Abstract
CCG-2961 incorporated 3 new agents, idarubicin, fludarabine and interleukin-2, into a phase 3 AML trial using intensive-timing remission induction/consolidation and related donor marrow transplantation or high-dose cytarabine intensifi-cation. Among 901 patients under age 21 years, 5-year survival was 52%, and event-free survival was 42%. Survival improved from 44% between 1996 and 1998 to 58% between 2000 and 2002 (P = .005), and treatment-related mortality declined from 19% to 12% (P = .025). Partial replacement of daunomycin with idarubicin in the 5-drug induction combination achieved a remission rate of 88%, similar to historical controls. Postremission survival was 56% in patients randomized to either 5-drug reinduction or fludarabine/cytarabine/idarubicin. For patients with or without a related donor, respective 5-year disease-free survival was 61% and 50% (P = .021); respective survival was 68% and 62% (P = .425). Donor availability conferred no benefit on those with inv(16) or t(8;21) cytogenetics. After cytarabine intensification, patients randomized to interleukin-2 or none experienced similar outcomes. Factors predictive of inferior survival were age more than 16 years, non-white ethnicity, absence of related donor, obesity, white blood cell count more than 100 000 × 109/L, −7/7q−, −5/5q−, and/or complex karyotype. No new agent improved outcomes; experience may have contributed to better results time.
Introduction
In the past 2 decades, cooperative group trials in pediatric acute myeloid leukemia (AML) have increased overall 5-year survival (OS) from approximately 30% to more than 50%.1-6 Intensification of dose, increased number of days of conventional induction chemotherapy, matched related donor bone marrow transplantation (MRD BMT) in first remission, and risk stratification of treatment have all contributed to this progress. Risk stratification typically classifies as favorable those patients with Down syndrome or with AML characterized by t(8;21), t(15;17), or inv(16) cytogenetic abnormalities and rapid early response to induction therapy. Unfavorable features include high white blood cell count, −7/7q−, −5/5q−, or complex cytogenetics, and slow or no early response.7-11 The emerging consensus is that patients with favorable AML do not benefit from MRD BMT in first remission.2,12
Since 1986, the Children's Cancer Group (CCG) explored a strategy to treat newly diagnosed AML using intensively timed 5-drug combination chemotherapy consisting of dexamethasone, cytarabine, thioguanine, etoposide, and rubidomycin (daunomycin) (DCTER) for remission induction followed by BMT for patients with matched related donors or intensively timed high-dose cytarabine/asparaginase (HidAC) after remission therapy for patients without related donors.13-15 Intensive timing involves administration of the second cycle of 5 drugs on day 10 regardless of remission status or blood counts. In the previous phase 3 trial, CCG-2891, intensively timed DCTER achieved an event-free survival (EFS) of 41% and OS of 49% at 5 years.
This manuscript describes the successor phase 3 trial, CCG-2961, built on the results of CCG-2891. The specific aims of CCG-2961 were achieving a remission induction rate of more than 85% following the first course of intensively timed therapy and EFS of 50% at 5 years. The study also aimed to validate previous prognostic factors and to define new ones. To accomplish these aims, this study introduced 3 new agents into the treatment program: idarubicin (IDA), fludarabine monophosphate (FAMP), and recombinant interleukin-2 (IL-2).
The selection of these new agents derived from contemporary studies in adults with AML and a series of phase 1 and 2 CCG pilot trials in children. Randomized trials showed IDA to be a more effective remission-inducing agent than rubidomycin in adults with AML16-20 and the CCG phase 1 trial defined pharmacokinetics of IDA in children.21 The CCG-2941 pilot replaced rubidomycin with IDA at 4:1 ratio in the 5-drug combination called IdaDCTER, but IdaDCTER caused excessive hematotoxicity and hepatotoxicity. An intensively timed IdaDCTER/DCTER hybrid had a toxicity profile similar to DCTER/DCTER in CCG-2891, but with a significantly higher proportion of patients with more than 5% marrow blasts on day 14.22 Thus, CCG-2961 adopted the IdaDCTER/DCTER hybrid for remission induction therapy.
Several studies demonstrated that the combination of FAMP and cytarabine (FAMP/AC)23-26 or FAMP/AC plus IDA were efficacious and tolerable in both adults and children with refractory or recurrent AML.27-30 After induction therapy, CCG-2961 compared FAMP/AC/IDA as described by Avramis et al to a second course of hybrid 5-drug IdaDCTER therapy.31 Because in CCG-2891 addition of granulocyte colony stimulating factor (G-CSF) to induction and consolidation therapy reduced hospital stay by 6 days,32 in courses 1 and 2 of CCG-2961 patients with less than 5% blasts in marrow on day 14 received G-CSF following chemotherapy until recovery of neutrophils.
In CCG AML trials since 1978, patients with matched, related family donors have had significantly better disease-free survival (DFS) and OS than those receiving chemotherapy.33 In patients without donors, intensively timed high-dose cytarabine/asparaginase (HidAC)-based intensification34 resulted in better outcomes than fractionated HidAC,13 and no maintenance therapy achieved better OS than a year or more of relatively low intensity maintenance chemotherapy.35 CCG-2961 therefore used HidAC and no maintenance therapy for patients without donors.34
Both preclinical and clinical trials suggested IL-2 had the characteristics of an agent that could reduce relapses in patients with AML who lacked matched, related donors by inducing an antileukemia immune response. In vitro IL-2 stimulates proliferation of antigen-specific T cells, enhances cytolytic activity of natural killer cells against AML targets, and induces multiple cytokines, including interferon gamma and tumor necrosis factor alpha.36-39 Case reports and small clinical trials in patients with recurrent AML36-39 showed remarkable reduction of tumor burden, successful induction of remission, and occasional long-term survival after single-agent IL-2 therapy.34-40 They also showed prolonged survival compared with historical controls in patients given IL-2 following autologous BMT for relapsed AML41-44 After the pilot study, CCG-2941 established that a single course of 4 days of high-dose and 10 days of low-dose infusion IL-2 was safe and tolerable in children with AML41,45 ; CCG-2961 randomized children without donors to one course of IL-2 or no further therapy after completing HIDAC.
Methods
CCG-2961 opened in August 1996. In October 1999, the Data Safety Monitoring Board (DSMB) suspended the trial because of concerns of treatment-related mortality (TRM) of more than 10%. An amended protocol opened in May 2000. CCG-2961 completed accrual in 2002. This study is registered at http://clinicaltrials.gov as NCT00002798.
Eligible patients were age one day to less than 21 years of age with French-American-British de novo AML subtypes M0-2 and M4-M7.46-48 Patients with acute promyelocytic leukemia, juvenile myelomonocytic leukemia, Down syndrome, constitutional marrow failure syndromes, and treatment-related AML were excluded. Patients with myelodysplastic syndrome and granulocytic sarcoma (N = 86) were eligible for registration but are not included in this manuscript. Institutional Review Boards at each participating center approved the study, and parents of patients signed a written informed consent as stipulated by the Declaration of Helsinki. The study chair, histopathologist, and statisticians reviewed eligibility. Of 1010 patients enrolled in CCG-2961, 23 were ineligible for the following reasons: ineligible diagnosis (n = 12), prior therapy (n = 4), and administrative issues (n = 7). Of 987 eligible patients, the 901 with de novo AML are the subject of this report.
Marrow morphology and histochemistry were reviewed centrally (D.R.B.) in 83% of patients. Favorable cytogenetics included t(8;21) and inv(16). Unfavorable cytogenetics were del(7), 7q−, del (5)or 5q−, or complex karyotypes defined as more than 3 structural and/or numerical abnormalities. Normal karyotype and all other abnormal karyotypes were considered standard. Central reviewers deemed 62% of karyotypes acceptable.
Treatment plan
Figure 1 shows the schema of the study and flow of the patients, and the legend describes details of drug dose and administration. Patients with less than 5% blasts after day 14 plus or minus 2 of induction received G-CSF until absolute neutrophil count (ANC) was more than 1000 × 109/L. Patients in complete or partial remission after course 1 were eligible for randomization to course 2 consolidation therapy consisting of a repetition of course 1 therapy or FAMP/AC/IDA.29,49 Complete remission (CR) was defined as less than 5% blasts with trilineage maturation and partial remission (PR) as 5% as to 29% blasts with at least moderate hypocellularity, with or without marrow recovery. Marrow recovery was defined as ANC more than 1000 mm3 and platelets more than 50 000 × 109/L. Patients in CR after consolidation were assigned to MRD BMT if they had a 5 of 6 or 6 of 6 HLA-compatible first-degree relative as a donor. Patients who did not have donors were assigned to HidAC.34,35 After course 3, those without donors were randomized to IL-2 or follow-up.41,45 Central nervous system prophylaxis consisted of intrathecal cytarabine or cytarabine, hydrocortisone, and methotrexate if blasts persisted after 3 lumbar punctures.14,15
Course 1 and Course 2: IdaDCTER is idarubicin 5 mg/m2 per day infused more than half an hour daily, cytarabine 200 mg/m2 /day and etoposide 100 mg/m2 per day both as continuous 96-hour infusions (CI), oral thioguanine 100 mg/m2 per day, and dexamethasone 6 mg/m2on days 0 to 3. On days 10 to 13, daunorubicin 20 mg/m2 day CI replaces idarubicin. Course 2: FAMP is fludarabine monophosphate, 10.5 mg/m2 loading dose, then 30.5 mg/m2 per 24 hours for a total of 48 hours, followed by beginning Ara-C 390 mg/m2 loading dose and 2400 mg/m2 per 24 hours continuous infusion for 72 hours and idarubicin 12 mg/m2 per day infused more than half an hour on days 0, 1, and 2 at 12.0 mg/m2 per day. G-CSF, 5 μg/kg per day, initiated in patients with less than 5% residual leukemic blasts in day 14 ± 1 marrow and continued until neutrophil recovery. HidAC is cytarabine 3 g/m2 as 3-hour infusions at hours 0 to 3, 12 to 15, 24 to 27, and 36 to 39 on days 0 and 7 followed by Escherichia coli L-asparaginase 6000 units/m2 intramuscularly at hour 42 given on days 1 and 8. Marrow transplantation cytoreduction consists of 16 doses of busulfan at 40 mg/m2 orally every 6 hours on days −9, −8, −7, and −6 and cyclophosphamide 50 mg/kg IV more than one hour on days −5,−4,−3, and −2; interleukin-2 is 9 × 106 IU/m2 per day CI day 0 to 3 and 1.6 × 106 IU/m2 per day on CI days 8 to 17. Central nervous system prophylaxis was intrathecal cytarabine on days 0 and 10 of course 1 and course 2 regimen A and weekly times 3 following recovering of counts after HidAC.14,61 In courses 1 and 2, G-CSF, 5 μg/m2 per day was started 48 hours after completion of chemotherapy and continued until the neutrophil count was more than 1500 × 109/L.
Course 1 and Course 2: IdaDCTER is idarubicin 5 mg/m2 per day infused more than half an hour daily, cytarabine 200 mg/m2 /day and etoposide 100 mg/m2 per day both as continuous 96-hour infusions (CI), oral thioguanine 100 mg/m2 per day, and dexamethasone 6 mg/m2on days 0 to 3. On days 10 to 13, daunorubicin 20 mg/m2 day CI replaces idarubicin. Course 2: FAMP is fludarabine monophosphate, 10.5 mg/m2 loading dose, then 30.5 mg/m2 per 24 hours for a total of 48 hours, followed by beginning Ara-C 390 mg/m2 loading dose and 2400 mg/m2 per 24 hours continuous infusion for 72 hours and idarubicin 12 mg/m2 per day infused more than half an hour on days 0, 1, and 2 at 12.0 mg/m2 per day. G-CSF, 5 μg/kg per day, initiated in patients with less than 5% residual leukemic blasts in day 14 ± 1 marrow and continued until neutrophil recovery. HidAC is cytarabine 3 g/m2 as 3-hour infusions at hours 0 to 3, 12 to 15, 24 to 27, and 36 to 39 on days 0 and 7 followed by Escherichia coli L-asparaginase 6000 units/m2 intramuscularly at hour 42 given on days 1 and 8. Marrow transplantation cytoreduction consists of 16 doses of busulfan at 40 mg/m2 orally every 6 hours on days −9, −8, −7, and −6 and cyclophosphamide 50 mg/kg IV more than one hour on days −5,−4,−3, and −2; interleukin-2 is 9 × 106 IU/m2 per day CI day 0 to 3 and 1.6 × 106 IU/m2 per day on CI days 8 to 17. Central nervous system prophylaxis was intrathecal cytarabine on days 0 and 10 of course 1 and course 2 regimen A and weekly times 3 following recovering of counts after HidAC.14,61 In courses 1 and 2, G-CSF, 5 μg/m2 per day was started 48 hours after completion of chemotherapy and continued until the neutrophil count was more than 1500 × 109/L.
In April 1998, the required platelet count recovery to proceed to course 2 or 3 of therapy was amended from 100 000 × 109/L to 75 000 × 109/L, and to 50 000 × 109/L in May 1999. Also in May 1999, graft versus host disease prophylaxis was modified to allow the institutions to use their standard regimen. Changes in the protocol after the suspension were as follows: (1) mandatory preemptive hospitalization during course 1 and during periods of anticipated neutropenia in courses 2 and 3 until the absolute phagocyte count was rising for 2 consecutive days; (2) at the time of the first fever in the patient with neutropenia, administration of empiric third-generation cephalosporin or comparable broad-spectrum antibiotic coverage until phagocyte recovery and empiric vancomycin for 24 to 48 hours until beta-lactam resistant Gram-positive infection had been ruled out; (3) preemptive administration of amphotericin B at more than 1 mg/kg after 72 hours of fever: (4) surveillance for fungus with computed tomography on neutrophil recovery; (5) dose reduction of FAMP and HidAC for renal compromise; (6) administration of intravenous immunoglobulin G for low immunoglobulin levels after course 1; and (7) proscription of glucocorticoid as an antiemetic or to treat rigors and deletion of dexamethasone in course 2, regimen A in patients who had presumed or documented fungal infection during course 1.
Statistical plan and analysis
The main outcome measures were remission status after courses 1 and 2 of chemotherapy, OS, EFS, DFS, and TRM. OS was defined as the time from study entry to death; EFS, as the time from study enrollment to failure, relapse, or death; and DFS, as the time from remission to relapse or death. The Kaplan-Meier method was used to calculate estimates of OS, EFS, and DFS.50 OS, EFS, and DFS were tested for significance using the log-rank statistic.51 TRM was defined as time from study entry to death resulting from nonprogressive disease where induction failures, relapses, and deaths resulting from progressive disease were competing events. Cumulative incidence estimates were used to determine TRM.52 Those lost to follow-up were censored at the last known point of study. Patients who withdrew before course 1 outcome determination were censored at the time of withdrawal in analyses of EFS and DFS; event and survival data were collected after withdrawal. EFS and DFS results when withdrawals before determination of induction were not censored were similar to those where withdrawals were censored and, hence, are not presented in the report. Confidence intervals were calculated according to Greenwood's formula.53 A Cox proportional hazards model was used to estimate hazard ratios for multivariate analyses.54 The significance of observed differences in proportions was tested using the χ2 test or Fisher exact test when data were sparse. The cumulative incidence for ANC recovery and platelet recovery was estimated by considering death during the phase of therapy as a competing event. A P value of less than .05 was set as a threshold for significance.
The study was designed to have 80% power to detect a 5% difference in remission rates between IdaDCTER and FAMP/AC/IDA intensification and to have adequate power to detect a 10% difference in DFS in the patients randomized to IL-2 or follow-up. All reported comparisons of randomized or biologic assignments were based on intention to treat. Standard factors analyzed for prognostic significance included age, white blood cell count, cytogenetic risk group, day 14 marrow response, and availability of a matched related donor. Additional prognostic variables identified in this study included ethnicity, body mass index, minimal residual disease as assessed by multichannel flow cytometry, and FLT internal tandem duplication (FLT3/ITD).55-58
This report analyzes data collected up to October 30, 2006, with a median follow-up of 56 months. To compensate for relatively early reporting of relapses and deaths, data were censored at 6 months before October 30, 2006.
Results
Table 1 lists the characteristics of the 901 eligible patients and of the 738 patients who participated in the first randomization after induction; Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) shows the flow of all patients in this study. Demographics are similar to previous CCG studies with the exception of an increase in Hispanic patients proportional to that in the general population and a modest relative reduction in black patients compared with the previous CCG-2891 study.55 There is also a reduction in the relative proportion of black patients after phase 1 but no apparent skew in the proportion of these patients randomized in phase 2. Of the patients with centrally reviewed cytogenetics, 25% had favorable, 5% had unfavorable, and 70% had standard karyotypes defined as normal or neither favorable nor unfavorable.
CCG-2961: demographic, clinical, and biologic characteristics of patients at study entry and at time of first randomization
| . | Study entry . | First randomization . | A vs B (P) . | ||||
|---|---|---|---|---|---|---|---|
| Regimen A: IdaDCTER DCTER . | Regimen B: FLU/AC/IDA . | ||||||
| N . | % . | N . | % . | N . | % . | ||
| N | 901 | — | 367 | — | 371 | — | — |
| Age, y | |||||||
| Range | 0.01-20.9 | — | 0.04-20.9 | — | 0.01-19.8 | — | — |
| Median | 9.5 | — | 9.1 | — | 9.6 | — | .350 |
| Male sex | 468 | 52 | 185 | 50 | 196 | 53 | .559 |
| Race | |||||||
| White | 583 | 66 | 240 | 67 | 249 | 68 | .754 |
| Black | 84 | 16 | 35 | 10 | 38 | 10 | .863 |
| Hispanic | 157 | 18 | 61 | 17 | 55 | 15 | .546 |
| Asian | 26 | 3 | 8 | 2 | 10 | 3 | .839 |
| Other | 34 | 4 | 15 | 4 | 13 | 4 | .812 |
| Unknown | 17 | — | 8 | — | 6 | — | — |
| Hepatomegaly | 288 | 32 | 116 | 32 | 108 | 29 | .526 |
| Splenomegaly | 279 | 31 | 114 | 31 | 102 | 28 | .324 |
| CNS positive | 52 | 6 | 16 | 4 | 22 | 6 | .430 |
| FAB | |||||||
| M0 | 55 | 6 | 25 | 6 | 21 | 5 | .634 |
| M1 | 150 | 17 | 64 | 18 | 58 | 16 | .596 |
| M2 | 249 | 28 | 112 | 31 | 105 | 29 | .592 |
| M4 | 206 | 23 | 71 | 20 | 92 | 25 | .082 |
| M5 | 158 | 18 | 64 | 18 | 60 | 16 | .742 |
| M6 | 20 | 2 | 6 | 2 | 9 | 2 | .609 |
| M7 | 47 | 5 | 19 | 5 | 18 | 5 | .986 |
| AML (NOS) | 16 | 2 | 6 | 1 | 8 | 2 | .795 |
| Cytogenetics | |||||||
| Normal | 125 | 22 | 48 | 21 | 60 | 25 | .393 |
| t(8;21) | 89 | 16 | 45 | 20 | 40 | 17 | .434 |
| Abnormal 16 | 49 | 9 | 18 | 8 | 23 | 10 | .650 |
| Abnormal 11 | 132 | 23 | 52 | 23 | 56 | 23 | .981 |
| t(6;9) | 10 | 2 | 3 | 1 | 4 | 2 | 1.000 |
| -7/7- | 22 | 4 | 10 | 4 | 3 | 1 | .072 |
| -5/5- | 7 | 1 | 3 | 1 | 2 | 1 | .677 |
| +8 | 33 | 6 | 10 | 4 | 13 | 5 | .779 |
| +21 | 9 | 2 | 2 | 1 | 6 | 2 | .287 |
| Other | 87 | 15 | 37 | 16 | 35 | 14 | .687 |
| Unknown | 338 | 38 | 139 | 38 | 129 | 35 | — |
| Complexity | |||||||
| 3 or less | 371 | 85 | 155 | 86 | 154 | 85 | .800 |
| More than 3 | 67 | 15 | 25 | 14 | 28 | 15 | — |
| Unfavorable* | 86 | 15 | 36 | 15 | 31 | 12 | .324 |
| Standard | 343 | 61 | 137 | 59 | 155 | 62 | .507 |
| Favorable† | 134 | 24 | 60 | 26 | 65 | 26 | .971 |
| . | Study entry . | First randomization . | A vs B (P) . | ||||
|---|---|---|---|---|---|---|---|
| Regimen A: IdaDCTER DCTER . | Regimen B: FLU/AC/IDA . | ||||||
| N . | % . | N . | % . | N . | % . | ||
| N | 901 | — | 367 | — | 371 | — | — |
| Age, y | |||||||
| Range | 0.01-20.9 | — | 0.04-20.9 | — | 0.01-19.8 | — | — |
| Median | 9.5 | — | 9.1 | — | 9.6 | — | .350 |
| Male sex | 468 | 52 | 185 | 50 | 196 | 53 | .559 |
| Race | |||||||
| White | 583 | 66 | 240 | 67 | 249 | 68 | .754 |
| Black | 84 | 16 | 35 | 10 | 38 | 10 | .863 |
| Hispanic | 157 | 18 | 61 | 17 | 55 | 15 | .546 |
| Asian | 26 | 3 | 8 | 2 | 10 | 3 | .839 |
| Other | 34 | 4 | 15 | 4 | 13 | 4 | .812 |
| Unknown | 17 | — | 8 | — | 6 | — | — |
| Hepatomegaly | 288 | 32 | 116 | 32 | 108 | 29 | .526 |
| Splenomegaly | 279 | 31 | 114 | 31 | 102 | 28 | .324 |
| CNS positive | 52 | 6 | 16 | 4 | 22 | 6 | .430 |
| FAB | |||||||
| M0 | 55 | 6 | 25 | 6 | 21 | 5 | .634 |
| M1 | 150 | 17 | 64 | 18 | 58 | 16 | .596 |
| M2 | 249 | 28 | 112 | 31 | 105 | 29 | .592 |
| M4 | 206 | 23 | 71 | 20 | 92 | 25 | .082 |
| M5 | 158 | 18 | 64 | 18 | 60 | 16 | .742 |
| M6 | 20 | 2 | 6 | 2 | 9 | 2 | .609 |
| M7 | 47 | 5 | 19 | 5 | 18 | 5 | .986 |
| AML (NOS) | 16 | 2 | 6 | 1 | 8 | 2 | .795 |
| Cytogenetics | |||||||
| Normal | 125 | 22 | 48 | 21 | 60 | 25 | .393 |
| t(8;21) | 89 | 16 | 45 | 20 | 40 | 17 | .434 |
| Abnormal 16 | 49 | 9 | 18 | 8 | 23 | 10 | .650 |
| Abnormal 11 | 132 | 23 | 52 | 23 | 56 | 23 | .981 |
| t(6;9) | 10 | 2 | 3 | 1 | 4 | 2 | 1.000 |
| -7/7- | 22 | 4 | 10 | 4 | 3 | 1 | .072 |
| -5/5- | 7 | 1 | 3 | 1 | 2 | 1 | .677 |
| +8 | 33 | 6 | 10 | 4 | 13 | 5 | .779 |
| +21 | 9 | 2 | 2 | 1 | 6 | 2 | .287 |
| Other | 87 | 15 | 37 | 16 | 35 | 14 | .687 |
| Unknown | 338 | 38 | 139 | 38 | 129 | 35 | — |
| Complexity | |||||||
| 3 or less | 371 | 85 | 155 | 86 | 154 | 85 | .800 |
| More than 3 | 67 | 15 | 25 | 14 | 28 | 15 | — |
| Unfavorable* | 86 | 15 | 36 | 15 | 31 | 12 | .324 |
| Standard | 343 | 61 | 137 | 59 | 155 | 62 | .507 |
| Favorable† | 134 | 24 | 60 | 26 | 65 | 26 | .971 |
— indicates not applicable.
*Unfavorable cytogenetics are del (7), 7q−, del (5), 5q−, and >3 nonrandom abnormalities. Some cells may have more than one unfavorable cytogenetic feature.
†Favorable cytogenetics are t(8;21), inv (16), or t(16;16); normal and all other abnormalities are standard.
Table 2 shows major outcomes for the entire study and by course of treatment. Eighty-eight percent of patients achieved CR after induction; of the remainder, half died and half had persistent or progressive AML; 7% withdrew; however, of these, 2% withdrew without assessment of marrow status. Of the 738 patients participating in the first randomization, 83% were in CR and 3% were in PR, 6% died, 5% had persistent or recurrent leukemia, and 3% were not evaluable for response at the end of consolidation. Seventy-five patients (10%) withdrew before the end of the phase. DFS at 5 years from randomization is 46 plus or minus 5% for IdaDCTER versus 49 plus or minus 5% for FAMP/AC/IDA (P = .361), and OS at 5 years is 59 plus or minus 5% for IdaDCTER versus 56 plus or minus 6% for FAMP/AC/IDA (P = .612). Figure 2 shows EFS from study entry is 42% plus or minus 3% and OS is 52% plus or minus 4% at 5 years. OS and EFS from the end of courses 1 and 2 are similar for those who did and did not withdraw. Although there is no significant difference between the 2 regimens in EFS and OS, FAMP/AC/IDA was associated with significantly fewer relapses but twice as many treatment-related deaths.
Outcomes at 5 years according to phase of therapy in CCG-2961
| . | . | Regimen A: IdaDCTER/DCTER . | Regimen B: Famp/AC/Ida . | P . | Donor . | No donor . | P . | IL-2 . | No IL-2 . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Phase | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 4 | 4 | 4 |
| N | 901 | 367 | 371 | — | 170 | 463 | — | 144 | 145 | — |
| Outcome | ||||||||||
| CR/PR | 88% | 88% | 89% | .895 | — | — | — | — | — | — |
| Die | 6% | 4% | 8% | .060 | 8% | 3% | .007 | 0% | 3% | .122 |
| Fail/relapse | 6% | 7% | 3% | .018 | 2% | 4% | .349 | 5% | 3% | .377 |
| Withdraw | 7% | 8% | 10% | — | — | — | — | — | — | — |
| Inevaluable | 2% | 3% | 3% | — | — | — | — | — | — | — |
| OS±2 SE | 52 ± 4% | 59 ± 5% | 56 ± 6% | .612 | 67 ± 8% | 62 ± 5% | .425 | 70 ± 8% | 73 ± 8% | .727 |
| EFS±2 SE | 42 ± 3% | 46 ± 5% | 49 ± 5% | .361 | — | — | — | — | — | — |
| DFS±2 SE | — | — | — | — | 60 ± 8% | 50 ± 5% | .021 | 51 ± 9% | 58 ± 8% | .489 |
| . | . | Regimen A: IdaDCTER/DCTER . | Regimen B: Famp/AC/Ida . | P . | Donor . | No donor . | P . | IL-2 . | No IL-2 . | P . |
|---|---|---|---|---|---|---|---|---|---|---|
| Phase | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 4 | 4 | 4 |
| N | 901 | 367 | 371 | — | 170 | 463 | — | 144 | 145 | — |
| Outcome | ||||||||||
| CR/PR | 88% | 88% | 89% | .895 | — | — | — | — | — | — |
| Die | 6% | 4% | 8% | .060 | 8% | 3% | .007 | 0% | 3% | .122 |
| Fail/relapse | 6% | 7% | 3% | .018 | 2% | 4% | .349 | 5% | 3% | .377 |
| Withdraw | 7% | 8% | 10% | — | — | — | — | — | — | — |
| Inevaluable | 2% | 3% | 3% | — | — | — | — | — | — | — |
| OS±2 SE | 52 ± 4% | 59 ± 5% | 56 ± 6% | .612 | 67 ± 8% | 62 ± 5% | .425 | 70 ± 8% | 73 ± 8% | .727 |
| EFS±2 SE | 42 ± 3% | 46 ± 5% | 49 ± 5% | .361 | — | — | — | — | — | — |
| DFS±2 SE | — | — | — | — | 60 ± 8% | 50 ± 5% | .021 | 51 ± 9% | 58 ± 8% | .489 |
— indicates not applicable.
Event-free survival and overall survival in CCG-2961. Kaplan-Meier plot of survival (OS) and event-free survival (EFS) from time on study.
Event-free survival and overall survival in CCG-2961. Kaplan-Meier plot of survival (OS) and event-free survival (EFS) from time on study.
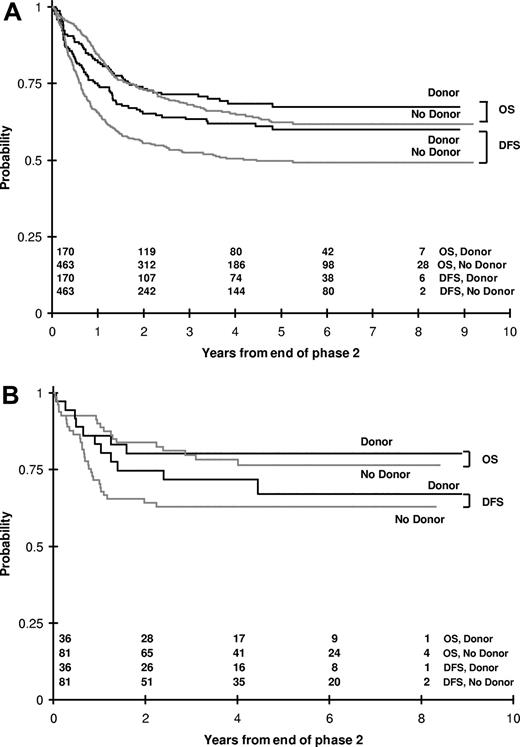
There were 170 patients assigned to MRD BMT and 463 to HidAC intensification. DFS for those with donors was 60 plus or minus 8% at 5 years, significantly better than the 50 plus or minus 5% of those without donors (P = .021), but OS at 5 years of those with and without donors was not significantly different (67% ± 8% vs 62% ± 5%, P = .425; Figure 3A). Among the 138 patients who had favorable cytogenetics, there were no significant differences in either DFS or OS among those with and without a donor (Figure 3B). Among 29 patients with unfavorable cytogenetics, by the end of course 2, 5 had not gone into remission, 5 died, and 5 had withdrawn. Of the 14 patients eligible for course 3, 7 had donors and 7 did not; 4 with donors and 1 without a donor are alive.
Outcomes in CCG-2961 according to donor availability. (A) Kaplan-Meier plot of OS and disease-free survival (DFS) from the time of entry to course 3 for those with and without matched related donors for marrow transplantation. (B) OS and DFS for those patients with favorable cytogenetics according to donor status.
Outcomes in CCG-2961 according to donor availability. (A) Kaplan-Meier plot of OS and disease-free survival (DFS) from the time of entry to course 3 for those with and without matched related donors for marrow transplantation. (B) OS and DFS for those patients with favorable cytogenetics according to donor status.
Of the 385 patients in continuous remission following HidAC chemotherapy intensification, 96 did not participate in randomization; of the remaining 289 patients, 144 were randomly assigned to IL-2 and 145 to no IL-2. Table 2 shows no TRM and no differences in DFS or OS between the 2 regimens. There was no significant difference in DFS or OS between those who had previously received IdaDCTER or FAMP/AC/IDA in course 2.
To determine whether the protocol changes instituted in May 2000 had reduced mortality, we compared outcomes before and after suspension (Table 3). This comparison showed a trend to higher EFS, DFS, and OS and a nonsignificant reduction in TRM after suspension. Before and after suspension 5-year TRM in the IDADCTER/IDADCTER regimen were 11 plus or minus 4% to 9 plus or minus 4% (P = .569) and in the FAMP regimen were 17 plus or minus 5% to 10 plus or minus 4% (P = .030). Conversely, the withdrawal rate in phase 2 increased in the IDADCTER/IDADCTER regimen from 6.3% to 11.2% (P = .144) and from 5.4% to 15.6% (P = .002) after suspension. The data were then examined according to the time on study based on the first 18 months, the second 18 months before suspension, and the 20 months after suspension. Table 3 shows a significant trend for improved EFS, DFS, OS, and TRM from the beginning to the end of the study. Comparisons of the cohort treated during the first 18 months to after suspension cohort show significant differences in all outcomes; the second 18-month cohort has outcomes intermediate between the first and last cohorts. Table 3 and Figure 4 document that improvement in outcomes preceded the mandated changes that came as a result of study suspension.
Outcomes by date and period of enrollment before and after suspension
| . | Before regimen A suspension . | Before regimen B suspension . | After regimen C suspension . | A vs C (P) . | B vs C (P) . | Trend (P) . |
|---|---|---|---|---|---|---|
| Date | September 1996 through March 1998 | March 1998 through October 1999 | May 2000 through December 2002 | — | — | — |
| Patients (N) | 205 | 290 | 406 | — | — | — |
| From study entry | ||||||
| OS ± 2 SE | 43 ± 7 (1.60) | 52 ± 6 (1.19) | 57 ± 6 (1.00) | <.001 | .14 | <.001 |
| EFS ± 2 SE | 34 ± 7 (1.42) | 42 ± 6 (1.13) | 46 ± 6 (1.00) | .002 | .250 | .002 |
| TRM ± 2 SE | 19 ± 5 (1.63) | 17 ± 4 (1.43) | 12 ± 3 (1.00) | .025 | .073 | .021 |
| After remission | ||||||
| OS ± 2 SE | 49 ± 8 (1.60) | 57 ± 6 (1.22) | 63 ± 6 (1.00) | .001 | .137 | .001 |
| DFS ± 2 SE | 40 ± 8 (1.42) | 46 ± 6 (1.19) | 51 ± 6 (1.00) | .006 | .138 | .006 |
| . | Before regimen A suspension . | Before regimen B suspension . | After regimen C suspension . | A vs C (P) . | B vs C (P) . | Trend (P) . |
|---|---|---|---|---|---|---|
| Date | September 1996 through March 1998 | March 1998 through October 1999 | May 2000 through December 2002 | — | — | — |
| Patients (N) | 205 | 290 | 406 | — | — | — |
| From study entry | ||||||
| OS ± 2 SE | 43 ± 7 (1.60) | 52 ± 6 (1.19) | 57 ± 6 (1.00) | <.001 | .14 | <.001 |
| EFS ± 2 SE | 34 ± 7 (1.42) | 42 ± 6 (1.13) | 46 ± 6 (1.00) | .002 | .250 | .002 |
| TRM ± 2 SE | 19 ± 5 (1.63) | 17 ± 4 (1.43) | 12 ± 3 (1.00) | .025 | .073 | .021 |
| After remission | ||||||
| OS ± 2 SE | 49 ± 8 (1.60) | 57 ± 6 (1.22) | 63 ± 6 (1.00) | .001 | .137 | .001 |
| DFS ± 2 SE | 40 ± 8 (1.42) | 46 ± 6 (1.19) | 51 ± 6 (1.00) | .006 | .138 | .006 |
Values are percentages ± standard error. Hazard ratio (HR) from multivariate regression is included in parentheses. Estimates are 5 years from study entry and 5 years after induction.
OS indicates overall survival; EFS, event-free survival; TRM, treatment-related mortality; DFS, disease-free survival; and —, not applicable.
Table 4 lists univariate hazard ratios for the prognostic factors typically used for risk stratification in pediatric AML: white blood cell count at diagnosis, cytogenetic risk group, age, and day 14 marrow response. In those with complete data, white blood cell count, age, race (white vs nonwhite), and cytogenetic risk groups were significant in both univariate and multivariate analyses. Age more than 16 years was associated with reduced OS and EFS. Availability of a related donor was not significant for those who have favorable cytogenetics (Figure 3B). Previous publications from CCG-2961 have identified other unfavorable prognostic factors that are not traditionally used in risk stratification: persistent residual disease in morphologic remission after course 1 as measured by multichannel flow cytometry,59 body mass index less than 10% or greater than 95%,56 black ethnicity,55 and FLT3/ITD and FLT3/ITD allelic ratio.58 Of note, because the first analysis of BMI 3 years ago, the extremes of BMI remain predictive of EFS, but only BMI more than 95th percentile for age is predictive of overall survival (Table 4).56
Univariate analysis of prognostic factors in CCG-2961
| . | N . | EFS . | OS . | ||||
|---|---|---|---|---|---|---|---|
| 5 years . | HR . | P . | 5 years . | HR . | P . | ||
| Age, y | |||||||
| Less than 2 | 193 | 39 ± 7 | 1.37 | .013 | 56 ± 7 | 1.11 | .477 |
| 2 to less than 10 | 275 | 46 ± 6 | 1 | — | 58 ± 6 | 1 | — |
| 10 to less than 16 | 310 | 42 ± 6 | 1.17 | .180 | 50 ± 6 | 1.27 | .058 |
| 16 or over | 123 | 34 ± 9 | 1.51 | .004 | 40 ± 9 | 1.74 | <.001 |
| WBC, × 109/L | |||||||
| Less than 50 | 639 | 45 ± 4 | 1 | — | 56 ± 4 | 1 | — |
| 50 to less than 100 | 118 | 33 ± 9 | 1.31 | .036 | 47 ± 10 | 1.31 | .059 |
| 100 or more | 143 | 32 ± 8 | 1.52 | <.001 | 41 ± 8 | 1.57 | <.001 |
| Race | |||||||
| White | 583 | 44 ± 4 | 1 | — | 56 ± 4 | 1 | — |
| Black | 84 | 30 ± 10 | 1.44 | .012 | 37 ± 11 | 1.60 | .002 |
| Hispanic | 157 | 39 ± 8 | 1.20 | .131 | 47 ± 8 | 1.34 | .024 |
| Asian | 26 | 50 ± 19 | 0.89 | .670 | 54 ± 19 | 1.11 | .726 |
| Other | 34 | 39 ± 17 | 1.26 | .310 | 51 ± 18 | 1.20 | .501 |
| Cytogenetic risk | |||||||
| Standard | 396 | 36 ± 5 | 1 | — | 47∥± 5 | 1 | — |
| Favorable* | 138 | 61 ± 8 | 0.49 | <.001 | 72 ± 8 | 0.45 | <.001 |
| Unfavorable† | 290 | 29 ± 17 | 1.40 | .149 | 39 ± 19 | 1.35 | .237 |
| Body mass index | |||||||
| Middle weight | 570 | 45 ± 4 | 1 | — | 54 ± 4 | 1 | — |
| Less than10% for age | 83 | 35 ± 11 | 1.41 | .023 | 51 ± 11 | 1.24 | .218 |
| More than 95% for age | 114 | 34 ± 9 | 1.35 | .022 | 44 ± 10 | 1.46 | .007 |
| Early response, day 14+1 marrow | 770 | — | 1.29 | .075 | — | 1.25 | .158 |
| . | N . | EFS . | OS . | ||||
|---|---|---|---|---|---|---|---|
| 5 years . | HR . | P . | 5 years . | HR . | P . | ||
| Age, y | |||||||
| Less than 2 | 193 | 39 ± 7 | 1.37 | .013 | 56 ± 7 | 1.11 | .477 |
| 2 to less than 10 | 275 | 46 ± 6 | 1 | — | 58 ± 6 | 1 | — |
| 10 to less than 16 | 310 | 42 ± 6 | 1.17 | .180 | 50 ± 6 | 1.27 | .058 |
| 16 or over | 123 | 34 ± 9 | 1.51 | .004 | 40 ± 9 | 1.74 | <.001 |
| WBC, × 109/L | |||||||
| Less than 50 | 639 | 45 ± 4 | 1 | — | 56 ± 4 | 1 | — |
| 50 to less than 100 | 118 | 33 ± 9 | 1.31 | .036 | 47 ± 10 | 1.31 | .059 |
| 100 or more | 143 | 32 ± 8 | 1.52 | <.001 | 41 ± 8 | 1.57 | <.001 |
| Race | |||||||
| White | 583 | 44 ± 4 | 1 | — | 56 ± 4 | 1 | — |
| Black | 84 | 30 ± 10 | 1.44 | .012 | 37 ± 11 | 1.60 | .002 |
| Hispanic | 157 | 39 ± 8 | 1.20 | .131 | 47 ± 8 | 1.34 | .024 |
| Asian | 26 | 50 ± 19 | 0.89 | .670 | 54 ± 19 | 1.11 | .726 |
| Other | 34 | 39 ± 17 | 1.26 | .310 | 51 ± 18 | 1.20 | .501 |
| Cytogenetic risk | |||||||
| Standard | 396 | 36 ± 5 | 1 | — | 47∥± 5 | 1 | — |
| Favorable* | 138 | 61 ± 8 | 0.49 | <.001 | 72 ± 8 | 0.45 | <.001 |
| Unfavorable† | 290 | 29 ± 17 | 1.40 | .149 | 39 ± 19 | 1.35 | .237 |
| Body mass index | |||||||
| Middle weight | 570 | 45 ± 4 | 1 | — | 54 ± 4 | 1 | — |
| Less than10% for age | 83 | 35 ± 11 | 1.41 | .023 | 51 ± 11 | 1.24 | .218 |
| More than 95% for age | 114 | 34 ± 9 | 1.35 | .022 | 44 ± 10 | 1.46 | .007 |
| Early response, day 14+1 marrow | 770 | — | 1.29 | .075 | — | 1.25 | .158 |
Data in “5 year” columns are percentages.
HR indicates hazard ratio; and —, not applicable.
*Favorable cytogenetic risk is t(8;21) and inv (16).
†Unfavorable is del(7), 7q−, del(5), 5q−, and more than 3 nonrandom abnormalities.
‡The day 14 marrow assessment was for less than 5% blasts; it is a time-dependent variable.
Tables 5 and 6 describes the serious or common toxicities of this study. Grade 4 fever and neutropenia were common in the first 3 courses. The study captured time to recovery of ANC more than 1000 × 109/L and platelet count of more than 50 000 × 109/L. In course 2, time to recovery of both neutrophils and platelets was significantly shorter in the FAMP/AC/IDA; however, compared with the IdaDCTER arm, FAMP/AC/IDA was associated with significantly more TRM attributed to infections. The median (range) of days to TRM were 49 days (16-131 days) in the IdaDCTER arm and 41 days (7-171 days) in FAMP/AC/IDA (P = .176). Death reports did not reveal an excess of fungal or viral infections in patients receiving FAMP/AC/IDA. Other common toxicities were NCI grade 3 and 4 hepatotoxicity,60 most often hyperbilirubinemia, gastrointestinal toxicity manifested as pain, diarrhea, nausea, vomiting, and pulmonary toxicity, which was not otherwise defined. The incidence of these toxicities increased in the after suspension populations but did not contribute to mortality. Hospital days increased before vs after suspension as mortality declined.
Toxicity in CCG-2961 according to phase and treatment assignment
| Toxicity by phase . | Phase 1: all . | Phase 2 . | A vs B (P) . | Phase 3 . | CT vs BMT (P) . | ||
|---|---|---|---|---|---|---|---|
| Regimen A . | Regimen B . | CT . | BMT . | ||||
| Number | 899 | 367 | 369 | — | 449 | 135 | — |
| Median days (no.) of ANC less than 109/L | 38 | 55 | 46 | <.001 | 38 | — | — |
| Median days (no.) of platelets more than 50×109/L | 37 | 56 | 50 | .043 | 37 | — | — |
| Median hospital days, no. | 36 | 38 | 31 | <.001 | 26 | 43 | <.001 |
| Grades 3 and 4 | |||||||
| Bilirubin | 23% | 22% | 18% | .251 | 16% | 35% | <.001 |
| Diarrhea | 30% | 22% | 22% | .969 | 9% | 19% | <.001 |
| Nausea/emesis | 24% | 23% | 28% | .118 | 13% | 40% | <.001 |
| Mucositis | 34% | 32% | 27% | .132 | 10% | 61% | <.001 |
| Glucose | 15% | 12% | 13% | .844 | 9% | 17% | .012 |
| Pulmonary (f) | 19% | 12% | 15% | .248 | 10% | 16% | .088 |
| Potassium | 21% | 27% | 20% | .017 | 13% | 16% | .391 |
| Any | 79% | 81% | 77% | .189 | 64% | 91% | <.001 |
| Toxicity by phase . | Phase 1: all . | Phase 2 . | A vs B (P) . | Phase 3 . | CT vs BMT (P) . | ||
|---|---|---|---|---|---|---|---|
| Regimen A . | Regimen B . | CT . | BMT . | ||||
| Number | 899 | 367 | 369 | — | 449 | 135 | — |
| Median days (no.) of ANC less than 109/L | 38 | 55 | 46 | <.001 | 38 | — | — |
| Median days (no.) of platelets more than 50×109/L | 37 | 56 | 50 | .043 | 37 | — | — |
| Median hospital days, no. | 36 | 38 | 31 | <.001 | 26 | 43 | <.001 |
| Grades 3 and 4 | |||||||
| Bilirubin | 23% | 22% | 18% | .251 | 16% | 35% | <.001 |
| Diarrhea | 30% | 22% | 22% | .969 | 9% | 19% | <.001 |
| Nausea/emesis | 24% | 23% | 28% | .118 | 13% | 40% | <.001 |
| Mucositis | 34% | 32% | 27% | .132 | 10% | 61% | <.001 |
| Glucose | 15% | 12% | 13% | .844 | 9% | 17% | .012 |
| Pulmonary (f) | 19% | 12% | 15% | .248 | 10% | 16% | .088 |
| Potassium | 21% | 27% | 20% | .017 | 13% | 16% | .391 |
| Any | 79% | 81% | 77% | .189 | 64% | 91% | <.001 |
ANC indicates absolute neutrophil count; NS, not significant; SGPT, serum glutamic pyruvic transaminase; GI, gastrointestinal; BP, blood pressure; PTT, partial thromboplastin time; and —, not applicable.
Toxicity in CCG-2961 according to period of enrollment
| Toxicity before and after suspension . | Phase 1 . | Phase 2 . | ||||
|---|---|---|---|---|---|---|
| Before . | After . | P . | Before . | After . | P . | |
| N | 495 | 404 | — | — | — | — |
| Grades 3 and 4 | ||||||
| Any liver toxicity | — | — | NS | 23.1% | 30.4% | .032 |
| SGPT | 7.9% | 14.1% | .004 | 8.6% | 17.3% | <.001 |
| Glucose | 11.3% | 18.8% | .002 | 9.6% | 15.8% | .015 |
| Any GI toxicity | — | — | NS | 49.9% | 58.4% | .026 |
| Nausea/emesis | 20.6% | 28.2% | .010 | 21.4% | 30.7% | .005 |
| Systolic BP | 5.9% | 2.5% | .021 | — | — | NS |
| Fibrinogen | 3.6% | 7.7% | .012 | — | — | NS |
| PTT | 1.8% | 5.0% | .014 | — | — | NS |
| Calcium | 4.8% | 9.7% | .007 | 1.7% | 6.1% | .003 |
| Fever | 8.9% | 5.2% | .046 | — | — | NS |
| Any toxicity | 77.2% | 80.9% | .195 | — | — | NS |
| Hospital days | ||||||
| Mean | 35.7 | 38.8 | <.001 | 34.2 | 39.6 | <.001 |
| Median | 35 | 37 | <.001 | 33 | 36 | — |
| ICU days | ||||||
| Mean | 4.5 | 4.6 | .875 | 3.1 | 3.3 | .683 |
| Median | 0 | 0 | .799 | 0 | 0 | — |
| Toxicity before and after suspension . | Phase 1 . | Phase 2 . | ||||
|---|---|---|---|---|---|---|
| Before . | After . | P . | Before . | After . | P . | |
| N | 495 | 404 | — | — | — | — |
| Grades 3 and 4 | ||||||
| Any liver toxicity | — | — | NS | 23.1% | 30.4% | .032 |
| SGPT | 7.9% | 14.1% | .004 | 8.6% | 17.3% | <.001 |
| Glucose | 11.3% | 18.8% | .002 | 9.6% | 15.8% | .015 |
| Any GI toxicity | — | — | NS | 49.9% | 58.4% | .026 |
| Nausea/emesis | 20.6% | 28.2% | .010 | 21.4% | 30.7% | .005 |
| Systolic BP | 5.9% | 2.5% | .021 | — | — | NS |
| Fibrinogen | 3.6% | 7.7% | .012 | — | — | NS |
| PTT | 1.8% | 5.0% | .014 | — | — | NS |
| Calcium | 4.8% | 9.7% | .007 | 1.7% | 6.1% | .003 |
| Fever | 8.9% | 5.2% | .046 | — | — | NS |
| Any toxicity | 77.2% | 80.9% | .195 | — | — | NS |
| Hospital days | ||||||
| Mean | 35.7 | 38.8 | <.001 | 34.2 | 39.6 | <.001 |
| Median | 35 | 37 | <.001 | 33 | 36 | — |
| ICU days | ||||||
| Mean | 4.5 | 4.6 | .875 | 3.1 | 3.3 | .683 |
| Median | 0 | 0 | .799 | 0 | 0 | — |
NS indicates not significant; and —, not applicable.
Discussion
Following a series of phase 1 and 2 investigations, CCG-2961 introduced idarubicin, fludarabine, and IL-2 into this large phase 3 trial in pediatric AML. With IDA partially replacing rubidomycin in the intensively timed IdaDCTER hybrid induction,14,22,61 day 14 response was superior, but remission induction rates were similar to those in CCG-2891. The improvement in EFS and OS in CCG-2961 over time could indicate that IDA was effecting more durable remissions once investigators had learned how to handle its toxicity. However, the use of historical controls rather than contemporary controls limits our ability to interpret the role of idarubicin. The BFM 93 study showed that in high-risk patients, IDA increased the proportion of patients with rapid early response on day 14, EFS, DFS, and OS, but the addition of an intensified HIDAC/mitoxantrone consolidation confounded the analysis of the impact of IDA on durability of remission.62 The successor BFM 98 study showed that among standard-risk patients IDA in induction did not confer significant increases in EFS or OS.12
The 6-drug IdaDCTER hybrid was compared with FAMP/AC/IDA in consolidation. One of the reasons to introduce a new regimen with higher-dose cytarabine was to determine whether changing strategy could improve the outcomes of the patients with slow early responses. The only significant differences between the 2 regimens were shorter duration of neutropenia in the FAMP/AC/IDA arm and paradoxically higher infectious mortality. FAMP/AC/IDA was not associated with excess of fungal or viral infections as might be expected with the highly immunosuppressive fludarabine.63 TRM offset a minor reduction in relapse in the FAMP/AC/IDA regimen, but random assignment of higher numbers of slow responding patients to this arm confounded assessment of whether introducing an alternative regimen killed cells resistant to the first regimen. Although FAMP/AC with or without IDA showed great promise in recurrent or refractory AML, it has not yet translated into improved outcomes in phase 3 trials. In the only randomized trial in relapsed AML, FAMP/AC compared unfavorably with MRC-10 induction therapy with cytarabine, rubidomycin, and etoposide.63
Compared with HidAC chemotherapy with or without IL-2, MRD BMT achieved a better DFS, but not a better OS in the entire study cohort. As several other studies in pediatric and adult AML have shown, MRD BMT in CCG-2961 did not effect significantly better DFS or OS than chemotherapy among patients with favorable cytogenetics.1-5 Hence, the Children's Oncology Group (COG) is no longer recommending MRD BMT in first remission for patients with favorable cytogenetics.
The CCG-2961 study was powered to show a 10% difference in DFS between IL-2 and no IL-2. IL-2 had also shown promise in vitro and recurrent or refractory AML. However, the CCG-2961 study showed that given in this dose and schedule in the setting of minimal residual disease, IL-2 did not improve DFS or OS pediatric in AML. A possible explanation may be the product: the manufacturer of IL-2 changed in 1994 after the initial provocative trial after autologous stem cell transplant.41 Using the new product, investigators at the Fred Hutchison Cancer Center could not repeat their earlier results.64 So far, there are no peer-reviewed follow-up trials confirming the benefits of similar or more intensive doses or schedules of IL-2. Thus, IL-2 as used in these studies probably does not have a role in AML therapy.
In pediatric patients treated on the MRC AML-10 trial, standardization of supportive care and experience reduced TRM from 18% at the beginning of the trial to 9% at the end. Similarly, CCG-2961 showed the progressive improvement in OS and EFS from the beginning to the end of the study. No such improvement took place in CCG-2891, a study marginally less toxic than CCG-2961.61 In CCG-2961, the improvement in EFS, OS, and DFS began before the DSMB suspension and continued after new supportive care guidelines were in place. TRM fell from 19% in the first 18 months to 12% in last 20 months, and EFS and DFS both improved by 12% and survival by 14%. Thus, the chemotherapy appears to have become more effective, an unlikely possibility. Perhaps subtle changes in practice over time contributed to a learning curve, a phenomenon well recognized in the surgical literature. Theoretically, a reduction in dose intensity would have further reduced TRM, but at the expense of increased treatment failure because refractory and recurrent disease was the main cause of death in this trial. These results suggest that neither reducing cytotoxic therapy nor increasing it would have substantially improved EFS and OS. In CCG-2961 TRM, prolonged hospitalizations and many documented infections probably contributed to a relatively high withdrawal rate and prompted the DSMB to suspend the study.
Outcomes in CCG-2961 appear marginally inferior to contemporary BFM and MRC trials. However, differences in outcomes may derive from differences in eligibility as much as from different treatment strategies. In CCG-2961, patients 16 to 21 years of age had inferior EFS and OS attributable to increased induction mortality. MRC and BFM pediatric trials do not enroll patients older than age 15 years. In the studies of the MRC-10 trial, patients 16 to 39 years of age have EFS and OS inferior to children but better than older adults receiving the same protocol therapy.65 CCG-2961 also excluded the favorable Down syndrome and acute promyelocytic leukemia subsets, yet they have been included in these other studies although excluded in subset analyses. In addition, ethnic differences are associated with different outcomes, and 28% of the patients in CCG-2961 belong to minorities with poorer outcomes than the white majority.55 Finally, nutritional status may differ in study cohorts. In CCG-2961, obesity was a prognostic factor; 14.8% of patients were obese, similar to the population at large.56 In the year 2000, in the United Kingdom, 4.8% of girls and 6.8% of boys 2 to 19 years of age were obese.66 These differences in patient populations may explain differing outcomes in CCG-2961 and the AML-MRC-10 study, Nonetheless, in 2001, when the COG AML Committee had to begin planning the successor to CCG-2961 and the previous POG phase 3 trial POG-9421,67 long-term outcomes and improvements CCG-2961 over time were unknown. Based on the excellent results of MRC AML-10, COG opted to test the feasibility of an MRC-based therapy plus gemtuzumab ozogamicin in pilot study AAML03P1 and in the current phase 3 trial, AAML0531.
In the past 2 decades, pediatric and adult cooperative groups have made substantial progress in the treatment of AML, principally through intensification of therapy. Nonetheless, the conclusions of this study as well as that of the most recent BFM and MRC pediatric trials are that, although intensifications of therapy introduced after 1995 have modestly improved outcomes, the improvements seem to derive from changes in supportive care and the effects of time as much as from the cancer therapy.2,12
IDA and FAMP are analogs of conventional therapy, and IL-2 is broad modifier of immune response. None of the 3 new agents introduced in this trial appeared to make a significant impact on EFS or OS. Refinement of risk stratification, different paradigms, and new agents are needed. The inherent biologic features of these diseases appear to have major effect on outcome. Small molecule therapeutics that target specific oncoproteins have markedly improved the outcomes of patients with acute promyelocytic leukemia and chronic myeloid leukemia. Based on these examples, molecular stratification and classification, according to presence or absence of specific mutations such as FLT3/ITD or Ras point mutations, offer a rationale for incorporating agents in existing chemotherapy regimens with novel mechanisms of action and nonoverlapping toxicities into existing chemotherapy regimens. In addition, randomized phase 2 designs testing 2 or more new biologic agents on a backbone of a cytotoxic combination of known efficacy may expedite introduction of new agents into pediatric trials and enhance identification of those of greatest potential to improve outcomes in a phase 3 setting.68,69
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christine Curran for typing the manuscript and Dr William Woods for critical review.
The work was supported by grants CA 13539 and CA 98543 from the National Institutes of Health and National Institutes of Health grants to Childrens Cancer Group Institutions: CCG grant CA 13539. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm. This work was also supported by the Yetta Dietch Novotny Chair in Clinical Oncology (B.J.L.).
Authorship
Contribution: B.J.L. designed and conducted the study, conducted eligibility analysis, and wrote the article; F.O.S. designed and conducted the study and reviewed conduct; J.F. designed and conducted the study and performed toxicity assessments; D.R.B. reviewed histology and eligibility; P.D. designed and conducted phase 2 and preformed amendments; S.F. designed the study and provided transplantation oversight; N.A.H. conducted eligibility analysis and reviewed and classified cytogenetics; C.A. designed the study and conducted chemotherapy intensification; R.J.A. conducted the study, performed infant subset, and edited the article; N.S. designed the study and performed management of infection; M.W. performed care guidelines and toxicity and edited the article; K.D. managed chloromas; K.S. designed the study and performed NF and del(7) cohorts; S.L.-F. designed the study and performed MDS cohorts; R.B.G. organized, reviewed, and prepared data and tables; and all authors reviewed and edited the manuscript. T.A.A. wrote the statistical section, wrote analyzed DMC and Progress Reports, and provided data oversight.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beverly Lange, MD, 4308 Wood Building, Children's Hospital of Philadelphia, Philadelphia, PA; e-mail: lange@email.chop.edu.