Resistance toward imatinib and other BCR/ABL tyrosine kinase inhibitors remains an increasing clinical problem in the treatment of advanced stages of chronic myeloid leukemia (CML). We recently have identified the heat shock protein 32 (Hsp32)/heme oxygenase-1 (HO-1) as a BCR/ABL-dependent survival molecule in CML cells. We here show that silencing Hsp32/HO-1 in CML cells by an siRNA approach results in induction of apoptosis. Moreover, targeting Hsp32/HO-1 by either pegylated zinc protoporphyrine (PEG-ZnPP) or styrene maleic acid-micelle–encapsulated ZnPP (SMA-ZnPP) resulted in growth inhibition of BCR/ABL-transformed cells. The effects of PEG-ZnPP and SMA-ZnPP were demonstrable in Ba/F3 cells carrying various imatinib-resistant mutants of BCR/ABL, including the T315I mutant, which exhibits resistance against all clinically available BCR/ABL tyrosine kinase inhibitors. Growth-inhibitory effects of PEG-ZnPP and SMA-ZnPP also were observed in the CML-derived human cell lines K562 and KU812 as well as in primary leukemic cells obtained from patients with freshly diagnosed CML or imatinib-resistant CML. Finally, Hsp32/HO-1–targeting compounds were found to synergize with either imatinib or nilotinib in producing growth inhibition in imatinib-resistant K562 cells and in Ba/F3 cells harboring the T315I mutant of BCR/ABL. In summary, these data show that HO-1 is a promising novel target in imatinib-resistant CML.
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disease defined by the presence of the Philadelphia chromosome, which is generated by the reciprocal translocation t(9;22).1,2 The resulting fusion of the c-ABL- and BCR genes generates the BCR/ABL oncogene, which encodes a 210-kDa oncoprotein, BCR/ABL, exhibiting constitutive tyrosine kinase (TK) activity.3 An important mechanism in the pathogenesis of CML appears to be enhanced survival and consequent accumulation of leukemic cells.4 Correspondingly, BCR/ABL has been described to promote expression of a number of antiapoptotic molecules in leukemic cells.5,,,–9
Heat shock protein 32 (Hsp32), also known as heme oxygenase-1 (HO-1), is a stress-related cytoprotective molecule that possesses antiapoptotic activity in various cells.10,11 We recently have shown that Hsp32/HO-1 is constitutively expressed in primary CML cells and that the BCR/ABL oncoprotein promotes expression of Hsp32/HO-1 in leukemic cells.12 Moreover, Hsp32 is considered to play an important role as a survival molecule in CML cells, as overexpression of HO-1 was found to inhibit apoptosis induced by the BCR/ABL tyrosine kinase inhibitor imatinib (STI571).12
During the past few years, imatinib has become frontline therapy in CML and has been shown to be superior in producing complete cytogenetic and molecular responses compared with other drugs.13,–15 However, resistance against imatinib can occur during therapy with imatinib, particularly in accelerated phase (AP) and blast phase (BP) CML, as well as in acute lymphoblastic leukemia (ALL), and can represent a serious clinical problem.16 In most patients, resistance against imatinib occurs from BCR/ABL point mutations.17,,–20 Other reasons for imatinib resistance include amplification of the BCR/ABL gene or the acquisition of additional pro-oncogenic hits.17,,–20 Novel BCR/ABL inhibitors such as nilotinib (AMN107) or dasatinib (BMS354825) have been shown to overcome imatinib resistance mediated by various BCR/ABL point mutations.21,–23 However, the T315I mutation (one of the frequently detected mutations) renders the BCR/ABL oncoprotein completely resistant against these novel tyrosine kinase (TK) inhibitors.24,25 Therefore, a number of new compounds and strategies using combinations of BCR/ABL inhibitors with each other or combinations between BCR/ABL inhibitors and other antileukemic compounds are currently under investigation.26
In the present study, we provide evidence that targeting of Hsp32/HO-1 leads to inhibition of growth of imatinib-sensitive as well as of imatinb-resistant CML cells. Moreover, we show that combinations of Hsp32/HO-1 inhibitors with imatinib or nilotinib can produce synergistic growth-inhibitory effects in imatinib-resistant cells. HO-1 may therefore represent an interesting novel molecular target in CML.
Methods
Reagents
Zinc-(II)-deuteroporphyrin-IX-2,4-bisethyleneglycol (ZnDPPIX)27 was purchased from Alexis Biochemicals (Lausen, Switzerland). Pegylated zinc protoporphyrin (PEG-ZnPP)28,29 and ZnPP encapsulated in the micelle of styrene maleic acid (SMA-ZnPP)30 were produced essentially as described.28,–30 Imatinib (STI571) and nilotinib (AMN107) were kindly provided by Dr Elisabeth Buchdunger and Dr Paul Manley (Novartis Pharma AG, Basel, Switzerland). RPMI 1640 medium and fetal calf serum (FCS) were purchased from PAA (Pasching, Austria); Iscove modified Dulbeccos medium (IMDM) from Gibco (Carlsbad, CA). Recombinant human (rh) granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from R&D Systems (Minneapolis, MN), rh interleukin-3 (IL-3) from Novartis, and rh erythropoietin from Roche (Basel, Switzerland).
Plasmids pLV-tTR-KRAB-dsRed, pWPT-GFP, psPAX2, pMD2G, pRSVrev, and pLVTHM were kindly provided by Dr Didier Trono (Department of Microbiology and Molecular Medicine, Faculty of Medicine, University of Geneva, Switzerland; http://tronolab.epfl.ch). pHHO-1 was kindly provided by Dr Shigeki Shibahara (Tohoku University School of Medicine, Sendai, Japan).31 To construct a lentiviral vector enabling doxycycline-dependent gene expression, the tetO cassette from pLVTHM was PCR-amplified and cloned into pWPT-GFP upstream of the cytomegalovirus (CMV) promoter. Then, the green fluorescent protein (GFP) cDNA was replaced by the HO-1 cDNA from pHHO-1. The resulting vector was termed pWPTet-HO-1.
Isolation and culture of primary CML cells
Primary leukemic cells were obtained from 14 patients with chronic phase (CP) CML, 5 with accelerated phase (AP) CML, and 4 with blast phase (BP) CML. Seven patients ([chronic phase] CP, n = 1; AP, n = 3; BP, n = 3) were found to have imatinib-resistant CML. The study was approved by the institutional review board (Medical University of Vienna), and informed consent was obtained in accordance with the Declaration of Helsinki prior to blood donation in each case. The characteristics of the patients are summarized in Table 1. Peripheral blood (pb) mononuclear cells (MNCs) and/or bone marrow (bm) MNCs were isolated by Ficoll density centrifugation. Isolated MNCs were cultured in RPMI 1640 medium containing 10% FCS in the presence or absence of GM-CSF (100 ng/mL) at 37°C for up to 7 days.
Patients' characteristics and response to Hsp32/HO-1-targeted drugs
| Patient no. . | Sex . | Age, y . | CML phase . | Imatinib-resistant . | WBC, G/L . | Hb g/dL . | Plt, G/L . | Cytogenetics . | BCR/ABL point mutations . | IC50 SMA-ZnPP . | IC50 PEG-ZnPP . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 47 | BP | Yes | 30.4 | 10.6 | 141 | t(9;22), i17, +8 | Glu279/Lys His396/Arg | 5 | n.t. |
| 2 | F | 46 | CP | No | 38.8 | 11.9 | 569 | t(9;22) | wt | 10 | n.t. |
| 3 | M | 49 | BP | No | 16.58 | 12.2 | 871 | t(9;22) | wt | 15 | n.t. |
| 4 | M | 59 | CP | No | 159.4 | 12.4 | 168 | t(9;22) | n.t. | 3 | n.t. |
| 5 | F | 80 | CP | No | 17.1 | 13.7 | 247 | t(9;22) | n.t. | n.t. | 2 |
| 6 | M | 67 | AP | Yes | 49.5 | 14.1 | 638 | t(9;22) | Glu255Lys | n.t. | 4 |
| 7 | M | 69 | AP | Yes | 19.1 | 8.4 | 132 | t(9;22) | wt | n.t. | 4 |
| 8 | F | 67 | CP | No | 27.9 | 13.2 | 178 | t(9;22) | wt | n.t. | 10 |
| 9 | M | 18 | AP | No | 37 | 11.8 | 114 | t(9;22), +8 | n.t. | n.t. | 13 |
| 10 | M | 66 | BP | Yes | 3.4 | 7.5 | 121 | t(9;22), i17, +8 | Val379Ile | n.t. | 20 |
| 11 | M | 74 | CP | No | 13.7 | 8.7 | 720 | t(9;22) | wt | n.t. | 6 |
| 12 | F | 58 | CP | No | 28.9 | 13.5 | 347 | t(9;22) | n.t. | n.t. | 3 |
| 13 | M | 65 | BP | Yes | 96.1 | 11.3 | 66 | t(9;22) | Thr315Ala Gly250Glu | 15 | 10 |
| 14 | M | 30 | CP | No | 156.8 | 12.2 | 171 | t(9;22) | wt | n.t. | 12 |
| 15 | M | 64 | CP | No | 342 | 8.9 | 478 | t(9;22) | n.t. | 7 | n.t. |
| 16 | F | 74 | CP | No | 103.5 | 10.5 | 717 | t(9;22) | n.t. | 5 | n.t. |
| 17 | M | 19 | CP | No | 96 | 14.6 | 500 | t(9;22) | n.t. | 6 | 18 |
| 18 | F | 45 | CP | No | 89.1 | 12.2 | 536 | t(9;22) | n.t. | n.t. | 12 |
| 19 | M | 73 | AP | No | 112.2 | 11.9 | 394 | t(9;22), t(2;10) | n.t. | 8 | n.t. |
| 20 | M | 68 | CP | No | 190.0 | 12.3 | 490 | t(9;22) | n.t. | 8 | n.t. |
| 21 | M | 67 | CP | No | 41.4 | 14.8 | 310 | t(9;22) | n.t. | 8 | 20 |
| 22 | M | 73 | CP | Yes | 21.1 | 13.7 | 754 | t(9;22) | Phe359Val | n.t. | 12 |
| 23 | M | 67 | AP | Yes | 16.2 | 9.9 | 69 | t(9;22) | Met244Val | 6 | 8 |
| Patient no. . | Sex . | Age, y . | CML phase . | Imatinib-resistant . | WBC, G/L . | Hb g/dL . | Plt, G/L . | Cytogenetics . | BCR/ABL point mutations . | IC50 SMA-ZnPP . | IC50 PEG-ZnPP . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 47 | BP | Yes | 30.4 | 10.6 | 141 | t(9;22), i17, +8 | Glu279/Lys His396/Arg | 5 | n.t. |
| 2 | F | 46 | CP | No | 38.8 | 11.9 | 569 | t(9;22) | wt | 10 | n.t. |
| 3 | M | 49 | BP | No | 16.58 | 12.2 | 871 | t(9;22) | wt | 15 | n.t. |
| 4 | M | 59 | CP | No | 159.4 | 12.4 | 168 | t(9;22) | n.t. | 3 | n.t. |
| 5 | F | 80 | CP | No | 17.1 | 13.7 | 247 | t(9;22) | n.t. | n.t. | 2 |
| 6 | M | 67 | AP | Yes | 49.5 | 14.1 | 638 | t(9;22) | Glu255Lys | n.t. | 4 |
| 7 | M | 69 | AP | Yes | 19.1 | 8.4 | 132 | t(9;22) | wt | n.t. | 4 |
| 8 | F | 67 | CP | No | 27.9 | 13.2 | 178 | t(9;22) | wt | n.t. | 10 |
| 9 | M | 18 | AP | No | 37 | 11.8 | 114 | t(9;22), +8 | n.t. | n.t. | 13 |
| 10 | M | 66 | BP | Yes | 3.4 | 7.5 | 121 | t(9;22), i17, +8 | Val379Ile | n.t. | 20 |
| 11 | M | 74 | CP | No | 13.7 | 8.7 | 720 | t(9;22) | wt | n.t. | 6 |
| 12 | F | 58 | CP | No | 28.9 | 13.5 | 347 | t(9;22) | n.t. | n.t. | 3 |
| 13 | M | 65 | BP | Yes | 96.1 | 11.3 | 66 | t(9;22) | Thr315Ala Gly250Glu | 15 | 10 |
| 14 | M | 30 | CP | No | 156.8 | 12.2 | 171 | t(9;22) | wt | n.t. | 12 |
| 15 | M | 64 | CP | No | 342 | 8.9 | 478 | t(9;22) | n.t. | 7 | n.t. |
| 16 | F | 74 | CP | No | 103.5 | 10.5 | 717 | t(9;22) | n.t. | 5 | n.t. |
| 17 | M | 19 | CP | No | 96 | 14.6 | 500 | t(9;22) | n.t. | 6 | 18 |
| 18 | F | 45 | CP | No | 89.1 | 12.2 | 536 | t(9;22) | n.t. | n.t. | 12 |
| 19 | M | 73 | AP | No | 112.2 | 11.9 | 394 | t(9;22), t(2;10) | n.t. | 8 | n.t. |
| 20 | M | 68 | CP | No | 190.0 | 12.3 | 490 | t(9;22) | n.t. | 8 | n.t. |
| 21 | M | 67 | CP | No | 41.4 | 14.8 | 310 | t(9;22) | n.t. | 8 | 20 |
| 22 | M | 73 | CP | Yes | 21.1 | 13.7 | 754 | t(9;22) | Phe359Val | n.t. | 12 |
| 23 | M | 67 | AP | Yes | 16.2 | 9.9 | 69 | t(9;22) | Met244Val | 6 | 8 |
CP indicates chronic phase; AP, accelerated phase; BP, blast phase; WBC, white blood cell count; Hb, hemoglobin; Plt, platelet count; wt, wild type BCR/ABL; and n.t., not tested.
In separate experiments, CD34-positive progenitor cells from patients with CML (n = 6) were enriched by FACS-sorting (on a FACSAria, Becton Dickinson, San Jose, CA) using a phycoerythrin (PE)–conjugated CD34-antibody (Becton Dickinson). After sorting, purified CD34+ cells (purity > 98%) were incubated with various concentrations (5–50 μM) of SMA-ZnPP or PEG-ZnPP at 37°C and 5% CO2, and then were subjected to 3H-thymidine incorporation assay, annexin V staining, or a clonogenic assay.
Cell lines and culture conditions
The human CML cell line K562 and murine Ba/F3 cells expressing either wild-type (wt) BCR/ABL (Ba/F3p210WT) or various imatinib-resistant mutants of BCR/ABL (Ba/F3p210T315I, Ba/F3p210E255K, Ba/F3p210M351T, Ba/F3p210Y253F, Ba/F3p210H396P) were kindly provided by Dr Michael W. Deininger (Oregon Health & Science University Cancer Institute, Portland, OR).32 Ba/F3p210 cells were maintained in RPMI 1640 medium with 10% FCS. Imatinib-resistant K562 cells (kindly provided by Dr James D. Griffin, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA)33 were maintained in RPMI 1640 medium containing 1 μM imatinib.
Generation of K562 cells with inducible expression of Hsp32/HO-1
To generate a K562 cell line with conditional Hsp32/HO-1 expression, we took advantage of a tetracycline-controlled hybrid protein, tTR-KRAB, in which the tetracycline repressor (tTR) from Escherichia coli Tn10 is fused to the KRAB domain of human Kox1.34,–36 In a first step, K562 cells were transduced with pLV-tTR-KRAB-dsRed. Transduced cells were cloned by limiting dilution and analyzed for expression of the tTR-KRAB-dsRed fusion protein by fluorescence microscopy. One single cell clone exhibiting stable expression of tTR-KRAB was then transduced with pWPTet-HO-1 to generate K562 cells with doxycycline-inducible expression of Hsp32/HO-1. Single cell clones were obtained by limiting dilution. To induce expression of Hsp32/HO-1, cells were cultured in the presence of doxycycline (1 μg/mL) for 24 hours. Expression of Hsp32/HO-1 was analyzed by Western blotting.
Recombinant lentiviruses were produced by transient transfection of HEK-293FT cells (Invitrogen, Carlsbad, CA) according to standard protocols.37,38 In brief, subconfluent HEK-293FT cells were cotransfected with a lentiviral vector carrying the gene of interest (pLV-tTR-KRAB-dsRed, pWPTet-HO-1) and with psPAX2, pMD2G, and pRSVrev by lipofectamine2000 (Invitrogen) according to published techniques.37,38 After 16 hours, medium was changed and recombinant lentivirus harvested 24 and 48 hours later. Supernatants were concentrated by ultracentrifugation at 100 000g for 90 minutes and resuspended in Episerf medium (Invitrogen).
Design and application of small interfering RNAs
Two small interfering RNAs (siRNAs) against HO-1 were designed according to guidelines published by Reynolds et al39 (sequence #1: 5′-AAGCUUUCUGGUGGCGACAGUdTdT-3′, sequence #2: 5′-AAGCAACAAAGUGCAAGUUCdTdT-3′). In addition, an HO-1 siRNA described by Zhang et al40 was used (sequence #3: 5′-GGAGAUUGAGCGCAACAAGdTdT-3′). siRNAs against HO-1 as well as a control siRNA against luciferase (5′-CUUACGCUGAGUACUUCGAdTdT) were synthesized in a 2′-deprotected, duplexed, desalted, and purified form (Dharmacon Research, Lafayette, CO). siRNAs (100–200 nM) were transfected into K562 cells using lipofectin (Invitrogen) according to the manufacturers' instructions. Eight hours after transfection, expression of HO-1 was determined by Western blotting. The percentage of apoptotic cells was determined by annexin V/propidium iodide staining.
Western blotting
Western blotting was performed as described.12 Lysates of K562 cells were separated under reducing conditions by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and then were transferred to a polyvinylidenefluoride (PVDF) membrane (Amersham, Amersham, United Kingdom) in buffer containing 25 mM Tris (tris(hydroxymethyl)aminomethane), 192 mM glycine, and 20% (v/v) methanol at 4°C. The membrane was blocked for 30 minutes in 5% bovine serum albumin (BSA) (Gibco). After incubation with a polyclonal rabbit anti–HO-1 antibody (Stressgen, Victoria, BC), the membrane was exposed to goat antirabbit IgG (Amersham). To confirm equal loading, membranes were reprobed with a rabbit anti-actin antibody (Sigma, St Louis, MO). Chemoluminescence was detected by exposure to CL-Xposure film (Pierce Biotechnology, Rockford, IL).
Determination of cell viability and apoptosis
The effects of Hsp32/HO-1 inhibitors on apoptosis were analyzed by flow cytometry, TUNEL assay, and light microscopy as well as by electron microscopy. Viability of leukemic cells, cell lines, and control cells (pb MNCs) was determined by trypan blue exclusion and expressed as percentage of (non)viable cells. Combined annexin V/propidium iodide staining was performed using the annexin V-FITC apoptosis detection kit (Alexis Biochemicals) as described.12 Cells were analyzed by flow cytometry on a FACSCalibur (Becton Dickinson). A TUNEL assay was performed as reported41 using the “In situ cell death detection kit” (Roche). Cells were analyzed with a Nikon Eclipse E 800 fluorescence microscope (Nikon, Tokyo, Japan). Electron microscopy was performed as described41 using K562 cells exposed to SMA-ZnPP (10 μM) or control medium for 48 hours. Sections were viewed under a JEOL 1200 EX II transmission electron microscope (JEOL, Tokyo, Japan). Apoptosis was defined according to conventional cytomorphologic criteria.42
HO-1 activity assay and determination of cellular uptake of PEG-ZnPP and SMA-ZnPP
K562 cells were treated with PEG-ZnPP or SMA-ZnPP (5 μM) for various time periods (0–48 hours). Thereafter, cells were recovered in suspension buffer (20 mM Tris-HCl, 0.25 M sucrose, pH 7.4), disrupted by sonication (20 watts, 60 s) and centrifuged at 4°C. HO-1 activity was measured spectrophotometrically as described.29,43 In select experiments, K562 cells transfected with HO-1 siRNA were subjected to an HO-1 activity assay. To provide sufficient survival in these experiments, cells were cultured in the presence of hemin (10 μM) for 4 hours prior to transfection with HO-1 siRNA. HO activity was expressed as nanomoles of bilirubin formed per milligram of protein per hour. To determine the kinetics of the cellular uptake of PEG-ZnPP and SMA-ZnPP, K562 cells were treated with SMA-ZnPP or PEG-ZnPP (5 μM) for various time periods. Thereafter, cells were harvested, washed with phosphate buffered saline (PBS), and resuspended in ethanol. After sonication (20 watts, 60 s), lysates were centrifuged and the concentration of ZnPP in supernatants determined spectrophotometrically.
3H-thymidine incorporation assay
To examine antiproliferative effects of ZnDPPIX, PEG-ZnPP, and SMA-ZnPP, BCR/ABL-positive cell lines (K562, KU812, imatinib-resistant K562, Ba/F3 cells expressing wt BCR/ABL or mutant forms of BCR/ABL) were cultured in 96-well microtiter plates (5 × 104 cells per well) in the absence or presence of various concentrations of imatinib, ZnDPPIX, PEG-ZnPP, or SMA-ZnPP for 48 hours. In select experiments, combinations of Hsp32/HO-1–targeting drugs (ZnDPPIX, PEG-ZnPP, SMA-ZnPP) and TK inhibitors (imatinib or nilotinib) were applied at a fixed ratio. Primary CML cells were kept in the absence or presence of GM-CSF (100 ng/mL) for 7 days before being exposed to inhibitors. After treatment, 1 μCi 3H-thymidine was added to each well. Twelve hours later, cells were harvested on filter membranes (Packard Bioscience, Meriden, CT) in a Filtermate 196 harvester (Packard Bioscience). Filters were air dried, and the bound radioactivity was measured in a β-counter (Top-Count NXT, Packard Bioscience). All experiments were performed in triplicate.
Clonogenic assay
To determine the effects of Hsp32/HO-1 inhibitors on clonogenic growth of CML progenitor cells, MNCs or CD34+ progenitor cells from CML patients (n = 3) or healthy donors (n = 3) were incubated with SMA-ZnPP (10 μM each) or were cultured in control medium for 24 hours. Thereafter, cells were examined for viability by trypan blue exclusion (more than 90% were viable cells in all experiments) and transferred to 30-mm culture plates. Cells (1–3 × 105 MNCs per well; 5 × 103 CD34+ cells) were maintained at 37°C and 5% CO2 in 0.8% methylcellulose, 30% FCS, 10% BSA, α-thioglycerol (10−4 mol/L) and IMDM supplemented with GM-CSF (10 ng/mL), IL-3 (5 ng/mL), and erythropoietin (2 U/mL). After a culture period of 14 days, cultures were examined under an inverted microscope (Olympus, Tokyo, Japan). Aggregates of greater than 40 nonerythroid cells were counted as CFU-GM, bursts containing more than 100 hemoglobinized cells as BFU-E, and mixed colonies as CFU-GEMM.
Mice
Nude mice (4 in each group) were injected subcutaneously with either Ba/F3p210wt or Ba/F3T315I cells (107 cells each flank). Starting on day 7, PEG-ZnPP (3 mM, 0.1 mL) was administered (equivalent to 5 mg of ZnPP/kg) intravenously 3 times a week over a period of 2 weeks (mice received a total of 6 injections). In control experiments, mice received physiological saline (0.1 mL) instead of the PEG-ZnPP solution. On day 30, mice were killed, and tumor nodules were excised and weighed.
Statistical analysis
To determine the level of significance in inhibition experiments, the paired Student t test was applied. Results were considered to be significantly different when the P value was less than .05. To characterize synergistic or antagonistic interactions between tyrosine kinase inhibitors (imatinib, nilotinib) and Hsp32/HO-1–targeting drugs (ZnDPPIX, PEG-ZnPP, SMA-ZnPP), leukemic cells were exposed to these drugs in various combinations (fixed ratio of drug concentrations). Drug interactions (additive versus synergistic) were determined by calculating combination index (CI) values using a commercially available software program (Calcusyn; Biosoft, Ferguson, MO).44 In these analyses, a CI value of 1.0 indicates an additive effect, whereas a CI value of less than 1.0 indicates synergism.
Results
Down-regulation of expression of Hsp32/HO-1 by siRNA leads to induction of apoptosis in K562 cells
We have previously shown that Hsp32/HO-1 is expressed in CML cells in a BCR/ABL-dependent manner.12 To investigate the functional role of this Hsp in CML cells, expression of Hsp32/HO-1 was specifically silenced in K562 cells using 3 different siRNAs. The siRNA-induced knockdown of Hsp32/HO-1 was found to be associated with a significant decrease in cell viability in K562 cells (Figure 1A). Loss of expression of Hsp32/HO-1 in these cells was confirmed by Western blotting (Figure 1B). Moreover, we were able to show that the siRNA-induced knockdown of Hsp32/HO-1 in hemin-treated K562 cells is associated with a substantial (> 50%) reduction of HO-1 activity (not shown). A control siRNA showed no effect on expression of Hsp32/HO-1 and no effect on survival in K562 cells (Figure 1A,B). All 3 siRNAs showed comparable effects on survival in K562 cells (Figure 1C).
Knockdown of expression of HO-1 in CML cells is followed by apoptosis. (A) K562 cells were left untreated (Control) or were transfected with a control siRNA against luciferase or an siRNA specific for HO-1 as indicated. 8 hours after transfection, the percentage of apoptotic cells (provided for each condition) was determined by combined annexin V/propidium iodide staining and FACS analysis. PI, propidium iodide. (B) K562 cells were incubated in control medium (Co) or were transfected with control siRNA against Luciferase (Luc) or with a HO-1-specific siRNA (HO-1) at 100 nM or 200 nM. 8 hours after transfection, expression of HO-1 was determined by Western blotting. Expression of β-actin is shown as loading control. (C) The effects are shown for 3 different siRNAs (nos. 1–3) specific for HO-1 on viability of K562 cells compared with untransfected cells (Co) or cells transfected with a control siRNA (Luc). Results represent the mean (± SD) of 3 independent experiments. *P < .05 compared with control (Co).
Knockdown of expression of HO-1 in CML cells is followed by apoptosis. (A) K562 cells were left untreated (Control) or were transfected with a control siRNA against luciferase or an siRNA specific for HO-1 as indicated. 8 hours after transfection, the percentage of apoptotic cells (provided for each condition) was determined by combined annexin V/propidium iodide staining and FACS analysis. PI, propidium iodide. (B) K562 cells were incubated in control medium (Co) or were transfected with control siRNA against Luciferase (Luc) or with a HO-1-specific siRNA (HO-1) at 100 nM or 200 nM. 8 hours after transfection, expression of HO-1 was determined by Western blotting. Expression of β-actin is shown as loading control. (C) The effects are shown for 3 different siRNAs (nos. 1–3) specific for HO-1 on viability of K562 cells compared with untransfected cells (Co) or cells transfected with a control siRNA (Luc). Results represent the mean (± SD) of 3 independent experiments. *P < .05 compared with control (Co).
Effects of pharmacologic inhibitors of HO-1 on growth of CML-derived cell lines
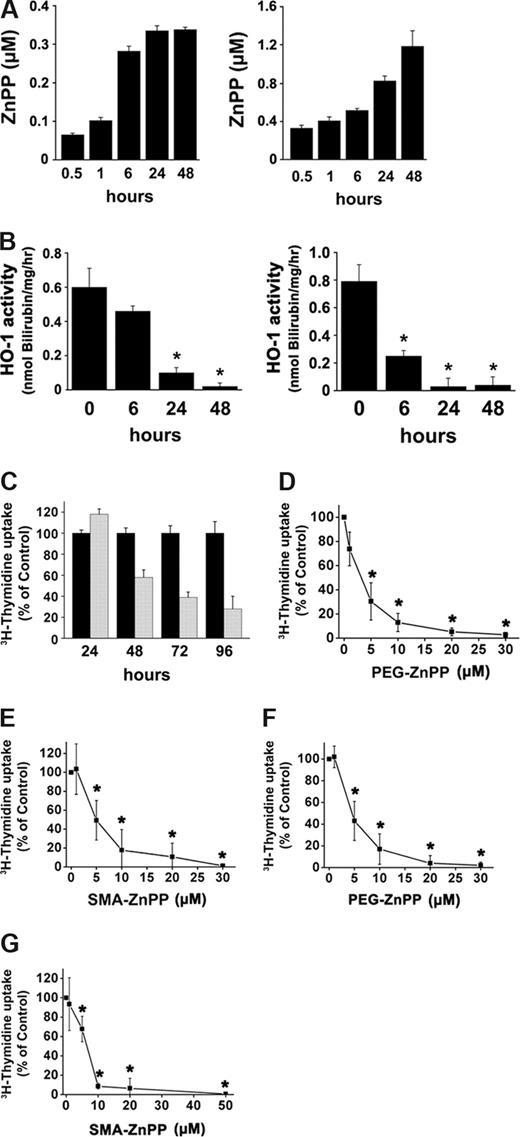
To evaluate the role of HO-1 as a potential therapeutic target in CML cells, ZnPP, a competitive inhibitor of HO-1, coupled to either polyethylene glycol, PEG (PEG-ZnPP),28 or to the copolymer of styrene-maleic acid, SMA (SMA-ZnPP)30 was applied. In addition, ZnDPPIX was used.27 In contrast to ZnDPPIX, PEG-ZnPP and SMA-ZnPP are water soluble compounds. In a first step, we investigated the kinetics of cellular uptake of PEG-ZnPP and SMA-ZnPP, and the resulting inhibition of HO-1 activity. As shown in Figure 2A and B, both compounds were found to reach their maximum inhibitory activity after 24 and 48 hours. Correspondingly, we found that drug uptake into K562 cells occurs within 48 hours (Figure 2A,B). We then determined the effects of these compounds on growth of CML cells. As assessed by 3H-thymidine uptake, all 3 Hsp32/HO-1–targeting drugs (ZnDPPIX, PEG-ZnPP, SMA-ZnPP) were found to inhibit proliferation of K562 cells and KU812 cells in a time- and dose-dependent manner. As exemplified for SMA-ZnPP and K562 cells in Figure 2C, maximum effects on proliferation of leukemic cells occurred after 72 hours. In Figure 2D through G, the dose-dependent effects of PEG-ZnPP and SMA-ZnPP on growth of K562 cells and KU812 cells are shown. The respective IC50 values ranged between 2 and 7 μM for PEG-ZnPP, and between 2 and 12 μM for SMA-ZnPP (Figure 2D-G; Table 2). The IC50 values for ZnDPPIX were 5 μM for KU812 cells and 8 μM for K562 cells (Table 2).
Pharmacologic inhibition of HO-1 suppresses growth of CML cell. (A,B) K562 cells were treated with PEG-ZnPP (left panel) or SMA-ZnPP (right panel) (5 μM each) for various time periods as indicated. Thereafter, cellular uptake of the compounds expressed as μM ZnPP incorporated in 106 input cells (A), and the HO-1 activity in treated cells (B) were determined as described in “Methods.” HO-1 activity is expressed as nanomoles of bilirubin formed per milligram of protein per hour (nmol Bilirubin/mg/hr). Results represent the mean (± SD) of 3 independent experiments. *P < .05 compared with untreated cells (timepoint 0). (C) Time-dependent inhibition of growth of K562 cells by SMA-ZnPP. K562 cells were incubated with SMA-ZnPP (10 μM; gray bars) or control medium (black bars) at 37°C for various time periods as indicated. Proliferation was measured by 3H-thymidine incorporation. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates of one representative experiment. (D-G) K562 cells (D,E) or KU812 cells (F, G) were incubated with various concentrations of PEG-ZnPP (D,F) or SMA-ZnPP (E, G) as indicated for 48 hours. After incubation, growth of cells was determined by 3H-thymidine incorporation. Results represent the mean (± SD) of 3 independent experiments. *P < .05 compared with control.
Pharmacologic inhibition of HO-1 suppresses growth of CML cell. (A,B) K562 cells were treated with PEG-ZnPP (left panel) or SMA-ZnPP (right panel) (5 μM each) for various time periods as indicated. Thereafter, cellular uptake of the compounds expressed as μM ZnPP incorporated in 106 input cells (A), and the HO-1 activity in treated cells (B) were determined as described in “Methods.” HO-1 activity is expressed as nanomoles of bilirubin formed per milligram of protein per hour (nmol Bilirubin/mg/hr). Results represent the mean (± SD) of 3 independent experiments. *P < .05 compared with untreated cells (timepoint 0). (C) Time-dependent inhibition of growth of K562 cells by SMA-ZnPP. K562 cells were incubated with SMA-ZnPP (10 μM; gray bars) or control medium (black bars) at 37°C for various time periods as indicated. Proliferation was measured by 3H-thymidine incorporation. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates of one representative experiment. (D-G) K562 cells (D,E) or KU812 cells (F, G) were incubated with various concentrations of PEG-ZnPP (D,F) or SMA-ZnPP (E, G) as indicated for 48 hours. After incubation, growth of cells was determined by 3H-thymidine incorporation. Results represent the mean (± SD) of 3 independent experiments. *P < .05 compared with control.
Response of leukemic cell lines to Hsp32/HO-1 inhibitors
| Cell line . | PEG-ZnPP (μM) . | SMA-ZnPP (μM) . | ZnDPPIX (μM) . | |||
|---|---|---|---|---|---|---|
| IC50 . | Range . | IC50 . | Range . | IC50 . | Range . | |
| K562 | 3 | 2–4 | 6 | 4–8 | 8 | 7–10 |
| KU812 | 5 | 2–7 | 7 | 2–12 | 5 | 4–6 |
| Imatinib-resistant K562 | 4 | 3–6 | 7 | 4–8 | 24 | 20–30 |
| BaF3p210wt | n.t | n.t | 16 | 5–28 | n.t | n.t |
| BaF3p210T315I | n.t | n.t | 8 | 5–10 | n.t | n.t |
| BaF3p210E255K | n.t | n.t | 10 | 5–13 | n.t | n.t |
| BaF3p210Y253F | n.t | n.t | 15 | 10–25 | n.t | n.t |
| BaF3p210M351T | n.t | n.t | 13 | 5–18 | n.t | n.t |
| BaF3p210H396P | n.t | n.t | 6 | 5–8 | n.t | n.t |
| Cell line . | PEG-ZnPP (μM) . | SMA-ZnPP (μM) . | ZnDPPIX (μM) . | |||
|---|---|---|---|---|---|---|
| IC50 . | Range . | IC50 . | Range . | IC50 . | Range . | |
| K562 | 3 | 2–4 | 6 | 4–8 | 8 | 7–10 |
| KU812 | 5 | 2–7 | 7 | 2–12 | 5 | 4–6 |
| Imatinib-resistant K562 | 4 | 3–6 | 7 | 4–8 | 24 | 20–30 |
| BaF3p210wt | n.t | n.t | 16 | 5–28 | n.t | n.t |
| BaF3p210T315I | n.t | n.t | 8 | 5–10 | n.t | n.t |
| BaF3p210E255K | n.t | n.t | 10 | 5–13 | n.t | n.t |
| BaF3p210Y253F | n.t | n.t | 15 | 10–25 | n.t | n.t |
| BaF3p210M351T | n.t | n.t | 13 | 5–18 | n.t | n.t |
| BaF3p210H396P | n.t | n.t | 6 | 5–8 | n.t | n.t |
Cell lines were cultured in control medium or in various concentrations of Hsp32/HO-1 inhibitors for 48 hours. Thereafter, proliferation of cells was measured by 3H-thymidine incorporation assay. IC50 values (μM) represent the mean from at least 3 independent experiments. The ranges of IC50 values (μM) are also provided.
n.t. indicates not tested.
Effects of HO-1 inhibitors on primary CML cells
In the next step, we investigated the effects of PEG-ZnPP and SMA-ZnPP on primary leukemic cells obtained from patients with CML (CP: n = 14; AP or BP: n = 9). Prior to incubation with PEG-ZnPP or SMA-ZnPP, cells were cultured in the presence of GM-CSF (100 ng/mL) for 7 days. In these experiments, both inhibitors were found to suppress in vitro growth of primary leukemic cells in a dose-dependent manner. In particular, PEG-ZnPP and SMA-ZnPP inhibited growth of leukemic cells obtained from patients with CP CML (Figure 3A,B) as well as leukemic cells from patients with AP or BP (Figure 3C,D). IC50 values ranged between 2 and 20 μM without a significant difference when comparing CP patients with AP/BP (Table 1). Unstimulated CML cells (kept in the absence of GM-CSF) were found to respond to Hsp32/HO-1 inhibitors in the same way as GM-CSF–stimulated cells (not shown).
PEG-ZnPP and SMA-ZnPP inhibit growth of primary CML cells. Isolated leukemic cells from patients with newly diagnosed CP CML (A,B) or advanced phase CML (C: AP; D: BP) were stimulated with GM-CSF (100 U/mL) for 7 days and then incubated with control medium (0), various concentrations of PEG-ZnPP (left panels), or various concentrations of SMA ZnPP (right panels) at 37°C for 48 hours. Thereafter, 3H-thymidine incorporation was determined. Results show the percentage of 3H-thymidine uptake compared with control (100%) and represent the mean (± SD) of triplicates in each patient. (E) Sorted pure CD34-positive progenitor cells from a patient with newly diagnosed CP CML were incubated with control medium (0) or various concentrations of SMA-ZnPP at 37°C for 48 hours. Thereafter, 3H-thymidine uptake was determined. Results are expressed as percent 3H-thymidine uptake compared with control (100%) and represent the mean (± SD) of triplicates. (F) CD34-positive progenitor cells from 3 CML patients were incubated with control medium (Co) or SMA-ZnPP (20 μM) for 48 hours. Thereafter, 3H-thymidine incorporation was determined. Results represent the mean (± SD) of 3 independent experiments (3 patients). *P < .05 compared with control. (G) Isolated CD34+ CML progenitor cells were cultured in control medium (left panel) or in the presence of SMA-ZnPP (10 μM) (right panel) for 4 days. Thereafter, the percentage of apoptotic cells was determined by annexin V staining and FACS analysis.
PEG-ZnPP and SMA-ZnPP inhibit growth of primary CML cells. Isolated leukemic cells from patients with newly diagnosed CP CML (A,B) or advanced phase CML (C: AP; D: BP) were stimulated with GM-CSF (100 U/mL) for 7 days and then incubated with control medium (0), various concentrations of PEG-ZnPP (left panels), or various concentrations of SMA ZnPP (right panels) at 37°C for 48 hours. Thereafter, 3H-thymidine incorporation was determined. Results show the percentage of 3H-thymidine uptake compared with control (100%) and represent the mean (± SD) of triplicates in each patient. (E) Sorted pure CD34-positive progenitor cells from a patient with newly diagnosed CP CML were incubated with control medium (0) or various concentrations of SMA-ZnPP at 37°C for 48 hours. Thereafter, 3H-thymidine uptake was determined. Results are expressed as percent 3H-thymidine uptake compared with control (100%) and represent the mean (± SD) of triplicates. (F) CD34-positive progenitor cells from 3 CML patients were incubated with control medium (Co) or SMA-ZnPP (20 μM) for 48 hours. Thereafter, 3H-thymidine incorporation was determined. Results represent the mean (± SD) of 3 independent experiments (3 patients). *P < .05 compared with control. (G) Isolated CD34+ CML progenitor cells were cultured in control medium (left panel) or in the presence of SMA-ZnPP (10 μM) (right panel) for 4 days. Thereafter, the percentage of apoptotic cells was determined by annexin V staining and FACS analysis.
To investigate the effects of Hsp32/HO-1 inhibitors on growth of CML progenitor cells, CD34-positive cells were enriched from untreated CP CML patients by FACS sorting (n = 3, purity > 98%). As shown in Figure 3E and F, growth of CD34-positive CML cells was found to be effectively inhibited by SMA-ZnPP. To further investigate the role of Hsp32/HO-1 in CML progenitors, primary leukemic cells from untreated CML patients (n = 3) were incubated with SMA-ZnPP (10 μM) or control medium for 24 hours in suspension and were then grown in methylcellulose for 14 days in the presence of IL-3, GM-CSF, and EPO. Preincubation with SMA-ZnPP was found to completely abolish growth of CML progenitor cells in this assay (Table 3). In contrast, growth of normal MNCs from healthy donors was not substantially affected by SMA-ZnPP (Table 3). The same results were obtained when CD34+ progenitor cells were used (Table 3). Again, growth of CD34+ CML cells was inhibited, whereas normal CD34+ progenitor cells were not suppressed by SMA-ZnPP (Table 3). Finally, we could show that SMA-ZnPP induces apoptosis in CD34+ CML progenitor cells (Figure 3G). Together, these data support the concept that HO-1 is an important survival molecule and interesting target in CML cells.
Inhibition of clonogenic growth of primary CML cells by SMA-ZnPP
| . | Control medium . | SMA-ZnPP . | ||||
|---|---|---|---|---|---|---|
| CFU-GM . | BFU-E . | CFU-GEMM . | CFU-GM . | BFU-E . | CFU-GEMM . | |
| CML (MNC) no. 1 | 29 | 133 | 3 | 0 | 0 | 0 |
| CML (MNC) no. 2 | 18 | 160 | 0 | 0 | 0 | 0 |
| CML (MNC) no. 3 | 17 | 59 | 0 | 0 | 0 | 0 |
| Healthy donor (MNC) no. 1 | 6 | 27 | 1 | 1 | 20 | 0 |
| Healthy donor (MNC) no. 2 | 9 | 36 | 2 | 2 | 15 | 1 |
| Healthy donor (MNC) no. 3 | 8 | 37 | 0 | 5 | 35 | 1 |
| Healthy donor (CD34+ cells) | 43 | 110 | 2 | 31 | 106 | 1 |
| CML (CD34+ cells) no. 1 | 12 | 107 | 3 | 0 | 0 | 0 |
| CML (CD34+ cells) no. 2 | 21 | 210 | 0 | 0 | 9 | 0 |
| CML (CD34+ cells) no. 3 | 18 | 22 | 0 | 2 | 5 | 0 |
| . | Control medium . | SMA-ZnPP . | ||||
|---|---|---|---|---|---|---|
| CFU-GM . | BFU-E . | CFU-GEMM . | CFU-GM . | BFU-E . | CFU-GEMM . | |
| CML (MNC) no. 1 | 29 | 133 | 3 | 0 | 0 | 0 |
| CML (MNC) no. 2 | 18 | 160 | 0 | 0 | 0 | 0 |
| CML (MNC) no. 3 | 17 | 59 | 0 | 0 | 0 | 0 |
| Healthy donor (MNC) no. 1 | 6 | 27 | 1 | 1 | 20 | 0 |
| Healthy donor (MNC) no. 2 | 9 | 36 | 2 | 2 | 15 | 1 |
| Healthy donor (MNC) no. 3 | 8 | 37 | 0 | 5 | 35 | 1 |
| Healthy donor (CD34+ cells) | 43 | 110 | 2 | 31 | 106 | 1 |
| CML (CD34+ cells) no. 1 | 12 | 107 | 3 | 0 | 0 | 0 |
| CML (CD34+ cells) no. 2 | 21 | 210 | 0 | 0 | 9 | 0 |
| CML (CD34+ cells) no. 3 | 18 | 22 | 0 | 2 | 5 | 0 |
Primary mononuclear cells (MNC) were isolated from the peripheral blood of untreated CML patients or from healthy controls by Ficoll density centrifugation and were then cultured in control medium or SMA-ZnPP (10 μM) for 24 hours. Thereafter, cells were cultured in methylcellulose in the presence of cytokines as described in the text for 14 days. Then, cultures were examined for the number of colonies by an inverted microscope. Results show the numbers of colonies per 105 cells (MNC seeded on day 0) or colonies per 5 x 103 CD34+ cells and represent the mean of duplicates in each donor.
Hsp32/HO-1–targeting drugs suppress the growth of imatinib-resistant leukemic cells
Imatinib inhibits growth of CML cells in vitro and in vivo and is widely used as a therapeutic agent in patients with CML.13,–15 However, an increasing number of patients with CML develop resistance against imatinib.20,45 To investigate growth-inhibitory effects of PEG-ZnPP on imatinib-resistant leukemic cells, we examined imatinib-resistant K562 cells, Ba/F3 cells expressing imatinib-resistant mutants of BCR/ABL, as well as primary leukemic cells obtained from patients with imatinib-resistant CML (n = 7). As shown in Figure 4A, all 3 Hsp32/HO-1–targeting drugs (ZnDPPIX, PEG-ZnPP, SMA-ZnPP) were found to inhibit proliferation of imatinib-resistant K562 cells. A similar effect was seen when SMA-ZnPP was applied on Ba/F3 cells carrying various imatinib-resistant BCR/ABL-mutants, including Ba/F3p210T315I, Ba/F3p210E255K, Ba/F3p210M351T, Ba/F3p210Y253F, and Ba/F3p210H396P (Figure 4B). The IC50 values for growth inhibition produced by PEG-ZnPP and SMA-ZnPP in imatinib-resistant Ba/F3p210 cells are shown in Table 2. Growth of parental Ba/F3 cells (not transformed with BCR/ABL) maintained in the presence of IL-3 (1 ng/mL) was not inhibited by Hsp32/HO-1–targeting drugs (data not shown).
Hsp32/HO-1-targeting drugs inhibit growth of imatinib-resistant CML cells. (A) Imatinib-resistant K562 cells were incubated in medium containing various concentrations of ZnDPPIX (left), PEG-ZnPP (middle), or SMA-ZnPP (right) for 48 hours. Growth-inhibitory effects were quantified by 3H-thymidine incorporation assay. Results show the percentage of 3H-thymidine uptake compared with medium control (100%) and represent the mean (± SD) of 3 independent experiments. *P < .05 compared with medium control. (B) Ba/F3 cells expressing wt BCR/ABL or various imatinib-resistant mutants of BCR/ABL were incubated with various concentrations of SMA-ZnPP for 48 hours. Thereafter, proliferation was measured by 3H-thymidine incorporation. Results are expressed as percent of control and represent the mean (± SD) of triplicates from one representative experiment. (C) Isolated leukemic cells from 3 patients with imatinib-resistant CML were treated with PEG-ZnPP for 48 hours. Thereafter, proliferation was measured by 3H-thymidine incorporation assay. Results are expressed as percent of control and represent the mean (± SD) of 3 independent experiments (3 patients). *P < .05 compared with control. (D) Isolated leukemic cells from a patient with imatinib-resistant CML carrying BCR/ABL T315I, were treated with PEG-ZnPP (left panel) or SMA-ZnPP (right panel) for 48 hours. Thereafter, 3H-thymidine incorporation was measured. Results show the percentage of 3H-thymidine uptake compared with control (100%) and represent the mean (± SD) of triplicates. (E) Ba/F3p210wt (left panel) and Ba/F3p210T315I cells (right panel) were injected subcutaneously into nude mice (4 in each group). 7 days after tumor inoculation, when visible tumors had developed, mice were treated with PEG-ZnPP (3 mM, 0.1 mL, equivalent to 5 mg of ZnPP/kg) intravenously 3 times a week over a period of 2 weeks. Mice in the control group received physiological saline instead of PEG-ZnPP. On day 30, mice were killed, and tumor nodules were excised and weighed.
Hsp32/HO-1-targeting drugs inhibit growth of imatinib-resistant CML cells. (A) Imatinib-resistant K562 cells were incubated in medium containing various concentrations of ZnDPPIX (left), PEG-ZnPP (middle), or SMA-ZnPP (right) for 48 hours. Growth-inhibitory effects were quantified by 3H-thymidine incorporation assay. Results show the percentage of 3H-thymidine uptake compared with medium control (100%) and represent the mean (± SD) of 3 independent experiments. *P < .05 compared with medium control. (B) Ba/F3 cells expressing wt BCR/ABL or various imatinib-resistant mutants of BCR/ABL were incubated with various concentrations of SMA-ZnPP for 48 hours. Thereafter, proliferation was measured by 3H-thymidine incorporation. Results are expressed as percent of control and represent the mean (± SD) of triplicates from one representative experiment. (C) Isolated leukemic cells from 3 patients with imatinib-resistant CML were treated with PEG-ZnPP for 48 hours. Thereafter, proliferation was measured by 3H-thymidine incorporation assay. Results are expressed as percent of control and represent the mean (± SD) of 3 independent experiments (3 patients). *P < .05 compared with control. (D) Isolated leukemic cells from a patient with imatinib-resistant CML carrying BCR/ABL T315I, were treated with PEG-ZnPP (left panel) or SMA-ZnPP (right panel) for 48 hours. Thereafter, 3H-thymidine incorporation was measured. Results show the percentage of 3H-thymidine uptake compared with control (100%) and represent the mean (± SD) of triplicates. (E) Ba/F3p210wt (left panel) and Ba/F3p210T315I cells (right panel) were injected subcutaneously into nude mice (4 in each group). 7 days after tumor inoculation, when visible tumors had developed, mice were treated with PEG-ZnPP (3 mM, 0.1 mL, equivalent to 5 mg of ZnPP/kg) intravenously 3 times a week over a period of 2 weeks. Mice in the control group received physiological saline instead of PEG-ZnPP. On day 30, mice were killed, and tumor nodules were excised and weighed.
In the next step, we investigated the effects of Hsp32/HO-1 inhibitors on growth of primary imatinib-resistant CML cells. In these experiments, we were able to show that PEG-ZnPP and SMA-ZnPP suppress the growth of leukemic cells obtained from patients with imatinib-resistant CML (Figure 4C; Table 1). Interestingly, growth inhibition by PEG-ZnPP and SMA-ZnPP was seen in all patients, including one patient exhibiting the T315I mutant of BCR/ABL (Figure 4D). IC50 values for all patients are shown in Table 1.
To show that pharmacologic targeting of Hsp32/HO-1 is associated with inhibition of growth of leukemic cells in vivo, Ba/F3p210wt as well as Ba/F3p210T315I cells were injected subcutaneously into nude mice. Treatment of mice with PEG-ZnPP resulted in a significantly decreased tumor growth compared with saline control (P < .05) (Figure 4E). Together, these data suggest that targeting of HO-1 in imatinib-resistant CML cells in vivo results in growth inhibition.
The growth-inhibitory effects of PEG-ZnPP and SMA-ZnPP on CML cells are associated with induction of apoptosis
Hsp32/HO-1 has been implicated as a survival-promoting molecule in various cell types and has been described to inhibit apoptosis.10 Therefore, we were interested to learn whether the growth-inhibitory effects of the Hsp32/HO-1 inhibitors used in this study are associated with induction of apoptosis. In a first step, we investigated the effects of PEG-ZnPP and SMA-ZnPP on K562 cells by electron microscopy. As exemplified for SMA-ZnPP in Figure 5A, we found that treatment with HO-1–targeting compounds leads to typical signs of apoptosis but does not induce necrotic cell death. Corresponding results were obtained in a TUNEL assay (Figure 5B) and by annexin V staining (Figure 5C). In both assays, the Hsp32/HO-1–targeting drugs were found to produce apoptosis in K562 cells. Drug-induced apoptosis was not accompanied by a rapid decrease in cell numbers at 48 hours (not shown), supporting the notion that apoptosis was the primary mechanism of reduced cell growth in these experiments. To provide further evidence that Hsp32/HO-1 inhibitors induce apoptosis by inhibition of Hsp32/HO-1, we generated K562 cells with doxycycline-inducible expression of Hsp32/HO-1. Using these cells, we were able to show that overexpression of Hsp32/HO-1 counteracts induction of apoptosis produced by SMA-ZnPP (Figure 5D,E). Finally, we found that Hsp32/HO-1–targeting drugs induce apoptosis in primary CML cells as determined by annexin V staining and light microscopy (Figure 5F,G). Together, these data show that growth inhibition induced by PEG-ZnPP and SMA-ZnPP in CML cells is associated with induction of apoptosis.
SMA-ZnPP induces apoptosis in CML cells. (A) K562 cells were incubated with control medium (left panel) or SMA-ZnPP (10 μM) (right panel) for 48 hours. Thereafter, cells were analyzed by electron microscopy as described in “Methods.” SMA-ZnPP was found to induce typical morphological signs of apoptosis (chromatin fragmentation/condensation). (B) K562 cells were incubated with control medium (left panel) or SMA-ZnPP (10 μM) (right panel) for 48 hours. Thereafter, cells were subjected to a Tunel assay. Apoptotic cells display bright green fluorescence. (C) K562 cells were incubated in control medium (Co) or in PEG-ZnPP or SMA-ZnPP (each 10 or 20 μM) at 37°C. After 48 hours, cells were subjected to annexin-V staining. Results show the percentage of annexin-V–positive cells and represent the mean ± SD of 3 independent experiments. *P < .05 compared with control. (D) K562-KRAB-HO-1 cells were cultured in control medium (Co) or in the presence of doxycycline (1 μg/ml) for 24 hours. Thereafter, expression of Hsp32/HO-1 was determined by Western blotting. β-actin is shown as loading control. (E) K562-KRAB-HO-1 cells were cultured in control medium (Co) or in the presence of doxycycline (1 μg/mL) for 24 hours. Thereafter, cells were kept in the absence or presence of SMA-ZnPP (10 μM) as indicated for 48 hours. Cell viability was determined by trypan blue exclusion test. Results show the percentage of trypan blue-positive cells and represent the mean (± SD) of 3 independent experiments. (F) Leukemic cells from untreated patients with CP CML were cultured in control medium or in the presence of SMA-ZnPP (10 μM or 30 μM) for 4 days. Thereafter, the percentage of apoptotic cells was determined by annexin-V staining and FACS analysis. Results represent the mean (± SD) of 3 patients. *P < .05. (G) Primary CML cells were cultured in the absence or presence of SMA-ZnPP (10 μM) for 5 days. Thereafter, the percentage of apoptotic cells was quantified on Wright-Giemsa-stained cytospin preparations. Results represent the mean (± SD) of 3 patients. *P < .05.
SMA-ZnPP induces apoptosis in CML cells. (A) K562 cells were incubated with control medium (left panel) or SMA-ZnPP (10 μM) (right panel) for 48 hours. Thereafter, cells were analyzed by electron microscopy as described in “Methods.” SMA-ZnPP was found to induce typical morphological signs of apoptosis (chromatin fragmentation/condensation). (B) K562 cells were incubated with control medium (left panel) or SMA-ZnPP (10 μM) (right panel) for 48 hours. Thereafter, cells were subjected to a Tunel assay. Apoptotic cells display bright green fluorescence. (C) K562 cells were incubated in control medium (Co) or in PEG-ZnPP or SMA-ZnPP (each 10 or 20 μM) at 37°C. After 48 hours, cells were subjected to annexin-V staining. Results show the percentage of annexin-V–positive cells and represent the mean ± SD of 3 independent experiments. *P < .05 compared with control. (D) K562-KRAB-HO-1 cells were cultured in control medium (Co) or in the presence of doxycycline (1 μg/ml) for 24 hours. Thereafter, expression of Hsp32/HO-1 was determined by Western blotting. β-actin is shown as loading control. (E) K562-KRAB-HO-1 cells were cultured in control medium (Co) or in the presence of doxycycline (1 μg/mL) for 24 hours. Thereafter, cells were kept in the absence or presence of SMA-ZnPP (10 μM) as indicated for 48 hours. Cell viability was determined by trypan blue exclusion test. Results show the percentage of trypan blue-positive cells and represent the mean (± SD) of 3 independent experiments. (F) Leukemic cells from untreated patients with CP CML were cultured in control medium or in the presence of SMA-ZnPP (10 μM or 30 μM) for 4 days. Thereafter, the percentage of apoptotic cells was determined by annexin-V staining and FACS analysis. Results represent the mean (± SD) of 3 patients. *P < .05. (G) Primary CML cells were cultured in the absence or presence of SMA-ZnPP (10 μM) for 5 days. Thereafter, the percentage of apoptotic cells was quantified on Wright-Giemsa-stained cytospin preparations. Results represent the mean (± SD) of 3 patients. *P < .05.
Hsp32/HO-1–targeting drugs synergize with imatinib and nilotinib in producing growth inhibition in imatinib-resistant BCR/ABL-transformed cells
In the next step, we examined cooperative drug effects on growth of CML cells using Hsp32/HO-1–targeting drugs and TK inhibitors. In these experiments, we found that ZnDPPIX and imatinib exert potent cooperative inhibitory effects on growth of imatinib-resistant K562 cells (Figure 6A). To show that ZnDPPIX and imatinib exert synergistic effects on cell growth, combination index (CI) values were calculated and were found to be below 1, indicating synergistic drug interactions (Figure 6B). In the next step, we examined cooperative effects between Hsp32/HO-1–targeting drugs (PEG-ZnPP and SMA-ZnPP) and TK inhibitors in Ba/F3 cells carrying BCR/ABL T315I. Again, synergistic growth inhibitory effects were obtained in these experiments (Figure 6C,D). The synergism between Hsp32/HO-1–targeting drugs and TK inhibitors also was demonstrable when cell viability of Ba/F3p210T315I cells was examined (Figure 6E). Moreover, we were able to confirm these cooperative drug effects in primary CML cells obtained from imatinib-resistant CML, including patients carrying the T315I BCR/ABL mutation (Figure 6F,G). In control experiments, neither the single drugs (imatinib up to 10 μM or Hsp32/HO-1–targeting drugs, up to 10 μM) nor any drug combination was found to affect the viability of normal blood MNCs (Figure 6H). Finally, a combination of imatinib (10 μM) and SMA-ZnPP (1 μM) did not inhibit clonogenic growth of CD34+ normal bone marrow progenitor cells in a control experiment (not shown). Together, our data suggest that Hsp32/HO-1–targeting drugs inhibit the growth of imatinib-resistant leukemic cells and synergize with imatinib and nilotinib in producing growth inhibition in these cells.
HO-1-targeting drugs synergize with BCR/ABL tyrosine kinase. (A) Imatinib-resistant K562 cells were grown in the absence of imatinib for 24 hours, and then were incubated with various concentrations of imatinib (■-■), ZnDPPIX (•-•), or a combination of imatinib and ZnDPPIX at a constant ratio of 1:200 (▴-▴) at 37°C for 48 hours. Thereafter, proliferation was measured by 3H-thymidine incorporation assay. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates. (B) Combination index (CI) values were determined for each fraction affected as described in “Methods.” A CI value of 1.0 indicates an additive effect, a CI greater than 1.0 indicates antagonism, whereas a CI less than 1.0 indicates synergism. (C) Ba/F3p210T315I cells were incubated with various concentrations of imatinib (■-■), SMA-ZnPP (•-•), or a combination of imatinib and SMA-ZnPP at a constant ratio of 1:0.1 (▴-▴). (D) Ba/F3p210T315I cells were incubated with various concentrations of nilotinib (■-■), SMA-ZnPP (•-•), or a combination of nilotinib and SMA-ZnPP at a constant ratio of 1:0.4 (▴-▴). After incubation at 37°C for 48 hours, cell growth was determined by 3H-thymidine incorporation. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates of one representative experiment for each drug combination. (E) Ba/F3p210T315I cells were cultured in control medium, imatinib (10 μM), PEG-ZnPP (10 μM), SMA-ZnPP (1 μM), or combinations of drugs as indicated, for 48 hours. Thereafter, cell viability was determined by trypan blue exclusion test. Results show the percentage of viable (trypan blue-negative) cells and represent the mean (± SD) of 3 independent experiments. (F) Primary leukemic cells from a patient carrying the BCR/ABL T315I mutation were cultured in control medium, PEG-ZnPP (5 μM), imatinib (5 μM), or a combination of both compounds (1:1 ratio) for 48 hours. Thereafter, proliferation was determined by 3H-thymidine incorporation. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates. (G) Primary leukemic cells from a patient carrying BCR/ABL T315I were cultured in control medium, SMA-ZnPP (7.5 μM), imatinib (0.75 μM), or a combination of both compounds (10:1 ratio) for 48 hours. Thereafter, proliferation was determined by 3H-thymidine incorporation. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates. (H) PB MNC from 3 healthy donors were cultured in control medium, imatinib (10 μM), PEG-ZnPP (10 μM), SMA-ZnPP (1 μM), or combinations of these drugs as indicated for 48 hours. Thereafter, cell viability was determined by trypan blue exclusion test. Results show the percentage of viable (trypan blue–negative) cells and represent the mean (± SD) of 3 independent experiments (3 donors).
HO-1-targeting drugs synergize with BCR/ABL tyrosine kinase. (A) Imatinib-resistant K562 cells were grown in the absence of imatinib for 24 hours, and then were incubated with various concentrations of imatinib (■-■), ZnDPPIX (•-•), or a combination of imatinib and ZnDPPIX at a constant ratio of 1:200 (▴-▴) at 37°C for 48 hours. Thereafter, proliferation was measured by 3H-thymidine incorporation assay. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates. (B) Combination index (CI) values were determined for each fraction affected as described in “Methods.” A CI value of 1.0 indicates an additive effect, a CI greater than 1.0 indicates antagonism, whereas a CI less than 1.0 indicates synergism. (C) Ba/F3p210T315I cells were incubated with various concentrations of imatinib (■-■), SMA-ZnPP (•-•), or a combination of imatinib and SMA-ZnPP at a constant ratio of 1:0.1 (▴-▴). (D) Ba/F3p210T315I cells were incubated with various concentrations of nilotinib (■-■), SMA-ZnPP (•-•), or a combination of nilotinib and SMA-ZnPP at a constant ratio of 1:0.4 (▴-▴). After incubation at 37°C for 48 hours, cell growth was determined by 3H-thymidine incorporation. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates of one representative experiment for each drug combination. (E) Ba/F3p210T315I cells were cultured in control medium, imatinib (10 μM), PEG-ZnPP (10 μM), SMA-ZnPP (1 μM), or combinations of drugs as indicated, for 48 hours. Thereafter, cell viability was determined by trypan blue exclusion test. Results show the percentage of viable (trypan blue-negative) cells and represent the mean (± SD) of 3 independent experiments. (F) Primary leukemic cells from a patient carrying the BCR/ABL T315I mutation were cultured in control medium, PEG-ZnPP (5 μM), imatinib (5 μM), or a combination of both compounds (1:1 ratio) for 48 hours. Thereafter, proliferation was determined by 3H-thymidine incorporation. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates. (G) Primary leukemic cells from a patient carrying BCR/ABL T315I were cultured in control medium, SMA-ZnPP (7.5 μM), imatinib (0.75 μM), or a combination of both compounds (10:1 ratio) for 48 hours. Thereafter, proliferation was determined by 3H-thymidine incorporation. Results are expressed as percent of medium control and represent the mean (± SD) of triplicates. (H) PB MNC from 3 healthy donors were cultured in control medium, imatinib (10 μM), PEG-ZnPP (10 μM), SMA-ZnPP (1 μM), or combinations of these drugs as indicated for 48 hours. Thereafter, cell viability was determined by trypan blue exclusion test. Results show the percentage of viable (trypan blue–negative) cells and represent the mean (± SD) of 3 independent experiments (3 donors).
Discussion
Recent data suggest that Hsp32/HO-1 plays an important role as an antiapoptotic molecule in various neoplastic cells.46,,–49 We have recently shown that CML cells constitutively express Hsp32/HO-1 and that the disease-related oncoprotein BCR/ABL up-regulates expression of Hsp32/HO-1 in Ba/F3 cells.12 In the present study, we show that Hsp32/HO-1 is a novel interesting target in CML cells. In particular, 3 Hsp32/HO-1–targeting agents, ZnDPPIX, PEG-ZnPP, and SMA-ZnPP, were found to inhibit growth of CML cells as well as Ba/F3 cells exhibiting wt BCR/ABL or imatinib-resistant mutants of BCR/ABL, including the T315I mutant that renders BCR/ABL resistant against all currently available TK inhibitors. Our study also shows that PEG-ZnPP and SMA-ZnPP synergize with imatinib and nilotinib (AMN107) in producing growth inhibition in BCR/ABL-transformed cells.
HO-1 is a heat shock protein (Hsp32) that recently has been identified as a survival factor in CML cells.12,50 It also has been described that the CML-specific oncoprotein BCR/ABL promotes expression of HO-1 in leukemic cells.12 The observation that HO-1 is expressed in CML cells independent of the phase of disease or resistance against imatinib prompted us to examine whether Hsp32/HO-1 can be employed as a molecular target in leukemic cells. As a first step, we confirmed the functional role of Hsp32/HO-1 as a survival-related molecule in CML cells by using 3 different HO-1–specific siRNAs. In these experiments, we were able to show that the siRNA-induced knockdown of Hsp32/HO-1 is associated with growth inhibition and with induction of apoptosis in K562 cells. These data provide solid evidence for the functional role of Hsp32/HO-1 as an important survival factor in CML cells.
During the past few years, pharmacologic inhibitors of Hsp32/HO-1 have been designed and used in experimental solid tumors.29,51,–53 Of particular interest are water-soluble conjugates of ZnPP, such as PEG-ZnPP or SMA-ZnPP, as these compounds might be developed for clinical application in patients with leukemias or solid tumors.29,30,49,52 In the present study, these 2 inhibitors were employed to target Hsp32/HO-1 in CML cells. Both inhibitors were found to down-regulate growth of K562 cells and KU812 cells as well as growth of primary CML cells at pharmacologically relevant drug concentrations.
An important observation was that the Hsp32/HO-1 inhibitors showed no effect on growth and viability of normal cells such as blood MNCs, endothelial cells, or fibroblasts.54 In this study, we were able to confirm these data and show that even when combined with high doses of imatinib, Hsp32/HO-1 inhibitors do not counteract viability in normal cells. In line with these observations, PEG-ZnPP did not produce major toxicity in mice in our study or in previous in vivo studies employing these HO-1–targeting drugs in experimental tumors.29 Moreover, enforced expression of Hsp32/HO-1 in K562 cells was found to revert the apoptosis-inducing effects of these compounds. Together, these observations suggest that conjugates of ZnPP might be rather specific inhibitors of Hsp32/HO-1 and that normal cells, which do not express Hsp32/HO-1 in a constitutive manner, are not influenced by these compounds.
Hsp32/HO-1 is a well-characterized survival factor that inhibits apoptosis in various cells.10,11 To define the mechanism of drug-induced growth inhibition, we tested whether targeting of HO-1 in CML cells was associated with signs of apoptosis. The results of our study show that the growth-inhibitory effects of both water-soluble HO-1 inhibitors (PEG-ZnPP and SMA-ZnPP) are associated with induction of apoptosis in CML cells. These data confirm reports on antineoplastic effects of PEG-ZnPP in solid tumors28,29,51 and are in line with the notion that Hsp32/HO-1 acts as an antiapoptotic factor in various neoplastic cells.
One important mechanism that may underlie the antiapoptotic effects of Hsp32/HO-1 involves the generation of bilirubin with consecutive neutralization of reactive oxygen species (ROS).10 In this regard, it is noteworthy that BCR/ABL has been reported to induce production of ROS in hematopoietic cells.55,56 More recently, production of ROS by BCR/ABL also has been implicated as a cause of oxidative DNA damage, resulting in mutations in the BCR/ABL kinase domain.57 It is therefore tempting to speculate that the antioxidative effects of Hsp32/HO-1 counteract ROS-induced cell damage in CML cells and that (pharmacologic) inhibition of Hsp32/HO-1 leads to increased oxidative stress and (finally) ROS-induced cell death in leukemic cells.
A major clinical challenge in the treatment of CML is the occurrence of resistance against imatinib and other BCR/ABL TK inhibitors.26,45 Therefore, a number of novel agents and pharmacologic approaches are currently under evaluation, with the ultimate goal to overcome drug resistance in these patients.26 In the current study, we were able to show that PEG-ZnPP and SMA-ZnPP inhibit growth of Ba/F3 cells exhibiting various imatinib-resistant mutants of BCR/ABL, including the T315I mutant that renders BCR/ABL resistant against all currently available BCR/ABL TK inhibitors including nilotinib and dasatinib.24,25 Moreover, targeting of Hsp32/HO-1 was found to result in inhibition of growth of Ba/F3 cells harboring the T315I mutant in vivo in a nude mouse model. Finally, our data show that the Hsp32/HO-1–targeting drugs applied suppress growth of primary leukemic cells obtained from patients with imatinib-resistant CML, including one patient carrying the T315I mutant of BCR/ABL in neoplastic cells. All in all, these data show that targeting of Hsp32/HO-1 may be an interesting novel approach to treat patients with drug-resistant CML.
An attractive strategy that may help to overcome drug resistance in CML is to combine various targeted drugs with each other or with other conventional drugs.26 The data of our study show that HO-1–targeting drugs synergize with imatinib and with nilotinib in producing growth inhibition in K562 cells and in Ba/F3 cells exhibiting the T315I-mutated variant of BCR/ABL. This observation suggests that targeting of HO-1 overcomes resistance against TK-inhibitors in these cells. In case of the T315I mutation of BCR/ABL, the observation that combinations of TK inhibitors and Hsp32/HO-1–targeting drugs produce synergistic antiproliferative effects was unexpected, as this mutation is considered to prevent the binding of TK inhibitors to BCR/ABL. One explanation for this phenomenon would be that the TK inhibitors interact with other targets (apart from BCR/ABL) expressed in leukemic cells. An alternative explanation would be that the remaining drug-binding capacity of BCR/ABL is sufficient to exert a pharmacodynamic or permissive activity and thus a synergistic effect when combining with other drugs. A similar phenomenon has recently been described by Tseng et al using combinations of imatinib and OSU-03012.58 Whether Hsp32/HO-1–targeting drugs and TK inhibitors synergize in vivo in producing growth inhibition in BCR/ABL-transformed cells remains to be determined in future studies.
In summary, our data show that Hsp32/HO-1 is a new interesting target in CML and that Hsp32/HO-1–targeting drugs induce apoptosis and inhibit the growth of leukemic cells expressing wt BCR/ABL or various imatinib-resistant mutants of BCR/ABL. Clinical studies are now warranted to show whether targeting of HO-1 alone or in combination with TK inhibitors may help to overcome drug resistance in patients with drug-resistant CML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich grant no. P-16412 and no. P-16865, the Austrian Federal Ministry for Science and Research (GENAU, GZ 200.136/1-VI/1/2005), and grant no. 17016076 for Cancer Research from the Ministry of Education, Science, Culture, and Sports of Japan.
Authorship
Contribution: M.M. established the research plan, performed key experiments, analyzed the data, and contributed to the drafting of the article. K.V.G., G.H., S.D., and W.F.P. performed 3H-thymidine incorporation assays, siRNA experiments, annexin-V/propidium iodide staining, and FACS analysis. E.J. performed experiments on clonogenic growth of CML cells. J.M., R.G.O., and V.S. performed animal experiments. H.M., K.G., and H.N. developed and synthesized PEG-ZnPP and SMA-ZnPP and performed the HO-1 activity assay. P.S. performed electron microscopy experiments and Tunel assay experiments. H.E. and C.S. contributed by designing and providing essential new reagents (HO-1-siRNA), analyzing data, and critically reviewing the manuscript. P.V. designed the study, established the project plan, provided logistic and budget support, and approved the data and the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthias Mayerhofer, Department of Internal Medicine I, Division of Hematology & Hemostaseology and Clinical Institute for Medical and Chemical Laboratory Diagnostics, Medical University of Vienna, Waehringer Guertel 18-20, A-1097 Vienna, Austria; e-mail: matthias.mayerhofer@meduniwien.ac.at.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal