leica DM IRBE; Leica Microsystems, Wetzlar, Germany).
To investigate the internal structures of ES-sacs, immunohistochemistry was carried out with serial 2-μm paraffin sections. Samples were fixed with 4% formaldehyde in PBS and stained with biotinylated UEA-I lectin, after which they were incubated with peroxidase-conjugated streptavidin (Nichirei, Tokyo, Japan). Peroxidase activity was visualized using 3′3-diaminobenzidine in PBS with 0.01% H2O2. Parallel sections were also stained with hematoxylin and eosin. An ECLIPSE50i light microscope (Nikon, Tokyo, Japan) was used for evaluation.
Expression of cell surface molecules was analyzed by flow cytometry (FACS Aria; Becton Dickinson Japan, Tokyo, Japan). To determine precise numbers of megakaryocytes, the cells were stained with antihuman CD41a, antihuman CD42a, and antihuman CD42b, and were accompanied with True Count Beads (BD Biosciences) when analyzed by flow cytometry.
Semiquantitative RT-PCR
On day 24 of culture, hematopoietic cells were sorted into CD34+/CD41a− and CD34+/CD41a+ fractions by flow cytometry and lysed with Trizol (Invitrogen). Total RNA was extracted as recommended by the manufacturer, after which cDNAs were obtained using a Thermo Script reverse-transcription–polymerase chain reaction (RT-PCR) system and oligo-dT primer (Invitrogen). Samples were normalized to intrinsic GAPDH. The following primer sequences (5′ to 3′) were used: for GAPDH, AAC AGC CTC AAG ATC ATC AGC (forward) and TTG GCA GGT TTT TCT AGA CGG (reverse); for GATA-1, TCA ATT CAG CAG CCT ATT CC (forward) and TTC GAG TCT GAA TAC CAT CC (reverse); for GATA-2, TGT TGT GCA AAT TGT CAG ACG (forward) and ACT TTG ACA GCT CCT CGA AGC (reverse); for FOG-1, GCC ACC GCA GTG ATC AAC AAA (forward) and AAG TGG CTG TAG AGG ATG TCC (reverse); for Fli-1, TAA GAA TAC AGA GCA ACG GCC (forward) and GGC ATG TAG GAG ATG TCA GAA (reverse); for NF-E2, ATG AGC TAT TGG CAA GGT ACC (forward) and TAC TCT TCA GGA GAG TAG CTG (reverse); for GPIbα, AAT CCA CTA CTG AAC CAA CCC (forward) and GGG TGG AGA AAA GGG TCA TTT (reverse).
Functional analysis of platelet activation
Platelet preparation.
Platelets in culture medium were gently collected, and a one-ninth volume of acid citrate dextrose solution (85 mM sodium citrate, 104 mM glucose, and 65 mM citric acid) was added. The modified medium containing the cells was then centrifuged at 150g for 10 minutes to eliminate any large cells. The supernatant was transferred to a new tube, 1 μM prostaglandin E1 and 1 U/mL apyrase were added to prevent platelet activation, and the mixture was centrifuged at 400g for 10 minutes to sediment a platelet pellet. The pellet was then resuspended in an appropriate volume of modified Tyrode-HEPES buffer at pH 7.4 (10 mM HEPES, 12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, and 1 mM MgCl2) and finally used after addition of 1 mM CaCl2.
Studies of agonist-mediated integrin αIIbβ3 activation.
To investigate integrin αIIbβ3 activation, 50-μL aliquots of hESC-derived platelets in buffer were incubated for 20 minutes at room temperature with PE-conjugated anti-CD42b and FITC-conjugated PAC-1 in the absence or presence of human thrombin or ADP. The binding of PAC-1 to platelets was quantified using flow cytometry. Nonspecific binding was determined in the presence of 10 μM tirofiban, a specific antagonist to human integrin αIIbβ3.20 Specific binding was defined as total minus nonspecific binding.
Confocal studies.
All observations of cytoskeletal changes in platelets were made using a confocal microscopic system (Leica TCS SP2; Leica Microsystems) equipped with a 63×/1.40 numeric aperture oil-immersion objective (Leica Microsystems). Images were assembled using Adobe Photoshop (Adobe Systems, San Jose, CA). Human washed platelets19 or hESC-derived platelets were spread on fibrinogen-coated cover glass in the absence or presence of an agonist (ADP or thrombin) that accelerated actin cytoskeletal changes. The cells were fixed, permeabilized, and stained with rhodamine-phalloidin to label F-actin (1:50 dilution) or an anti-CD41a antibody (10 μg/mL) followed by Alexa 488–conjugated secondary antibody (1:500 dilution).
Results and discussion
Multipotent hematopoietic progenitors that efficiently generate mature megakaryocytes are enriched inside ES-sacs
Using the protocol depicted in Figure 1Ai,15 Gaur et al successfully generated megakaryocytes with high ploidy from H9 hESCs, but no release of platelets was observed.16 We also failed to obtain large numbers of platelet-like particles from hESCs17 when following this basic protocol, or even after application of additional hematopoietic cytokines, including SCF, IL-6, and IL-11 (data not shown). During the course of these studies, however, we noticed the appearance of inflated sac-like structures in cultures maintained for 2 weeks without additional reseeding procedures (Video S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). What is more, some of these sac-like structures contained round, hematopoietic-like cells inside; those, we termed embryonic stem cell–derived sacs (ES-sacs).
Human ESC-derived sac-like structure (ES-sac). (A) Schematic diagrams of 2 different in vitro differentiation protocols for human embryonic stem cell (hESC)–derived megakaryocytes and platelets. (i) Megakaryocytes are generated on OP-9 stromal cells, as described previously.16 (ii) Megakaryocytes are generated from the cells within ES-sacs on days 14 to 15. (B) Photomicrographs showing undifferentiated hESCs (day 0) and differentiated stages on C3H10T1/2 cells (days 4, 7, and 14). On day 14, ES-sacs appeared. Original magnification, 40×. A high-magnification view (day 14) shows an ES-sac containing numerous bright, spherical cells (200×). VEGF indicates vascular endothelial growth factor; TPO, thrombopoietin.
Human ESC-derived sac-like structure (ES-sac). (A) Schematic diagrams of 2 different in vitro differentiation protocols for human embryonic stem cell (hESC)–derived megakaryocytes and platelets. (i) Megakaryocytes are generated on OP-9 stromal cells, as described previously.16 (ii) Megakaryocytes are generated from the cells within ES-sacs on days 14 to 15. (B) Photomicrographs showing undifferentiated hESCs (day 0) and differentiated stages on C3H10T1/2 cells (days 4, 7, and 14). On day 14, ES-sacs appeared. Original magnification, 40×. A high-magnification view (day 14) shows an ES-sac containing numerous bright, spherical cells (200×). VEGF indicates vascular endothelial growth factor; TPO, thrombopoietin.
The protocol used to obtain ES-sacs required stromal cells, such as OP-9 or C3H101/2 cells. While OP-9 and C3H10T1/2 cells generated equal numbers of ES-sacs (data not shown), we primarily used C3H10T1/2 cells in the subsequent studies summarized in the results.
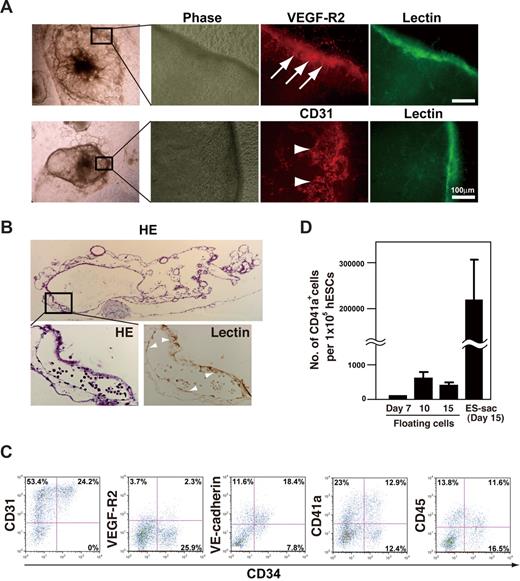
Each ES-sac consisted of a morphologically distinct external layer that enveloped several thousand spherical cells (Figure 1B). Immunohistochemical studies of ES-sacs revealed that the cells in the external layer preferentially expressed VEGF-R2, CD31, CD34, and UEA-1 lectin-binding activity (UEA-1+), indicating differentiation along an endothelial cell lineage (Figure 2A arrows).21 Hematoxylin-eosin staining revealed that ES-sacs contain multiple cystic structures demarcated by UEA-1+ septa (arrowheads in the higher-magnification view of Figure 2B). To characterize the spherical cells within ES-sacs, they were stained with anti-CD31, anti-CD34, anti–VEGF-R2, anti–VE-cadherin (a hematoendothelial cell marker),2 anti-CD41a (an early hematopoietic progenitor and megakaryocyte marker),22 and anti-CD45 (a panhematopoietic cell marker) and then analyzed by flow cytometry. Most spherical cells within ES-sacs expressed CD31 (Figure 2C), whereas only approximately one-third of the CD31+ cells expressed CD34. VEGF-R2, VE-cadherin, CD41a, and CD45 were present on 8%, 70%, 50%, and 41% of CD34+ cells, respectively (Figure 2C). Moreover, these cells effectively formed multilineage colonies under semisolid liquid conditions (Figure S1).
Structure of ES-sacs and hematopoietic progenitors within ES-sacs. (A) Immunohistochemical staining of an ES-sac on day 15. The ES-sac expresses UEA-1 lectin-binding activity, CD31, and VEGF-R2, which is indicative of endothelial differentiation (arrows). Many of the cells inside ES-sacs also express CD31 (arrowheads). (B) Parallel sections of an ES-sac stained with hematoxylin-eosin or UEA-I lectin. The structure is composed of multiple cystic regions in which cellular septa express UEA-1 lectin-binding activity (arrowheads, large magnification view). Top image, 40×; bottom 2 images, 200×. (C) Representative flow-cytometry dot plots of surface molecule expression on the cells inside ES-sacs on day 15. The y-axes indicate CD31, VEGF-R2, vascular endothelial (VE)–cadherin, CD41a (integrin αIIb), or CD45; the x-axes indicate CD34 expression. Numbers on plots are the percentages of total cells within each quadrant. (D) Numbers of CD41a+ cells per starting 105 hESCs. Using protocol 2, CD41a+ cells were obtained more efficiently from cells within ES-sacs on day 15 (adherent cells) than through the use of floating cells on day 7, 10, or 14. Error bars represent SD.
Structure of ES-sacs and hematopoietic progenitors within ES-sacs. (A) Immunohistochemical staining of an ES-sac on day 15. The ES-sac expresses UEA-1 lectin-binding activity, CD31, and VEGF-R2, which is indicative of endothelial differentiation (arrows). Many of the cells inside ES-sacs also express CD31 (arrowheads). (B) Parallel sections of an ES-sac stained with hematoxylin-eosin or UEA-I lectin. The structure is composed of multiple cystic regions in which cellular septa express UEA-1 lectin-binding activity (arrowheads, large magnification view). Top image, 40×; bottom 2 images, 200×. (C) Representative flow-cytometry dot plots of surface molecule expression on the cells inside ES-sacs on day 15. The y-axes indicate CD31, VEGF-R2, vascular endothelial (VE)–cadherin, CD41a (integrin αIIb), or CD45; the x-axes indicate CD34 expression. Numbers on plots are the percentages of total cells within each quadrant. (D) Numbers of CD41a+ cells per starting 105 hESCs. Using protocol 2, CD41a+ cells were obtained more efficiently from cells within ES-sacs on day 15 (adherent cells) than through the use of floating cells on day 7, 10, or 14. Error bars represent SD.
Collectively, the results suggest the cells inside ES-sacs include multipotent hematopoietic progenitors. By contrast, hESC-derived clumps within cultures that did not form ES-sacs (they appeared in the absence of round cells) failed to differentiate into hematopoietic cells. Thus, our new protocol (protocol 2, summarized in Figure 1Aii) enabled hESCs to develop into hematopoietic progenitors, once they formed an ES-sac structure (Figure 2C). Indeed, when the cells inside ES-sacs were harvested, reseeded onto feeder cells in the presence of TPO, and cultured according to protocol 2, megakaryocytes were generated with much greater efficiency than has been seen with other in vitro methods, including protocol 1 and the floating cell method with protocol 2 (Figure 2D). This is noteworthy in that we recently succeeded in using the floating cells but not adherent cells to efficiently generate megakaryo-cytes and platelets from monkey ES cells (CMK6).23 There is thus a clear difference in the optimal methodologies between CMK6 ESCs and hESCs.
In addition, we found that exogenous administration of VEGF significantly increased the number of ES-sacs (Figure 3A). While the synergistic action of IGF-II plus VEGF is required for more efficient production of hematopoietic progenitors from CMK6 ESCs,23 VEGF (up to 20 ng/mL), by itself, induced ES-sacs from hESCs as efficiently as VEGF plus IGF-II (Figure 3A). On the other hand, administration of BMP-4, which is known to be a potent accelerator for hESC-derived hematopoiesis through embryoid bodies,2 inhibited the positive action of VEGF on production of ES-sacs and hematopoietic progenitors (Figure 3A). To test whether VEGF specifically acts on the formation of ES-sacs, we next examined the effects of antihuman VEGF neutralizing antibody (bevacizumab).18 For these experiments, 20 ng/mL VEGF was preincubated with 500 ng/mL bevacizumab for 2 hours at 37°C. As shown in Figure 3B, bevacizumab completely reversed the VEGF-induced increase in ES-sac formation seen on day 15, as well as the production of platelets seen on day 24 (Figure 3Bi,ii).
VEGF promoted the generation of ES-sacs and subsequent platelets. (A) The effects of cytokine/growth factor(s) on production of ES-sacs via protocol 2. VEGF increased the numbers of ES-sacs generated, while BMP-4 inhibited the effect of VEGF on the ES-sac production. (B) Bevacizumab, an anti-VEGF neutralizing antibody, reversed the VEGF-induced increase in ES-sac formation on day 15 (i) or platelets on day 24 (ii), confirming that the phenomenon is VEGF dependent. (C) PlGF, but not bFGF, acted additively with VEGF to increase ES-sac formation. The number of ES-sacs obtained with no additional cytokine/factor(s) was assigned a value of 1.0 (control), and all results are means (± SD) of 3 independent experiments.
VEGF promoted the generation of ES-sacs and subsequent platelets. (A) The effects of cytokine/growth factor(s) on production of ES-sacs via protocol 2. VEGF increased the numbers of ES-sacs generated, while BMP-4 inhibited the effect of VEGF on the ES-sac production. (B) Bevacizumab, an anti-VEGF neutralizing antibody, reversed the VEGF-induced increase in ES-sac formation on day 15 (i) or platelets on day 24 (ii), confirming that the phenomenon is VEGF dependent. (C) PlGF, but not bFGF, acted additively with VEGF to increase ES-sac formation. The number of ES-sacs obtained with no additional cytokine/factor(s) was assigned a value of 1.0 (control), and all results are means (± SD) of 3 independent experiments.
We also found that VEGF increased the numbers of c-Mpl–expressing cells within ES-sacs on day 15 (Figure S2). Because it is well known that signaling mediated by the TPO/c-Mpl axis is indispensable for megakaryopoiesis,24,25 we suggest that augmented expression of c-Mpl mediated via a VEGF signaling pathway increases megakaryopoiesis and thrombopoiesis to levels not seen in the absence of VEGF (Figure 3A,B), which is consistent with a recent report.26
Because the cells making up ES-sacs manifest characteristics similar to those of endothelial cells (Figure 2A,B), and because bFGF and PlGF, like VEGF, are necessary for endothelial cell growth,27-29 we next assessed the respective capacities of bFGF or PlGF to promote the generation of ES-sacs and hematopoietic progenitors. Interestingly, bFGF at concentrations of 2.5 or 10 ng/mL exerted a dose-dependent inhibitory effect, while PlGF at 50 ng/mL tended to increase the generation of ES-sacs (Figure 3C) and platelets (data not shown). Moreover, whereas bFGF inhibited VEGF-augmented production of ES-sacs, PlGF acted additively with VEGF to further augment ES-sac production (Figure 3C).
We also compared generation of ES-sacs and platelets from the KhES-1, KhES-2, and KhES-3 lines, and found that the induction efficiencies of ES-sacs differed. The number of ES-sacs generated from KhES-3 was several-fold higher than those from KhES-1 or KhES-2 (data not shown). We therefore used KhES-3 cells for most of the results shown here.
Hematopoietic progenitors inside ES-sacs efficiently yield megakaryocytes
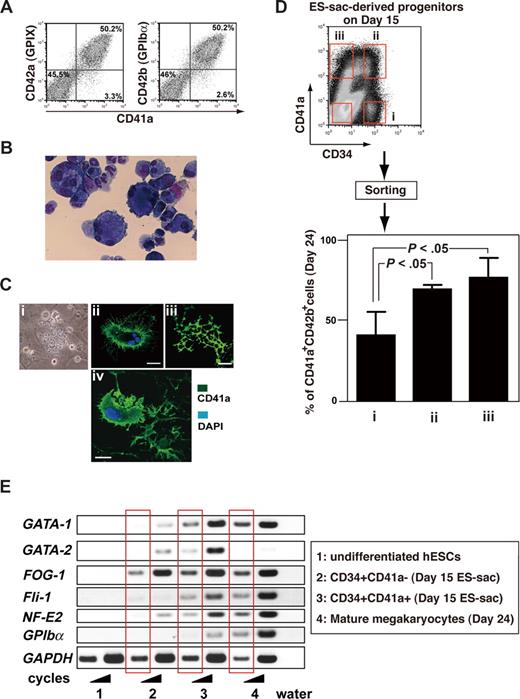
Coculture for an additional 7 to 9 days after day 14 on fresh, but irradiated, feeder cells in the presence of 100 ng/mL TPO (Figure 1Aii) promoted differentiation of many hematopoietic progenitors inside the ES-sacs into mature, proplatelet-forming megakaryocytes, as evidenced by cell surface markers revealed by flow cytometric analyses and cytospin-preparation staining (Figure 4A,B). Fifty percent to 60% of cells consistently expressed CD41a, CD42a, and CD42b, which is indicative of mature megakaryocytes.30,31 These hESC-derived megakaryocytes showed polyploidy upon May-Giemsa staining (Figure 4B) and proplatelet formation upon immunohistochemical staining (Figure 4C). In addition, some megakaryocytes appeared to be shedding their cytoplasmic membranes (Figure 4Civ). These morphologic features demonstrate that megakaryocytes cultured in vitro from hESCs possess the demarcation membrane system necessary for the generation of platelets.32
Hematopoietic progenitors within ES-sacs efficiently generate megakaryocytes. (A) Dot plots on flow cytometry showing CD41a (integrin αIib; x-axis) and CD42a (GPIX; y-axis) expression (left panel) or CD41a (x-axis) and CD42b (GPIbα; y-axis) expression (right panel) on day 24. Fifty percent to 60% of the cells appeared to be mature megakaryocytes. Numbers on plots are the percentages of total cells within each quadrant. (B) Floating cells on days 23 to 24 were stained with Hemacolor (Diagnostica Merck, Darmstadt, Germany). (C) Formation of megakaryocytes bearing proplatelets (i-iii) or a megakaryocyte shedding its cytoplasmic membrane for direct releases of platelets (iv). (i) Representative phase contrast photomicrograph. (ii-iv) Immunohistochemical staining demonstrating expression of CD41a (green); nuclei were stained using 4,6-diamino-2-phenylindole (DAPI; blue). Bar represents 20 μm. (D) Top panel: Hematopoietic progenitors within ES-sacs on day 15 were stained for CD34 and CD41a and sorted using the indicated gates (i, ii, iii, and iv). Bottom panel: Percentages of CD41a+/CD42b+ megakaryocytes among the hematopoietic cells on day 24 that were derived from CD34+/CD41a−, CD34+/CD41a+, or CD34−/CD41a+ hematopoietic progeni-tors within ES-sac on day 15. Error bars represent SD. (E) Undifferentiated hESCs (no. 1), cells sorted on day 15 (no. 2 or no. 3), or mature megakaryocytes on day 24 (no. 4) were collected and prepared as described in “Methods.” Extracted RNAs were used for semiquantitative RT-PCR. The bands in the red squares were obtained with fewer PCR cycles.
Hematopoietic progenitors within ES-sacs efficiently generate megakaryocytes. (A) Dot plots on flow cytometry showing CD41a (integrin αIib; x-axis) and CD42a (GPIX; y-axis) expression (left panel) or CD41a (x-axis) and CD42b (GPIbα; y-axis) expression (right panel) on day 24. Fifty percent to 60% of the cells appeared to be mature megakaryocytes. Numbers on plots are the percentages of total cells within each quadrant. (B) Floating cells on days 23 to 24 were stained with Hemacolor (Diagnostica Merck, Darmstadt, Germany). (C) Formation of megakaryocytes bearing proplatelets (i-iii) or a megakaryocyte shedding its cytoplasmic membrane for direct releases of platelets (iv). (i) Representative phase contrast photomicrograph. (ii-iv) Immunohistochemical staining demonstrating expression of CD41a (green); nuclei were stained using 4,6-diamino-2-phenylindole (DAPI; blue). Bar represents 20 μm. (D) Top panel: Hematopoietic progenitors within ES-sacs on day 15 were stained for CD34 and CD41a and sorted using the indicated gates (i, ii, iii, and iv). Bottom panel: Percentages of CD41a+/CD42b+ megakaryocytes among the hematopoietic cells on day 24 that were derived from CD34+/CD41a−, CD34+/CD41a+, or CD34−/CD41a+ hematopoietic progeni-tors within ES-sac on day 15. Error bars represent SD. (E) Undifferentiated hESCs (no. 1), cells sorted on day 15 (no. 2 or no. 3), or mature megakaryocytes on day 24 (no. 4) were collected and prepared as described in “Methods.” Extracted RNAs were used for semiquantitative RT-PCR. The bands in the red squares were obtained with fewer PCR cycles.
We then investigated which cells inside ES-sacs have the potential to differentiate into the megakaryocyte lineage. Data from earlier reports using human bone marrow– or umbilical cord blood–derived cells suggest that the development of hematopoietic progenitors into mature megakaryocytes is characterized and defined by the expression of CD34 and CD41a.33,34 We therefore isolated cells from inside ES-sacs on day 15, stained them with anti-CD34 and anti-CD41a antibodies, and divided them into CD34+/CD41a−, CD34+/CD41a+, CD34−/CD41a+, and CD34−/CD41a− subpopulations using a cell sorter (Figure 4D top panel of dot plots on flow cytometry). Equal numbers of the sorted cells in each fraction were then transferred onto new feeder cells and maintained for an additional 9 days in the presence of 100 ng/mL TPO. Over that 9-day period, the CD34+/CD41a+ and CD34−/CD41a+ populations continued to develop in culture, so that 80% to 90% of the differentiated nonadherent cells were CD41a+/CD42b+ on day 24. This means that CD41a+ cells within ES-sacs on day 15 were able to differentiate into mature megakaryocytes in our culture system. On the other hand, from the CD34+/CD41a− population, only 40% of nonadherent cells were CD41a+/CD42b+ megakaryocytes on day 24 (Figure 4D), which suggests that the CD34+/CD41a− population has the potential to differentiate into other lineages in addition to the megakaryocytic lineage. Furthermore RT-PCR analysis revealed that the development of CD34+/CD41a− progenitors into CD41a+ populations may reflect their expression of GATA-1, FOG-1, Fli-1, and/or NF-E2 (Figure 4E), which are required for megakaryopoiesis.35 Taken together, these results suggest that ES-sacs contain heterogeneous populations of cells at different stages during which CD34 and/or CD41a may be expressed, and that CD41a+ cells preferentially differentiate into the megakaryocyte lineage, which is consistent with the developmental behavior observed for hematopoietic cells in bone marrow and umbilical cord blood.33,34
ES-sac–derived megakaryocytes generate platelets
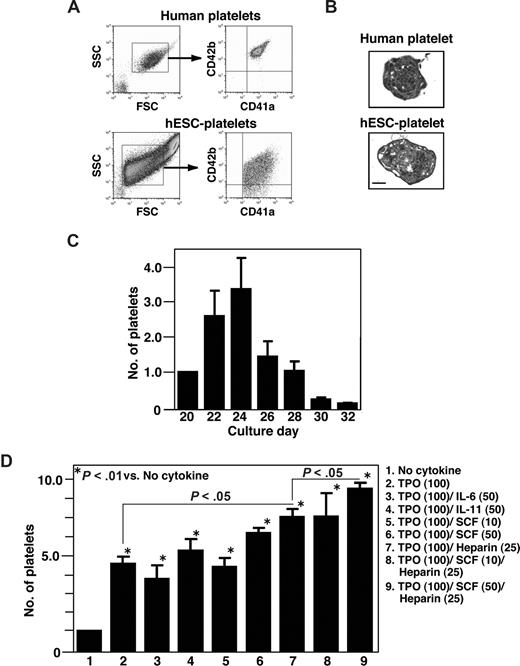
When the surface markers of hESC-derived platelet-like particles collected on day 24 were examined by flow cytometry using the same forward- and side-scatter gates used for plasma-derived adult human platelets, the CD41a+/CD42b+ particles were detected in the culture supernatants (Figure 5A). Subsequent electron microscopic examination of the cytosolic structures of hESC-derived platelets revealed normal microtubules but fewer granules than were seen in plasma-derived human platelets (Figure 5B). Platelets generated in culture supernatant increased in number up to day 24, but thereafter their numbers declined in the presence of TPO alone (Figure 5C). To test whether other cytokines/mediators also affect platelet production, we examined the effects of the combinations shown in Figure 5D.25,36,37 Heparin (25 U/mL), SCF (50 ng/mL), and TPO (100 ng/mL) were the most effective stimulators of platelet production via protocol 2, whereas IL-6 and IL-11 had no effect on production, which is quite different from what is seen with mouse ESC-derived platelets (H. Nishikii, K.E., N. Tamura, K. Hattori, B. Heissig, T. Kanaji, A.S., S. Goto, J. Wave, H. Nakaushi, manuscript submitted). On average, 4.8 (± 0.2) × 106 platelets were generated from an initial 105 hESCs (KhES-3) on day 24 in a cocktail of TPO, SCF, and heparin. This can be considered the lower limit of the yield, as platelets are inevitably lost during the collection and purification procedures. C3H10T1/2 and OP-9 stromal cells proved equally efficacious in their ability to support platelet generation (data not shown).
Characterization of platelets from hESCs. (A) Surface markers of hESC-derived platelets were examined by flow cytometry using the same forward- and side-scatter gates used for human plasma-derived adult platelets. Most small particles positive for CD41a (x-axis) were also positive for CD42b (y-axis; lower panel). (B) Transmission electron micrographs of fresh plasma-derived platelets or of hESC-derived platelets on day 23. Bar represents 1μm. (C) Numbers of platelets released into the culture supernatant from day 20 to day 32. The graph shows the relative number when the total number on day 20 per initial 105 hESCs was assigned a value of 1.0. Platelets were collected every other day from culture days 20 to 32, and the cells were counted by flow cytometry using True Count Beads. Data are means (± SD) from 3 independent experiments. (D) Numbers of CD41a+ platelets counted in the absence or presence of TPO (100 ng/mL) alone and in combination with SCF (25 or 50 ng/mL), IL-6 (50 mg/mL), IL-11 (50 mg/mL), and/or heparin (25 U/mL). The graph shows the relative platelet number when the total number of platelets yielded without cytokine was assigned a value of 1.0. Data are means (± SD) from more than 3 independent experiments.
Characterization of platelets from hESCs. (A) Surface markers of hESC-derived platelets were examined by flow cytometry using the same forward- and side-scatter gates used for human plasma-derived adult platelets. Most small particles positive for CD41a (x-axis) were also positive for CD42b (y-axis; lower panel). (B) Transmission electron micrographs of fresh plasma-derived platelets or of hESC-derived platelets on day 23. Bar represents 1μm. (C) Numbers of platelets released into the culture supernatant from day 20 to day 32. The graph shows the relative number when the total number on day 20 per initial 105 hESCs was assigned a value of 1.0. Platelets were collected every other day from culture days 20 to 32, and the cells were counted by flow cytometry using True Count Beads. Data are means (± SD) from 3 independent experiments. (D) Numbers of CD41a+ platelets counted in the absence or presence of TPO (100 ng/mL) alone and in combination with SCF (25 or 50 ng/mL), IL-6 (50 mg/mL), IL-11 (50 mg/mL), and/or heparin (25 U/mL). The graph shows the relative platelet number when the total number of platelets yielded without cytokine was assigned a value of 1.0. Data are means (± SD) from more than 3 independent experiments.
Platelet production from mature megakaryocytes is likely influenced by multiple factors within the bone marrow microenvironment.32,38 Thus, while our new protocol introduces a number of intercellular or intratissue mediators that were lacking in earlier in vitro coculture systems, additional developments would be expected to increase the efficiency of platelet generation, thereby further advancing the process toward the ultimate aim of use in a clinical setting.
hESC-derived platelets can activate integrin and undergo integrin-dependent actin cytoskeletal changes
Stimulating platelets with an agonist changes the conformation of integrin αIIbβ3 and promotes clustering. This inside-out activation of αIIbβ3 promotes the binding of its ligands,39 principally fibrinogen, but also von Willebrand factor. These ligands in turn promote outside-in signaling to induce the cytoskeletal changes and spreading required for production of stable platelet thrombi in vivo.39 To explore the functionality of hESC-derived platelets, we used flow cytometry to examine αIIbβ3 activation following stimulation with the major platelet agonists, thrombin and ADP. Platelet-sized particles were collected from culture supernatants and incubated with antihuman CD42b (GPIbα) antibody and with PAC-1, an antibody that mimics the specific fibrinogen binding to human αIIbβ3 in the activated state.19 Administration of ADP induced concentration-dependent increases in PAC-1 binding to hESC-derived platelets (Figure 6A,B). The binding was specific for αIIbβ3, as 10 μM tirofiban, a selective inhibitor to human αIIbβ3,20 reversed the agonist-induced PAC-1 binding. Similar results for PAC-1 binding were observed when platelets were stimulated with thrombin (data not shown).
Integrin activation and actin cytoskeletal reorganization in hESC-derived platelets. (A) Representative dot plots for platelets binding FITC-conjugated PAC-1 in the absence (left panel) or presence (middle panel) of 50 μM ADP. The right panel shows inhibition of PAC-1 binding by 10 μM tirofiban. Numbers on plots are the percentages of total cells within each quadrant. (B) Binding of FITC-conjugated PAC-1 to hESC-derived platelets was quantified in the absence and presence of ADP by flow cytometry. Some specimens were also incubated with tirofiban. Data depict means (± SD) from more than 3 independent experiments. (C) hESC-derived platelets spreading on fibrinogen-coated cover glass in the absence and presence of 50 μM ADP or 1.0 U/mL thrombin. Cells were fixed, permeabilized, and stained with rhodamine-phalloidin to label F-actin (red) and anti-CD41a antibody followed by Alexa 488–conjugated secondary antibody (green). αIIbβ3-dependent formation of stress fibers, lamellipodia, and filopodia was observed. Bar represents 10μm.
Integrin activation and actin cytoskeletal reorganization in hESC-derived platelets. (A) Representative dot plots for platelets binding FITC-conjugated PAC-1 in the absence (left panel) or presence (middle panel) of 50 μM ADP. The right panel shows inhibition of PAC-1 binding by 10 μM tirofiban. Numbers on plots are the percentages of total cells within each quadrant. (B) Binding of FITC-conjugated PAC-1 to hESC-derived platelets was quantified in the absence and presence of ADP by flow cytometry. Some specimens were also incubated with tirofiban. Data depict means (± SD) from more than 3 independent experiments. (C) hESC-derived platelets spreading on fibrinogen-coated cover glass in the absence and presence of 50 μM ADP or 1.0 U/mL thrombin. Cells were fixed, permeabilized, and stained with rhodamine-phalloidin to label F-actin (red) and anti-CD41a antibody followed by Alexa 488–conjugated secondary antibody (green). αIIbβ3-dependent formation of stress fibers, lamellipodia, and filopodia was observed. Bar represents 10μm.
Stable thrombus formation requires actin reorganization through αIIbβ3 integrin outside-in signaling.19 To observe integrin-mediated changes in the actin cytoskeleton, such as the assembly of filopodia, lamellipodia, and/or stress fibers, hESC-derived platelets were allowed to adhere to fibrinogen-coated cover glass and then stained for F-actin and labeled with anti-CD41a antibodies. As shown in Figure 6C, hESC-derived platelets formed filopodia, even in the absence of an agonist. In addition, they also formed lamellipodia and actin stress fibers when stimulated with 50 μM ADP or 1.0 U/mL thrombin. Thus our hESC-derived platelets exhibit a number of major functional responses established for plasma-derived platelets, at least in these in vitro assays.
In the present study, we report the establishment of a novel culture method for generating ES-sacs—unique, balloonlike structures that provide a microenvironment for the generation and differentiation of hematopoietic progenitors. Cells within ES-sacs are enriched in progenitors with multilineage differentiation potential, enabling megakaryocytes and platelets to be more efficiently produced in vitro than reported previously.16 Here we tested 2 different protocols for the production of platelets from hESCs (Figure 1Ai,ii). Protocol 2 differs from that of Gaur et al (protocol 1) in which megakaryocytes, but not platelets, were generated after multiple rounds of passaging hESC-derived progenitors grown on OP-9 cells.16 Our finding that generation of ES-sacs requires continuous culture for at least 2 weeks, without passaging, suggests protocol 1 does not allow sufficient time for cells to develop into ES-sacs. Moreover, the fact that ES-sacs appear to provide a suitable environment for highly prolific hematopoietic progenitors suggests the generation of ES-sacs may be indispensable for efficient production of fully differentiated hematopoietic cells, including platelets (Figure 4,5; Figure S1). It was previously reported that protocol 1 has the potential to generate 1 to 4 × 104 megakaryocytes, but not platelets, from 105 hESCs.16 By contrast, protocol 2 consistently generated more than 2 to 5 × 105 platelet-producing megakaryocytes, improving the efficiency by at least one order of magnitude (Figure 1Aii). Still, the megakaryocytes in this system yielded fewer platelets than megakaryocytes do in vivo, where approximately 2000 platelets can be generated per megakaryocyte.38 This may indicate that some stimulus of thrombopoiesis (eg, shear flow40 ) is lacking in the in vitro environment. Further improvement of our method will be required before we are able to obtain numbers of functional platelets that are sufficient for posttransfusion in vivo functional studies and, ultimately, clinical application. Genetic manipulation may prove to be a useful means of overcoming this challenge, as is exemplified by HoxB4 overexpression in ESC-derived hematopoietic stem cells.41 Nevertheless, our study provides a setting for future molecular studies of human thrombopoiesis and for further development of culture methods to generate platelets from hESCs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs N. Nakatsuji and H. Suemori at Kyoto University for providing human khES-1, khES-2, and khES-3 cell lines; Drs Y. Inagaki and H. Miyazaki at Kirin for providing an antibody against human c-Mpl; Drs M. Otsu and Y. Nakamura for valuable discussion; and Drs A. Knisely and M. Mahaut-Smith for critical reading of the paper.
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Authorship
Contribution: N.T., K.E., and H. Nakauchi designed the research and wrote the paper; K.E. edited the paper; N.T., H. Nishikii, J.U., H.T., A.S., and K.E. performed experiments and analyzed data; T.H. provided critical information for the novel methodology.
Conflict-of-interest disclosure: N.T., H. Nishikii, K.E., and H. Nakauchi have applied for a patent related to the methodology described in the present work. The other authors declare no competing financial interests.
Correspondence: Koji Eto, Laboratory of Stem Cell Therapy, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minatoku, Tokyo 108-8639, Japan; e-mail: keto@ims.u-tokyo.ac.jp; or Hiromitsu Nakauchi, Laboratory of Stem Cell Therapy, The Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minatoku, Tokyo 108-8639, Japan; e-mail: nakauchi@ims.u-tokyo.ac.jp.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal