Semaphorins and their receptors (plexins) have pleiotropic biologic functions, including regulation of immune responses. However, the role of these molecules inside the immune system and the signal transduction mechanism(s) they use are largely unknown. Here, we show that Semaphorin3A (Sema3A) triggers a proapoptotic program that sensitizes leukemic T cells to Fas (CD95)-mediated apoptosis. We found that Sema3A stimulation provoked Fas translocation into lipid raft microdomains before binding with agonistic antibody or FasL (CD95L). Disruption of lipid rafts reduced sensitivity to Fas-mediated apoptosis in the presence of Sema3A. Furthermore, we show that plexin-A1, together with Sema3A-binding neuropilin-1, was rapidly incorporated into membrane rafts after ligand stimulation, resulting in the transport of actin-linking proteins into Fas-enriched rafts. Cells expressing a dominant-negative mutant of plexin-A1 did not show Fas clustering and apoptosis on Sema3A/Fas costimulation. This work identifies a novel biologic function of semaphorins and presents an unexpected signaling mechanism linking semaphorin to the tumor necrosis factor family receptors.
Introduction
The semaphorin family comprises soluble and membrane-bound proteins that function during neuronal development, organogenesis, angiogenesis, and cancer progression.1,2 This family has also attracted the attention of immunologists as novel regulators of immune cell responses.3 Various members of semaphorins act as amplifiers of the immune response,4 whereas others, the secreted semaphorins of class 3 in particular, may negatively control immune functions.5 Previously, we have found that Semaphorin-3A (Sema3A), a prototype member of secreted semaphorins of class 3, has the potential to inhibit T-cell functions by promoting growth arrest and/or blocking proinflammatory cytokine secretion.6 The downstream pathways by which Sema3A exerts its action on T cells are still unclear, but they are likely to involve a receptor complex formed from one of 2 neuropilin (NP) proteins and one of 4 Plexin-A proteins. NPs serve as the primary ligand binding sites and Plexin-As as the signal transducing components.7,8
Recent evidence indicated that Sema3A/NP/PlexA signaling may regulate cell apoptosis.9,10 Indeed, on treatment with recombinant human Sema3A, neurons were found to undergo apoptosis. Moreover, marked and prolonged protection from dopamine-induced apoptosis was achieved by cotreatment with function-blocking anti-Sema3A antibodies. Therefore, Sema3A has been proposed to act as an autocrine and paracrine “amplifier” signal for neuronal cell death. Apoptosis is a pivotal process in immunologic development that is frequently curbed in lymphoid leukemias. Interestingly, the potential role of Sema3A in the regulation of apoptosis in immune cells has not been investigated so far. To address this question, we assayed the potential regulatory function of Sema3A in Fas-induced apoptosis of human leukemic cells.
Fas (CD95/APO-1) is the prototype of “death receptors” in the tumor necrosis factor (TNF) receptor superfamily. It has been implicated in a wide range of apoptosis-based physiologic processes (eg, T-cell–dependent cytotoxicity, deletion of autoreactive T and B cells, activation-induced cell death, tumor surveillance, immune privilege, angiogenesis) and pathologic degenerative diseases (eg, autoimmunity, fulminant hepatitis, and neurodegeneration).11,12 On ligation with its cognate ligand FasL, or with agonistic antibodies, Fas rapidly recruits the Fas-associated death domain protein (FADD) and procaspase 8, forming the so-called death inducing signaling complex (DISC). The clustering of procaspase-8 molecules in the DISC complex results in their activation by self-cleavage, triggering downstream effector caspases and eventually leading to apoptosis.13 In certain cells (indicated as type I), Fas is localized in detergent-resistant plasma membrane microdomains called lipid rafts, and Fas-induced apoptosis depends on signaling pathways restricted to these microdomains.14 In contrast, in other cell types (ie, type II), Fas is excluded from lipid rafts and its signaling depends on the clustering with selected coreceptors.15,–17 Lipid rafts are cholesterol-rich structures where adapters and kinases required for signal transduction are localized, including glycosylphosphatidyl-inositol–linked proteins and molecules connecting the actin cytoskeleton to the plasma membrane (the so-called ERM proteins).18 The formation of membrane platforms where Fas and its signal transducers are brought in close proximity is thought to increase DISC formation and therefore potentiate Fas signaling.15,–17 Here, we show a novel function of Sema3A, mediated by plexins, whereby it increases Fas translocation into membrane rafts, sensitizing leukemic cells to Fas-mediated apoptosis.
Methods
Cell culture and apoptosis
The human leukemic cell lines HUT78 and HUT78.B119 were kindly provided by R. Testi (University of Rome, Tor Vergata, Italy). The other cell lines were obtained from American Type Culture Collection (Manassas, VA) and grown as described previously in RPMI-1640 culture medium supplemented with 10% heat-inactivated fetal calf serum (FCS).6 Bone marrow aspirates, obtained from patients at the initial diagnosis and after signing informed consent, were kindly provided by the Clinic of Hematology of the Polytechnic University of Marche (Ancona, Italy). Mononuclear cells isolated by Ficoll-Hypaque density gradient centrifugation, consisting mostly of leukemia blasts (> 90%), were washed in phosphate-buffered saline (PBS), resuspended in cell culture medium, and used immediately for experimentation. Death receptor-mediated apoptosis was induced with rhFasL (rhsSuperFasLigand), TNF-related apoptosis-inducing ligand (TRAIL), anti-Fas mAb APO1-1 (Alexis Biochemicals, Milan, Italy), or cytotoxic anti-Fas CH11 antibody (Upstate Biotechnology, Milan, Italy). Human recombinant Sema-3A fused to human Fc fragment (Sema3A-Fc) were purchased from R&D Systems (Minneapolis, MN) and added simultaneously to anti-Fas stimuli. For the NP1 functional blocking test, the blocking anti-NP1 antibody, a generous gift of Dr M. Tessier-Lavigne (Howard Hughes Medical Institute, Stanford University, Stanford, CA), was previously described.6 The control antibody was a rabbit polyclonal anti-NP1 antibody (clone H286, Santa Cruz Biotechnology, Santa Cruz, CA) with a nonblocking function.6 The caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp(Ome)-fluoromethylketone (zVAD-fmk), the de novo RNA inhibitor actinomycin D, and the protein synthesis inhibitor cycloheximide were from Sigma (Milan, Italy). To disrupt lipid rafts, cells (5 × 105) were pretreated with 15 μg/mL methyl-β-cyclodextrin (MBCD; Sigma) for 1 hour at 37°C in serum-free medium before Sema3A-Fc and Fas stimuli.
For quantitative determination of apoptosis, cells (5 × 105) were fixed overnight in 70% ethanol at 4°C. Cells were then incubated for 1 hour with 1 mg/mL RNase A and 20 μg/mL propidium iodide at room temperature, and analyzed with a FACScan flow cytometer (BD Biosciences, Milan Italy) as described previously.20 Apoptotic cells were calculated as the percentage of cells in the sub-G1 region in cell-cycle analysis. Apoptotic cells were also identified using an annexin-V apoptosis detection kit (BD Biosciences PharMingen).
Immunofluorescence flow cytometry
Cell surface expression of Fas and NP1 was analyzed by immunofluorescence flow cytometry in 4 × 105 cells as described previously20 in a FACSCalibur flow cytometer (BD Biosciences) using anti-Fas DX2 mAb (a gift of Dr R. Testi) and specific antibody against NP1 (Clone H286, Santa Cruz Biotechnology). FAM-VAD-fmk was obtained from Cell Technology (Mountain View, CA). Isotype-matched control antibodies were included in each staining.
Isolation of lipid rafts
Lipid rafts were isolated from 107 cells by nonionic detergent lysis and centrifugation on discontinuous sucrose gradients exactly as described previously15 ; 1-mL fractions were collected from the top of the gradient, and 20 μL of each fraction was subjected to SDS-PAGE, immunoblotting, and enhanced chemiluminescence detection. Location of lipid rafts was determined using cholera toxin B subunit conjugated to horseradish peroxidase (anti-GM1; Sigma) or by blotting fractions for the tyrosine kinase Lck (obtained from Santa Cruz Biotechnology). Proteins were identified using the following specific antibodies: anti-48-kDa Fas (C-20), anti-120-kDa-NP-1 (H286), anti-195-kDa-plexin-A1 (H-60), anti-30-kDa RhoGDI (K-21) rabbit polyclonal antibodies and anti-21-kDa RhoA mAb (Santa Cruz Biotechnology); anti-29-kDa FADD (clone-1) and anti-80-kDa ezrin (clone-18) mAb (BD Transduction Laboratories, Lexington, KY); and anti-55-kDa procaspase-8 (Ab-3) mAb (Calbiochem, San Diego, CA). Prestained protein molecular mass standards (Bio-Rad, Milan, Italy) were run in parallel.
Confocal and immunofluorescence microscopy
To confocal microscopy, cells were settled onto poly-L-lysine-coated slides and analyzed with a Zeiss LSM 510 laser scan confocal microscope (Oberkochen, Germany) for membrane raft and protein visualization as described.15,16 Colocalization assays were analyzed by excitation of the corresponding fluorochromes in the same section. Negative controls, lacking the primary antibody or using an irrelevant antibody, showed no staining. To immunofluorescence, cells (3 × 106) were washed and spun onto glass slides at 150g for 2 minutes. The cells were then fixed and exposed to purified anti-Fas (DX2) and anti-plexin-A1 or anti-NP1 antibodies for 1 hour. Then, they were incubated for 30 minutes with the secondary antibodies Cy3-conjugated goat antirabbit IgG and FITC-conjugated goat antimouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). F-actin filaments were stained with phalloidin-TRITC and nuclei were stained with DAPI (Sigma). Each slide was mounted with Fluoromount G (Electron Microscopy Sciences, Hatfield, PA). Images were acquired and processed on a fluorescence microscope with 40× objective. Image analysis and merging of images were done with Adobe PhotoShop 7.0 software (Adobe Systems, San Jose, CA).
Real-time reverse-transcribed polymerase chain reaction
Real-time reverse-transcribed polymerase chain reaction (RT-PCR) analysis was done in a Chromo4 sequence detector (Bio-Rad) as previously described.6 The primers and probes of Plexin-A1, -A2, -A3, and -A4 genes were determined by Laboratory Tools software analysis of Stratagene (Milan, Italy). As positive control for human plexin-A1 and -A4, we used cDNAs derived from peripheral blood mononuclear cells of healthy donors. Details of sequences and thermal cycle conditions are available on request. Data were acquired and analyzed with the sequence detector Chromo4 software.
Immunoprecipitation and immunoblot analysis
Immunoprecipitation and immunoblots were performed from whole cell lysates as previously described.20,–22 Briefly, cells were lysed with EB buffer (20 mM Tris-HCl at pH 7.4, 5 mM EDTA, 150 mM sodium chloride, 10% glycerol, and 1% Triton X-100) in the presence of 1 μg/mL leupeptin, 3 μg/mL aprotinin, 1 μg/mL pepstatin, 2 mM phenylmethylsulphonyl fluoride, and 1 mM sodium orthovanadate. After immunoprecipitation with antibodies against NP1 and plexin-A1 (all by Santa Cruz Biotechnology), high-stringency washes were performed (EB buffer containing 1 M lithium chloride). Western blots were then performed, and appropriate antibodies were detected using enhanced chemiluminescence (Amersham, Milan, Italy). In some experiments, blots were reprobed with an antiactin monoclonal antibody (Santa Cruz Biotechnology) as loading control.
Dominant-negative plexin-A1 transfectants and RNA interference
Dominant-negative plexin-A1 expression lentiviral vector has been described previously8,21 and was produced in 293T packaging cells, transiently cotransfected with a mixture of transfer, envelope, and core-packaging plasmids. Conditioned media containing the vector was harvested 48 hours after transfection and incubated with a fresh culture of sparse Jurkat cells, in the presence of 8 μg/μL Polybrene (Sigma) for 16 hours. The infected cells were previously exposed to 32 μM genistein for 3 hours to enhance lentiviral vector entry.23 Genistein was purchased from Calbiochem (Milan, Italy) and diluted in dimethyl sulfoxide (DMSO) before use. The final concentration of DMSO in the cultures never exceeded 0.2% (vol/vol). The plexin construct included a vesicular stomatitis virus (VSV) tag, detectable with an anti-VSV-G monoclonal antibody (V-5507; Sigma) by immunoblotting.
Four siRNA sequences specific for human plexin-A1 were selected, synthesized, and annealed by the manufacturer (Dharmacon, Milan, Italy). Transfection was performed using RNAiFect (QIAGEN, Milan, Italy) according to the manufacturer's protocol. After 48 hours of incubation, the resulting cells were harvested, washed, and used for subsequent experiments. Transfection efficiencies were determined using fluorescein-labeled nonsilencing RNA (35%-45%).
Statistical analysis
All values were expressed as mean plus or minus SEM of no less than triplicate measurements of 3 independent experiments. Comparison of results between different groups was performed by one-way analysis of variance, paired t test, and ANOVA using StatView 5.0 (NET Engineering, Pavia, Italy; P less than or equal to .05 was considered statistically significant).
Results
Proapoptotic activity of Sema3A in association with Fas stimulation
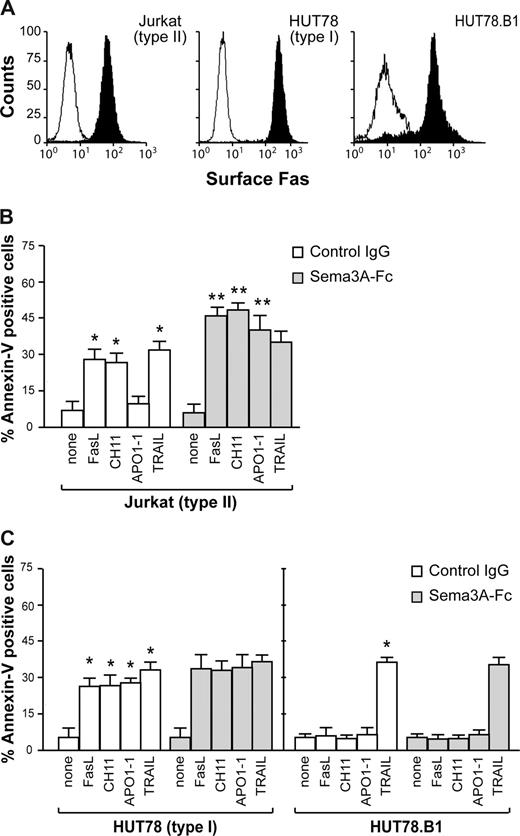
The proapoptotic activity of Sema-3A was assessed in a variety of human tumor cells. We first used Fas-sensitive T leukemia cells Jurkat (as type II cell line) and HUT78 (as type I cell line), as well as the Fas-resistant HUT78.B1 cells, previously derived from HUT78.14 As reported for type I and type II cells,15 Jurkat cells expressed less Fas on their cell surface than HUT78 or HUT78.B1 cells (Figure 1A). rhFasL or the agonistic cytotoxic CH11 anti-Fas antibody induced apoptosis in Jurkat and HUT78 cells, but not in HUT78.B1 cells (Figure 1B,C). In response to APO1-1, an IgG1 isotype switch variant of anti-Fas antibody that induces apoptosis in type I but not in type II cells,15 HUT78 cells underwent apoptosis, whereas Jurkat and HUT78.B1 cells were almost completely resistant (Figure 1B,C). In contrast, all of the cells were sensitive to TRAIL (Figure 1B,C). When Jurkat (type II) cells were treated with rhFasL, CH11, or APO1-1, the costimulation with Sema3A strongly increased the sensitivity to cell death (Figure 1B). Sema3A was less effective in sensitizing HUT78 (type I) cells, whereas it had no effect on HUT78.B1 cells (Figure 1C). Moreover, Sema3A did not increase the sensitivity to cell death induced by TRAIL (Figure 1B,C). These data show that Sema3A sensitizes some cell types to die via anti-Fas stimuli.
Fas-induced cell death varies with cell type in the presence of Sema3A. (A) Jurkat, HUT78, and HUT78.B1 cells were assayed by flow cytometry for their respective cell surface content of Fas. (B,C) Jurkat, HUT78, or HUT78.B1 cells were stimulated with 50 ng/mL rhFasL, 200 ng/mL CH11, 150 ng/mL APO1-1, and 50 ng/mL TRAIL, in the presence of soluble control IgG or Sema3A-Fc (150 ng/mL) for 7 hours. Cell death was assessed by staining using FITC-annexin V. Data are mean plus or minus SEM of 3 independent experiments (*P < .05, vs none; **P < .05, vs its respective death receptor ligand).
Fas-induced cell death varies with cell type in the presence of Sema3A. (A) Jurkat, HUT78, and HUT78.B1 cells were assayed by flow cytometry for their respective cell surface content of Fas. (B,C) Jurkat, HUT78, or HUT78.B1 cells were stimulated with 50 ng/mL rhFasL, 200 ng/mL CH11, 150 ng/mL APO1-1, and 50 ng/mL TRAIL, in the presence of soluble control IgG or Sema3A-Fc (150 ng/mL) for 7 hours. Cell death was assessed by staining using FITC-annexin V. Data are mean plus or minus SEM of 3 independent experiments (*P < .05, vs none; **P < .05, vs its respective death receptor ligand).
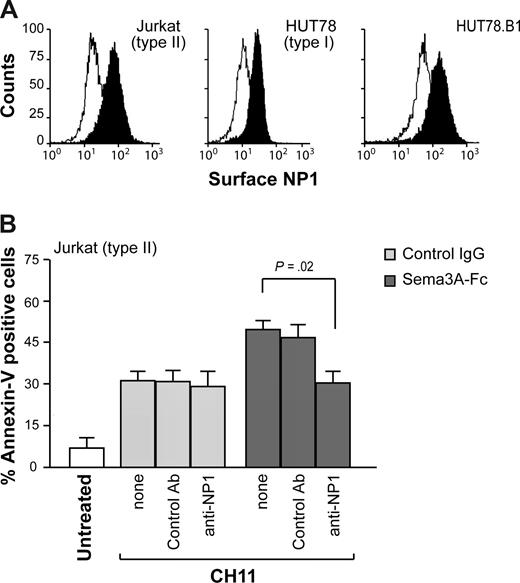
We next investigated the involvement of Sema3A-receptor NP1 in the proapoptotic activity of Sema3A. Jurkat, HUT78, and HUT78.B1 expressed similar protein levels of NP1 on their cell surface (Figure 2A). However, the addition of blocking antibodies directed against NP1 (anti-NP1) abrogated the sensitizing effect mediated by Sema3A but had no effect on Fas-induced cell death (Figure 2B). These data suggest that Sema3A, through NP1 receptor, enhances the death signal induced by Fas activation, especially in Jurkat (type II) cells.
The proapoptotic activity of Sema3A is dependent by NP1. (A) Surface expression of NP1 for Jurkat, HUT78, or HUT78.B1 cells. (B) Jurkat cells were incubated with CH11 plus Sema3A-Fc or control IgG for 7 hours, and cell death was determined as described in Figure 1. Alternatively, cells were first incubated with the blocking anti-NP1 antibody or a control Ab. Statistical analysis is shown.
The proapoptotic activity of Sema3A is dependent by NP1. (A) Surface expression of NP1 for Jurkat, HUT78, or HUT78.B1 cells. (B) Jurkat cells were incubated with CH11 plus Sema3A-Fc or control IgG for 7 hours, and cell death was determined as described in Figure 1. Alternatively, cells were first incubated with the blocking anti-NP1 antibody or a control Ab. Statistical analysis is shown.
Sema3A increases Fas-mediated caspase activation in type II, but not in type I, cells
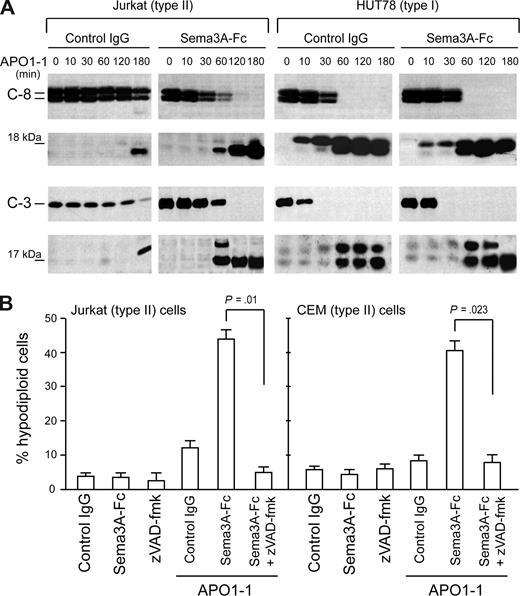
We then analyzed the effect of Sema3A cotreatment on the cleavage of procaspase-8 and -3, which is the hallmark of Fas-induced apoptosis.11,–13 In a time-course experiment, we observed full cleavage of caspase-8 and -3 after only 2 hours of Sema3A/anti-Fas costimulation in Jurkat cells (Figure 3A), whereas APO1-1 alone was only partly effective at this stage. In contrast, we did not find a substantial shift in the activation of caspase-8 and -3 in HUT78 cells. In H9 T lymphoma cells, another type I cell line, there was no change in caspase activation after Sema-3A cotreatment; whereas in the type II CEM T leukemia cells, we found complete cleavage of caspase-8 and -3, similar to Jurkat cells (data not shown). Consistently, the enhancement of Fas-mediated apoptosis by Sema3A was blocked by preincubating Jurkat or CEM cells with the broad-range caspase inhibitor zVAD-fmk (Figure 3B). Therefore, the observed differences in Fas-mediated cell death in the presence of Sema3A in type II cells seem to correlate with the intracellular activation of caspases.
Sema3A cosignal differentially increases the Fas-mediated caspase activation in cell lines. (A) Jurkat (left) or HUT78 (right) cells were stimulated with 150 ng/mL APO1-1 in the presence of control IgG or Sema3A-Fc (150 ng/mL) for indicated times. Activation of caspase 8 and caspase 3 was analyzed by immunoblotting. The 18-kDa and 17-kDa bands correspond to active cleavage forms of caspase-8 and -3, respectively. The results shown are representative of 3 independent experiments. (B) Jurkat or CEM (type II) cells were incubated for 7 hours as described in panel A with or without zVAD-fmk (20 μM). Cell death was assessed by flow cytometry with propidium iodide.
Sema3A cosignal differentially increases the Fas-mediated caspase activation in cell lines. (A) Jurkat (left) or HUT78 (right) cells were stimulated with 150 ng/mL APO1-1 in the presence of control IgG or Sema3A-Fc (150 ng/mL) for indicated times. Activation of caspase 8 and caspase 3 was analyzed by immunoblotting. The 18-kDa and 17-kDa bands correspond to active cleavage forms of caspase-8 and -3, respectively. The results shown are representative of 3 independent experiments. (B) Jurkat or CEM (type II) cells were incubated for 7 hours as described in panel A with or without zVAD-fmk (20 μM). Cell death was assessed by flow cytometry with propidium iodide.
Increased Fas-mediated apoptosis by Sema3A does not require new protein synthesis or Rap1 activity
We next examined whether Sema3A could induce Fas up-regulation on the cell surface, resulting in higher receptor density and signaling potential. However, Sema3A did not increase Fas expression on the cell surface in Jurkat cells, after up to 6 hours of treatment (at the stage in which the increased sensitivity to apoptosis was observed; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). To assess whether the proapoptotic activity of Sema3A was dependent on new protein synthesis, we pretreated Jurkat cells with actinomycin D or cycloheximide. Neither actinomycin D nor cycloheximide was able to inhibit the increased sensitivity to cell death mediated by Sema3A (Figure S1B).
Moreover, because we have previously shown that the small G protein Rap1 is involved in NP1-mediated Sema3A inhibitory signaling in T cells,6 we asked whether the same signal transducer could be implicated in the regulation of Fas-induced apoptosis. We expressed a dominant-negative Rap1 (Rap1N17) in Jurkat cells; however, Sema3A cotreatment promoted apoptosis in cells expressing Rap1N17 in a comparable manner as in cells transfected with an empty vector (Figure S1C). Therefore, the proapoptotic signaling of Sema3A occurred independently of Rap1 activation.
Sema3A stimulation recruits Fas into the lipid rafts, where semaphorin receptor NP1 is localized
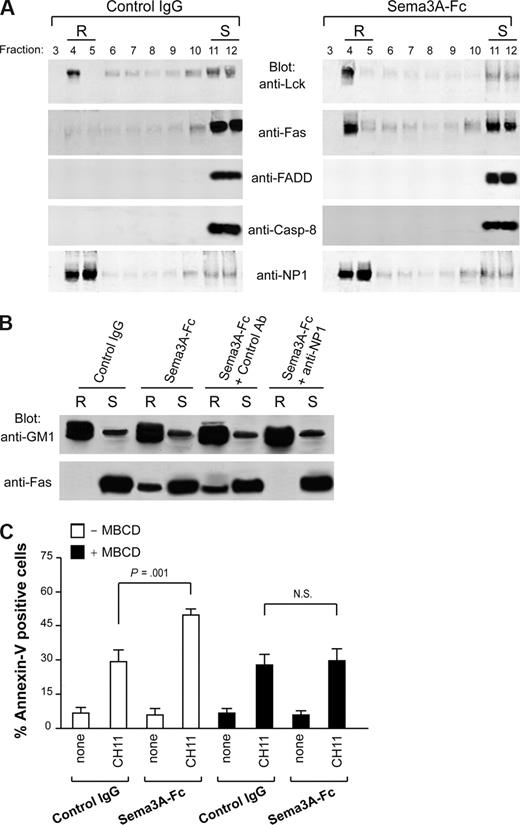
In the absence of the cognate ligand, redistribution of Fas on lipid rafts is a potential mechanism to up-regulate its signaling in type II cells.15,–17 Therefore, we asked whether Sema3A may enhance Fas-mediated apoptosis in Jurkat (type II) cells by Fas redistribution in lipid rafts. The membrane proteins from Jurkat cell lysates were separated by sucrose gradient ultra-centrifugation and analyzed by Western blotting as previously described.15 Fractions containing the lipid rafts4,5 were identified by the presence of the tyrosine kinase Lck (Figure 4A). In unstimulated cells, Fas was not found in lipid rafts, whereas after a 60-minute treatment with Sema3A, a marked proportion of it translocated into lipid rafts (Figure 4A). Neither FADD nor procaspase-8 was recruited into lipid rafts on Sema3A stimulation (Figure 4A), consistent with the notion that Fas relocalization into lipid rafts in type II cells occurs before any stimulation with death receptor ligands.15,16 The Sema3A receptor NP1 was markedly present in lipid rafts before Sema3A treatment, and its localization did not change on stimulation (Figure 4A). Also in this case, Fas translocation from soluble (S, fraction 11) to insoluble lipid raft (R, fraction 4) was NP1 dependent because it was entirely abrogated in the presence of anti-NP1 blocking antibody (Figure 4B). The raft-associated glycosphingolipid GM1 showed localization of the raft fraction in these experiments (Figure 4B). Therefore, a portion of NP1 is distributed into lipid rafts, and its stimulation with Sema3A elicits the recruitment of Fas into lipid rafts.
Sema3A stimulation induces redistribution of Fas into lipid rafts. (A) Jurkat cells (107) were treated with control IgG or Sema3A-Fc for 60 minutes and subjected to density gradient fractionation. Fractions were immunoblotted with antibodies for Lck, Fas, FADD, caspase-8, and NP1. Fractions corresponding to lipid raft (R) and soluble (S) proteins are indicated. (B) Jurkat cells were preincubated with the blocking anti-NP1 antibody or a control Ab. Then, cells were treated as in panel A. Fractions 4 (R) and 11 (S) were immunoblotted with antibodies specific for GM1 and Fas. Data in panels A and B are representative of at least 3 independent experiments. (C) Jurkat cells were stimulated with Sema3A-Fc for 60 minutes, depleted of cholesterol with 15 μg/mL MBCD for 10 minutes at 37°C, and then stimulated with CH11 for 6 hours. Cell death was determined by flow cytometry with annexin V staining. P value is shown.
Sema3A stimulation induces redistribution of Fas into lipid rafts. (A) Jurkat cells (107) were treated with control IgG or Sema3A-Fc for 60 minutes and subjected to density gradient fractionation. Fractions were immunoblotted with antibodies for Lck, Fas, FADD, caspase-8, and NP1. Fractions corresponding to lipid raft (R) and soluble (S) proteins are indicated. (B) Jurkat cells were preincubated with the blocking anti-NP1 antibody or a control Ab. Then, cells were treated as in panel A. Fractions 4 (R) and 11 (S) were immunoblotted with antibodies specific for GM1 and Fas. Data in panels A and B are representative of at least 3 independent experiments. (C) Jurkat cells were stimulated with Sema3A-Fc for 60 minutes, depleted of cholesterol with 15 μg/mL MBCD for 10 minutes at 37°C, and then stimulated with CH11 for 6 hours. Cell death was determined by flow cytometry with annexin V staining. P value is shown.
As previously described,16 the disruption of lipid rafts by cholesterol depletion using MBCD did not affect Fas-mediated apoptosis in Jurkat cells (Figure 4C). However, by pretreating with MBCD, we completely abrogated Sema3A-mediated amplification of Fas apoptosis (Figure 4C). These results indicated that Fas translocation into lipid rafts induced by an NP1-dependent Sema3A signaling is required to increase Fas-mediated apoptosis.
Sema3A costimulation enhances Fas-induced apoptosis in primary leukemic cells
Our data indicated that Sema3A can sensitize some leukemic cell lines to Fas-mediated apoptosis. To extend our findings to primary leukemic cells, we studied bone marrow cells derived from patients with acute myeloblastic leukemia or acute promyelocytic leukemia (M2 or M3, respectively, following the FAB classification). These M2 and M3 leukemia cells were positive for Fas and NP1 (> 60% and > 50% positive cells, respectively; data not shown), and underwent apoptosis after Fas stimulation (Figure S2A). Consistent with that seen in Jurkat cells, primary leukemia cells were sensitized to Fas-induced cell death by treatment with Sema3A, and this effect was inhibited by MBCD (Figure S2A). Staining of leukemia cells with the fluorescent caspase substrate FAM-VAD-fmk showed that a higher percentage of cells receiving anti-Fas and Sema3A cotreatment contained more activated caspases than cells receiving anti-Fas alone. Consistently, this effect was blocked by MBCD pretreatment (Figure S2B). Therefore, Sema3A and Fas stimuli may act in synergy to enhance caspase activation and apoptosis in primary leukemic cells.
The Sema3A coreceptor plexin-A is involved in enhancing Fas-mediated cell death
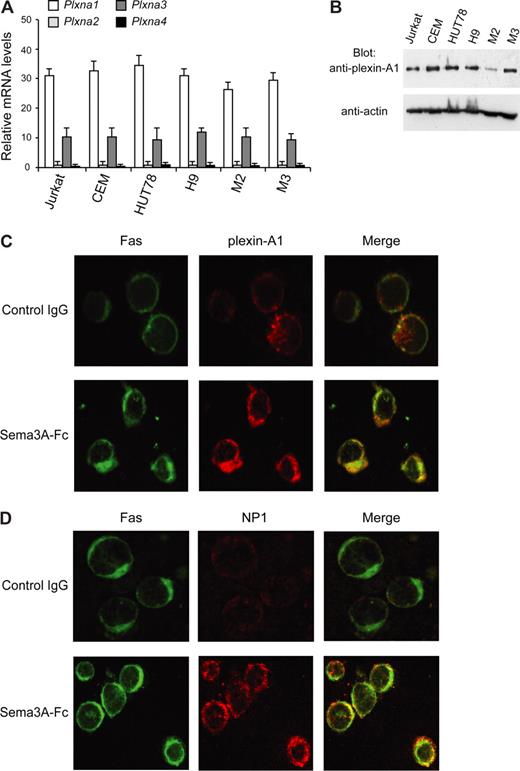
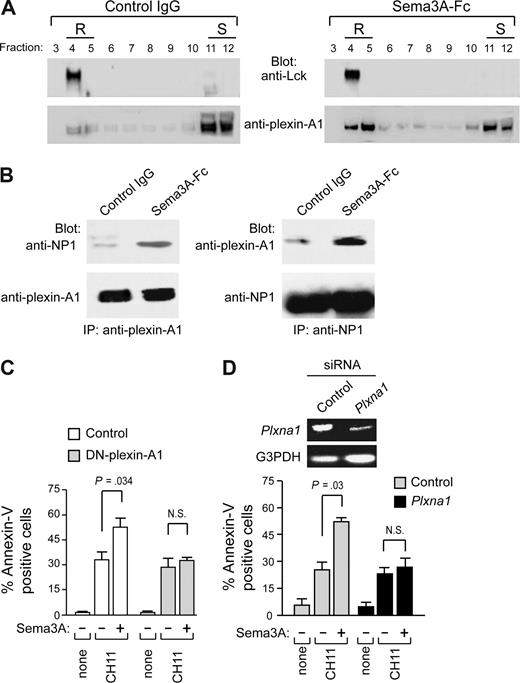
In addition to NP1, members of the plexin-A family (plexin-A1, -A2, -A3, and -A4) serve as components of receptor complexes for Sema3A.8 We found that plexin-A1 and -A3 mRNAs are expressed in cell lines HUT78 and H9 (type I), Jurkat, and CEM (type II), as well as in primary M2 and M3 leukemic cells. In contrast, plexin-A2 and -A4 were virtually absent in these cells (Figure 5A). Plexin-A1 was expressed more abundantly than plexin-A3, and it was confirmed at the protein level by immunoblotting (Figure 5B). Moreover, by immunofluorescence microscopy, plexin-A1 and NP1 were found to colocalize with Fas in Sema3A-treated Jurkat cells, but not in untreated cells (Figure 5C,D). A substantial proportion of plexin-A1 translocated into lipid rafts in cells stimulated with Sema3A (Figure 6A), and the association of plexin-A1 with NP1 was increased in response to Sema3A (Figure 6B). Because we found that NP1 is constitutively present in these membrane microdomains (Figure 4), our data indicate that Sema3A enhances NP1/plexin-A1 complex formation into lipid rafts.
Plexins expression in leukemic cells. (A) Total RNA (50 ng/μL) was isolated from the indicated cells, and real-time PCR was done with primers and probes specific for plexin-A1, -A2, -A3, and -A4 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Plexin mRNA expression was normalized to GAPDH for each sample. (B) Total lysates (50 μg) from indicated cell lines were immunoblotted (Blot Ab) with antiplexin-A1 antibody. Expression of actin was used as loading control. (C,D) Colocalization of Fas (green) with either plexin-A1 (C) or NP1 (D) (red) in Sema3A-treated Jurkat cells in contrast to cells treated with control IgG (original magnification, ×1600).
Plexins expression in leukemic cells. (A) Total RNA (50 ng/μL) was isolated from the indicated cells, and real-time PCR was done with primers and probes specific for plexin-A1, -A2, -A3, and -A4 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Plexin mRNA expression was normalized to GAPDH for each sample. (B) Total lysates (50 μg) from indicated cell lines were immunoblotted (Blot Ab) with antiplexin-A1 antibody. Expression of actin was used as loading control. (C,D) Colocalization of Fas (green) with either plexin-A1 (C) or NP1 (D) (red) in Sema3A-treated Jurkat cells in contrast to cells treated with control IgG (original magnification, ×1600).
Involvement of Plexin in Sema3A signaling. (A) Jurkat cells were treated with control IgG or Sema3A-Fc for 60 minutes and subjected to density gradient fractionation. For anti-Lck immunoblots, 10 μg of protein was loaded per lane; and for antiplexin-A1 immunoblots, 40 μg of protein was loaded per lane. (B) Jurkat cells (107 cells per test) were treated with control IgG or Sema3A-Fc (150 ng/mL) for 5 minutes and lysates were prepared. Equivalent amounts of whole-cell lysates were either immunoprecipitated with antiplexin-A1 antibody (IP, antiplexin-A1) or anti-NP1 antibody (IP, anti-NP1). Immune complexes were then immunoblotted (Blot Ab) as indicated. Data in panels A and B are representative of 3 experiments. (C) The Jurkat cell line was engineered by lentiviral-mediated gene-transfer of a truncated form of plexin-A1, lacking its cytoplasmic domain (DN-plexin-A1). These cells or control Jurkat cells were incubated with CH11 or CH11 plus Sema3A-Fc for 6 hours. (D) In addition, Jurkat cells transfected with siRNA specific for plexin-A1 or nonsilencing siRNA were cultured with control IgG or Sema3A-Fc for 6 to 8 hours. Plexin-A1 expression in RNAi-treated Jurkat cells was determined by RT-PCR analysis. Cell death was quantified by flow cytometry with annexin V staining. P values were also shown. N.S., not significant.
Involvement of Plexin in Sema3A signaling. (A) Jurkat cells were treated with control IgG or Sema3A-Fc for 60 minutes and subjected to density gradient fractionation. For anti-Lck immunoblots, 10 μg of protein was loaded per lane; and for antiplexin-A1 immunoblots, 40 μg of protein was loaded per lane. (B) Jurkat cells (107 cells per test) were treated with control IgG or Sema3A-Fc (150 ng/mL) for 5 minutes and lysates were prepared. Equivalent amounts of whole-cell lysates were either immunoprecipitated with antiplexin-A1 antibody (IP, antiplexin-A1) or anti-NP1 antibody (IP, anti-NP1). Immune complexes were then immunoblotted (Blot Ab) as indicated. Data in panels A and B are representative of 3 experiments. (C) The Jurkat cell line was engineered by lentiviral-mediated gene-transfer of a truncated form of plexin-A1, lacking its cytoplasmic domain (DN-plexin-A1). These cells or control Jurkat cells were incubated with CH11 or CH11 plus Sema3A-Fc for 6 hours. (D) In addition, Jurkat cells transfected with siRNA specific for plexin-A1 or nonsilencing siRNA were cultured with control IgG or Sema3A-Fc for 6 to 8 hours. Plexin-A1 expression in RNAi-treated Jurkat cells was determined by RT-PCR analysis. Cell death was quantified by flow cytometry with annexin V staining. P values were also shown. N.S., not significant.
To determine the role of plexin-A1 in Sema3A-mediated signaling, we generated a clone from the leukemic Jurkat cell line that expresses a previously characterized plexin-A1 dominant-negative form8,21 (DN-plexin-A1). The control and DN-plexin-A1–transfected cells displayed similar sensitivities to Fas stimulation alone. However, the DN-plexin-A1 cells were less sensitive to Fas/Sema3A cotriggering than control cells (Figure 6C). We also selectively knocked down plexin-A1 gene expression. RNAi against plexin-A1 reduced the proapoptotic activity of Sema3A on Jurkat cells (Figure 6D). These results suggest that NP1 and plexin-A1 are functional receptor components for Sema3A.
Fas relocalization induced by Sema3A requires actin cytoskeleton
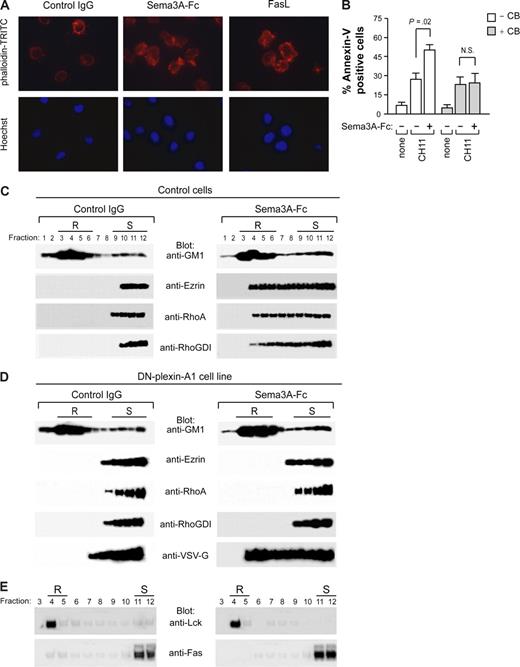
We next asked how Fas is concentrated in lipid rafts by Sema3A/NP/PlexA signaling. Clustered rafts are often bound to the cytoskeleton, and Sema3A signaling is thought to alter actin reorganization in immune cells.24,25 To investigate whether Sema3A could modify the actin cytoskeleton in human leukemic cells, Jurkat cells treated with Sema3A were stained with phalloidin-TRITC for F-actin and with Hoechst 33 258 to assess nuclear morphology and visualized by fluorescence microscopy. Similar to FasL, Sema3A caused both cell shape change and a redistribution of the actin cytoskeleton (Figure 7A). In addition, by pretreating Jurkat cells with the microfilament-disrupting agent cytochalasin-B, we prevented the sensitization to Fas-induced apoptosis mediated by Sema3A (Figure 7B).
Plexin-mediated Sema3A stimulation induces cytoskeleton reorganization and redistribution of actin-linking proteins into lipid rafts. (A) Jurkat cells were incubated with control IgG or Sema3A-Fc (150 ng/mL) for 30 minutes. As positive control, Jurkat cells were also incubated with 50 ng/mL rhFasL. Actin morphology was visualized by staining with phalloidin-TRITC. Nuclei were stained with Hoechst 33 258 (AX10 microscope, Carl Zeiss, Oberkochen, Germany, original magnification, ×1600). (B) Jurkat cells were pretreated with 5 μg/mL cytochalasin B (CB) for 30 minutes and then incubated with anti-Fas or anti-Fas plus Sema3A-Fc for 6 hours. Cell death was quantified by flow cytometry with annexin V staining. P values were also shown. N.S., not significant. (C-E) Control or dominant negative plexin-A1 expressing cells (DN-plexin-A1) were treated with control IgG or Sema3A-Fc for 60 minutes and subjected to density gradient fractionation. (C,D) Fractions were immunoblotted with antibodies for GM1, Ezrin, RhoA, and RhoGDI. Western blots were also probed with anti-VSV-G antibody to detect the VSV-G epitope tag of plexin construct. (E) Fractions derived from DN-plexin-A1 were immunoblotted with antibodies for Lck and Fas. Lipid raft (R) and soluble (S) fractions are indicated.
Plexin-mediated Sema3A stimulation induces cytoskeleton reorganization and redistribution of actin-linking proteins into lipid rafts. (A) Jurkat cells were incubated with control IgG or Sema3A-Fc (150 ng/mL) for 30 minutes. As positive control, Jurkat cells were also incubated with 50 ng/mL rhFasL. Actin morphology was visualized by staining with phalloidin-TRITC. Nuclei were stained with Hoechst 33 258 (AX10 microscope, Carl Zeiss, Oberkochen, Germany, original magnification, ×1600). (B) Jurkat cells were pretreated with 5 μg/mL cytochalasin B (CB) for 30 minutes and then incubated with anti-Fas or anti-Fas plus Sema3A-Fc for 6 hours. Cell death was quantified by flow cytometry with annexin V staining. P values were also shown. N.S., not significant. (C-E) Control or dominant negative plexin-A1 expressing cells (DN-plexin-A1) were treated with control IgG or Sema3A-Fc for 60 minutes and subjected to density gradient fractionation. (C,D) Fractions were immunoblotted with antibodies for GM1, Ezrin, RhoA, and RhoGDI. Western blots were also probed with anti-VSV-G antibody to detect the VSV-G epitope tag of plexin construct. (E) Fractions derived from DN-plexin-A1 were immunoblotted with antibodies for Lck and Fas. Lipid raft (R) and soluble (S) fractions are indicated.
Ezrin, a major component of the ERM protein family (ezrin, radixin, moesin), can interact with Fas and mediate Fas cell membrane localization during Fas-induced apoptosis.18 We found that ezrin as well as actin dynamics regulatory proteins, such as RhoA and RhoGDI, were accumulated in lipid rafts following Sema3A stimulation (Figure 7C). In comparison, the amount of ezrin, RhoA, or RhoGDI redistributed in the lipid rafts was very faint when the DN-plexin-A1 cell line was stimulated with Sema3A alone (Figure 7D). In addition, Fas relocalization into lipid rafts was not observed in these cells on Sema3A treatment (Figure 7E). All together, these findings indicate that PlexinA-driven Fas translocation into lipid rafts sensitizes leukemic cells to apoptosis and that this effect seems to require actin-linking proteins accumulation in membrane rafts and cytoskeleton reorganization.
Discussion
The recruitment of Fas to lipid rafts, elicited by independent nonapoptotic receptor signaling, represents a noncompletely understood pathway that modulates Fas-induced cell death.14,–16 Semaphorins play a central role in axonal guidance, and emerging evidence points to diverse functions of several semaphorins (including Sema3A) in the immune system. For instance, class IV semaphorins (eg, Sema4D and Sema4A) play crucial roles in the reciprocal stimulation between T cells and APCs, both in vitro and in vivo.26 In addition, Sema3A inhibits the migration of human monocytes in response to cytokine stimulation27 as well as T-cell proliferation and cytokine production under stimulating conditions.6 In this study, we identify a novel biologic function for semaphorins in immune cells and an unexpected signaling mechanism, namely, the coupling of Sema3A to a death receptor.
A pivotal role of secreted semaphorins in the regulation of neuronal cell death has been previously established,28 although the implicated pathways have not been characterized as yet. The present study demonstrates that secreted Sema3A and its receptor NP1 are important determinants of leukemic cells sensitivity to Fas-mediated death signals (Figures 1,2). De novo protein synthesis is not essential for the proapoptotic activity of Sema3A, but the redistribution and clustering of membrane-bound Fas into lipid raft microdomains are a pivotal step for cell death signaling (Figure 4).
Membrane rafts could serve to generate high local concentration of Fas, as platforms for coupling adaptor and effector proteins required for Fas downstream signaling, facilitating, and amplifying signaling processes by transient local assembly of various cross-interacting molecules.15,–17 This is of particular importance in Fas-mediated signal transduction because death receptors lack enzymatic activity and the pathway is triggered by protein–protein interactions. The presence of Fas in lipid rafts is restricted to cells previously described as type I, which show more efficient formation of death signaling complexes and greater sensitivity to Fas stimuli. In peripheral T cells, it has been shown that the membrane distribution of Fas is dynamically regulated by antigen receptor signaling and possibly by other signals.15,–17 This can increase Fas-mediated apoptosis in type II cells and may be important to promote the clonotypic elimination of chronically stimulated T cells. As some leukemic primary cells are described as type II cells, in which Fas signaling is dependent on receptor-mediated clustering in membrane microdomains,18 our observations could be also relevant for apoptotic pathways in leukemia cells. Notably, Sema3A expression is reduced in primary leukemic cells, whereas they express the receptor NP1 at high levels.29,30 Therefore, the Sema3A-dependent regulatory mechanism described here may be relevant to curb uncontrolled T-cell proliferation.
The intracellular domain of NP1 is short and apparently unable to mediate functional responses to Sema-3A.1 Plexin-A1 is a coreceptor for Sema-3A in the nervous system,2,–4 and it has a signaling role in immune responses.31,32 We detected plexin-A1 expression in our cells and a dominant negative mutant of plexin-A1 or siRNA against plexin-A1 blocked the proapoptotic activity of Sema3A. Therefore, Sema-3A may act through plexin-A1 in leukemic cells.
Interestingly, our data show the translocation of plexin-A1 into membrane rafts on Sema3A stimulation, whereas a remarkable fraction of NP1 appears to be constitutively localized in these membrane microdomains. Because lipid rafts are specialized structures involved in several biologic processes, such as apoptosis, synaptic transmission, adhesion, and migration,15,,–18 the clustering of NP1 and Plexin-A1 into these microdomains on the cell surface may lead to a more effective Sema3A signaling. The clustering of NP1 and plexin-A1 on the cell surface, on Sema3A stimulation, had been shown previously in neuronal cells33 ; however, the identity of the implicated membrane microdomains was not known. Notably, we found that Plexin-A1 is rapidly incorporated into lipid rafts, after a few minutes of incubation with Sema3A (Figure 6), and this process precedes Fas clustering (A.C. et al, unpublished data, 2008). Thus, we propose that Sema3A/NP1/Plexin signaling rearranges membrane rafts, promoting receptor clustering and Fas redistribution.
How Plexin can affect Fas translocation into lipid rafts is presently unknown. This signal is distinct from the Rap-1–dependent pathway leading to inhibition of T-cell proliferation and cytokine production.6 It has been demonstrated that the cytoplasmic domain of plexins carries an intrinsic R-Ras GAP activity,34 and several groups have reported that plexin-associated effector molecules control cytoskeletal dynamics and integrin function through monomeric G proteins.35 Cytoskeletal rearrangements or changes in the interaction between Fas and components of the actin cytoskeleton may contribute to Fas translocation into lipid rafts.36 Our findings indicate the involvement of actin-network remodeling triggered by plexins in the translocation of Fas into lipid rafts (Figure 7). Alternatively, posttranslational modification of Fas may also mediate translocation, and this point is currently under investigation.
In conclusion, the data presented here indicate that Sema3A plays a relevant role by bolstering Fas-mediated apoptosis in human leukemic cells. Moreover, because NP1 and Sema3A are constitutively expressed in human thymus in both thymic epithelial cells and CD4/CD8-defined thymocytes,37 our data suggest a possible involvement of Sema3A in T-cell homeostasis, a process in which the role of tumor necrosis factor family receptors is well documented.11,12
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr M. Tessier-Lavigne (Howard Hughes Medical Institute, Stanford University, Stanford, CA) for providing the blocking anti-NP1 antibody and Dr L. Capparuccia (Institute for Cancer Research and Treatment [IRCC], Candiolo, Torino), Dr G. Fulgenzi (Polytechnic University of Marche, Ancona, Italy), and Dr M. Fanelli (Center of Biotechnology, University of Urbino, Fano, Italy) for technical assistance.
This work has been supported by grants from the Italian Association for Cancer Research (A.P., A.C.) and the Ministry of University and the Ministry of Health (A.P.). S.M. and R.L. were supported by a fellowship from Italian Association for Cancer Research and Italian Foundation for Cancer Research, respectively.
Authorship
Contribution: S.M. performed cell-culture experiments, generated figures, and helped to write the manuscript; A.P. analyzed the data, helped to design the study, and helped to write the manuscript; R.L. assisted with experiments and provided expertise in molecular biology; R.T., M.M., and M.R.R. provided methodologic expertise and helped to design some experiments; L.T. analyzed the data, helped to design the study, and helped to write the manuscript; and A.C. (principal investigator) designed the experiments, interpreted and analyzed data, and drafted and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alfonso Catalano, Dipartimento di Patologia Molecolare, Politecnica delle Marche, Via Tronto 10/A, 60100, Ancona, Italy; e-mail: catgfp@yahoo.it or a.catalano@univpm.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal