Sustained engagement of the B-cell receptor (BCR) increases apoptosis resistance in chronic lymphocytic leukemia (CLL) B cells, whereas transient stimulation usually has an opposite effect. The antiapoptotic BCR signal has been associated with prolonged activation of the PI3K/Akt and MEK/ERK pathways, which are key regulators of survival and proliferation in various cell types. To further define the relative contribution of the Akt and ERK kinases in regulating CLL B-cell survival, we introduced constitutively active mutants of Akt and MEK in primary CLL B cells and evaluated changes in the expression of relevant pro- and antiapoptotic proteins. Sustained activation of Akt resulted in increased leukemic cell viability and increased expression of the antiapoptotic proteins Mcl-1, Bcl-xL, and X-linked inhibitor of apoptosis protein (XIAP), thus largely recapitulating the effects of sustained BCR stimulation. Constitutively active MEK2 also up-regulated XIAP, but did not show a significant impact on leukemic cell survival. Down-regulation of Mcl-1 by siRNA treatment induced rapid and potent apoptosis in CLL B cells and blocked the antiapoptotic effect of sustained BCR stimulation, whereas down-regulation of Bcl-xL and XIAP did not affect leukemic cell viability. These data demonstrate that Akt and Mcl-1 are major components of a survival pathway that can be activated in CLL B cells by antigen stimulation.
Introduction
Certain features of the B-cell receptor (BCR) have recently emerged as major prognostic factors in chronic lymphocytic leukemia (CLL).1,2 In particular, the absence of somatic mutations in the immunoglobulin variable region genes and expression of the protein tyrosine kinase ZAP-70 are strongly associated with an aggressive clinical course, and both features have been correlated with a greater capacity of CLL B cells to transmit BCR-derived signals.3,,,,,,,–11 In addition, CLL B cells from the prognostically unfavorable subset express higher levels of BCR-inducible genes, indicating that they have received recent antigenic stimulation in vivo.12 A role for antigenic selection in the pathogenesis of CLL is further supported by the identification of similar or virtually identical leukemic immunoglobulins in a substantial proportion of patients with CLL.13,–15 Altogether, these studies indicate that signaling through the BCR plays an important role at both early and late stages of CLL pathogenesis, including the initial clonal expansion and during subsequent disease progression.
Engagement of the BCR in vitro can have opposing effects on CLL B-cell survival. Stimulation with soluble anti-IgM antibodies elicits only transient signaling, leading to leukemic cell apoptosis in the majority of patients.16,,–19 In contrast, immobilized anti-IgM antibodies induce sustained BCR signaling, a decrease in spontaneous and chemotherapy-induced apoptosis, and increased expression of the antiapoptotic protein Mcl-1.18,19 In terms of activation of downstream signaling pathways, sustained engagement of the BCR results in more efficient degradation of the NF-κB inhibitor IκB and significantly prolonged activation of the Akt and ERK kinases.18
The magnitude and duration of Akt and ERK signaling are important determinants of the cellular response in many different cell types. For example, sustained activation of ERK is required for proliferation of fibroblasts, whereas transient activation does not induce cell-cycle entry.20,21 Similarly, proliferation and survival of activated murine T and B cells is dependant on sustained antigen receptor engagement and sustained activation of the PI3K/Akt pathway.22,23 Sustained activation of Akt is also required to drive cell-cycle progression of CLL B cells stimulated with CpG oligonucleotides.24 Altogether, these studies suggest that the duration of Akt and/or ERK kinase activation may largely determine the different survival response of CLL B cells following transient and sustained BCR stimulation.
The PI3K/Akt and Raf/MEK/ERK pathways control the expression and function of many proteins that are essential for cell survival. These include members of the Bcl-2 family of proteins, such as proapoptotic Bcl-2 antagonist of cell death (BAD) or antiapoptotic Mcl-1, which can be inhibited or up-regulated by Akt, respectively.25,–27 In addition, Akt can increase the expression of NF-κB target genes, such as Bcl-xL and A1, by activating the IκB kinase (IKK) to induce the degradation of IκB.28,29 Akt can also prevent apoptosis by directly phosphorylating and inactivating caspase-9 or by inducing the expression of distal negative regulators of apoptosis, such as the X-linked inhibitor of apoptosis protein (XIAP).30,,–33 Expression of XIAP can also be up-regulated by ERK in some myeloid and monocytic leukemia cell lines.34,35 In addition, ERK has been implicated in the posttranslational regulation of Bim, another proapoptotic Bcl-2 family protein.36 Altogether, these examples illustrate the complexity of cell-survival regulation by Akt and ERK, which, depending on cell type, can be mediated by different pro- and antiapoptotic proteins.
The increased survival of CLL B cells in vivo is considered primarily a consequence of inappropriate expression of Bcl-2 family proteins. Essentially all CLL B cells express high levels of Bcl-2 and considerable but variable levels of Mcl-1, Bcl-xL, Bax, and Bim.37,,–40 High levels of Mcl-1 and an increased ratio of Bcl-2 to Bax have also been associated with poor treatment responses.37,38,41 In addition, XIAP is expressed at high levels in CLL B cells and is up-regulated by prosurvival signals, indicating that this protein may also contribute to apoptosis resistance through caspase inhibition.41,42
To further dissect the molecular pathways that transduce antiapoptotic signals downstream of the BCR, we investigated the effects of sustained activation of the Akt and ERK kinases in primary CLL cells. Changes in the expression of several antiapoptotic proteins were noted, among which most prominent was the up-regulation of Mcl-1 by the Akt kinase. Inhibition of Mcl-1 by siRNA treatment induced leukemic cell apoptosis and suppressed the antiapoptotic effect of sustained BCR engagement, indicating that Akt and Mcl-1 are major components of a survival pathway that can be activated in CLL B cells by antigen stimulation.
Methods
Patients, CLL B-cell samples, cell lines, and culture conditions
Peripheral blood samples were collected from patients with B-CLL that had been diagnosed according to standard morphologic and immunophenotyping criteria. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki, and approval for the study was obtained from the institutional human research committee at the Catholic University Medical School in Rome.
Mononuclear cells were separated from the peripheral blood of patients with CLL or from normal tonsils by Ficoll gradient centrifugation (Amersham Biosciences, Uppsala, Sweden). B cells were purified by negative selection using anti-CD3, anti-CD14, and anti-CD16 mouse monoclonal antibodies (kindly provided by Prof F. Malavasi, Turin, Italy) and Dynabeads coated with pan anti-mouse antibody (Dynal Biotech, Oslo, Norway). The purity of the selected B-cell populations was evaluated by staining with anti-CD5 R-phycoerythrin (R-PE)–conjugated and anti-CD19 fluorescein (FITC)–conjugated antibodies (BD Biosciences, Franklin Lakes, NJ), followed by flow cytometric analysis on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The purity of the CLL B-cell samples was more than 98%, whereas the purity of the normal tonsillar B cells was more than 75%.
CLL B cells, the Burkitt lymphoma cell line BJAB and the human B-CLL cell line MEC1 (DSMZ, Braunschweig, Germany) were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM l-glutamine, and 1 mM sodium pyruvate (Invitrogen, Carlsbad, CA) at 37°C in a humified atmosphere containing 5% CO2. The B104 B-cell lymphoma was maintained under the same conditions, except for the presence of 50 μM β-mercaptoethanol (Sigma-Aldrich, St Louis, MO). The pancaspase inhibitor Z-VAD-fmk (BD Biosciences) was used at 100 μM.
BCR stimulations were performed on 107 CLL B cells with 2 × 107 Dynabeads M-450 Epoxy (Dynal Biotech) coated with 20 μg goat anti–human IgM (Southern Biotechnology Associates, Birmingham, AL), as previously described.18 Stimulations were done in 1 mL complete medium for the indicated times. The coating procedure was done according to the instructions of the manufacturer of the Dynabeads (Dynal Biotech).
Transfection of plasmid DNA, mRNA, and siRNA
Myristoylated Akt (myr.Akt), which is constitutively active because of the presence of the myristoylated signal peptide of Lck at the N-terminus, was cloned in the mammalian expression vector pcDNA3. The constitutively active (CA) MEK2 construct (pUSEamp-MEK2S222D/S226D), which contains an HA tag, was purchased from Upstate Technology (Lake Placid, NY). Plasmid DNAs were purified with the Plasmid Maxi Kit (Qiagen, Hilden, Germany).
Empty pcDNA3, myr.Akt, or CA MEK2 vectors were introduced into purified CLL B cells using the Nucleofector system (Amaxa Biosystems Cologne, Germany). Typically, 1.2 × 107 cells were resuspended in 100 μL Cell Line Nucleofector Solution V and mixed with 3 μg plasmid DNA. To evaluate transfection efficiency, the same number of cells were transfected with the green fluorescent protein (GFP)–expression vector pmaxGFP (Amaxa Biosystems). Nucleofections were done using the Amaxa Nucleofector II device with the U-013 program. CLL cells were collected and processed for flow cytometry or immunoblotting analysis at various time points ranging from 3 to 48 hours after nucleofection.
For nucleofection with mRNA, the 3 vectors (empty pcDNA3, pcDNA3-myrAkt, and pUSEamp–CA MEK2) were digested with restriction enzymes that cut approximately 1200 to 1500 bp downstream of the T7 promoter. In vitro transcription, capping, and poly(A) tailing were then performed with the mMESSAGE mMACHINE T7 Ultra Kit (Ambion, Austin, TX) according to the manufacturer's instructions. The mRNAs were purified with the MEGAclear kit (Ambion), analyzed by agarose gel electrophoresis, and quantified with a ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Nucleofections were performed with 3 μg mRNA, using the same conditions as for the plasmid DNAs.
Silencing of Mcl-1, Bcl-xL, and XIAP was performed with 1 μg of the specific ON-TARGETplus SMARTpool siRNA (Dharmacon, Lafayette, CO). The nontargeting ON-TARGETplus siCONTROL pool was used as a negative control. The siRNAs were nucleofected into CLL and BJAB cells under the same conditions already described for plasmid DNA and mRNA. Nucleofection of MEC1 and B104 cells was performed with Cell Line Nucleofector Solution L and the C-005 program of the Amaxa Nucleofector II device. Transfection efficiency was assessed using the siGLO Red siRNA (Dharmacon).
Analysis of cell viability and apoptosis
Cell survival was analyzed by double staining with FITC-conjugated Annexin V and propidium iodide (PI) using the ApoAlert Annexin V kit (BD Biosciences) according to the manufacturer's instructions. Briefly, 106 cells were washed once with phosphate-buffered saline (PBS) and resuspended in 200 μL binding buffer with 0.5 μg/mL Annexin V–FITC and 2 μg/mL PI. After incubation for 10 minutes at room temperature in a light-protected area, the samples were analyzed by flow cytometry. Data analysis was performed with the CellQuest software (Becton Dickinson). Cell viability was measured as the percentage of Annexin V– and PI–double-negative cells.
Immunoblot analysis
Cell pellets were lysed in ice-cold 1% Nonidet P-40 (NP-40) lysis buffer containing 1:40 dilution of protease inhibitor cocktail for mammalian cells and 1:70 dilution of phosphatase inhibitor cocktail 2 (both from Sigma-Aldrich). The protein samples were separated by 10% SDS-PAGE and transferred on Immobilon-P polyvinilidene difluoride membranes (Millipore, Bedford, MA). Membranes were blotted at 4°C with the following antibodies: phospho-AktSer473, phospho-ERKThr202/Tyr204, Akt, ERK, pGSK3α/βSer21/9, Bcl-2, XIAP, Bim, cyclin D2, cyclin D3, PARP, p27KIP1, rabbit IgG–horseradish peroxidase (HRP), mouse IgG HRP-linked (Cell Signaling Technology, Danvers, MA), Mcl-1, Bax, cyclin E, cyclin A (Santa Cruz Biotechnology, Santa Cruz, CA), Bcl-xL (BD Biosciences), HA-peroxidase (Roche Diagnostics, Monza, Italy), and β-actin (Sigma-Aldrich). Immunodetection was done with the ECL Plus enhanced-chemiluminescence detection system (Amersham Biosciences) and BioMax MR films (Eastman Kodak, Rochester, NY). Protein bands were quantified by laser densitometry and analyzed with ImageQuant software (Amersham Biosciences). In some experiments, chemiluminescence imaging and band intensity measurements were performed with the Gel Logic 2200 Imaging System (Eastman Kodak).
Real-time PCR analysis
Total cellular RNA was isolated from CLL B cells using the Trizol reagent (Invitrogen) according to the manufacturer's instructions. First-strand cDNAs were generated from 500 ng of total RNA by using the T-primed first strand Kit Ready-To-Go (Amersham Biosciences). Real-time quantitative polymerase chain reaction (PCR) was performed on the Sequence Detection System ABI Prism 7700 (Applied Biosystems) using TaqMan gene expression assays for MCL1 and actin, according to the manufacturer's recommendations (Applied Biosystems). Samples were run in triplicate, and relative quantification was performed with the comparative Ct method.
Statistical analysis
Changes in leukemic cell viability and in the expression of Mcl-1, Bcl-xL, and XIAP after nucleofection with the various vectors or siRNAs were evaluated with the paired t test. Statistical analyses were performed using the SigmaStat 3.1 program (Systat Software, Richmond, CA).
Results
Nucleofection of primary CLL B cells with myr.AKT and CA MEK2 results in sustained activation of the Akt and ERK kinases
To investigate the relative contribution of the PI3K/Akt and MEK/ERK pathways in regulating leukemic cell growth and survival, we introduced expression vectors encoding for myr.Akt and CA MEK2 in purified primary CLL B cells by nucleofection. Nucleofection with empty pcDNA3 vector was used as a control for nonspecific effects of the transfection procedure. Transfection efficiency was assessed with a GFP-expression vector and ranged from 40% to more than 60%, based on the number of viable GFP+ cells evaluated 5 hours after nucleofection (data not shown).
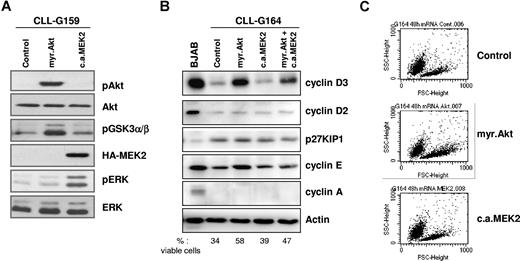
Expression of transgenic Akt and transgenic MEK2 was evaluated by immunoblotting of cell lysates collected 48 hours after nucleofection, using antibodies specific for phosphorylated Akt or HA-tagged MEK2. As shown in Figure 1A, both transgenes were efficiently expressed in CLL B cells. To determine whether the 2 kinases are functional, we investigated the activation status of signaling molecules that are immediately downstream of Akt and MEK2. Expression of myr.Akt resulted in phosphorylation of its substrate GSK3α/β, whereas ERK was phosphorylated in cells transfected with CA MEK2. These experiments showed that both kinases are active for prolonged periods following gene transfer into primary CLL B cells.
Constitutively active Akt and MEK2 activate downstream signaling pathways after nucleofection in primary CLL B cells. (A) Immunoblotting analysis of phospho-Akt, phosphoGSK3α/β, phospho-ERK, and HA-tagged MEK2 in CLL B cells collected 48 hours after transfection with empty pcDNA3 vector (Control), myr.Akt, or CA MEK2. Total Akt and total ERK served as loading controls. One representative CLL sample (G159) is shown. (B) Analysis of cell cycle–regulatory proteins 48 hours after nucleofection with empty pcDNA3 vector (Control), myr.Akt, CA MEK2, or cotransfection of Akt and MEK2. The Burkitt lymphoma cell-line BJAB was used as a control for the expression of the various proteins. Actin served as a loading control. The percentage of viable cells was determined by Annexin V/PI staining and is indicated at the bottom of the panel. (C) Flow cytometric analysis shows an increase in leukemic cell size 48 hours after nucleofection with myr.Akt.
Constitutively active Akt and MEK2 activate downstream signaling pathways after nucleofection in primary CLL B cells. (A) Immunoblotting analysis of phospho-Akt, phosphoGSK3α/β, phospho-ERK, and HA-tagged MEK2 in CLL B cells collected 48 hours after transfection with empty pcDNA3 vector (Control), myr.Akt, or CA MEK2. Total Akt and total ERK served as loading controls. One representative CLL sample (G159) is shown. (B) Analysis of cell cycle–regulatory proteins 48 hours after nucleofection with empty pcDNA3 vector (Control), myr.Akt, CA MEK2, or cotransfection of Akt and MEK2. The Burkitt lymphoma cell-line BJAB was used as a control for the expression of the various proteins. Actin served as a loading control. The percentage of viable cells was determined by Annexin V/PI staining and is indicated at the bottom of the panel. (C) Flow cytometric analysis shows an increase in leukemic cell size 48 hours after nucleofection with myr.Akt.
Since both Akt and ERK are involved in cell-cycle progression, we investigated the expression of several cell cycle–regulatory proteins in transfected CLL B cells. Expression of myr.Akt induced a substantial increase in the levels of cyclin D3 in all investigated patients (Figure 1B). In addition, cyclin E was induced in some patients but remained undetectable in others, possibly because of differences in the activation status of the transfected CLL B-cell samples. Flow cytometric analysis showed that constitutive activation of Akt also increases the size of the leukemic cells, indicating an increased metabolic activity (Figure 1C). None of these changes were observed following transfection with CA MEK2. Expression of other investigated cell cycle–regulatory proteins, including p27KIP1, cyclin D2, and cyclin A, was not affected by transfection of myr.Akt, CA MEK2, or cotransfection of both transgenes. Together, these data confirm that Akt is an important regulator of the cell cycle in CLL B cells, but also show that sustained activation of Akt either alone or together with ERK is insufficient to drive progression of CLL B cells to later stages of the cell cycle.24
Sustained activation of Akt and ERK results in the up-regulation of Mcl-1, Bcl-xL, and XIAP
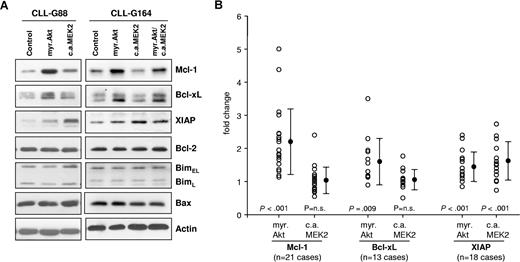
We next investigated the effect of sustained Akt and ERK activation on the levels of several apoptosis-regulatory proteins that have been implicated in the pathogenesis of CLL. As shown in Figure 2, expression of myr.Akt induced a substantial increase in the levels of Mcl-1 and a moderate increase in Bcl-xL, whereas the levels of Bcl-2, Bim, and Bax remained unchanged. A moderate increase in XIAP was also observed in most experiments, but this protein was more strongly up-regulated by constitutively active MEK2. Expression of CA MEK2 did not induce a consistent change in the levels of any other investigated protein. Cotransfection of Akt and MEK2 showed a similar increase in Mcl-1, Bcl-xL, and XIAP as the single transfections. The data from all experiments are summarized in Figure 2B and show that Mcl-1 is reproducibly up-regulated in CLL B cells following sustained activation of Akt. The effects on the expression of Bcl-xL and XIAP were more variable, although up-regulation of both proteins by Akt or up-regulation of XIAP by MEK2 was observed in most CLL B-cell samples.
The MEK/ERK and Akt pathways induce distinct antiapoptotic proteins in CLL B cells. (A) Immunoblotting analysis of Mcl-1, Bcl-xL, XIAP, Bcl-2, Bim, and Bax in CLL B cells collected 48 hours after transfection with empty pcDNA3 vector (Control), myr.Akt, or CA MEK2. Actin was used as a loading control. A total of 2 representative CLL B-cell samples are shown. (B) Summary of changes in Mcl-1, Bcl-xL, and XIAP protein levels 48 hours after nucleofection with myr.Akt or CA MEK2. For quantification, band intensities were first normalized to the respective actin signal and then calculated as fold change relative to the control nucleofection, which was set to 1.0. The individual responses of the patients are shown as ○. ● represents means of 21 different experiments for Mcl-1, 13 experiments for Bcl-xL, and 18 experiments for XIAP. Error bars indicate standard deviation. P values indicate the significance of the change in expression with respect to cells transfected with the control vector.
The MEK/ERK and Akt pathways induce distinct antiapoptotic proteins in CLL B cells. (A) Immunoblotting analysis of Mcl-1, Bcl-xL, XIAP, Bcl-2, Bim, and Bax in CLL B cells collected 48 hours after transfection with empty pcDNA3 vector (Control), myr.Akt, or CA MEK2. Actin was used as a loading control. A total of 2 representative CLL B-cell samples are shown. (B) Summary of changes in Mcl-1, Bcl-xL, and XIAP protein levels 48 hours after nucleofection with myr.Akt or CA MEK2. For quantification, band intensities were first normalized to the respective actin signal and then calculated as fold change relative to the control nucleofection, which was set to 1.0. The individual responses of the patients are shown as ○. ● represents means of 21 different experiments for Mcl-1, 13 experiments for Bcl-xL, and 18 experiments for XIAP. Error bars indicate standard deviation. P values indicate the significance of the change in expression with respect to cells transfected with the control vector.
We also performed a second set of experiments in which in vitro transcribed mRNA was used instead of plasmid DNA for transgene expression. These experiments were performed because a recent report suggested that nucleofection of plasmid DNA may be more toxic to CLL B cells that nucleofection of mRNA.43 However, we did not observe a difference between the mRNA and DNA experiments, either in terms of transgene expression or in terms of induction of antiapoptotic proteins (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
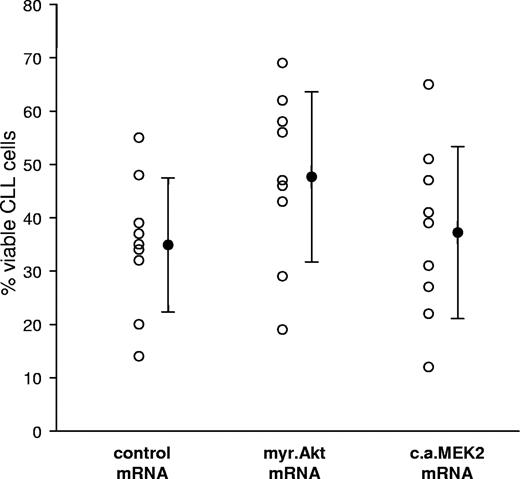
The mRNA nucleofections were also used to investigate the effect of constitutive activation of Akt and MEK2 on CLL B-cell survival. In pilot experiments, we observed that the nucleofection procedure per se induces variable levels of apoptosis, which was sample dependent and ranged from a few to more than 30% apoptotic cells (Figure S2A). Induction of apoptosis was not related to introduction of the transgenic RNA, since nucleofections with or without RNA yielded the same CLL cell viability (Figure S2B). We therefore investigated the viability of leukemic B cells nucleofected with myr.Akt, CA MEK2, or control pcDNA3 mRNA. To further minimize the possibility of nonspecific RNA effects, the 3 mRNAs were prepared to be of almost identical length. Comparison of leukemic cell viability 48 hours after nucleofection showed a consistent and highly significant increase in the percentage of viable cells transfected with myr.Akt relative to cells transfected with the control mRNA (P ≤ .001; Figure 3). The effect of CA MEK expression was again more variable. Although apoptosis protection was observed in certain patients, the difference with respect to the control mRNA transfection was not significant when all patients were considered together (P = .235).
Sustained activation of the Akt kinase promotes leukemic cell survival. Leukemic cell viability was determined 48 hours after nucleofection with pcDNA3 (Control), myr.Akt, or CA MEK2 mRNA by Annexin V/PI staining. ○ represents the response of individual patients. Mean values are indicated by ●. Error bars indicate standard deviation. A total of 9 experiments were performed.
Sustained activation of the Akt kinase promotes leukemic cell survival. Leukemic cell viability was determined 48 hours after nucleofection with pcDNA3 (Control), myr.Akt, or CA MEK2 mRNA by Annexin V/PI staining. ○ represents the response of individual patients. Mean values are indicated by ●. Error bars indicate standard deviation. A total of 9 experiments were performed.
Mcl-1 down-regulation induces potent apoptosis in CLL B cells
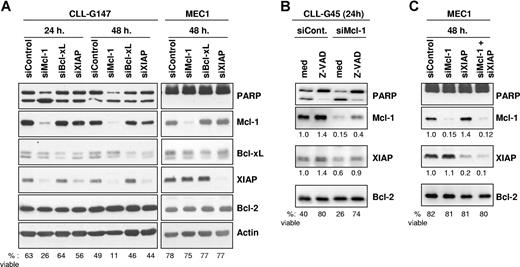
We next evaluated the relative importance of Mcl-1, Bcl-xL, and XIAP for CLL B-cell survival by specifically down-regulating expression of these proteins with siRNAs. Down-regulation of Mcl-1 and XIAP in CLL B cells was very rapid and efficient, resulting in a 90% and 70% reduction in the respective protein levels by 24 hours following nucleofection (Figure 4A). At 48 hours, the levels of Mcl-1 and XIAP had additionally decreased to 2% and 15% with respect to CLL cells transfected with the scrambled siRNA control. Silencing of Bcl-xL was somewhat less efficient, presumably due to the greater stability of this protein. However, a significant reduction in the levels of this protein was also achieved, which amounted to approximately 30% of the levels present in CLL cells transfected with the siRNA control.
Silencing of Mcl-1, Bcl-xL, and XIAP by RNA interference in primary CLL B cells and in the MEC1 cell line. (A) Leukemic B cells were nucleofected with Mcl-1, Bcl-xL, XIAP, or control siRNAs and analyzed by immunoblotting after 24 and 48 hours. Experiments with 1 representative CLL B-cell sample (G147) and with the CLL cell line MEC1 are shown. The percentage of viable cells was determined for each sample by Annexin V/PI staining and is indicated at the bottom of the panel. Induction of apoptosis was also evidenced by increased cleavage of the caspase 3 substrate PARP in CLL cells transfected with the Mcl-1–specific mRNA. (B) CLL cells were nucleofected with control or Mcl-1–specific siRNA and cultured with or without the pancaspase inhibitor Z-VAD-fmk (100 μM). Expression of Mcl-1 and XIAP was evaluated by immunoblotting 24 hours after the nucleofection. The levels of Mcl-1 and XIAP were normalized to the levels of Bcl-2, which served as a loading control, and are presented as fold change relative to the control nucleofection. The percentage of viable cells is indicated at the bottom of the panel. One representative experiment out of 3 with similar results is shown. (C) MEC1 cells were cotransfected with the Mcl-1 and XIAP siRNAs to evaluate the effect of simultaneous inhibition of both proteins. Apoptosis induction and expression of Mcl-1 and XIAP was evaluated as in panel B.
Silencing of Mcl-1, Bcl-xL, and XIAP by RNA interference in primary CLL B cells and in the MEC1 cell line. (A) Leukemic B cells were nucleofected with Mcl-1, Bcl-xL, XIAP, or control siRNAs and analyzed by immunoblotting after 24 and 48 hours. Experiments with 1 representative CLL B-cell sample (G147) and with the CLL cell line MEC1 are shown. The percentage of viable cells was determined for each sample by Annexin V/PI staining and is indicated at the bottom of the panel. Induction of apoptosis was also evidenced by increased cleavage of the caspase 3 substrate PARP in CLL cells transfected with the Mcl-1–specific mRNA. (B) CLL cells were nucleofected with control or Mcl-1–specific siRNA and cultured with or without the pancaspase inhibitor Z-VAD-fmk (100 μM). Expression of Mcl-1 and XIAP was evaluated by immunoblotting 24 hours after the nucleofection. The levels of Mcl-1 and XIAP were normalized to the levels of Bcl-2, which served as a loading control, and are presented as fold change relative to the control nucleofection. The percentage of viable cells is indicated at the bottom of the panel. One representative experiment out of 3 with similar results is shown. (C) MEC1 cells were cotransfected with the Mcl-1 and XIAP siRNAs to evaluate the effect of simultaneous inhibition of both proteins. Apoptosis induction and expression of Mcl-1 and XIAP was evaluated as in panel B.
Down-regulation of Mcl-1 resulted in apoptosis induction, as evidenced by the increased cleavage of the caspase-3 substrate PARP in CLL cells transfected with the Mcl-1-specific siRNA relative to CLL cells transfected with the siRNA control. In contrast, transfection of siRNAs specific for Bcl-xL and XIAP did not increase the level of cleaved PARP. These changes were consistent with the data obtained by Annexin V/PI staining, which showed that only Mcl-1 down-regulation induces a substantial decrease in CLL cell viability. Down-regulation of Mcl-1 also resulted in a significant decrease in the viability of B104 lymphoma B cells (70% viable cells at 24 hours after transfection with control siRNA, 9% after transfection with Mcl-1–specific siRNA), whereas the CLL cell line MEC1 and the Burkitt lymphoma BJAB were resistant (Figure 4A; data not shown).
Transfection of CLL B cells with Mcl-1 siRNA also resulted in down-regulation of XIAP, whereas the levels of Bcl-xL and Bcl-2 remained unchanged. The decrease in XIAP appeared to be secondary to cell death rather than a nonspecific effect of the Mcl-1 siRNA, considering that Mcl-1 silencing had no effect on the levels of XIAP in the apoptosis-resistant MEC1 and BJAB cells (Figure 4A; data not shown). This explanation was further supported by the experiment in Figure 4B, which showed that blocking CLL cell death with the pancaspase inhibitor Z-VAD-fmk prevents down-regulation of XIAP following transfection with Mcl-1–specific siRNA. Moreover, coinhibition of Mcl-1 and XIAP in MEC1 cells did not induce apoptosis, indicating that simultaneous inhibition of both proteins is not a prerequisite for apoptosis induction (Figure 4C).
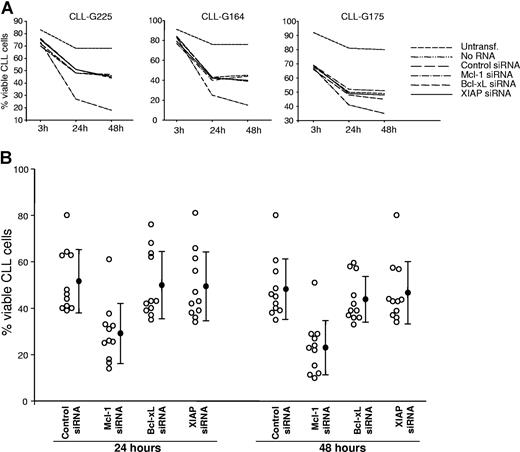
The sensitivity of CLL B cells to Mcl-1, Bcl-xL, and XIAP down-regulation was further investigated by Annexin V/PI staining in a larger series of patients with CLL. Time course experiments showed that Mcl-1 down-regulation induces rapid apoptosis, resulting in a more than 40% reduction in viability already by 24 hours following the nucleofection (Figure 5). At 48 hours, the percentage of viable cells had additionally decreased to less than 50% relative to CLL B cells transfected with the siRNA control (mean percentage of viable cells: Mcl-1 siRNA, 23%; control siRNA, 48%; P ≤ .001), whereas no significant cytotoxicity occurred in CLL B cells transfected with XIAP (47%; P = .119) or Bcl-xL siRNA (44%; P = .051). These experiments demonstrated that of the 3 antiapoptotic proteins up-regulated by Akt and ERK in CLL B cells, only Mcl-1 is essential for leukemic cell survival.
Mcl-1 down-regulation induces rapid apoptosis in primary CLL B cells. (A) Time course experiments comparing the viability of CLL cells nucleofected with control, Mcl-1, Bcl-xL, and XIAP siRNA. Untransfected samples (untransf.) and samples transfected without RNA (no RNA) were used to evaluate apoptosis induced by the nucleofection procedure. Viability was determined by Annexin V/PI staining at 3, 24, and 48 hours after nucleofection. (B) Purified CLL B cells from 11 patients were transfected with the indicated siRNAs. Cell viability was evaluated after 24 and 48 hours. ○ represents the percentage of viable cells in individual cases. Mean values are indicated by ●, error bars indicate standard deviation.
Mcl-1 down-regulation induces rapid apoptosis in primary CLL B cells. (A) Time course experiments comparing the viability of CLL cells nucleofected with control, Mcl-1, Bcl-xL, and XIAP siRNA. Untransfected samples (untransf.) and samples transfected without RNA (no RNA) were used to evaluate apoptosis induced by the nucleofection procedure. Viability was determined by Annexin V/PI staining at 3, 24, and 48 hours after nucleofection. (B) Purified CLL B cells from 11 patients were transfected with the indicated siRNAs. Cell viability was evaluated after 24 and 48 hours. ○ represents the percentage of viable cells in individual cases. Mean values are indicated by ●, error bars indicate standard deviation.
To determine whether the marked dependence on Mcl-1 expression is a general feature of CLL B cells or only a characteristic of certain CLL subsets, we investigated the sensitivity to Mcl-1 siRNA-induced apoptosis in a large series of patients with CLL with defined clinical and biological features (Table 1). This analysis revealed that all CLL B cells are sensitive to Mcl-1 down-regulation, regardless of clinical course, Ig VH gene mutation status, ZAP-70 and CD38 expression, and the presence of high-risk cytogenetic abnormalities.
Percentage of viable CLL B-cells 48 hours after nucleofection with control or Mcl-1 siRNA
| Patient . | Age/sex . | Clinical stage at dg. . | % CD38† . | % ZAP-70† . | VH gene (% hom.) . | FISH . | TTP* months . | Prior treatment . | % live with siControl . | % live with siMcl-1 . |
|---|---|---|---|---|---|---|---|---|---|---|
| G194 | 81 / m | A / 0 | 1 | 2 | Mut. V4–39 (91.2) | No abnormality | 36+ | no | 29% | 8% |
| G158 | 50 / m | A / 0 | 1 | 1 | Mut. V3–23 (92.7) | n.d. | 24+ | no | 15% | 7% |
| G159 | 73 / m | A / 0 | 3 | 1 | Mut. V1–46 (94.0) | No abnormality | 20+ | no | 66% | 33% |
| G53 | 67 / m | B / II | 1 | 8 | Mut. V3–53 (95.0) | No abnormality | 58+ | no | 40% | 14% |
| G160 | 64 / m | A / 0 | 20 | 57 | Unm V3–11 (100) | No abnormality | 42+ | no | 39% | 19% |
| G181 | 71 / m | B / II | 1 | 10 | Unm. V3–74 (100) | tris12 | 11 | no | 53% | 24% |
| G147 | 86 / f | A / 0 | 34 | 41 | Unm. V3–30 (100) | del11q22 | 16+ | no | 49% | 11% |
| G165 | 88 / f | A / I | 30 | 30 | Unm. V1–46 (100) | n.d. | 4+ | no | 56% | 29% |
| G130 | 73 / m | A / 0 | 1 | 3 | Mut. V4–34 (93.7) | No abnormality | 84+ | no | 61% | 22% |
| G109 | 70 / m | A / 0 | 3 | 5 | Mut. V3–53 (88.0) | del13q14 | 63+ | no | 35% | 15% |
| G212 | 65 / f | A / 0 | 1 | 1 | Unm. V3–30 (100) | No abnormality | 16+ | no | 45% | 27% |
| G28 | 58 / m | B / II | 3 | 5 | Mut. V3–74 (94.4) | del17p13 | 48 | yes | 14% | 5% |
| G61 | 71 / m | A / 0 | 2 | 8 | n.d. | No abnormality | 117+ | no | 42% | 10% |
| G213 | 70 / m | C / IV | 8 | 0 | Mut. V3–48 (95.6) | del17p13 | 48 | yes | 23% | 10% |
| G18 | 73 / m | B / II | 1 | 45 | Unm. V3–09 (100) | No abnormality | 66 | yes | 39% | 14% |
| G113 | 45 / f | A / 0 | 1 | 4 | Mut. V3–53 (90.7) | No abnormality | 24 | no | 60% | 23% |
| G169 | 58 / f | A / 0 | 1 | 3 | Mut. V3–72 (95.6) | del13q14 | 28+ | no | 55% | 23% |
| G4 | 72 / m | A / 0 | 6 | 78 | Unm. V3–73 (100) | del17p13 | 30 | yes | 80% | 51% |
| G29 | 65 / f | B / II | 1 | 13 | Mut. V4–34 (95.1) | No abnormality | 3 | yes | 38% | 12% |
| G34 | 68 / m | A / 0 | 4 | 29 | Unm. V1–69 (99.6) | del13q14 | 44 | yes | 32% | 15% |
| G42 | 50 / m | A / 0 | 1 | 13 | Mut. V3–23 (91.9) | No abnormality | 51+ | no | 39% | 24% |
| G45 | 56 / m | B / I | 30 | 35 | Unm. V3–11 (100) | del11q22 | 40 | yes | 40% | 21% |
| G63 | 79 / m | A / I | n.d. | 22 | Mut. V3–23 (90.8) | n.d. | 72 | yes | 61% | 33% |
| G164 | 68 / m | B / II | 73 | 41 | Mut. V3–49 (94.2) | del17p13; tris12 | 1 | yes | 36% | 15% |
| G175 | 65 / m | A / 0 | 1 | 31 | Unm V1–69 (100) | No abnormality | 6+ | no | 45% | 35% |
| G211 | 52 / m | A / 0 | 12 | 1 | n.d. | No abnormality | 38+ | no | 36% | 13% |
| G225 | 68 / m | B / II | 1 | 5 | n.d. | del13q14 | 6+ | no | 47% | 18% |
| G228 | 74 / f | B / I | 2 | 4 | n.d. | tris12 | 24+ | no | 63% | 37% |
| G229 | 59 / m | A / 0 | 43 | 7 | n.d. | n.d. | 96+ | no | 46% | 23% |
| G230 | 58 / f | A / 0 | 1 | 5 | Mut. 1–02 (93.4) | n.d. | 16+ | no | 40% | 29% |
| Patient . | Age/sex . | Clinical stage at dg. . | % CD38† . | % ZAP-70† . | VH gene (% hom.) . | FISH . | TTP* months . | Prior treatment . | % live with siControl . | % live with siMcl-1 . |
|---|---|---|---|---|---|---|---|---|---|---|
| G194 | 81 / m | A / 0 | 1 | 2 | Mut. V4–39 (91.2) | No abnormality | 36+ | no | 29% | 8% |
| G158 | 50 / m | A / 0 | 1 | 1 | Mut. V3–23 (92.7) | n.d. | 24+ | no | 15% | 7% |
| G159 | 73 / m | A / 0 | 3 | 1 | Mut. V1–46 (94.0) | No abnormality | 20+ | no | 66% | 33% |
| G53 | 67 / m | B / II | 1 | 8 | Mut. V3–53 (95.0) | No abnormality | 58+ | no | 40% | 14% |
| G160 | 64 / m | A / 0 | 20 | 57 | Unm V3–11 (100) | No abnormality | 42+ | no | 39% | 19% |
| G181 | 71 / m | B / II | 1 | 10 | Unm. V3–74 (100) | tris12 | 11 | no | 53% | 24% |
| G147 | 86 / f | A / 0 | 34 | 41 | Unm. V3–30 (100) | del11q22 | 16+ | no | 49% | 11% |
| G165 | 88 / f | A / I | 30 | 30 | Unm. V1–46 (100) | n.d. | 4+ | no | 56% | 29% |
| G130 | 73 / m | A / 0 | 1 | 3 | Mut. V4–34 (93.7) | No abnormality | 84+ | no | 61% | 22% |
| G109 | 70 / m | A / 0 | 3 | 5 | Mut. V3–53 (88.0) | del13q14 | 63+ | no | 35% | 15% |
| G212 | 65 / f | A / 0 | 1 | 1 | Unm. V3–30 (100) | No abnormality | 16+ | no | 45% | 27% |
| G28 | 58 / m | B / II | 3 | 5 | Mut. V3–74 (94.4) | del17p13 | 48 | yes | 14% | 5% |
| G61 | 71 / m | A / 0 | 2 | 8 | n.d. | No abnormality | 117+ | no | 42% | 10% |
| G213 | 70 / m | C / IV | 8 | 0 | Mut. V3–48 (95.6) | del17p13 | 48 | yes | 23% | 10% |
| G18 | 73 / m | B / II | 1 | 45 | Unm. V3–09 (100) | No abnormality | 66 | yes | 39% | 14% |
| G113 | 45 / f | A / 0 | 1 | 4 | Mut. V3–53 (90.7) | No abnormality | 24 | no | 60% | 23% |
| G169 | 58 / f | A / 0 | 1 | 3 | Mut. V3–72 (95.6) | del13q14 | 28+ | no | 55% | 23% |
| G4 | 72 / m | A / 0 | 6 | 78 | Unm. V3–73 (100) | del17p13 | 30 | yes | 80% | 51% |
| G29 | 65 / f | B / II | 1 | 13 | Mut. V4–34 (95.1) | No abnormality | 3 | yes | 38% | 12% |
| G34 | 68 / m | A / 0 | 4 | 29 | Unm. V1–69 (99.6) | del13q14 | 44 | yes | 32% | 15% |
| G42 | 50 / m | A / 0 | 1 | 13 | Mut. V3–23 (91.9) | No abnormality | 51+ | no | 39% | 24% |
| G45 | 56 / m | B / I | 30 | 35 | Unm. V3–11 (100) | del11q22 | 40 | yes | 40% | 21% |
| G63 | 79 / m | A / I | n.d. | 22 | Mut. V3–23 (90.8) | n.d. | 72 | yes | 61% | 33% |
| G164 | 68 / m | B / II | 73 | 41 | Mut. V3–49 (94.2) | del17p13; tris12 | 1 | yes | 36% | 15% |
| G175 | 65 / m | A / 0 | 1 | 31 | Unm V1–69 (100) | No abnormality | 6+ | no | 45% | 35% |
| G211 | 52 / m | A / 0 | 12 | 1 | n.d. | No abnormality | 38+ | no | 36% | 13% |
| G225 | 68 / m | B / II | 1 | 5 | n.d. | del13q14 | 6+ | no | 47% | 18% |
| G228 | 74 / f | B / I | 2 | 4 | n.d. | tris12 | 24+ | no | 63% | 37% |
| G229 | 59 / m | A / 0 | 43 | 7 | n.d. | n.d. | 96+ | no | 46% | 23% |
| G230 | 58 / f | A / 0 | 1 | 5 | Mut. 1–02 (93.4) | n.d. | 16+ | no | 40% | 29% |
TTP indicates time to progression; n.d., not determined; +, cases that have not progressed during follow-up; and dg, diagnosis.
The antiapoptotic effect of sustained BCR signaling is dependent on Mcl-1 expression
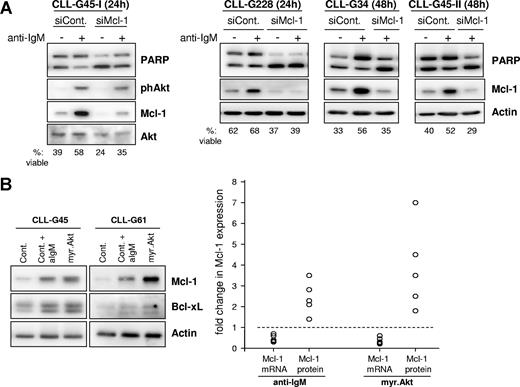
Previous studies by us and others have shown that CLL B cells from some patients survive longer in culture in the presence of immobilized anti-IgM antibodies.18,19 This effect was attributed to sustained BCR signaling and was associated with up-regulation of Mcl-1.18 To further explore the possibility that Mcl-1 is responsible for the antiapoptotic effect of sustained BCR signaling, we transfected CLL B cells with Mcl-1 or control siRNA and stimulated them for 24 or 48 hours with immobilized anti-IgM antibodies (Figure 6A). Anti-IgM stimulation resulted in phosphorylation of Akt, which was associated with a substantial increase in Mcl-1 protein levels and reduced apoptosis in CLL cells transfected with the control siRNA. In contrast, anti-IgM stimulation did not reduce apoptosis in CLL cells transfected with Mcl-1 siRNA, demonstrating that the prosurvival effect of sustained BCR signaling is mediated at least in part by up-regulation of Mcl-1.
The antiapoptotic effect of sustained BCR signaling is mediated by posttranscriptional regulation of Mcl-1. (A) CLL B cells were transfected with control or Mcl-1 siRNA and stimulated for 24 or 48 hours with immobilized anti-IgM antibodies. Protection from apoptosis was evaluated by PARP cleavage and Annexin V/PI staining. A total of 4 independent experiments are shown. (B) Comparison of changes in Mcl-1 mRNA and protein levels induced by sustained BCR engagement and sustained activation of Akt. CLL B cells from 5 patients were nucleofected with control or myr.Akt mRNA. Cells nucleofected with control mRNA were either left unstimulated or were stimulated with immobilized anti-IgM antibody. All samples were collected 24 hours after nucleofection and analyzed by immunoblotting (left panel) and by real-time RT-PCR. Mcl-1 mRNA and protein levels were normalized to actin and are expressed as fold change relative to corresponding levels in CLL cells transfected with control mRNA, which were set to 1 (dashed line).
The antiapoptotic effect of sustained BCR signaling is mediated by posttranscriptional regulation of Mcl-1. (A) CLL B cells were transfected with control or Mcl-1 siRNA and stimulated for 24 or 48 hours with immobilized anti-IgM antibodies. Protection from apoptosis was evaluated by PARP cleavage and Annexin V/PI staining. A total of 4 independent experiments are shown. (B) Comparison of changes in Mcl-1 mRNA and protein levels induced by sustained BCR engagement and sustained activation of Akt. CLL B cells from 5 patients were nucleofected with control or myr.Akt mRNA. Cells nucleofected with control mRNA were either left unstimulated or were stimulated with immobilized anti-IgM antibody. All samples were collected 24 hours after nucleofection and analyzed by immunoblotting (left panel) and by real-time RT-PCR. Mcl-1 mRNA and protein levels were normalized to actin and are expressed as fold change relative to corresponding levels in CLL cells transfected with control mRNA, which were set to 1 (dashed line).
To further explore the mechanism of Mcl-1 up-regulation in CLL cells, we investigated the relative levels of Mcl-1 mRNA and protein after stimulation with immobilized anti-IgM or transfection with myr.Akt. As shown in Figure 6B, immobilized anti-IgM and myr.Akt induced a similar increase in Mcl-1 protein levels. Interestingly, a decrease rather than an increase in Mcl-1 mRNA levels was observed in both cases, suggesting that regulation of Mcl-1 through the BCR and Akt kinase is entirely dependent on posttranscriptional mechanisms.
Comparison of apoptosis-regulatory proteins in primary CLL B cells and Mcl-1-resistant B-cell lines
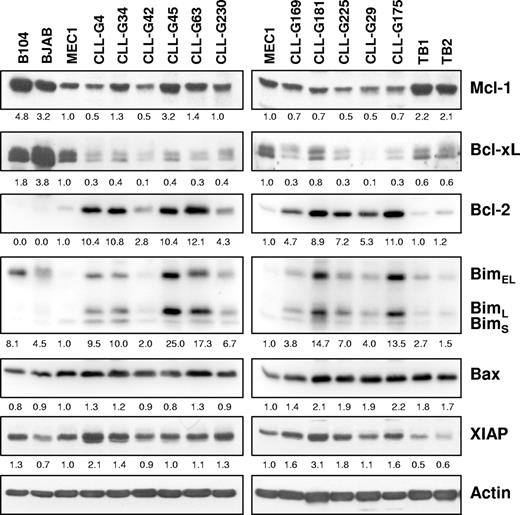
The resistance of BJAB and especially MEC1 B cells to Mcl-1 siRNA-induced apoptosis was intriguing, considering that MEC1 is one of the rare established CLL B-cell lines. A possible explanation for this resistance was that BJAB and MEC1 cells lack proapoptotic proteins that are regulated by Mcl-1. To investigate this possibility, we compared the levels of proapoptotic Bim and Bax in the 2 resistant cell lines with those in freshly isolated CLL B cells and in the Mcl-1 siRNA-sensitive B104 cell line. Normal tonsillar B cells were also included for comparison. In addition, we determined the levels of Mcl-1, Bcl-xL, Bcl-2, and XIAP in the same samples (Figure 7). A difference was noted in terms of Bim expression, which was typically higher in CLL and B104 cells than in MEC1 and normal tonsillar B cells. In addition, Bim in BJAB cells was detected mainly as a higher-molecular-weight band (Figure 7; data not shown), which based on size should correspond to the phosphorylated protein that associates less efficiently with Bax and is therefore less proapoptotic.36 Although these data indicate that inadequate Bim expression may be responsible for the resistance of MEC1 and BJAB cells to Mcl-1 down-regulation, it should be noted that the levels of Bim also varied between different CLL samples and did not always correlate with the rate of apoptosis induced by Mcl-1 siRNA (Figure 7; Table 1). For example, samples G169 and G29 were highly sensitive to Mcl-1 silencing, although they expressed lower levels of Bim than most other CLL samples. In contrast, sample G175 with high levels of Bim showed a lower apoptotic rate, indicating that other factors may also be responsible for the marked sensitivity of CLL B cells to Mcl-1 down-regulation.
Expression of apoptosis-regulatory proteins in primary CLL B cells and B-cell lines. Immunoblotting analysis was performed with purified CLL B cells from 11 patients, purified normal tonsillar B cells (TB1 and TB2), and the B104, BJAB, and MEC1 cell lines. Actin was used as a loading control. Normalized levels of each protein are expressed as fold change relative to the levels in the MEC1 cell line.
Expression of apoptosis-regulatory proteins in primary CLL B cells and B-cell lines. Immunoblotting analysis was performed with purified CLL B cells from 11 patients, purified normal tonsillar B cells (TB1 and TB2), and the B104, BJAB, and MEC1 cell lines. Actin was used as a loading control. Normalized levels of each protein are expressed as fold change relative to the levels in the MEC1 cell line.
Discussion
Recent studies have shown that sustained BCR engagement results in prolonged activation of the Akt and ERK kinases in CLL B cells and a reduction in spontaneous and chemotherapy-induced apoptosis.18,19 Although both kinases can promote survival in other cell types, their relative contribution to the regulation of apoptosis resistance in CLL B cells has not been directly assessed. We now show that sustained activation of the Akt kinase confers increased apoptosis resistance to CLL B cells, which is largely dependent on up-regulation of the antiapoptotic protein Mcl-1.
We used a recently developed technology for the delivery of transgenes in primary CLL B cells to investigate the effects of sustained Akt and ERK activation on the expression of several apoptosis-regulatory proteins. We focused on proteins that have already been implicated in CLL pathogenesis or that are regulated by Akt and ERK in other cell types. The most significant change induced by constitutively active Akt was increased expression of Mcl-1, which was accompanied by a lesser up-regulation of Bcl-xL and XIAP and increased leukemic cell viability. These changes paralleled the effects of stimulation with immobilized anti-IgM antibodies (Figure 6B; data not shown). In addition, transfection of constitutively active Akt provoked an increase in the size of the leukemic cells and in the levels of cyclin D3, consistent with a role for Akt in regulating not only leukemic cell survival but also cell-cycle progression. Transfection of constitutively active MEK2 resulted in increased expression of XIAP, but did not show a consistent change in leukemic cell viability. Expression of other investigated proteins, including Bcl-2, Bim, and Bax, was not affected by transfection of either or both active kinases. Together, these experiments showed that sustained activation of the Akt kinase largely recapitulates the effects of sustained BCR stimulation in terms of leukemic cell survival and expression of antiapoptotic proteins.
Increased expression of Mcl-1, Bcl-xL, and XIAP has also been observed following stimulation of CLL cells with other survival signals. For instance, the levels of Mcl-1 increase in the presence of CD40 ligand, vascular-endothelial growth factor (VEGF), or dendritic cells.42,44,–46 In addition, CD40 ligand can induce Bcl-xL, whereas the levels of XIAP can be increased by VEGF stimulation.42,44,45 Since induction of Mcl-1, Bcl-xL, and XIAP by the various survival signals is often concomitant in CLL B cells, it is difficult to discern their relative contribution to apoptosis resistance. To more directly address this issue, we evaluated survival of CLL B cells following specific down-regulation of each of these proteins. A significant decrease in leukemic cell viability was observed following down-regulation of Mcl-1, confirming previous studies with antisense oligonucleotides or more recently with siRNA that CLL cell survival is dependent on Mcl-1 expression.46,47 In contrast, leukemic cell viability was not affected by down-regulation of XIAP and showed only a modest but not significant decrease following down-regulation of Bcl-xL. Together, these experiments demonstrate that of the 3 antiapoptotic proteins up-regulated by Akt and ERK, only Mcl-1 is essential for CLL B-cell survival.
To more directly assess the role of Mcl-1 in BCR-mediated apoptosis resistance, we investigated the effect of Mcl-1 down-regulation in leukemic cells stimulated with immobilized anti-IgM antibodies. The prosurvival effect of sustained BCR stimulation was completely abrogated in the absence of Mcl-1, providing evidence that the antiapoptotic BCR signal is largely dependent on Mcl-1 induction.
Treatment with Mcl-1 siRNA induced apoptosis in all investigated CLL B-cell samples but did not affect the viability of MEC1 and BJAB cells, indicating that B-lymphocytes are not uniformly sensitive to Mcl-1 down-regulation. To provide a possible explanation for this difference, we compared expression of several apoptosis-regulatory proteins in apoptosis-sensitive and apoptosis-resistant B cells. The most prominent difference related to expression of Bim. This protein was present at high levels in most primary CLL B-cell samples, but was either not expressed or was predominantly detected as a phosphorylated and presumably inactive protein in the 2 resistant cell lines. Since one of the best known functions of Mcl-1 is to sequester Bim and thereby prevent Bax and Bak activation,48,–50 it would seem reasonable to postulate that high levels of Bim may be responsible for the marked dependence of CLL cells on Mcl-1 expression. However, in a very recent study, Del Gaizo Moore et al51 observed that most of Bim in CLL B cells is already complexed with Bcl-2, indicating that Mcl-1 may also increase the apoptosis resistance of CLL B cells through mechanisms that do not involve direct interactions with Bim.
Comparative analysis of Mcl-1 mRNA and protein revealed that sustained BCR signaling and Akt activation regulate Mcl-1 expression entirely through posttranscriptional mechanisms. Interestingly, Smit et al44 recently reported that induction of Mcl-1 by CD40 is also regulated by posttranscriptional events, and noted higher levels of Mcl-1 protein in lymph nodes than in the peripheral blood compartment despite similar levels of Mcl-1 mRNA in the 2 leukemic cell populations. Considering that Akt can also be activated by CD40 in CLL cells,52 it is likely that a common pathway downstream of this kinase regulates Mcl-1 expression in response to various survival signals from the microenvironment. Collectively, these findings indicate that targeting of the Akt/Mcl-1 pathway may inhibit multiple environmental signals that promote leukemic cell survival in vivo and therefore may provide a novel strategy for the treatment of CLL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by The Leukemia & Lymphoma Society Translational Research Program (grant 6043-06 to D.G.E.).
Authorship
Contribution: P.G.L. and S.G. participated in designing the study, performed research, analyzed data, and contributed to the writing of the manuscript; L.L., S.S., and G.L. provided patient material and clinical data, performed data analysis, and contributed to the writing of the manuscript; and D.G.E. designed and supervised the study, performed research and data analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dimitar Efremov, ICGEB Outstation-Monterotondo, CNR Campus Adriano Buzzati-Traverso, Via E Ramarini 32, I-00016 Monterotondo Scalo, Rome, Italy; e-mail: efremov@icgeb.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal