Abstract
Our recent study demonstrated that a novel proteasome inhibitor NPI-0052 triggers apoptosis in multiple myeloma (MM) cells, and importantly, that is distinct from bortezomib (Velcade) in its chemical structure, effects on proteasome activities, and mechanisms of action. Here, we demonstrate that combining NPI-0052 and bortezomb induces synergistic anti-MM activity both in vitro using MM cell lines or patient CD138+ MM cells and in vivo in a human plasmacytoma xenograft mouse model. NPI-0052 plus bortezomib–induced synergistic apoptosis is associated with: (1) activation of caspase-8, caspase-9, caspase-3, and PARP; (2) induction of endoplasmic reticulum (ER) stress response and JNK; (3) inhibition of migration of MM cells and angiogenesis; (4) suppression of chymotrypsin-like (CT-L), caspase-like (C-L), and trypsin-like (T-L) proteolytic activities; and (5) blockade of NF-κB signaling. Studies in a xenograft model show that low dose combination of NPI-0052 and bortezomib is well tolerated and triggers synergistic inhibition of tumor growth and CT-L, C-L, and T-L proteasome activities in tumor cells. Immununostaining of MM tumors from NPI-0052 plus bortezomib–treated mice showed growth inhibition, apoptosis, and a decrease in associated angiogenesis. Taken together, our study provides the preclinical rationale for clinical protocols evaluating bortezomib together with NPI-0052 to improve patient outcome in MM.
Introduction
Normal cellular homeostasis requires balanced regulation of protein synthesis and degradation. Intracellular protein degradation occurs for the most part via a multisubunit complex called the proteasome.1-4 Earlier studies by Ciechanover, Hershko, and Rose et al demonstrated that ATP-dependent conjugation of proteins with polypeptide (ubiquitin) mediates protein degradation.5-11 The 26S multisubunit proteasome complex12-15 contains 19S units flanking a barrel-shaped 20S proteasome core.16-18 The 19S units of the 26S proteasome complex regulate entry of ubiquitinated proteins into the 20S core chamber.2,19,20 Protein ubiquitination is facilitated through several enzymatic reactions involving E1 and E2 ubiquitin enzymes as well as E3 ubiquitin ligase.21,22 Once the ubiquitinated proteins are recognized by the 19S regulatory subunits of the proteasome complex, they are degraded into small peptides by 3 major proteasomal activities residing within the 20S core complex (ie, chymotrypsin-like [CT-L], trypsin-like [T-L], and caspase-like [C-L] activities).23-26
Proteasomes regulate many normal cellular processes, such as cell cycle, inflammation, transcription, DNA replication, and apoptosis via proteolysis of key enzymes and regulatory proteins. Deregulation of the ubiquitin-proteasome signaling (UPS)27 pathway is linked to the pathogenesis of various human diseases,4,28,29 and therefore proteasome inhibitors offer great promise as therapeutic agents.
The dipeptidyl boronic acid bortezomib/PS-341 (Velcade; Millennium Pharmaceuticals, Cambridge, MA), a reversible inhibitor of CT-L,30 exhibited remarkable antitumor activity against the 60 NCI tumor cell line panel. Bortezomib is the first in class proteasome inhibitor approved by the US Food and Drug Administration (FDA) for the treatment of relapsed and relapsed/refractory multiple myeloma (MM) and mantle cell lymphoma.4,31-33 Even though bortezomib therapy is a major advance (43% objective response rates),32,33 it has been associated with possible off-target toxicities and the development of drug-resistance.34-38
Our recent study39 characterized a novel proteasome inhibitor NPI-0052, a small molecule derived from the fermentation of the marine Gram-positive actinomycete Salinospora tropica.40 NPI-0052 induces apoptosis in MM cells resistant to conventional and bortezomib therapies without significantly affecting normal lymphocyte viability.39 Importantly, NPI-0052 is distinct from bortezomib in its chemical structure, effects on proteasome inhibition profiles, and mechanisms of action.39 For example, biochemical and genetic studies showed that NPI-0052, in contrast to bortezomib, relies more on FADD–caspase-8–mediated cell death signaling in MM cells. In vivo studies using human MM xenografts show that NPI-0052 is well tolerated, prolongs survival, and reduces tumor recurrence.39 Similar antitumor activity by NPI-0052 has been reported in chronic lymphocytic leukeumia (CLL) and colon cancer cells.41,42 Our preclinical data provided the basis for the ongoing phase 1 clinical trial of NPI-0052 in patients with relapsed/refractory MM.
A recent series of elegant studies using an in vitro protein model system demonstrate that simultaneous inhibition of multiple proteasome activities is a prerequisite for significant (ie, greater than 50%) proteolysis.25 Moreover, MM and leukemia cell lines exhibit a differential pattern of qualitative and quantitative constitutive proteasome activity,43,44 suggesting a differential requirement to inhibit proteasome activity. Our prior study showed that bortezomib predominantly inhibits proteasome CT-L and more recently defined C-L activities.39 A recent study showed that the epoxyketone-based irreversible proteasome inhibitor PR-171, like bortezomib, predominantly blocks CT-L activity.45 Importantly, NPI-0052 blocks all 3 (CT-L, T-L, and C-L) proteasome activities.39 It is therefore likely that NPI-0052, by virtue of its ability to block all 3 proteasome activities, can be combined with bortezomib or PR-171 to confer a broader proteasome inhibition at lower and potentially safer doses. These findings provide the rationale for combining NPI-0052 with bortezomib to achieve optimal proteasome inhibition and potent antitumor activity while allowing for the use of lower doses of bortezomib to reduce toxicity.
In the present study, we characterized the effects of NPI-0052 and bortezomib combinations against MM cell lines and primary patient cells resistant to conventional and novel therapies. In both in vitro and in vivo MM xenograft models, combined NPI-0052 and bortezomib inhibits growth of MM cells and overcomes drug resistance, setting the stage for potential clinical trials of combination therapy to improve patient outcome in MM.
Methods
Cell culture
MM.1S (dexamethasone [Dex]–sensitive), MM.1R (Dex-resistant), RPMI-8226, doxorubicin (Dox)–resistant (Dox-40), U266, OPM2, and INA6-6 (IL-6–dependent) human MM cell lines were maintained as previously described.39 MM cells were freshly isolated from patients relapsing after multiple prior therapies including Dex, melphalan, thalidomide, or bortezomib. Tumor cells were purified by CD138+ selection46 using the Auto MACS magnetic cell sorter (Miltenyi Biotec, Auburn, CA). Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Cells were treated with NPI-0052 (Nereus Pharmaceuticals, San Diego, CA), and bortezomib (Millennium Pharmaceuticals, Cambridge, MA). Peripheral blood mononuclear cells (PBMNCs) from healthy donors were maintained in culture medium.39
Cell viability and apoptosis assays
Cell viability was assessed by MTT (Chemicon International, Temecula, CA) assay, as previously described.46 Cell death was quantified using a live and dead cell assay from Molecular Probes (Carlsbad, CA). Briefly, 106 cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or their combination; stained with ethidium homodimer and calcein-AM; and then analyzed under a fluorescence microscope. Annexin V/propidium iodide (PI) staining assays were performed as previously described.46 A TUNEL apoptosis detection kit (Upstate/Millipore, Billerica, MA) was used to measure apoptosis in murine tumor sections.
In vitro migration and capillary-like tube structure formation assays
Western blotting
Immunoblot analysis was performed using antibodies to caspase-8, caspase-9, caspase-3 (Cell Signaling, Beverly, MA), PARP, Bcl-2, BIM, Hsp-27, Hsp-70, Hsp-90, CHOP (CEBP homology protein), phospho-eIF2-α, phospho-JNK, actin, or tubulin (BD Bioscience Pharmingen, San Diego, CA). Blots were then developed by enhanced chemiluminesence (ECL; Amersham, Arlington Heights, IL).
In vitro and in vivo proteasome activity assays
20S proteasome activity assays were performed using fluorogenic peptide substrates, as previously described.39,49 In vivo comparative analysis of proteasome activities was performed in tumors from treated mice. At 90 minutes after administration of drugs, the animals were anesthetized and tumors were excised, followed by determination of ex vivo proteasome activity.
Human plasmacytoma xenograft model
All animal studies were approved by the Dana Farber Cancer Institute Institutional Animal Care and Use Committee. The xenograft tumor model was performed as previously described.39,50 CB-17 severe combined immunodeficiency (SCID) mice (n = 24; Taconic, Gemantown, NY) were subcutaneously inoculated with 5.0 × 106 MM.1S cells in 100 μL serum-free RPMI-1640 medium. When tumors were measurable approximately 3 weeks after MM cell injection, mice were treated with vehicle or drugs at various doses on a twice-a-week schedule for 4 consecutive weeks. Tumor size was measured every third day in 2 dimensions using calipers, and tumor volume was calculated using the following formula: V = 0.5a × b2, where “a” and “b” are the long and short diameter of the tumor, respectively. Animals were killed when their tumors reached 2 cm3. In situ detection of apoptosis, IHC determination of proliferation, and assessment of MVD Apoptotic cells in xenograted MM tumors were identified by TUNEL assays using the ApopTag in situ apoptosis detection system (Serologicals, Norcross, GA)47 and immunohistochemical (IHC) staining for caspase-3 activation and hematoxylin and eosin (H&E) staining. Ki-67 was determined by automated IHC staining to quantify proliferation.47 Microvessel density (MVD), a marker for tumor angiogenesis, was quantified by IHC staining of factor VIII.47 VEGFR1/FLT-1 expression was examined by IHC staining with specific VEGFR1 Abs (Abcam, Cambridge, MA), as previously described.51 IHC section analysis was done using microscopy (Leica DM LB).
Statistical analysis
The Wilcoxon signed-rank test was performed to compare proliferation in untreated and treated patient cells, and the Jonchkeere-Terpstra (J-T) trend test was used to measure viability of lymphocytes and cell lines. Statistical significance of differences observed in NPI-0052–, bortezomib-, or NPI-0052 plus bortezomib–treated mice compared with control groups was determined using a Student t test. The minimal level of significance was a P value less than .05. Tumor growth inhibition was determined using the SigmaPlot analysis software (Systat Software, San Jose, CA). Isobologram analysis was performed usin the CalcuSyn software program (Biosoft, Ferguson, MO, and Cambridge, United Kingdom). A combination index (CI) less than 1.0 indicates synergism, and a CI of 1 indicates additive activity.52
Results
Combined low doses of NPI-0052 and bortezomib trigger synergistic anti-MM activity
For these studies, we used NPI-0052 and bortezomib at concentrations lower than their respective IC50 for each cell line. MM.1S, U266, RPMI-8226, INA-6, OPM-2, Dox-40, and MM.1R cells were treated for 24 hours with low doses of NPI-0052, bortezomib, or the combination and analyzed for viability by MTT assay. A more significant decrease in viability of all cell lines was noted in response to treatment with combined low doses of NPI-0052 and bortezomib than with either agent alone (P < .05, n = 3; Figure 1A-G). Isobologram analysis52 confirmed that combined NPI-0052 plus bortezomib triggered synergistic anti-MM activity in MM cell lines, albeit with differential kinetics. The combination ratio of agents varied for each cell line to achieve a similar degree of cell death. Shown in Figure 1 are representative results from minimally toxic and maximally additive concentrations of each agent: for example, treatment of U266 MM cells with low doses of 7 nM NPI-0052 (IC50 = 24 nM) and 1 nM bortezomib (IC50 = 30 nM) triggers 43% growth inhibition, whereas no significant growth inhibition was observed using either of these agents alone at these low concentrations (Figure 1B). These data confirm and extend our prior findings demonstrating synergistic anti-MM activity of NPI-0052 plus bortezomib.
Combination of low doses of NPI-0052 and bortezomib induces synergistic MM cell death. MM.1S (A), U266 (B), RPMI-8226 (C), INA-6 (D), OPM-2 (E), Dox-40 (F), or MM.1R (G) cells were treated with NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib and assessed for viability using MTT assays. The concentrations of drugs, either alone or in combination, were as follows: MM.1S cells: 1 nM NPI-0052, 3 nM bortezomib, or NPI-0052 (1 nM) plus bortezomib (3 nM); U266 cells: 7 nM NPI-0052, 1 nM bortezomib, or NPI-0052 (7 nM) plus bortezomib (1 nM); RPMI-8226 cells: 10 nM NPI-0052, 10 nM bortezomib, or NPI-0052 (10 nM) plus bortezomib (10 nM); INA-6 cells: 5 nM NPI-0052, 5 nM bortezomib, or NPI-0052 (5 nM) plus bortezomib (5 nM); OPM-2 cells: 5 nM NPI-0052, 7 nM bortezomib, or NPI-0052 (5 nM) plus bortezomib (7 nM); Dox-40 cells: 5 nM NPI-0052, 10 nM bortezomib, or NPI-0052 (5 nM) plus bortezomib (10 nM); and MM.1R cells: 5 nM NPI-0052, 3 nM bortezomib, or NPI-0052 (5 nM) plus bortezomib (3 nM). Data presented are means plus or minus SD (n = 3; P < .005 for all cell lines). A CI of less than 1 indicates synergy. (H) MM.1S cells were treated with the indicated concentrations of NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib for 12 hours and assessed for apoptosis using annexin V/PI staining assays by flow cytometry. Percentage of apoptotic cells (annexin V+/PI−) were as follows: control cells, 3.5%; NPI-0052–treated cells, 3.6%; bortezomib-treated cells, 5%; and NPI-0052 plus bortezomib–treated cells, 25%. Data presented are means plus or minus SD (n = 2; P < .04). (I) MM.1S cells were treated with the indicated concentrations of NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib for 12 hours and assessed for cell death using a live and dead cell assay using fluorescent microscopy (Leica Microsystems AF600, Bannockburn, IL); (live cells are green; dead cells are red). Data presented are means plus or minus SD (n = 3; P < .05).
Combination of low doses of NPI-0052 and bortezomib induces synergistic MM cell death. MM.1S (A), U266 (B), RPMI-8226 (C), INA-6 (D), OPM-2 (E), Dox-40 (F), or MM.1R (G) cells were treated with NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib and assessed for viability using MTT assays. The concentrations of drugs, either alone or in combination, were as follows: MM.1S cells: 1 nM NPI-0052, 3 nM bortezomib, or NPI-0052 (1 nM) plus bortezomib (3 nM); U266 cells: 7 nM NPI-0052, 1 nM bortezomib, or NPI-0052 (7 nM) plus bortezomib (1 nM); RPMI-8226 cells: 10 nM NPI-0052, 10 nM bortezomib, or NPI-0052 (10 nM) plus bortezomib (10 nM); INA-6 cells: 5 nM NPI-0052, 5 nM bortezomib, or NPI-0052 (5 nM) plus bortezomib (5 nM); OPM-2 cells: 5 nM NPI-0052, 7 nM bortezomib, or NPI-0052 (5 nM) plus bortezomib (7 nM); Dox-40 cells: 5 nM NPI-0052, 10 nM bortezomib, or NPI-0052 (5 nM) plus bortezomib (10 nM); and MM.1R cells: 5 nM NPI-0052, 3 nM bortezomib, or NPI-0052 (5 nM) plus bortezomib (3 nM). Data presented are means plus or minus SD (n = 3; P < .005 for all cell lines). A CI of less than 1 indicates synergy. (H) MM.1S cells were treated with the indicated concentrations of NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib for 12 hours and assessed for apoptosis using annexin V/PI staining assays by flow cytometry. Percentage of apoptotic cells (annexin V+/PI−) were as follows: control cells, 3.5%; NPI-0052–treated cells, 3.6%; bortezomib-treated cells, 5%; and NPI-0052 plus bortezomib–treated cells, 25%. Data presented are means plus or minus SD (n = 2; P < .04). (I) MM.1S cells were treated with the indicated concentrations of NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib for 12 hours and assessed for cell death using a live and dead cell assay using fluorescent microscopy (Leica Microsystems AF600, Bannockburn, IL); (live cells are green; dead cells are red). Data presented are means plus or minus SD (n = 3; P < .05).
We next examined whether NPI-0052 plus bortezomib–induced decrease in viability is due to apoptosis. NPI-0052 plus bortezomib, but not either agent alone, triggered significant apoptosis in MM.1S cells, as determined by annexin V/PI staining assays. (P < .05, n = 3; Figure 1H). Assessment of cell death by live and dead cell assays demonstrated similar results (Figure 1I).
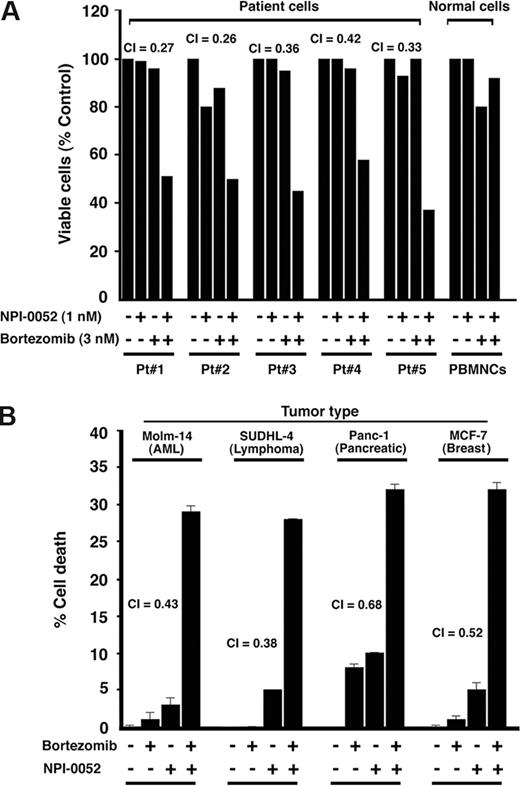
To determine whether NPI-0052 plus bortezomib similarly affect purified patient MM cells, tumor cells from 5 patients with MM who relapsed after multiple prior therapies, including thalidomide (patient 1), bortezomib (patients 2 and 3), and Dex/melphalan (patients 4 and 5) were treated for 24 hours with NPI-0052 (1 nM) plus bortezomib (3 nM), and then analyzed for viability. A significant decrease in viability of all patient MM cells was noted (P < .005; Figure 2A). Of note, 2 of 5 patients studied were refractory to bortezomib therapy, and 2 were resistant to Dex and melphalan therapies. Patients were considered refractory to bortezomib therapy when they have relapsed within 6 months after or progressed on bortezomib therapy. NPI-0052 plus bortezomib–triggered decrease in viability of MM patient cells was due to apoptosis, as evidenced by DNA fragmentation (data not shown). Taken together, these findings suggest that NPI-0052 plus bortezomib induces synergistic anti-MM activity and overcomes drug resistance in both MM cell lines and purified patient MM cells. Importantly, the low-dose combination of NPI-0052 (1 nM) plus bortezomib (3 nM) did not trigger significant decrease in viability of normal PBMNCs (P = .22 from J-T trend test) or PHA/IL-2–stimulated normal PBMCs (Figure 2A). Higher concentrations of bortezomib (10 nM), but not of NPI-0052 (10 nM), induced a modest (20%) decrease in normal cell viability, as previously reported.39
Combined NPI-0052 and bortezomib triggers synergistic antitumor activity in MM patient (CD138+) cells and other cancer cell types. (A) Purified patient MM cells (CD138+) were treated with the indicated concentrations of NPI-0052, bortezomib (3 nM), or combined NPI-0052 plus bortezomib and assessed for viability using MTT assays. Data represent means plus or minus SD of triplicate samples (P < .05 for all patient samples). A CI of less than 1 indicates synergy. PBMNCs from healthy donors were treated with indicated concentrations of NPI-0052, bortezomib, or NPI-0052 plus bortezomib and then analyzed for viability. Data are means plus or minus SD of 3 independent experiments (P = .25 from J-T test for trend). (B) MTT assays were performed after incubation of leukemia, lymphoma, pancreatic, and breast cancer cells with NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib. The concentrations of agents for cell lines were as follows: Molm-14, SUDHL-4, and Panc-1 cells: 7 nM bortezomib, 7 nM NPI-0052, or NPI-0052 (7 nM) plus bortezomib (7 nM); MCF-7 cells: 10 nM bortezomib, 3 nM NPI-0052, or NPI-0052 (3 nM) plus bortezomib (10 nM). Data presented are means plus or minus SD (n = 2; P < .05 for all cell lines).
Combined NPI-0052 and bortezomib triggers synergistic antitumor activity in MM patient (CD138+) cells and other cancer cell types. (A) Purified patient MM cells (CD138+) were treated with the indicated concentrations of NPI-0052, bortezomib (3 nM), or combined NPI-0052 plus bortezomib and assessed for viability using MTT assays. Data represent means plus or minus SD of triplicate samples (P < .05 for all patient samples). A CI of less than 1 indicates synergy. PBMNCs from healthy donors were treated with indicated concentrations of NPI-0052, bortezomib, or NPI-0052 plus bortezomib and then analyzed for viability. Data are means plus or minus SD of 3 independent experiments (P = .25 from J-T test for trend). (B) MTT assays were performed after incubation of leukemia, lymphoma, pancreatic, and breast cancer cells with NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib. The concentrations of agents for cell lines were as follows: Molm-14, SUDHL-4, and Panc-1 cells: 7 nM bortezomib, 7 nM NPI-0052, or NPI-0052 (7 nM) plus bortezomib (7 nM); MCF-7 cells: 10 nM bortezomib, 3 nM NPI-0052, or NPI-0052 (3 nM) plus bortezomib (10 nM). Data presented are means plus or minus SD (n = 2; P < .05 for all cell lines).
Combination of NPI-0052 and bortezomib trigger apoptosis in leukemic (Molm-14), lymphoma (SUDHL-4), pancreatic (Panc-1), and breast cancer (MCF-7) cells
As seen in Figure 2B, NPI-0052 plus bortezomib decreased the viability of Molm-14, SUDHL-4, Panc-1, and MCF-7 cells. The decrease in viability was due to apoptosis, as determined by annexin V/PI staining and flow cytometry. The percentage of apoptotic cell population (annexin V+/PI−) in each case were as follows: Molm-14: NPI-0052 alone, 7.2%; bortezomib alone, 10.5%; and NPI-0052 plus bortezomib, 26.7%; SUDHL-4 cells: NPI-0052 alone, 5.5%; bortezomib alone, 4.8%; and NPI-0052 plus bortezomib, 20.9%; Panc-1 cells: NPI-0052 alone, 10.9%; bortezomib alone, 9.8%; and NPI-0052 plus bortezomib, 25.6%; and MCF-7 cells: NPI-0052 alone, 8.3%; bortezomib alone, 13.4%; NPI-0052 plus bortezomib, 32.8% (CI < 1 in all cell lines; n = 3).
Combined low doses of NPI-0052 and bortezomib block both migration of MM cells and angiogenesis
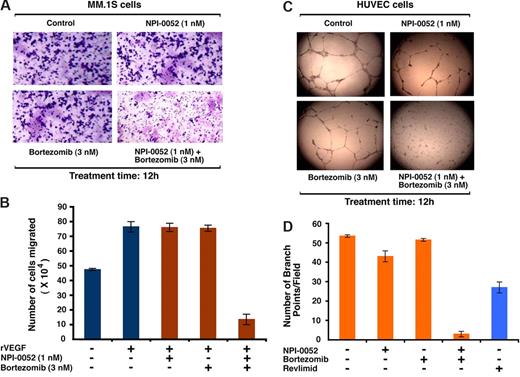
We and others have shown that migration and angiogenesis play an important role in progression of MM.53,54 The effect of NPI-0052 plus bortezomib was examined on these events using Transwell insert systems and in vitro tubule formation assays.47 Serum alone markedly increased MM.1S cell migration; importantly, NPI-0052 (1 nM) plus bortezomib (3 nM) significantly inhibit serum-dependent MM.1S cell migration, as reflected by a decrease in the number of crystal violet–stained cells (Figure 3A). These results were further confirmed by quantification of cell migration in response to treatment with serum (58 × 104) versus NPI-0052 plus bortezomib (12 × 104; P < .05, n = 2). No significant inhibition of migration occurred in cells treated with low doses of either agent alone (Figure 3A). NPI-0052 plus bortezomib at the concentrations tested in the migration assays did not affect survival of MM cells (viability greater than 95%). Similarly, the combination of NPI-0052 plus bortezomib, but not either agent alone, significantly blocked recombinant vascular-endothelial growth factor (rVEGF)–induced migration of MM.1S cells (Figure 3B; P < .05, n = 3).
Combined low doses of NPI-0052 and bortezomib block migration and tubule formation. (A) Growth factor–deprived MM.1S cells were either pretreated with indicated concentrations of NPI-0052, bortezomib, or a combination of NPI-0052 and bortezomib. Cells were then plated on a fibronectin-coated polycarbonate membrane in a modified Boyden chamber and exposed for 8 hours to serum containing medium in the lower chamber. The migrated cells on the bottom face of the membrane were fixed with 90% ethanol and stained with crystal violet (magnification: 10×/0.25 NA oil). A total of 3 randomly selected fields were examined for cells that had migrated from top to bottom chambers. Image is representative of 2 experiments with similar results. (B) MM.1S cells were treated with the indicated concentrations of NPI-0052, bortezomib, or NPI-0052 plus bortezomib for 4 hours (viability greater than 95%), washed, and then treated for 24 hours with rVEGF (10 ng/mL), followed by analysis in a Transwell migration assay. The bar graph represents quantification of migrated cells. Data represents means plus or minus SD of 2 independent experiments (P < .05 for samples treated with rVEGF alone versus NPI-0052 plus bortezomib–treated cells). (C) HUVECs were treated with the indicated concentrations of NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib and assessed for in vitro angiogenesis using Matrigel capillary-like tube structure formation assays (magnification: 4×/0.10 NA oil media: EBM-2). Data represent means plus or minus SD (n = 3; P < .05). The in vitro angiogenesis is reflected by capillary tube branch formation (dark brown). Image is representative of 3 experiments with similar results. (D) HUVECs were treated with the indicated concentrations of NPI-0052, bortezomib, combined NPI-0052 plus bortezomib, or lenalidomide (5 μM) and assessed for in vitro angiogenesis using Matrigel capillary-like tube structure formation assays as in panel C. The bar graph represents quantification of in capillary-like tube structure formation in response to indicated agents: branch points in several random view fields/well were counted, values were averaged, and statistically significance differences were measured using the Student t test. Error bars represent standard deviation.
Combined low doses of NPI-0052 and bortezomib block migration and tubule formation. (A) Growth factor–deprived MM.1S cells were either pretreated with indicated concentrations of NPI-0052, bortezomib, or a combination of NPI-0052 and bortezomib. Cells were then plated on a fibronectin-coated polycarbonate membrane in a modified Boyden chamber and exposed for 8 hours to serum containing medium in the lower chamber. The migrated cells on the bottom face of the membrane were fixed with 90% ethanol and stained with crystal violet (magnification: 10×/0.25 NA oil). A total of 3 randomly selected fields were examined for cells that had migrated from top to bottom chambers. Image is representative of 2 experiments with similar results. (B) MM.1S cells were treated with the indicated concentrations of NPI-0052, bortezomib, or NPI-0052 plus bortezomib for 4 hours (viability greater than 95%), washed, and then treated for 24 hours with rVEGF (10 ng/mL), followed by analysis in a Transwell migration assay. The bar graph represents quantification of migrated cells. Data represents means plus or minus SD of 2 independent experiments (P < .05 for samples treated with rVEGF alone versus NPI-0052 plus bortezomib–treated cells). (C) HUVECs were treated with the indicated concentrations of NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib and assessed for in vitro angiogenesis using Matrigel capillary-like tube structure formation assays (magnification: 4×/0.10 NA oil media: EBM-2). Data represent means plus or minus SD (n = 3; P < .05). The in vitro angiogenesis is reflected by capillary tube branch formation (dark brown). Image is representative of 3 experiments with similar results. (D) HUVECs were treated with the indicated concentrations of NPI-0052, bortezomib, combined NPI-0052 plus bortezomib, or lenalidomide (5 μM) and assessed for in vitro angiogenesis using Matrigel capillary-like tube structure formation assays as in panel C. The bar graph represents quantification of in capillary-like tube structure formation in response to indicated agents: branch points in several random view fields/well were counted, values were averaged, and statistically significance differences were measured using the Student t test. Error bars represent standard deviation.
We next used in vitro capillary-like tube structure formation assays to determine whether NPI-0052 plus bortezomib triggers antiangiogenic effects. In vitro angiogenesis was measured using Matrigel capillary-like tube structure formation assays: human vascular endothelial cells (HUVECs) seeded onto Matrigel differentiate and form capillary-like tube structures similar to in vivo neovascularization, and are dependent upon cell-matrix interaction, cellular communication, and cellular motility.47 This assay therefore provides evidence for antiangiogenic effects of drugs/agents. HUVECs were seeded in 96-well culture plates precoated with Matrigel; treated with vehicle (DMSO), NPI-0052 (1 nM), bortezomib (3 nM), or NPI-0052 (1 nM) plus bortezomib (3 nM) for 8 hours; and then examined for tube formation using an inverted microscope (Leica Microsystems). As seen in Figure 3C, tubule formation was markedly decreased in the NPI-0052 plus bortezomib–treated cells, but not after treatment with either agent alone. In order to clearly demonstrate the magnitude of changes in tubule formation, we compared the effects of NPI-0052 (1 nM) plus bortezomib (3 nM) with the known antiangiogenic agent lenalidomide (5 μM). Our data show that combination of low doses of bortezomib plus NPI-0052 trigger more potent inhibition of in vitro capillary-like tube formation than lenalidomide alone (Figure 3D; P = .021 for NPI-0052 plus bortezomib–treated cells versus lenalidomide-treated cells; n = 2). Taken together, these findings suggest that combination of low doses of NPI-0052 and bortezomib block migration and angiogenesis.
Mechanisms mediating anti-MM activity of NPI-0052 plus bortezomib
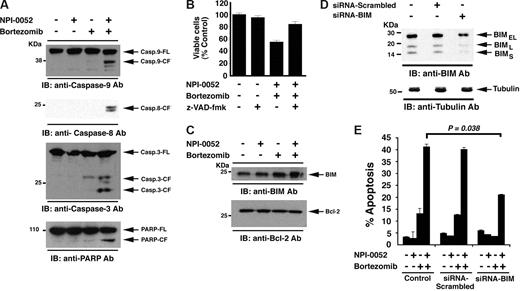
Our earlier studies showed that higher doses of NPI-0052 (7-24 nM) and bortezomib (5-15 nM) efficiently triggered both intrinsic and extrinsic cell death pathways in various MM cell lines. Here, we asked whether the combination of low concentrations of each agent retains the ability to induce extrinsic and/or intrinsic apoptotic signaling pathways. Our results show that NPI-0052(1 nM) plus bortezomib (3 nM), but not NPI-0052 (1 nM) or bortezomib (3 nM) alone, induces activation of caspase-9 (intrinsic), casapse-8 (extrinsic), and caspase-3, followed by cleavage of PARP, a hallmark of apoptosis (Figure 4A). Importantly, pretreatment of MM.1S cells with a pancaspase inhibitor (z-VAD-fmk) significantly blocked NPI-0052 plus bortezomib–induced apoptosis (P < .05, n = 3; Figure 4B).
Mechanisms of NPI-0052 plus bortezomib–induced apoptosis. (A) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours and harvested; total proteins were then subjected to immunoblot analysis with anti–caspase-9, anti–caspase-8, anti–caspase-3, or anti–PARP Abs. FL indicates full length; CF denotes cleaved fragment. Blots shown are representative of 3 independent experiments. (B) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 24 hours in the presence or absence of pancaspase inhibitor z-VAD-fmk and then assessed for viability. Shown are the means plus or minus SD (error bar) of 3 independent experiments (P < .005). (C) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours and harvested; total proteins were then subjected to immunoblot analysis with anti-BIM or anti–Bcl-2 Abs. (D) MM.1S cells were transfected with siRNA BIM or scrambled siRNA for 48 hours and harvested; total protein extracts were then subjected to immunoblot analysis with anti-BIM or antitubulin Abs. Blots shown are representative of 2 independent experiments. (E) MM.1S cells were transfected with siRNA BIM or scrambled siRNA for 48 hours, followed by 12-hour of treatment with indicated agents and analysis for apoptosis by annexin V/PI staining. Error bars represent standard deviation.
Mechanisms of NPI-0052 plus bortezomib–induced apoptosis. (A) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours and harvested; total proteins were then subjected to immunoblot analysis with anti–caspase-9, anti–caspase-8, anti–caspase-3, or anti–PARP Abs. FL indicates full length; CF denotes cleaved fragment. Blots shown are representative of 3 independent experiments. (B) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 24 hours in the presence or absence of pancaspase inhibitor z-VAD-fmk and then assessed for viability. Shown are the means plus or minus SD (error bar) of 3 independent experiments (P < .005). (C) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours and harvested; total proteins were then subjected to immunoblot analysis with anti-BIM or anti–Bcl-2 Abs. (D) MM.1S cells were transfected with siRNA BIM or scrambled siRNA for 48 hours and harvested; total protein extracts were then subjected to immunoblot analysis with anti-BIM or antitubulin Abs. Blots shown are representative of 2 independent experiments. (E) MM.1S cells were transfected with siRNA BIM or scrambled siRNA for 48 hours, followed by 12-hour of treatment with indicated agents and analysis for apoptosis by annexin V/PI staining. Error bars represent standard deviation.
Prior studies have established that the BH3-only Bcl-2 family protein BIM regulates caspase-9 activation by modulating Cyto-c/Smac release from mitochondria to the cytosol.55 Our data show that combined NPI-0052 plus bortezomib, but not either agent alone, significantly up-regulates BIM protein levels (3-fold increase) without altering Bcl-2 protein levels (Figure 4C). To determine whether BIM mediates NPI-0052 plus bortezomib–induced apoptosis, we knocked down BIM expression using an siRNA strategy. The functional specificity of BIM siRNA was evident from a marked decrease in protein levels of all 3 isoforms of BIM (Figure 4D). Importantly, transfection of siRNA BIM, but not negative control (scrambled) siRNA, significantly inhibits NPI-0052 plus bortezomib–induced apoptosis in MM.1S cells (Figure 4E). These findings suggest that NPI-0052 plus bortezomib–triggered apoptosis is mediated, at least in part, via BIM.
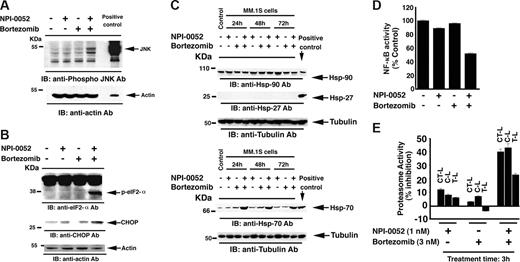
Stress-induced apoptosis is associated with activation of c-Jun NH(2)-terminal kinase (JNK/SAPK).56-58 As seen in Figure 5A, treatment of MM.1S cells with low concentrations of NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours triggered JNK activation. The extent of JNK activation was similar to that observed with IC50 concentrations of each agent at 24 hours (7 nM of NPI-0052 or 5 nM for bortezomib). We also compared JNK activation at concentrations equivalent to the combined doses (ie, 1 nM NPI-0052 plus 3 nM bortezomib versus 4 nM of each agent). No significant JNK activity was observed in response to 4 nM of each agent (data not shown). Together, these findings demonstrate that NPI-0052 plus bortezomib–induced apoptosis is associated with the activation of caspase cascade, BIM, and JNK.
Effects of NPI-0052 plus bortezomib on Hsp's and proteasomal activities. (A) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours and harvested; total proteins were then subjected to immunoblot analysis with anti–phospho-JNK or anti–actin Abs. Blots shown are representative of 3 independent experiments. (B) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours and harvested; total proteins were then subjected to immunoblot analysis with anti–phospho-eIF2-α, anti–CHOP/GADD153, or anti–actin Abs. Blots shown are representative of 3 independent experiments. (C) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 24, 48, and 72 hours and harvested; total proteins were then subjected to immunoblot analysis with anti–Hsp-90, anti–Hsp-27, or anti–Hsp-70 Abs. Lysates from HeLA cells served a positive control in immunobloting with Hsp Abs. Blots shown are representative of 2 independent experiments. (D) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 24 hours and harvested; nuclear extracts were then analyzed for NF-κB activity by using a p65 enzyme-linked immunosorbent assay (ELISA) kit (Active Motif, Carlsbad, CA). (E) MM.1S cells were treated with indicated concentrations of NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib for 30 minutes and harvested; cytosolic extracts were then analyzed for CT-L, C-L, and T-L proteasomal activities. The data are represented as percentage of inhibition compared with vehicle control. Data are presented as means plus or minus SD. (n = 3; P < .05).
Effects of NPI-0052 plus bortezomib on Hsp's and proteasomal activities. (A) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours and harvested; total proteins were then subjected to immunoblot analysis with anti–phospho-JNK or anti–actin Abs. Blots shown are representative of 3 independent experiments. (B) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 12 hours and harvested; total proteins were then subjected to immunoblot analysis with anti–phospho-eIF2-α, anti–CHOP/GADD153, or anti–actin Abs. Blots shown are representative of 3 independent experiments. (C) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 24, 48, and 72 hours and harvested; total proteins were then subjected to immunoblot analysis with anti–Hsp-90, anti–Hsp-27, or anti–Hsp-70 Abs. Lysates from HeLA cells served a positive control in immunobloting with Hsp Abs. Blots shown are representative of 2 independent experiments. (D) MM.1S cells were treated with NPI-0052 (1 nM), bortezomib (3 nM), or combined NPI-0052 (1 nM) plus bortezomib (3 nM) for 24 hours and harvested; nuclear extracts were then analyzed for NF-κB activity by using a p65 enzyme-linked immunosorbent assay (ELISA) kit (Active Motif, Carlsbad, CA). (E) MM.1S cells were treated with indicated concentrations of NPI-0052, bortezomib, or combined NPI-0052 plus bortezomib for 30 minutes and harvested; cytosolic extracts were then analyzed for CT-L, C-L, and T-L proteasomal activities. The data are represented as percentage of inhibition compared with vehicle control. Data are presented as means plus or minus SD. (n = 3; P < .05).
Effects of NPI-0052 plus bortezomib on ER stress response, heat shock proteins, and NF-κB
Previous studies have shown that bortezomib or NPI-0052 alone at higher concentrations induces unfolded protein response (UPR).29,59-61 We asked whether combined low doses of NPI-0052 and bortezomib similarly induce UPR. Our results show that even at low doses, the combination of NPI-0052 and bortezomib retained its ability to induce endoplasmic reticulum (ER) stress response, evidenced by increased eIf2-α kinase activity and protein levels of its target protein CHOP/GADD153 (Figure 5B).
Multiple prior studies link bortezomib-induced apoptosis with the up-regulation of heat shock proteins (Hsp's).29,59-61 We therefore next examined the effect of combined low doses of NPI-0052 and bortezomib on 3 Hsp's: Hsp-27, Hsp-70, and Hsp-90. MM.1S cells were treated with NPI-0052 (1 nM) plus bortezomib (3 nM) for 24 hours, 48 hours, and 72 hours, and protein lysates were subjected to immunoblot analysis with anti–Hsp-90, anti–Hsp-27, and anti–Hsp-70 Abs. NPI-0052 plus bortezomib triggered an increase in Hsp-70 protein levels, but not Hsp-27 or Hsp-90 protein levels, even at longer exposure times (48 hours and 72 hours; Figure 5C). Similarly, a shorter exposure time (12 hours) triggered Hsp-70, but not Hsp-27 or Hsp-90 (data not shown). Our prior studies showed that IC50 concentrations of bortezomib (5 nM) trigger all 3 Hsp's. Thus, while a higher dose of bortezomib alone is able to induce Hsp-27, Hsp-70, and Hsp-90, a lower-dose combination of bortezomib with NPI-0052 only triggers Hsp-70. These findings suggest that apoptosis triggered by combination of low doses of bortezomib and NPI-0052 involves only Hsp-70, and not Hsp-90 or Hsp-27. The lack of Hsp-90 or Hsp-27 induction may be due to differential signaling pathways triggered by a higher dose of bortezomib alone versus its combination with NPI-0052. Furthermore, NPI-0052 plus bortezomib–induced ER stress response (as shown in Figure 5B) and apoptosis likely involves Hsp-70, but not Hsp-27 and Hsp-90. These findings provide evidence of distinct mechanism of action of combined NPI-0052 plus bortezomib versus each agent alone. Our findings have clinical implications: Hsp's confer drug resistance, and prior studies showed that higher concentrations of bortezomib induce all 3 Hsp's.29,62 Based on these findings, bortezomib has been combined with Hsp-90 inhibitor in clinical trials to overcome Hsp-mediated drug resistance. Our present finding that combined low-dose bortezomib with NPI-0052 does not induce 2 of the 3 Hsp's suggests that drug resistance may be less frequent in patients given combined low-dose regimens.
We and others have shown that proteasome inhibitors block NF-κB, a major growth and survival pathway in MM cells.39,63-65 Combined low doses of NPI-0052 (1 nM) and bortezomib (3 nM), but not either agent alone, showed synergistic inhibition of NF-κB activity, evidenced by decrease in nuclear translocation of p65 subunit (CI = 0.35; n = 3; Figure 5D).
Effects of NPI-0052 plus bortezomib on CT-L, C-L, and T-L proteolytic activities
Although proteasome inhibitors induce apoptotic signaling cascades, the primary target of these agents is the proteasome. The proteolytic activity of proteasomes is mediated by 3 active sites: CT-L, T-L, and C-L.23-26,29,66 Our prior study showed that NPI-0052 blocks all 20S proteasomal activities in MM cells, while bortezomib primarily inhibits CT-L and to a lesser degree C-L activity.39 Treatment of MM.1S cells for 30 minutes with a low dose (1 nM) of NPI-0052 inhibits 13% of CT-L, 8% of C-L, and 6% of T-L activity, whereas bortezomib (3 nM) only inhibits 3% of CT-L and 7% of C-L activities, as measured by activity specific fluorogenic peptide substrates.49 Combined low doses of these agents result in a significant block in all 3 proteasomal activities: 40%, 43%, and 20% inhibition in CT-L, C-L, and T-L activities, respectively (Figure 5E). Longer (3 hours) exposure also induced synergistic inhibition in CT-L, C-L and T-L activities (data not shown). Our data suggest that the combination of even low doses of NPI-0052 and bortezomib can effectively target all 3 proteasome activities and trigger potent anti-MM activity.
NPI-0052 and bortezomib synergize to suppress human MM cell growth in vivo
Having shown that combined NPI-0052 plus bortezomib induces synergistic apoptosis in MM cells in vitro, we next examined the in vivo efficacy of low-dose combination NPI-0052 and bortezomib treatment using a human plasmacytoma xenograft mouse model.46,50 Our prior studies have shown that either NPI-0052 or bortezomib alone are effective anti-MM agents in this xenograft model.39,50 To assess for synergistic cytotoxicity in vivo, we first used low doses of either NPI-0052 (0.025 mg/kg, 0.050 mg/kg, or 0.075 mg/kg) or bortezomib (0.25 mg/kg or 0.50 mg/kg). As seen in Figure 6A, low doses of either agent had minimal effect on the growth of tumors, which increased as in control mice. Importantly, when NPI-0052 was combined with bortezomib, there was a significant (75%) reduction (P = .03) in tumor growth relative to untreated mice (Figure 6A; inset). As an additional control, we also treated mice with the maximum tolerated dose (MTD) of NPI-0052 and bortezomib (0.15 mg/kg and 1 mg/kg, respectively). As in our previous study,39 a significant reduction in tumor growth was noted in these cohorts (data not shown). Importantly, the extent of tumor growth inhibition was similar in mice treated with low-dose combination NPI-0052 plus bortezomib versus mice treated with the MTD of either NPI-0052 or bortezomib. Furthermore, the combination did not have increased toxicity, evidenced by lack of weight loss and neurologic changes even after 4 weeks of NPI-0052 plus bortezomib treatment (data not shown). These findings suggest that combining NPI-0052 with bortezomib markedly reduces tumor growth and is well tolerated in vivo.
Combination of low doses of NPI-0052 plus bortezomib inhibits human plasmacytoma growth in immunodeficient beige-nude-xid (BNX) mice. (A) Average and standard deviation of tumor volume (mm3) from group of mice (n = 3/group) versus time (days) when tumor was measured. Mice were treated with vehicle, NPI-0052 (intravenously), bortezomib (intravenously), or NPI-0052 plus bortezomib (intravenously) at the indicated doses on a twice-a-week schedule for 30 days. A significant delay in tumor growth in NPI-0052 plus bortezomib–treated mice was noted compared with vehicle-treated control mice (P = .04). Bars indicate means plus or minus SE. Inset shows tumors resected from control (vehicle) and NPI-0052 (0.025 mg/kg) plus or minus bortezomib (0.25 mg/kg)–treated mice. (B-D) Micrographs show apoptotic cells in tumors sectioned on day 30 (endpoint) from untreated or NPI-0052 (0.025 mg/kg) plus bortezomib (0.25 mg/kg)–treated mice as identified by a TUNEL assay (TUNEL-positive cells are dark brown) and caspase-3 cleavage (dark brown cells), as well as H&E and Ki-67 staining. Shown photographs are representative of similar observations in 3 different mice receiving same treatment. (B,C) Bar graphs show the quantification of TUNEL-positive cells and caspase-3 cleavage activity in tumors from NPI-0052 plus bortezomib–treated mice. Apoptotic tumor cells were enumerated in nonnecrotic areas of each section using light microscopy (magnification, 40×/0.75 NA oil). Eror bars represent standard deviation. Arrows: Immunostained tumor section of mice receiving indicated combined doses of agents.
Combination of low doses of NPI-0052 plus bortezomib inhibits human plasmacytoma growth in immunodeficient beige-nude-xid (BNX) mice. (A) Average and standard deviation of tumor volume (mm3) from group of mice (n = 3/group) versus time (days) when tumor was measured. Mice were treated with vehicle, NPI-0052 (intravenously), bortezomib (intravenously), or NPI-0052 plus bortezomib (intravenously) at the indicated doses on a twice-a-week schedule for 30 days. A significant delay in tumor growth in NPI-0052 plus bortezomib–treated mice was noted compared with vehicle-treated control mice (P = .04). Bars indicate means plus or minus SE. Inset shows tumors resected from control (vehicle) and NPI-0052 (0.025 mg/kg) plus or minus bortezomib (0.25 mg/kg)–treated mice. (B-D) Micrographs show apoptotic cells in tumors sectioned on day 30 (endpoint) from untreated or NPI-0052 (0.025 mg/kg) plus bortezomib (0.25 mg/kg)–treated mice as identified by a TUNEL assay (TUNEL-positive cells are dark brown) and caspase-3 cleavage (dark brown cells), as well as H&E and Ki-67 staining. Shown photographs are representative of similar observations in 3 different mice receiving same treatment. (B,C) Bar graphs show the quantification of TUNEL-positive cells and caspase-3 cleavage activity in tumors from NPI-0052 plus bortezomib–treated mice. Apoptotic tumor cells were enumerated in nonnecrotic areas of each section using light microscopy (magnification, 40×/0.75 NA oil). Eror bars represent standard deviation. Arrows: Immunostained tumor section of mice receiving indicated combined doses of agents.
We next investigated the effect of the drug combination on in vivo apoptosis using TUNEL staining, cleavage of caspase-3, and H&E staining of paraffin-embedded sections of xenografted tumors. The peripheral necrotic regions of the sections from the xenografts were excluded for quantification of cell death. As seen in Figure 6B (top and bottom panels), either NPI-0052 or bortezomib alone at low doses triggered a very modest increase in the number of TUNEL-positive cells (brown color) compared with tumors from control cohorts. However, the combination dramatically increased the number of TUNEL-positive cells compared with either treatment alone (Figure 6B). Similarly, NPI-0052 plus bortezomib triggered robust caspase activation in tumors (Figure 6C). In agreement with these data, a significant decrease in Ki-67+ cells was noted in tumor sections from NPI-0052 plus bortezomib–treated mice relative to tumors from mice receiving either treatment alone (Figure 6D).
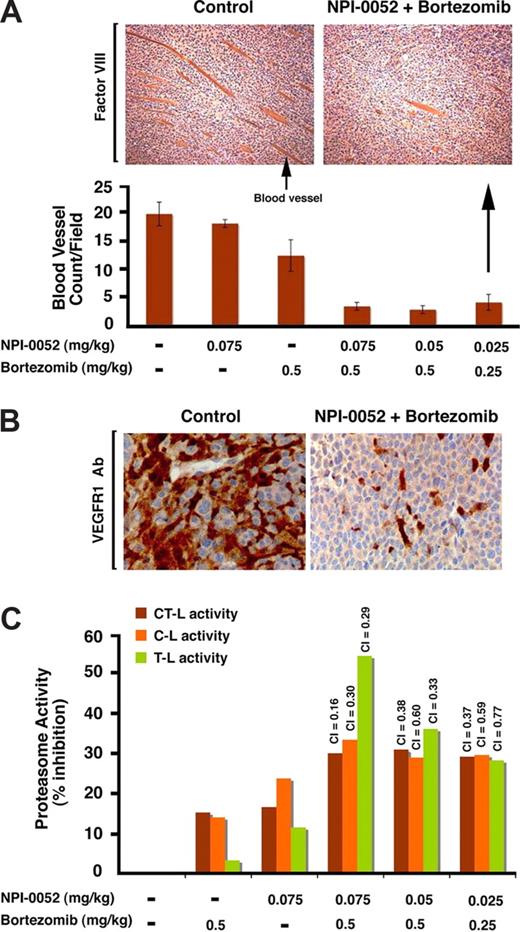
In vitro data indicate antiangiogenic activity of NPI-0052 plus bortezomib, and we therefore next evaluated paraffin-embedded sections of xenografted tumors harvested from mice treated with NPI-0052 and bortezomib for factor VIII staining, a marker of angiogenesis. As seen in Figure 7A, low doses of NPI-0052 or bortezomib alone triggered a very modest decrease in the number of factor VIII–positive cells compared with sections from control-treated tumors, whereas the combination dramatically decreased the number of factor VIII–positive cells compared with treatment with either agent alone (Figure 7A). Since VEGFR1/FLT-1 is highly expressed in MM cells and plays a crucial role in angiogenesis in MM,48,54 we examined the sections of xenografted tumors harvested from mice treated with NPI-0052 and bortezomib for VEGFR1 expression. IHC analysis showed that low doses of NPI-0052 or bortezomib markedly decrease the number of VEGFR1+ cells compared with sections from control-treated tumors (Figure 7B). These findings suggest a likely mechanism mediating NPI-0052 plus bortezomib–triggered inhibition of migration and angiogenesis.
Combination of low doses of NPI-0052 plus bortezomib inhibits neovascularization and proteasomal activities in vivo in xenografted MM tumors. (A) Top panel shows blood vessels in tumor sections from untreated or NPI-0052 (0.025 mg/kg) plus bortezomib (0.25 mg/kg)–treated mice, as identified by staining with a marker for angiogenesis factor VIII. Photograph is representative of similar observations in 3 different mice receiving same treatment. Bottom panel shows the quantification of blood vessels in tumor sections from mice treated with indicated doses of NPI-0052, bortezomib, or combined NPI-0052 plus or minus bortezomib. Blood vessels were enumerated in nonnecrotic areas of each section using light microscopy (magnification, 40×/0.75 NA oil). Error bars represent standard deviation. Arrows: Arrow in left hand panel indicates blood vessel. Arrow in right hand panel shows immunostained tumor section of mice receiving indicated doses of agents. (B) VEGFR1 expression in tumor sections from untreated or NPI-0052 (0.025 mg/kg) plus bortezomib (0.25 mg/kg)–treated mice as identified by IHC staining with VEGFR1 Abs. Photograph is representative of similar observations in 2 different mice receiving same treatment. (C) NPI-0052 plus bortezomib triggers synergistic inhibition of CT-L, C-L, and T-L proteasomal activities in vivo in xenografted MM tumors. For the analysis of proteasome activities in tumors, mice were treated with NPI-0052 (intravenously), bortezomib (intravenously), or NPI-0052 plus bortezomib (as in panel A) and killed after 90 minutes of drug administration; tumors were then examined for CT-L, C-L, and T-L proteasome activity. The data represent percentage of inhibition compared with vehicle control–treated animals from 2 independent experiments with similar results. A CI less than 1 indicates synergy.
Combination of low doses of NPI-0052 plus bortezomib inhibits neovascularization and proteasomal activities in vivo in xenografted MM tumors. (A) Top panel shows blood vessels in tumor sections from untreated or NPI-0052 (0.025 mg/kg) plus bortezomib (0.25 mg/kg)–treated mice, as identified by staining with a marker for angiogenesis factor VIII. Photograph is representative of similar observations in 3 different mice receiving same treatment. Bottom panel shows the quantification of blood vessels in tumor sections from mice treated with indicated doses of NPI-0052, bortezomib, or combined NPI-0052 plus or minus bortezomib. Blood vessels were enumerated in nonnecrotic areas of each section using light microscopy (magnification, 40×/0.75 NA oil). Error bars represent standard deviation. Arrows: Arrow in left hand panel indicates blood vessel. Arrow in right hand panel shows immunostained tumor section of mice receiving indicated doses of agents. (B) VEGFR1 expression in tumor sections from untreated or NPI-0052 (0.025 mg/kg) plus bortezomib (0.25 mg/kg)–treated mice as identified by IHC staining with VEGFR1 Abs. Photograph is representative of similar observations in 2 different mice receiving same treatment. (C) NPI-0052 plus bortezomib triggers synergistic inhibition of CT-L, C-L, and T-L proteasomal activities in vivo in xenografted MM tumors. For the analysis of proteasome activities in tumors, mice were treated with NPI-0052 (intravenously), bortezomib (intravenously), or NPI-0052 plus bortezomib (as in panel A) and killed after 90 minutes of drug administration; tumors were then examined for CT-L, C-L, and T-L proteasome activity. The data represent percentage of inhibition compared with vehicle control–treated animals from 2 independent experiments with similar results. A CI less than 1 indicates synergy.
Since combined low doses of NPI-0052 and bortezomib inhibit proteasome activities in MM cell lines, we next asked whether this combination alters the proteasome activity profile in the xenografted tumors. Tumors were excised after 90 minutes of the last dose administration and examined for CT-L, C-L, and T-L proteasome activity. Treatment with combined low doses of NPI-0052 (0.025 mg/kg, 0.050 mg/kg, or 0.075 mg/kg) and bortezomib (0.25 mg/kg or 0.50 mg/kg), but not with either agent alone, significantly inhibited all 3 proteasome activities (Figure 7B), confirming that the ability of NPI-0052 to synergize with bortezomib in vivo is associated with inhibition of proteasome activity in tumors. Together, these findings demonstrate potent in vivo anti-MM activity of NPI-0052 combined with bortezomib at doses that are well tolerated in a murine model, supporting the potential clinical evaluation of combined NPI-0052 plus bortezomib treatment in MM.
Discussion
Our preclinical and clinical studies led to the FDA approval of bortezomib for the treatment of relapsed and relapsed/refractory MM.32,33,67,68 However, as with other agents, dose-limiting toxicities and the development of resistance limits its long-term utility.34-38 Our recent study showed another novel proteasome inhibitor, NPI-0052, to also have anti-MM cytotoxicity.39 NPI-0052 is currently being evaluated in patients with MM in a phase 1 clinical trial. Here, we examined whether 2 proteasome inhibitors that are distinct in their chemical structure, mechanisms of action, and effects on proteasomal activities can be combined at low doses to enhance anti-MM activity, reduce toxicity, and overcome drug resistance.
We first show that the combination of low doses of NPI-0052 and bortezomib induces apoptosis even in MM cells resistant to conventional and bortezomib therapies without affecting normal lymphocyte viability. For example, combined low doses of NPI-0052 (5 nM) and bortezomib (7 nM) triggers a degree of apoptosis in OPM-2 MM cells that is only achievable at much higher doses of either agent alone (26 nM and 20 nM for NPI-0052 and bortezomib, respectively). Likewise, we observed synergistic growth inhibition induced by NPI-0052 and bortezomib in MM cell lines and patient cells, including those resistant to anti-MM therapies such as Dex or Dox. Mechanistic studies show that NPI-0052 plus bortezomib–induced apoptosis is associated with activation of the caspase cascade, BIM, JNK, Hsp-70, and ER stress response. However, the overall effective cell killing by NPI-052 plus bortezomib may involve activation of other apoptotic signaling pathways. Our data also demonstrate synergistic cytotoxicity of NPI-0052 and bortezomib on lymphoma, leukemic, and solid tumor cells that are relatively resistant to bortezomib, thereby suggesting clinical applicability of this therapeutic regimen beyond MM.
Importantly, the synergistic anti-MM activity of NPI-0052 and bortezomib is also observed in vivo in a human MM xenograft mouse model. NPI-0052 and bortezomib were used in vivo at doses 6 and 4 times lower, respectively, than has been reported to be inhibitory on tumor growth in order to demonstrate synergistic anti-MM activity. No significant tumor growth inhibition was noted at these low doses for either agent alone, but a marked growth inhibitory effect was observed with combined NPI-0052 and bortezomib. The combination of low-dose NPI-0052 and bortezomib is not associated with any toxicity, since no differences in body weight and overall appearance was noted in mice. The remarkable anti-MM activity of NPI-0052 plus bortezomib in vivo was confirmed by IHC analysis of tumors harvested from control and NPI-0052 plus bortezomib–treated mice using several molecular markers of growth inhibition (Ki-67), apoptosis (TUNEL, caspase-3 cleavage, and H&E staining) and associated angiogenesis (factor VIII staining and VEGFR1 expression). Therefore, these findings demonstrate a dual effect of combining NPI-0052 and bortezomib: decreased proliferation and increased apoptosis.
Direct analysis of tumor cells from mice showed synergistic blockade of all 3 proteasome activities in NPI-0052 plus borte-zomib–treated mice, but not in mice treated with low doses of either agent alone. Similar to our earlier results,50 examination of whole blood and spleen showed a greater degree of proteasome inhibition than observed in tumors. These in vivo findings, coupled with our in vitro data showing minimal toxicity of combined NPI-0052 plus bortezomib against normal cells (Figure 2A), confirm that MM cells are more sensitive to proteasome inhibition than normal cells.
Interestingly, while only NPI-0052 is known to inhibit T-L activity, its combination with bortezomib blocks T-L activity even more. In this context, prior studies have reported that proteasome active sites allosterically regulate each other.69,70 For example, a biochemical study showed that occupancy of C-L sites induces the T-L activity of proteasomes.69 It is likely that blockade of one proteasomal activity may affect the function of other proteasomal activities. While a definitive evidence for the role of individual proteasomal activity awaits further genetic studies involving specific inhibition of beta subunits, it is clear from our in vivo data that even 30% to 40% proteasome inhibition, albeit of all 3 activities, is sufficient to trigger significant anti-MM activity.
The clinical observation that bortezomib therapy can be associated with toxicity and resistance, coupled with our present preclinical findings demonstrating that low doses of bortezomib together with NPI-0052 trigger a similar or more potent anti-MM effect in vitro and in vivo without increased toxicity, suggests the promise of combination treatment strategies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Anthony Letai and Martin Sattler for BIM constructs and transfection assays.
This investigation was supported by National Institutes of Health (NIH) grants CA 50947, CA 78373, and CA10070; a Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.); The Myeloma Research Fund; and LeBow Family Fund to Cure Myeloma.
Authorship
Contribution: D.C. designed research, analyzed data, and wrote the manuscript; A.S. performed most of the experiments and interpreted data; M.B., K.P., and T.H. helped in MTT and migration assays and interpretation of data; P.R. and N.M. provided clinical samples; M.A.P. provided vital new agents and proteasome inhibitor NPI-0052; and K.C.A. analyzed data and wrote the manuscript.
Conflict-of-interest disclosure: M.A.P. is an employee of Nereus Pharmaceuticals. The other authors declare no competing financial interests.
Correspondence: Dharminder Chauhan; M561, Dana-Farber Cancer Institute, 44 Binney Street, Boston, MA 02115; e-mail: dharminder_chauhan@dfci.harvard.edu.
References
Author notes
D.C. and A.S. contributed equally to this work.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal