Abstract
The core-binding factor (CBF)–associated leukemia fusion protein CBFβ-SMMHC impairs myeloid and lymphoid differentiation. By inhibiting RUNX function, the fusion oncoprotein predisposes specifically to acute myeloid leukemia in both patients and mouse models. We have shown that Cbfβ-SMMHC expression leads to a sustained reduction of circulating B lymphocytes in the mouse. In this study, we demonstrate that the activation of Cbfβ-SMMHC reduces pre–pro-B cells approximately 3-fold and pre-B cells more than 10-fold and that this differentiation block is cell-autonomous. The reduction of pre–pro-B cells coincided with an increase in apoptosis in this population. The number of common lymphoid progenitors (CLPs) were not affected; however, the expression of critical early B-cell factors Ebf1, Tcfe2a, and Pax5 was significantly reduced. In addition, Cbfβ-SMMHC reduced Rag1 and Rag2 expression and impaired V(D)J recombination in the CLPs. Furthermore, CLPs expressing Cbfβ-SMMHC also show inhibition of B cell–specific genes Cd79a, Igll1, VpreB1, and Blk. These results demonstrate that CBF/RUNX function is essential for the function of CLPs, the survival of pre–pro-B cells, and the establishment of a B lineage–specific transcriptional program. This study also provides a mechanistic basis for the myeloid-lineage bias of CBFβ-SMMHC–associated leukemia.
Introduction
The core-binding factor (CBF) is a heterodimeric transcription factor that regulates specific pathways during development and differentiation.1 The CBF consists of a DNA-binding α subunit (encoded by one of 3 genes: RUNX1, RUNX2, and RUNX3) and a common non-DNA-binding β subunit (CBFβ). CBFβ increases the affinity of RUNX proteins for DNA2,3 and protects them from proteolysis.4 The CBF is essential for embryonic multilineage hematopoiesis because hematopoietic stem cells (HSCs) failed to emerge from the hemogenic endothelium in Runx1-null embryos,5-7 and definitive hematopoiesis was impaired in Runx1-null or Cbfb-null embryos.8-10 In adult hematopoiesis, CBF regulates expression of genes involved in myeloid and lymphoid differentiation.11
The CBFB and RUNX1 genes are frequent targets of recurrent chromosomal rearrangements and mutations in human leukemia, including acute myeloid leukemia (AML) and acute lymphoblastic leukemia. For example, the chromosome 16 inversion inv(16)(p13; q22) is found in approximately 10% of patients with AML.12 This inversion disrupts the CBFB and smooth muscle myosin heavy chain MYH11 genes to create the CBFB-MYH11 fusion gene,13 and the encoded CBFβ-SMMHC protein acts as a dominant repressor of CBF function. Studies in the mouse using a Cbfb-MYH11 knock-in allele showed that heterozygous Cbfb-MYH11 knock-in embryos exhibit identical phenotypes to those of Runx1- and Cbfb-null embryos.8,10,14
Using a conditional Cbfb-MYH11 knock-in allele (Cbfb56M), we have shown that Cbfβ-SMMHC impairs myeloid differentiation in adult hematopoiesis, creating an abnormal myeloid progenitor population that can be the cell of origin of leukemia stem cells.15 Consistent with the specific association of inv(16) with human AML, Cbfβ-SMMHC expression predisposes for AML development in the mouse.15-17 Despite its specificity for myeloid malignancy, Cbfβ-SMMHC expression severely affects lymphoid differentiation, inducing a significant reduction of B lymphocytes.12 CBF cooperates with the transcription factors Ebf1 and E2A in the regulation of proteins that participate in the B-cell differentiation program, including Blk (encodes the B cell–specific src-family tyrosine kinase), Igll1 (also called lambda5, and encodes the immunoglobulin surrogate light chain), and Cd79a (also called mb-1 or Iga, and encodes the B-cell/pre-B-cell receptor component immunoglobulin (Ig)-α).18-21 However, the alterations in B-cell development caused by inhibition of CBF function are poorly understood.
Here we study the consequence of Cbfβ-SMMHC expression during B-cell fate decisions in early hematopoietic progenitors using multiparametric flow cytometry and molecular analyses in the bone marrow (BM) from Cbfb+/56M/Mx1Cre conditional knock-in mice. Expression analysis of B-cell differentiation factors in the uncommitted common lymphoid progenitors (CLPs) using qPCR analysis shows that transcription of early factors Ebf1 and E2A as well as downstream B-cell factors Cd79a, Igll1, VpreB1, and Blk is reduced. We show that introduction of an Ebf1-expressing retrovirus rescues the differentiation deficiency induced by Cbfβ-SMMHC. Furthermore, we used a reporter transgenic mouse to show that V(D)J recombination in CLPs was reduced in the presence of Cbfβ-SMMHC. This study demonstrates that Cbfβ-SMMHC reduces the expression of Ebf1 and E2A in the CLPs and that it impairs the differentiation and survival of pre–pro-B cells and pro-B cells, resulting in a dramatic arrest in B-cell development. This study also provides a rationale for the myeloid specificity observed in inv(16) leukemia.
Methods
Mice
The conditional Cbfb-MYH11 knock-in mice in 129SvEv background (Cbfb+/56M or Cbfb56M/56M)12 were crossed with the Mx1-Cre transgenic mice (generously provided by Klaus Rajewsky, Harvard Medical School22 ). To assess V(D)J recombinase activity, Cbfb+/56M/Mx1-Cre mice were crossed with H2-SVEX transgenic mice.23,24 To induce expression of Cbfβ-SMMHC, mice were intraperitoneally injected with 3 doses of 200 μg/dose pIpC (In VivoGen, San Diego, CA) every other day at 4 weeks of age. BM cells from restored Cbfb+/56M and similarly treated Cbfb+/56M control littermates were analyzed 4 to 6 weeks after induction. All mice were treated in accordance with federal and state government guidelines and the University of Massachusetts Medical School Institutional Animal Care and Use Committee.
Noncompetitive repopulation assay
Unfractionated BM cells (106) from Cbfb+/56M/Mx Cre (floxed) or control mice (test mice: 129Sv/Ev; Ly9.1+) were transplanted into lethally irradiated (13 gy) wild-type C57BL/6 (Ly9.2+/Ly5.2+) mice. Engraft-ment of test cells was confirmed before administering 7 doses of pIpC (200 μg/dose) every other day, 2 weeks after transplantation. FITC-Ly9.1 (BD Biosciences, San Jose, CA) was used to determine the donor–derived population by flow cytometric analysis. Peripheral blood was analyzed every 2 weeks for 14 weeks. The BM progenitor cells were analyzed by flow cytometry at 16 weeks.
Flow cytometry and cell sorting
BM cells isolated from femurs and tibias were resuspended in biotin-, flavin-, and phenol red-deficient RPMI 1640 (Invitrogen, Carlsbad, CA) staining media containing 10 mM Hepes, pH 7.2, 0.02% sodium azide, 1 mM EDTA, 3% newborn calf serum, and treated 10 minutes on ice with 2.4G2 Fc block (BD Biosciences). Cells were incubated for 20 minutes on ice with primary antibodies, washed, and biotin-stained cells were incubated with secondary reagents for 15 minutes on ice. After 2 washes, cells were resuspended in 1 μg/mL propidium-iodide to exclude dead cells. Primary antibodies included CD24 (clone 30-F1) cascade blue or FITC, CD19 PE-Cy5 or biotin, B220 APC or biotin, CD43 phycoerythrin (PE), Ly6C biotin or FITC, DX5 biotin or FITC, and IgM (clone 331) biotin or FITC, AA4.1 APC or PE-Cy7, Sca1 (E13-161.7) FITC or PE-Cy5, IL-7R PE-Cy7 or PE, c-kit (2B8)-APC, CD229.1 (Ly-9.1) (30C7)-FITC, CD45.1 (Ly-5.1) (A20)-PE, CD3 (17A2), CD4 (L3T4), CD8 (53-6.7), Gr-1 (R86-8C5), Mac-1 (M1/70), Ter119 (Ly-76) conjugated with biotin (BD Biosciences; eBiosciences, San Diego, CA; Southern Biotechnology Associates, Birmingham, AL; CALTAG Laboratories, Burlingame, CA or purified from hybridoma supernatants and conjugates to fluorescent dye according to the manufacturer's protocol). Secondary reagents used were streptavidin (SA)-PE-Cy7, SA-APC-Cy7 (BD Biosciences) or SA-pacific blue (Molecular Probes/Invitrogen). Flow cytometry was performed on a 3-laser, 9-detector or a 12-detector LSR II (BD Biosciences) and analyzed with FlowJo software (Tree Star, Ashland, OR). Sorting was performed using a 3-laser, 7-detector DIVA FACSVantage (BD Biosciences) or a 3-laser, 7-detector MoFlo (DAKO Cytomation, Fort Collins, CO). The purity of sorted populations was 90% to 95%. Phenotypic progenitor populations were defined as CLP (Lin−/IL-7R+/Sca-1lo/AA4.1+ or Lin−/IL-7R+/c-kitINT/AA4.1+), pre–pro-B (Ly6C−/DX5−/IgM−/B220+/CD43+/CD24−/CD19− or Ly6C−/DX5−/IgM−/B220+/CD43+/AA4.1+/CD19−), pro-B (Ly6C−/DX5−/IgM−/B220+/CD43+ /CD24+/CD19+), and pre-B (Ly6C−/DX5−/IgM−/B220+/CD43−) as previously described.22-25
In vitro differentiation assay
Sorted CLPs were cultured on OP-9-MIGR126 stromal cells (generously provided by Juan Carlos Zuniga-Pflucker, Sunnybrook Research Institute and University of Toronto) supplemented with IL-7 (10 ng/mL) and Flt3 ligand (10 ng/mL) (PeproTech, Rocky Hill, NJ) for 4 days at 37°C with 5% CO2. Every 24 hours, cells were recovered to assess differentiation by cell- surface antigen staining and flow cytometry as well as qPCR analysis of the percentage of CbfbMYH11 restored cells. For the rescue experiments, BM cells were isolated from restored Cbfb+/56M/Mx1Cre or Cbfb+/56M control mice 3 days after treatment with 150 mg/kg 5-fluorouracil (Sigma, St Louis, MO) and transduced with MIG, MIG-Ebf1, or E47 retrovirus (generously provided by Harinder Singh and Babara Kee, University of Chicago) in the presence of 5 μg/mL polybrene (American Bioanalytical, Natick, MA) by spin-infection for 90 minutes followed by incubation at 37°C. Twenty-four hours after transduction, cells were plated on OP9-MIGR1 stromal cells supplemented with IL-7 (10 ng/mL) and Flt3 ligand (10 ng/mL) and cultured for 4 days. The differentiation status and the percentage of Cbfb+/MYH11 restored cells were analyzed every 24 hours as described above.
Quantitative PCR analysis of the percentage of CbfbMYH11 restored cells
Genomic DNA was isolated from differentiating CLPs using QIAamp DNA micro kit (Qiagen, Valencia, CA) following the protocol provided by the manufacturer. The relative proportion of restored allele was de-termined by qPCR using primers specific for the restored allele (fwd: 5′-CAGTGCTCTTGCTAGTGGATC-3′; rev: 5′-GCTCAACAGTATCAAGA-GTCG-3′) and the floxed allele (fwd: 5′-AGGCTCTCGATGAGCTGAT-GCTTTG-3′; rev: 5′-CTCCAGAAGAAGATGTTGGCGACC-3′) and compared with BM samples showing equal intensity of restored and floxed allele in Southern blot analysis (set as 50%). Q-PCR was performed in ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA) using Power SYBR Green PCR master mix (Applied Biosystems) containing 0.2 μM primer following manufacturer's suggestion.
Apoptosis analysis
BM cells were isolated and analyzed by flow cytometry as described above and apoptosis analysis was performed using FITC–annexin V and propidium-iodide (PI) staining according to manufacturer suggested procedures (BD Biosciences).
Quantitative RT-PCR
RNA from sorted BM populations was extracted with Trizol reagent (Invitrogen) according to manufacturer's protocol. First-strand cDNA was generated using 1 μg total RNA, 200 U SuperScript III reverse transcriptase (Invitrogen) and 0.5 μg oligo dT primer in a 20-μL reaction. Quantitative RT-PCR was performed using Power SYBR Green PCR master mix (Applied Biosystems) containing 0.2 μM gene-specific primers and detected in ABI PRISM 7000 sequence detection system (Applied Biosystems) according to manufacturer's instructions. Gene specific primers included Rag1-fw 5′-CATTCTAGCACTCTGGCCGG-3′ and Rag1-rv 5′-TCATCGGGTGCAGAACTGAA-3′, Rag2-fw 5′-TTAATTCCTGGCTTGGCCG-3′ and Rag2-rv 5′-TTCCTGCTTGTGGATGTGAAAT-3′, Ebf1-fw 5′-GCTGTGGCAACCGAAATGAG-3′ and Ebf1-rv 5′-CCGTGCTTGGAGT-TATTGTGGAC-3′, Tcfe2a-fw 5′-CTAGCCCCTCAACGCCTGTG-3′ and Tcfe2a-rv 5′-CGGTGCCAACAGCGTGGCT-3′, Flt3-fw 5′-ATCCTTCCCCAACCTGACTT-3′ and Flt3-rv 5′-TTGCCACCCATGTTCTGATA-3′, Pax5-fw 5′-TCCTCGGACCATCAGGACAG-3′ and Pax5-rv 5′-CCTGTTGATGGAGCTGACGC-3′, Sfpi1-fw 5′-CCTCCATCGGATGACTTG-3′ and Sfpi1-rv 5′-GTGTGCGGAGAAATCCCA-3′, Igll1-fw 5′-TGTGAAGTTCTCCTCCTGCTG-3′ and Igll1-rv 5′-ACCACCAAAGTACCTGGGTAG-3′, CD79a-fw 5′-CATCTTGCTGTTCTGTGCAGTG-3′ and CD79a-rv 5′-TTCTCATTTTGCCACCGTTTC-3′, Blk-fw 5′-TGGTCACCAGAGAGCCCATTTACA-3′ and Blk-rv 5′-TGTCAATCAGCCTTGGAAGGGACA-3′, VpreB1-fw 5′-CGTCTGTCCTGCTCATGCT-3′ and VpreB1-rv 5′-ACGGCACAGTAATACACAGCC-3′. Samples were normalized to β-actin transcript levels (Actb-fw 5′-CCCTAAGGCCAACCGTGAA-3′ and Actb-rv 5′-CAGCCTGGATGGCTACGTACAAG-3′), and the relative expression levels (RELs) were determined by the standard curve method.
Single-cell RT-PCR
Single cells were sorted into wells containing 4 μL lysis buffer containing 0.4% NP40, 25 μM DTT, 0.25 U RNasin, and 65 μM dNTP. For reverse transcription reactions, 4 μL of 2× Sensiscript reverse transcription reaction mix (Qiagen) was added to each well and incubated at 37°C for 60 minutes. Each sample was amplified with 2 rounds of PCR using Cbfb56M floxed allele specific primers (1st PCR fwd: 5′-GGCGTGATTTCATATGCGCGATTG-3′, rev: 5′-TCGAAATTGCCGTCAACCAAGCTC-3′; nested PCR fwd: 5′-AGGCTCTCGATGAGCTGATGCTTTG-3′, rev: 5′-CTCCAGAAGAAGATGTTGGCGACC-3′), CbfbMYH11 restored allele specific primers (1st PCR fwd: 5′-GCAGGCAAGGTATACTTGAAGG-3′, rev: 5′-CGTGAAGCTGTCTCTGCAGTTG-3′; nested PCR fwd: 5′-CCATGATTCTGAATGGAGTCTGTG-3′, rev: 5′-CTCTTCTCCTCATTCTGCTC-3′) and β-actin primers (1st PCR fwd: 5′-CTAGGCACCAGGGTGTGATGG-3′, rev: 5′-TCTCTTTGATGTCACGCACGA-3′; nested fwd: 5′-CGAGGCCCAGAGCAAGAGAG-3′, rev: 5′-CGGTTGGCCTTAGGGTTCAG-3′). PCR reactions were carried out in 25 μL reaction mix containing 0.2 mM dNTP, 1.5 mM MgCl2, and 1 U Taq polymerase (New England Biolabs, Ipswich, MA). Each reaction consisted of a denaturation at 94°C for 5 minutes, followed by 35 cycles of amplification (for 1st PCR: 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 35 seconds; for nested PCR: 94°C for 30 seconds, 62°C for 30 seconds, 72°C for 35 seconds) and a final extension step at 72°C for 5 minutes.
Results
Cbfβ-SMMHC impairs CLPs to pre-pro B-cell transition
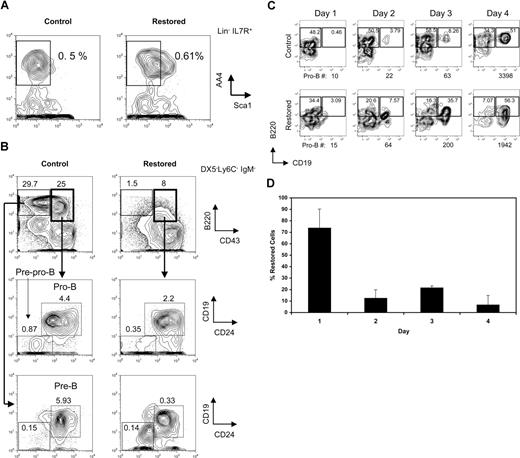
The leukemia fusion protein Cbfβ-SMMHC inhibits CBF function during hematopoietic differentiation. We have previously reported that mice expressing Cbfβ-SMMHC show a marked reduction in circulating B cells. To define the developmental point that is blocked by the fusion protein, B-cell progenitors from mice carrying a conditional Cbfb56M knock-in allele were analyzed by flow cytometry.12 In this model, the endogenous expression of Cbfβ from the Cbfb56M allele is switched to Cbfβ-SMMHC after Cre activation via the inducible Mx1Cre transgene. Expression of endogenous Cbfβ-SMMHC was “restored” in 4- to 5-week-old Cbfb+/56M/Mx1Cre mice by pIpC treatment, and B-cell progenitor populations in the BM were analyzed 4 to 6 weeks later. The CLP emerges from HSCs and multipotent progenitors, exhibits robust generation of B-lineage cells and retains some T- and NK-cell potential.25,27,28 In mouse strains that are not congenic for the Thy1.1 marker, such as 129SvEv, the CLPs can be defined as Lin−/IL-7R+/Sca-1lo/AA4.1+.28,29 The analysis of this compartment revealed that the frequency of CLPs in “restored” mice was unaltered relative to controls in 3 independent experiments (mean controls, 0.025% ± 0.022% of whole BM; mean “restored,” 0.038% ± 0.019%; a representative example is shown in Figure 1A). Similar results were obtained when using the markers used to enumerate CLPs in the C57BL/Ka strain (Lin−/c-kit+/IL7R+/Sca1lo; data not shown). Analysis of the earliest B-cell progenitors derived from CLPs, the pre–pro-B cells (DX5−Ly6C−IgM−B220+CD43+CD19−CD24−) as previously characterized,25 revealed significant reduction of approximately 3-fold (Figure 1B middle row; mean controls, 0.69% ± 0.29%; mean “restored,” 0.21% ± 0.12%; P = .02). Similar reduction of the subsequent pro-B-cell progenitors (DX5−Ly6C−IgM−B220+CD43+CD19+CD24+) was observed (mean controls, 2.68% ± 1.6%; mean “restored,” 1.23% ± 0.86%; P = .04). Furthermore, the pre-B-cell compartment (DX5−Ly6C−IgM−B220+CD43−CD19+CD24+) was markedly reduced more than 10-fold (Figure 1B bottom row; mean controls, 6.66% ± 0.64%; mean restored, 0.58% ± 0.25%; P ≤ .001).
Cbfβ-SMMHC impairs early B-lineage differentiation as early as the commitment step from CLPs to pre–pro-B cells. (A) Flow cytometric analysis of CLP cells (Lin−/IL-7R+/Sca-1lo/AA4.1+) in whole BM from wild-type control and restored Cbfb+/56M/Mx1Cre mice. Representative results of 3 independent experiments are shown. (B) Flow cytometric analysis of pre–pro-B (Ly6C−/DX5−/IgM−/B220+/CD43+/CD24−/CD19−), pro-B (Ly6C−/DX5−/IgM−/B220+/CD43+/CD24+/CD19+), and pre-B (Ly6C−/DX5−/IgM−/B220+/CD43−) cells in whole BM from control and restored Cbfb+/56M/Mx1Cre mice. Representative results of 3 independent experiments are shown. (C) Flow cytometric analysis of a time course of sorted CLPs from wild-type control or restored Cbfb+/56M/Mx1Cre mice cultured on OP9-MIGR1 stromal cells supplemented with IL-7 and Flt3 ligand. The number of pro-B cells produced at each time point is shown below each panel. Shown are representative results from 2 independent experiments. Numbers on plots are percentages of total cells. (D) Percentage of cells retaining the restored Cbfb-MYH11 allele over the 4-day culture, analyzed by qPCR. Shown are means plus or minus SD from 2 independent experiments, each performed in duplicate.
Cbfβ-SMMHC impairs early B-lineage differentiation as early as the commitment step from CLPs to pre–pro-B cells. (A) Flow cytometric analysis of CLP cells (Lin−/IL-7R+/Sca-1lo/AA4.1+) in whole BM from wild-type control and restored Cbfb+/56M/Mx1Cre mice. Representative results of 3 independent experiments are shown. (B) Flow cytometric analysis of pre–pro-B (Ly6C−/DX5−/IgM−/B220+/CD43+/CD24−/CD19−), pro-B (Ly6C−/DX5−/IgM−/B220+/CD43+/CD24+/CD19+), and pre-B (Ly6C−/DX5−/IgM−/B220+/CD43−) cells in whole BM from control and restored Cbfb+/56M/Mx1Cre mice. Representative results of 3 independent experiments are shown. (C) Flow cytometric analysis of a time course of sorted CLPs from wild-type control or restored Cbfb+/56M/Mx1Cre mice cultured on OP9-MIGR1 stromal cells supplemented with IL-7 and Flt3 ligand. The number of pro-B cells produced at each time point is shown below each panel. Shown are representative results from 2 independent experiments. Numbers on plots are percentages of total cells. (D) Percentage of cells retaining the restored Cbfb-MYH11 allele over the 4-day culture, analyzed by qPCR. Shown are means plus or minus SD from 2 independent experiments, each performed in duplicate.
To estimate the fraction of CLPs and early B-cell progenitors expressing Cbfβ-SMMHC in restored Cbfb+/56M/Mx1Cre mice, the efficiency of Cre–mediated activation of Cbfβ-SMMHC expression was tested by single-cell RT-PCR analysis of Cbfb-MYH11 transcripts in sorted populations. As shown in Table 1, more than 75% of single CLP cells expressed the Cbfb-MYH11 transcript, whereas a progressively smaller fraction of later B-cell progenitors expressed this transcript, including 47% of pre–pro-B, 13% of pro-B, and less than 3% of pre-B cells.
Percentage of cells expressing Cbfb-MYH11
| Cells . | Cc/Bc . | Percentage . |
|---|---|---|
| CLP | 70/91 | 77 |
| Pre–pro-B | 16/34 | 47 |
| Pro-B | 4/31 | 13 |
| Pre-B | 0/33 | <3 |
| Cells . | Cc/Bc . | Percentage . |
|---|---|---|
| CLP | 70/91 | 77 |
| Pre–pro-B | 16/34 | 47 |
| Pro-B | 4/31 | 13 |
| Pre-B | 0/33 | <3 |
Cc indicates the number of Cbfb-MYH11-positive cells; Bc, total number of beta-actin positive cells.
The decreasing proportion of cells that express the Cbfb-MYH11 transcript as B-cell differentiation proceeds in vivo from pre–pro-B to pro-B to pre-B likely reflects a biologic selection for cells that escape Cre-mediated deletion and do not express Cbfb-MYH11 transcript. To further evaluate this, CLPs from restored Cbfb+/56M/Mx1Cre mice were sorted and analyzed in an in vitro differentiation assay in which they were cocultured with OP9 stromal cells and supplemented with interleukin 7 (IL-7) and Flt3 ligand.30 CLPs from both restored and control mice readily generate B220+CD19+ pro-B cells after 4 days of culture (Figure 1C). Strikingly, on day 1, 70% of the cells isolated from cultures initiated with restored CLPs have the restored Cbfb-MYH11 allele, whereas on day 4, fewer than 10% of the cells still retain this allele (Figure 1D). These data are consistent with a strong selection against B-lineage cells expressing the Cbfb-MYH11 transcript.
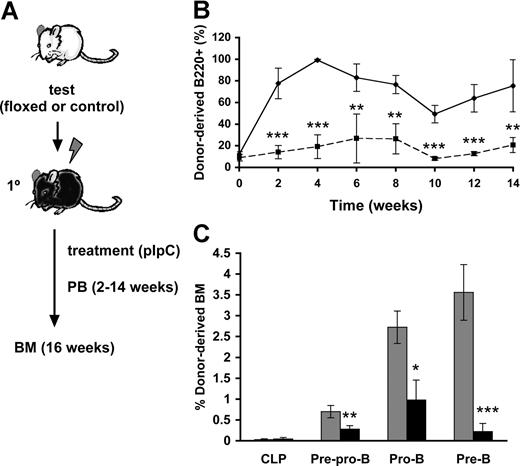
To assess whether the observed block of Cbfβ-SMMHC on B-cell differentiation is cell-autonomous, we performed a time-course analysis of B-cell differentiation using a noncompetitive repopulation assay (Figure 2A). Lethally irradiated wild-type mice were transplanted with 106Cbfb+/56M/Mx1Cre (floxed) or wild-type (control) unfractionated BM cells. Similar engraftment of floxed and control groups in the recipient mice was confirmed in the recipients 2 weeks after transplantation. The transplanted recipients (floxed and control) were then treated with 7 doses of pIpC to ensure maximum induction of Cbfβ-SMMHC expression. Flow cytometric analysis of peripheral blood using a time-course sampling over 2 to 14 weeks after pIpC treatment showed a profound reduction in the contribution of restored donor-derived B220+ B cells (Figure 2B), and this reduction was maintained during the entire course of the experiment. Analysis of BM progenitors from control and restored groups at 16 weeks posttreatment showed similar donor-derived contribution to CLPs, whereas restored-derived pre–pro-B cells and pro-B cells were reduced by 2- to-3-fold, and pre-B cells by approximately 15-fold (Figure 2C and data not shown). The impairment in differentiation observed in primary Cbfβ-SMMHC restored mice is precisely replicated in this repopulation assay, demonstrating that the Cbfβ-SMMHC–mediated early B-cell differentiation block is indeed cell-autonomous.
Cbfβ-SMMHC repression of early B-cell program is cell-autonomous. (A) Flow chart depicting the noncompetitive repopulation assay used in this study. BM cells from Cbfb+/56M/Mx1Cre floxed or control mice were transplanted into lethally irradiated wild-type recipients, and treated with pIpC 2 weeks later. Contribution of donor cells to peripheral blood was analyzed biweekly up to 14 weeks. BM progenitor populations were analyzed at 16 weeks. (B) Time course of control- (♦) or restored- (■) derived B220+ B cells in the peripheral blood shown as mean plus or minus SD (n = 5; **P < .01; ***P < .001). (C) Contribution of control- (▩) and Cbfβ-SMMHC restored- (■) cells in various progenitor populations, including CLP, pre–pro-B, pro-B, and pre-B cells in the BM 16 weeks after induction. Shown are mean plus or minus SD (n = 5; *P < .05; **P < .01; ***P < .001).
Cbfβ-SMMHC repression of early B-cell program is cell-autonomous. (A) Flow chart depicting the noncompetitive repopulation assay used in this study. BM cells from Cbfb+/56M/Mx1Cre floxed or control mice were transplanted into lethally irradiated wild-type recipients, and treated with pIpC 2 weeks later. Contribution of donor cells to peripheral blood was analyzed biweekly up to 14 weeks. BM progenitor populations were analyzed at 16 weeks. (B) Time course of control- (♦) or restored- (■) derived B220+ B cells in the peripheral blood shown as mean plus or minus SD (n = 5; **P < .01; ***P < .001). (C) Contribution of control- (▩) and Cbfβ-SMMHC restored- (■) cells in various progenitor populations, including CLP, pre–pro-B, pro-B, and pre-B cells in the BM 16 weeks after induction. Shown are mean plus or minus SD (n = 5; *P < .05; **P < .01; ***P < .001).
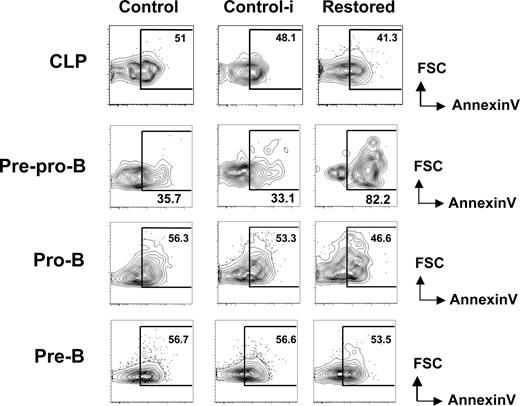
We reasoned that the hindrance of early B-cell differentiation by Cbfβ-SMMHC could result from an increase in apoptosis. To address this, we analyzed the proportion of early apoptotic cells within early B-cell progenitors in restored mice and wild-type untreated (control) and pIpC treated wild-type (control-i) mice using annexin V and PI staining. The percentage of apoptotic CLPs was unchanged between groups (Figure 3 top row). In contrast, annexin V+ PI− population was increased 2- to 3-fold within the pre-pro-B-cell compartment of Cbfβ-SMMHC restored mice (Figure 3 second row). The increase in early apoptotic population coincides precisely with the developmental block, suggesting that Cbfβ-SMMHC affects the differentiation and the survival of pre–pro-B cells.
Increased apoptosis in pre–pro-B cells induced by Cbfβ-SMMHC. Flow cytometric analysis for annexin V staining analysis in live (PI−) BM CLPs, pre–pro-B, pro-B, and pre-B populations from control (Cbfb+/56M), control-i (pIpC injected Cbfb+/56M), and restored (pIpC injected Cbfb+/56M/Mx1Cre) mice. Shown are representative data from 3 independent experiments. Numbers on plots are percentages of displayed cells.
Increased apoptosis in pre–pro-B cells induced by Cbfβ-SMMHC. Flow cytometric analysis for annexin V staining analysis in live (PI−) BM CLPs, pre–pro-B, pro-B, and pre-B populations from control (Cbfb+/56M), control-i (pIpC injected Cbfb+/56M), and restored (pIpC injected Cbfb+/56M/Mx1Cre) mice. Shown are representative data from 3 independent experiments. Numbers on plots are percentages of displayed cells.
Taken together, these results show that Cbfβ-SMMHC expression does not block differentiation before CLPs. Rather, its expression impairs B lineage differentiation as early as the commitment step between CLPs and pre–pro-B cells and profoundly blocks production or survival of pro-B and pre–B-cell progenitor populations.
Cbfβ-SMMHC deregulates B lineage specifying transcription program
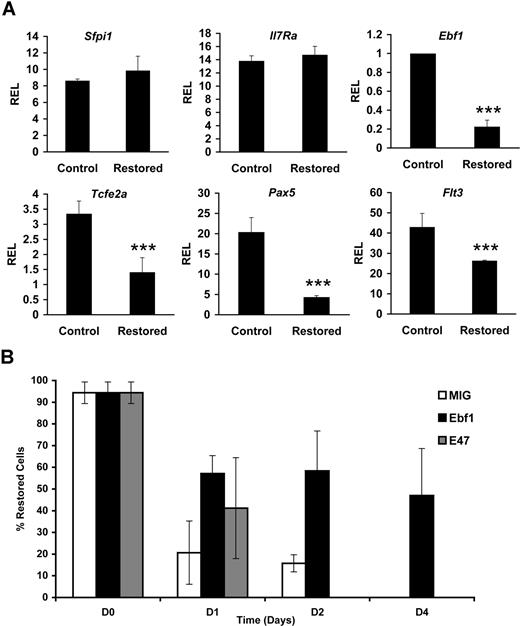
Given the finding that Cbfβ-SMMHC expression elicits a marked reduction of the first committed B-lineage progenitors, pre–pro-B cells, we tested the expression status of proteins that participate in B-cell fate decisions in the CLP compartment. Early stages of B-cell differentiation represent a tightly regulated program in which multipotent progenitors give rise to pro-B cells that successfully rearrange and express an immunoglobulin heavy chain and signal through the pre–B-cell antigen receptor complex, permitting transition to the pre–B-cell stage. Critical transcription factors that regulate this process include E2A (encoded by the Tcfe2a gene), Ebf1, and PU.1 (encoded by the Sfpi1 gene), as well as signaling from cytokine receptors Flt3 and IL-7R.31-37 Ebf1 is a critical factor for the activation of the B lineage–specific gene program, including the regulation of the lineage commitment factor Pax5.38,39 The expression of these regulatory factors in CLPs sorted from control and restored Cbfb+/56M/Mx1Cre mice was analyzed using qPCR. We found a 60%–80% reduction in Ebf1, Tcfe2a, and Pax5 transcript levels (Figure 4A). The expression of Flt3 transcript was reduced 40%, although detection of the receptor at the cell surface was reduced modestly (data not shown). The expression of Il-7r or Sfpi1 transcripts was not changed. Together, these results demonstrate that Cbfβ-SMMHC inhibits expression of the B-lineage transcriptional program in the CLP cells.
Cbfβ-SMMHC hinders B lineage–specifying transcriptional programs. (A) Relative expression levels of B lineage–specifying factor transcripts Sfpi-1, Il7r, Ebf1, Tcfe2a, Pax5, and Flt3 in control or Cbfβ-SMMHC restored CLPs analyzed by qRT-PCR. Shown are means plus or minus SD (n = 3; ***P < .001) from 3 to 5 independent sorting experiments, each performed by duplicate. (B) Percentage of cells retaining the restored Cbfb-MYH11 alleles analyzed by qPCR. Cells were transduced with MIG (□; 26% GFP+), Ebf1 (■; 34% GFP+) or E47 (▩; 24% GFP+) and differentiated on OP9-MIGR1 stromal cells over a period of 4 days. Shown are the representative means plus or minus SD data of 2 independent experiments, each performed in duplicate.
Cbfβ-SMMHC hinders B lineage–specifying transcriptional programs. (A) Relative expression levels of B lineage–specifying factor transcripts Sfpi-1, Il7r, Ebf1, Tcfe2a, Pax5, and Flt3 in control or Cbfβ-SMMHC restored CLPs analyzed by qRT-PCR. Shown are means plus or minus SD (n = 3; ***P < .001) from 3 to 5 independent sorting experiments, each performed by duplicate. (B) Percentage of cells retaining the restored Cbfb-MYH11 alleles analyzed by qPCR. Cells were transduced with MIG (□; 26% GFP+), Ebf1 (■; 34% GFP+) or E47 (▩; 24% GFP+) and differentiated on OP9-MIGR1 stromal cells over a period of 4 days. Shown are the representative means plus or minus SD data of 2 independent experiments, each performed in duplicate.
Because Ebf1 and E47 are critical factors that specify B-cell development, we tested whether restoration of Ebf1 or E47 expression could rescue B-cell differentiation. To test this hypothesis, 5-FU–treated BM progenitor cells from restored Cbfb+/56M; Mx1Cre mice were transduced with MIG, Ebf1, or E47 retrovirus and tested in an in vitro differentiation culture on OP9 stromal cells supplemented with IL-7 and Flt3 ligand. Within 4 days of culture, B220+CD19+ pro-B cells can be readily produced from both control and restored BM progenitor cells (data not shown). Consistent with the strong selection against Cbfβ-SMMHC–expressing cells, MIG-transduced cells showed a drastic reduction in the percentage of cells retaining the restored Cbfb-MYH11 allele and was practically undetectable by day 4 (Figure 4B). Restoration of Ebf1 expression rescued the deficiency as the restored Cbfb-MYH11 allele was retained in 60% of cells at day 2 and 47% at day 4. Restoration of E47 expression was unable to rescue the defect. These results suggest that Cbfβ-SMMHC–induced impairment of B-cell differentiation is, at least in part, because of the repression of Ebf1 expression.
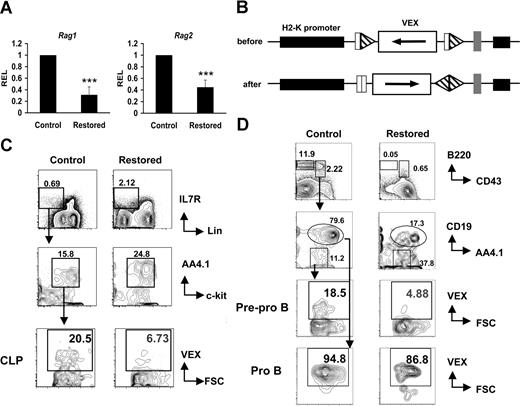
Cbfβ-SMMHC reduces Rag1/2 expression and V(D)J recombinase activity
In developing lymphocytes, Ig and T-cell receptor genes are assembled by sequence-specific V(D)J recombination of variable (V), diversity (D), and joining (J) gene segments, initiated by the products of recombinase activating genes 1 and 2 (Rag1 and Rag2). Because expression of the Rag locus is regulated by E2A,35 we reasoned that the reduction in transcript levels of these factors should affect Rag expression and the function of V(D)J recombinase in CLP cells. The expression of Rag1 and Rag2 genes was analyzed by qRT-PCR in RNA samples from sorted restored and control CLP cells. We found that Rag1 expression is 75% reduced and Rag2 expression is 50% reduced in restored CLPs (Figure 5A). To directly assess V(D)J recombinase activity, we crossed Cbfb56M/56M/Mx1Cre mice with the H2-SVEX recombination substrate reporter transgenic line in which VEX serves as a sensitive reporter of V(D)J recombinase activity (Figure 5B).23,24 We found that the percentage of VEX-positive cells was reduced approximately 70% in both the CLP cells (Figure 5C) and in pre–pro-B cells (Figure 5D) of restored mice. Our results showing that CLPs and pre–pro-B cells expressing Cbfβ-SMMHC have reduced V(D)J recombinase activity, but no reduction occurs in pro-B cells, are consistent with our finding that Cbfβ-SMMHC is expressed in higher proportions of CLPs and pre–pro-B cells, and only a small fraction of pro-B cells express Cbfβ-SMMHC. These data provide functional evidence suggesting that the reduced expression of Ebf1 and E2A inhibited Rag1/2 expression, compromising V(D)J rearrangement in CLPs and pre–pro-B cells expressing Cbfβ-SMMHC.
Cbfβ-SMMHC hinders Rag1 and Rag2 expression, and V(D)J recombinase activity. (A) Relative expression levels of Rag1 and Rag2 in sorted CLPs by qRT-PCR. Shown are mean plus or minus SD (n = 3; *** P < .001) from at least 2 independent sorting experiments. (B) Illustration of the transgene H2-SVEX used in this study. The VEX reporter (white rectangle) in the antisense orientation flanked by V(D)J recombination signal sequences (triangles) that direct inversional recombination is driven by the murine H2K promoter (black rectangle). (C) Analysis of recombination reporter VEX expression in BM CLP populations from Cbfb+/56M/Mx1Cre/H2-SVEX mice (restored) or control mice. (D) Analysis of recombination reporter VEX expression in BM pre–pro-B and pro-B populations from Cbfb+/56M/Mx1Cre/H2-SVEX mice (restored) or control mice. The data represent results from at least 3 independent experiments. Numbers on plots are percentages of displayed cells.
Cbfβ-SMMHC hinders Rag1 and Rag2 expression, and V(D)J recombinase activity. (A) Relative expression levels of Rag1 and Rag2 in sorted CLPs by qRT-PCR. Shown are mean plus or minus SD (n = 3; *** P < .001) from at least 2 independent sorting experiments. (B) Illustration of the transgene H2-SVEX used in this study. The VEX reporter (white rectangle) in the antisense orientation flanked by V(D)J recombination signal sequences (triangles) that direct inversional recombination is driven by the murine H2K promoter (black rectangle). (C) Analysis of recombination reporter VEX expression in BM CLP populations from Cbfb+/56M/Mx1Cre/H2-SVEX mice (restored) or control mice. (D) Analysis of recombination reporter VEX expression in BM pre–pro-B and pro-B populations from Cbfb+/56M/Mx1Cre/H2-SVEX mice (restored) or control mice. The data represent results from at least 3 independent experiments. Numbers on plots are percentages of displayed cells.
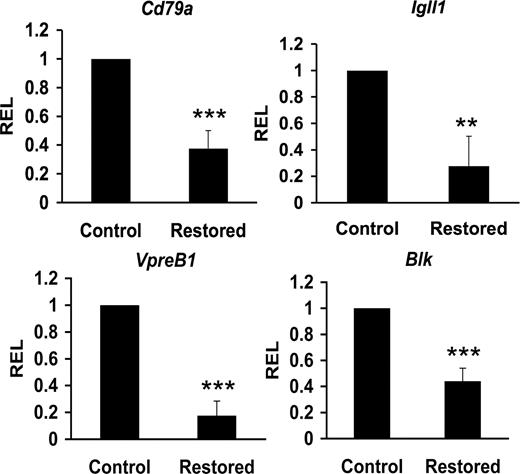
Cbfβ-SMMHC represses expression of B cell–specific factors
The combinatorial effect of early B-cell factors E2A, EBF, and Pax5 form a circuitry that interact with CBF to coregulate target genes acting in the progression and commitment of B-cell progenitors, including Cd79a (also called mb-1), Igll1 (also called lambda5), and Blk genes.18,21,40-42 These factors also regulate VpreB1 expression independent of CBF.43 We reasoned that Cbfβ-SMMHC–mediated reduction of Tcfe2a, Ebf1, and Pax5 expression could also repress expression of their target genes in CLPs from restored mice. Sorted CLPs from control and restored Cbfb56M/Mx1Cre mice were used to quantify the expression levels by qRT-PCR. This analysis revealed a 50% to 85% reduction of Cd79a1, Igll5, VpreB1, and Blk transcripts in CLPs expressing Cbfβ-SMMHC (Figure 6). These results provide evidence that Cbfβ-SMMHC also inhibits expression of B cell–specific transcripts as early as in the CLP compartment. Furthermore, these data provide a molecular mechanism for the dramatic arrest in B-cell development; impaired V(D)J recombination and reduced expression of pre-BCR components are predicted to inhibit the pro-B to pre–B-cell transition.
Cbfβ-SMMHC reduces expression of B lineage–specific factors. Relative expression levels of B lineage–specific transcripts Cd79a (mb-1), Igll1 (lambda5), VpreB1, and Blk in control or Cbfβ-SMMHC restored CLPs analyzed by qRT-PCR. Shown are mean plus or minus SD (n = 3; ** P < .01; *** P < .001) from 3 to 5 independent sorting experiments, each performed by duplicate.
Cbfβ-SMMHC reduces expression of B lineage–specific factors. Relative expression levels of B lineage–specific transcripts Cd79a (mb-1), Igll1 (lambda5), VpreB1, and Blk in control or Cbfβ-SMMHC restored CLPs analyzed by qRT-PCR. Shown are mean plus or minus SD (n = 3; ** P < .01; *** P < .001) from 3 to 5 independent sorting experiments, each performed by duplicate.
Discussion
This study shows that expression of the acute myeloid leukemia fusion protein Cbfβ-SMMHC, a repressor of CBF function, alters initiation of the B-cell developmental program in CLPs by hindering the expression of critical transcription factors, and rendering CLPs with reduced V(D)J recombination and deficient activation of B cell–specific factors. In addition, B-cell differentiation is blocked at the earliest B cell–committed progenitors, the pre–pro-B cells, causing reduction in the cell number and increases in cell death.
The CBF subunits Runx1 and Cbfβ are expressed in all stages of B-cell differentiation.44-46 B-cell maturation is significantly reduced in mice lacking Runx1 or expressing Cbfβ-SMMHC,14,47,48 and the number of Runx1−/− CLPs were reduced 7-fold.47 We find that expression of Cbfβ-SMMHC does not significantly change the number or the apoptotic status of CLP cells. This difference is not the result of inefficient Cre-mediated activation of the fusion gene because approximately 75% of CLPs do express Cbfβ-SMMHC. Instead, Cbfβ-SMMHC expression may cause an incomplete repression of CBF function. Consistent with this finding, inv(16)-positive cells can be detected in a fraction of CD19+ B cells in the BM of patients with AML subtype M4Eo,49 indicating that CBFβ-SMMHC-expressing B-cell progenitors could also be present in humans.
We observed that Cbfβ-SMMHC reduces the frequency of pre-pro B cells in vivo approximately 3-fold, concomitant with a 3-fold increase in apoptosis, demonstrating that the CLP to pre–pro-B–cell transition is tightly regulated by CBF. In addition, the pro-B to pre–B-cell transition seems to be another likely CBF regulatory point as we found a 15-fold reduction of pre-B cells. These progenitors are probably also undergoing apoptosis but this effect may have been undetected because of the reduced number of Cbfβ-SMMHC–positive pro-B cells remaining in the compartment. Future studies using conditional Cbfb knock-out mice in combination with B cell–specific Cre-transgenics, such as CD19-Cre, will prove useful to further define the precise sites of CBF regulation in the B-cell progenitors. Alternatively, as indicated by our findings with cultured CLPs that differentiated in vitro, the persistence of small numbers of undeleted/non–Cbfβ-SMMHC expressing cells in the Mx1Cre system may reflect a very strong biologic selection or advantage of undeleted cells that could be present in any Cre-mediated system.
The understanding of the regulatory networks that act during B-cell development is evolving. This process includes hierarchical regulatory circuits of cytokine receptors and transcription factors.50 We analyzed the expression of transcripts for the transcription factors E2A, Ebf1, and Pax5, and cytokine receptors IL7R and Flt3, which are critical for the development of CLPs and the subsequent B-lineage committed progenitors from the multipotential progenitor cells. Our findings that Cbfβ-SMMHC–positive CLPs showed a 2-fold reduction in Tcfe2a and a 5-fold reduction in Ebf1 transcript levels and the rescue of B-cell differentiation in OP9 coculture observed in addition to Ebf1 by retroviral transduction suggests that CBF may regulate the expression of these factors before B-cell commitment. The observation that E47 ectopic expression was insufficient to rescue differentiation supports the idea that, although E47 is upstream of Ebf1, CBF function may be required for expression of both factors. Future studies should focus on the molecular mechanism of CBF function in the E47-Ebf regulatory network before B-cell commitment. Consistent with our findings, mice lacking Tcfe2a or Ebf1 have no B cells and heterozygous mice have reduced B cells.33-35 The process of V(D)J rearrangement, catalyzed by Rag1 and Rag2, is essential for lymphoid differentiation. B lineage–specific regulation of V(D)J recombinase activity is established in CLPs and the Tcfe2a gene product E47 is required for Rag1 expression and V(D)J recombination, reflecting E2A-dependent regulation of the B-specific Erag enhancer of the Rag locus.23,24,36,51 We found that the V(D)J recombinase activity and expression of both Rag1 and Rag2 were reduced in CLPs expressing Cbfβ-SMMHC. This phenotype and the defect in Tcfe2a expression mark a functional deficiency of CLPs that is consistent with our previous results indicating Rag expression in CLPs requires the E2A-encoded factor E47, and probably Ebf1.52,53 Therefore, as E2A promotes expression of Ebf1, it will be of interest to determine whether CBF factors directly regulates Ebf1 in CLPs and whether the observed recombination defect is, at least in part, because of the regulation of Rag via Ebf1.
It has been proposed that, although E2A is expressed at low levels in the HSC/multipotent progenitors, its up-regulation in CLPs enables Rag1/2 and Ebf1 gene expression and rearrangement of the IgH locus.36,50 Thus, our findings suggest that CBF could, directly or indirectly, be involved in Tcfe2a up-regulation. E2A and EBF are primary B-cell fate determinants required for the activation of the B lineage–specific gene program from the CLPs, including expression of the lineage commitment factor Pax5.38,39,54 Our finding that Cbfβ-SMMHC also reduces the expression of Pax5 transcript suggests that CBF may directly or indirectly regulate Pax5 expression in CLPs. Furthermore, because E47-deficient mice have a marked reduction in pre–pro-B cells and essentially lack pro-B cells,36 reduction of E2A via the action of Cbfβ-SMMHC may explain much of the B-cell developmental arrest that we observed.
B-cell differentiation beyond the pro–B-cell stage requires the expression of the pre-BCR complex components including Cd79a, Igll1, and VpreB1, and the src-related kinase Blk. Cbfβ-SMMHC could be executing a dual inhibition on the expression of the pre-BCR components by repressing transcription factors E2A, EBF1, and PAX5 and also blocking CBF function on the cis-regulatory regions of their target genes. Transition to the pre–B-cell stage also requires the ordered and productive DNA rearrangement of the Ig heavy chain (IgH). Normal pre-BCR assembly is a critical developmental checkpoint that promotes expansion and survival and differentiation of pro-B cells that have successfully rearranged an IgH locus. It is likely that the reduced generation of pro-B and pre-B cells could result from the lower expression of the pre-BCR components and reduced V(D)J rearrangement.
Clinically, human inv(16)–positive leukemic samples that express CBFβ-SMMHC have been exclusively associated with acute myeloid leukemia. Two lines of evidence support the HSC origin of the inv(16) rearrangement: first, the cytogenetic abnormality can be detected in CD34+CD38− cells,55,56 and second, a case of inv(16)-positive AML has been reported that relapsed with a pro–B-cell acute lymphoblastic leukemia.57 However, inv(16) can be detected in only a small proportion of CD19+ B cells in the BM of patients with inv(16) positive AML,49 suggesting that the presence of inv(16) may have a negative impact on the differentiation and survival of B-cell lineage. Our study in the mouse provides evidence that Cbfβ-SMMHC impairs the development of CLPs into B-cell lineage and induces an increase in apoptosis in pre–pro-B cells. These results provide a mechanistic basis for the model that Cbfβ-SMMHC blocks differentiation of early B-cell, T-cell, and myeloid progenitors, activating apoptosis of lymphoid progenitors while creating a leukemia precursor in the myeloid compartment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors appreciate the superb technical assistance of Erin Cloherty and Heather Paquin.
This work was supported by National Institutes of Health grants R01 CA096983 (L.H.C.) and R01 AI043534 (R.M.G.) and the Ruth L. Kirschstein National Research Service Award F32CA101571 (Y.-H.K.).
Authorship
Contribution: Y.-H.K. designed and performed experiments, analyzed experimental results, and helped write the manuscript; R.M.G. participated in the design of experiments, performed experiments, and helped write the manuscript; and L.H.C. participated in the design and interpretation of the experiments and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lucio H. Castilla, Program in Gene Function and Expression, University of Massachusetts Medical School, 364 Plantation Street, LRB Room 622, Worcester, MA 01605; e-mail: Lucio.Castilla@umassmed.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal