The polycomb group (PcG) gene BMI1 has been identified as one of the key epigenetic regulators of cell fates during different stages of development in multiple murine tissues. In a clinically relevant model, we demonstrate that enforced expression of BMI1 in cord blood CD34+ cells results in long-term maintenance and self-renewal of human hematopoietic stem and progenitor cells. Long-term culture-initiating cell frequencies were increased upon stable expression of BMI1 and these cells engrafted more efficiently in NOD-SCID mice. Week 5 cobblestone area-forming cells (CAFCs) were replated to give rise to secondary CAFCs. Serial transplantation studies in NOD-SCID mice revealed that secondary engraftment was only achieved with cells overexpressing BMI1. Importantly, BMI1-transduced cells proliferated in stroma-free cytokine-dependent cultures for more than 20 weeks, while a stable population of approximately 1% to 5% of CD34+ cells was preserved that retained colony-forming capacity. Whereas control cells lost most of their NOD-SCID engraftment potential after 10 days of ex vivo culturing in absence of stroma, NOD-SCID multilineage engraftment was retained by overexpression of BMI1. Thus, our data indicate that self-renewal of human hematopoietic stem cells is enhanced by BMI1, and we classify BMI1 as an intrinsic regulator of human stem/progenitor cell self-renewal.
Introduction
Cellular memory induced by chromatin modifications can be maintained through subsequent cell divisions by the opposing effects of transcriptional activators of Trithorax (Trx) proteins and repressors of the polycomb proteins (PcG).1 Two functionally different PcG complexes have been identified. Polycomb repressive complex (PRC) 2, also termed as “initiation” complex, consists of several subunits, including EED, SU(Z)12, and EZH2, functions as a histone methyltransferase that specifically trimethylates histone 3 on lysine residue 27 (H3K27) resulting in compaction of the chromatin and subsequent gene silencing.2 PRC1 which is referred to as “maintenance” complex consists of various subunits, including BMI1, RING1A/B, CBX, MEL18, and MPH/RAE28.2 This complex does not possess methyltransferase activity itself, and the only observed enzymatic activity is ubiquitinylation of lysine 119 of H2A via its RING subdomains.3 However, it has recently been demonstrated that DNA methyltransferase (Dnmt1) is required for proper assembly of PRC1 complex and that it can associate with BMI1 via Dmap1 and thus regulate DNA and histone methylation.4,5 These observations suggest that not only PRC2, but also PRC1, might affect the epigenetic state of cells via methylation in a more direct fashion.
Polycomb target genes were mapped in genome-wide screens in human embryonic fibroblasts,6 murine embryonic stem cells,7,8 and Drosophila melanogaster.9,10 In embryonic fibroblasts, PRC1, PRC2, and H3K27-3me co-occupied genes that tend to be involved in embryonic development and cell fate decisions.6 In ES cells, SU(Z)12, EED, and H3K27-3me co-occupied genes that appear to regulate early developmental stages of neurogenesis, hematopoiesis, and cell fate specification.7,8 In Drosophila, PcG preferentially bind to developmental genes, such as Wingless, Hedgehog, and Notch.9,10 In hematopoietic stem cells (HSCs), it has been demonstrated that PcG proteins are involved in the regulation of stem-cell self-renewal. We have recently reported that EZH2 prevents murine stem-cell exhaustion.11 Others have reported that targeted deletion of BMI1 in murine HSCs impaired their competitive repopulation capacity, whereas its overexpression enhanced symmetric cell divisions of HSCs and consequently led to augmented HSC self-renewal.12,–14 In senescence screens, it was observed that the p16INK4a/p19ARF locus is repressed by BMI1, which was required to bypass senescence of embryonic fibroblasts.15 Targeted deletion of BMI1 in HSCs resulted in an increase in the expression of p16 and p19.16 Deletion of p16 and p19 in bmi1−/− HSCs partially, but not completely, restored the self-renewal. In contrast, overexpression of BMI1 could increase progenitor levels in absence of p16 and p19, indicating that other targets of BMI1 exist as well.13,16
The expression of the PcG genes in mouse HSCs varies throughout the hematopoietic hierarchy. EZH2 and EED are more ubiquitously expressed,17 MEL18, CBX, and MPH1/REA28 are expressed at rather low levels in HSCs but increase upon differentiation, and BMI1 is selectively expressed in HSCs.17,18 This is suggestive for the notion that BMI1 can participate in the self-renewal and growth mechanisms at the earliest stages of hematopoiesis.
Although these data are intriguing, they are based on murine experimental systems, and whether they reflect functional properties of the Polycomb group of genes in the human system is unclear. Several genes that have been shown to induce self-renewal in mouse HSCs have no or very modest effects in human stem cells. Treatment of cells with WNT3a protein or overexpression of modified β-catenin can expand mouse cells more than 100-fold, yet no significant effects on human HSCs have been observed.19,20 Ex vivo expansion and self-renewal of murine long-term repopulating HSCs were observed when the hoxb4 gene was overexpressed in murine cells or when the TAT-HOXB4 fusion protein was developed.21,22 However, ex vivo and in vivo expansion of human CB CD34+ cells by HOXB4 overexpression or direct delivery of HOXB4 had remarkably lower effects compared with the murine cells.23,–25
In a clinically relevant model, we wished to assess whether BMI1 could induce expansion of human cord blood (CB) cells. We stably introduced BMI1 in stem and progenitor cells derived from umbilical CB, and we assayed the biologic effects on the hematopoiesis ex vivo and in vivo. We show that constitutive expression of BMI1 in human CB cells results in prolonged maintenance of the stem-cell pool and enhances self-renewal of human stem and progenitor cells.
Methods
Primary cell isolation
Neonatal CB was obtained after informed consent from healthy full-term pregnancies from the obstetrics departments of the Sophia Hospital in Zwolle, the University Medical Center in Groningen and the Martini Hospital, Groningen. After Ficoll separation of mononuclear cells, CD34+ cells were enriched using magnetically activated cell sorting CD34 progenitor kit (Miltenyi Biotec, Nijmegen, The Netherlands). The purity of the CB CD34+ cell ranged between 90% and 95%, as assessed by flow cytometry.
Cell lines and ex vivo culture of primary cells
293T human embryonic kidney cells and PG13 cells were grown in DME medium (BioWhittaker, Veriers, Belguim) supplemented with 10% FCS (Sigma, Zwijndrecht, The Netherlands), 200 mM glutamine, and penicillin and streptomycin (all from Sigma). MS-5 murine stromal cells were grown in αMEM (BioWhittaker) supplemented with 10% fetal calf serum (FCS), 200 mM glutamine, and penicillin and streptomycin. For the MS-5 coculture experiments and long-term culture-initiating (LTC-IC) assays, cells were grown in 12.5% heat-inactivated FCS, 12.5% heat-inactivated horse serum (Sigma), penicillin, and streptomycin, 200 mM glutamine, 57.2 μM β-mercaptoethanol, and 1 μM hydrocortisone (Sigma). The stroma-independent culture assays were performed in IMD medium (PAA Laboratories, Pasching, Austria) supplemented with 20% FCS, 200 mM glutamine, penicillin, and streptomycin, and the following cytokines: SCF (100 ng/mL), Flt3 ligand (100 ng/mL, both from Amgen, Thousand Oaks, CA), thrombopoietin (TPO, 100 ng/mL; Kirin, Tokyo, Japan), interleukin-3 (IL-3, 10 ng/mL), and IL-6 (10 ng/mL; both from Gist-Brocades, Delft, The Netherlands).
Immunoblotting and cytospins
A total of 5 × 105 cells were lysed to prepare whole cell extracts. Western blot analysis was performed by standard protocols and as previously described.26 Antibody against BMI1 (Upstate Biotechnology, Charlottesville, VA) was used in a 1:1000 dilution and anti-GFP antibody (Santa Cruz Biotechnology, Heerhugowaard, The Netherlands) was used in a 1:300 dilution. May-Grünwald Giemsa staining was used to analyze cytospins. Pictures were taken on Olympus BX50 microscope (Olympus Nederland BV, Zoeterwoude, The Netherlands) with 40×/0.60 objective.
RNA extraction and real-time polymerase chain reaction analysis
Total RNA was isolated from 1 × 105 cell using RNeasy kit from QIAGEN (Venlo, The Netherlands) and was reverse-transcribed using M-MuLV reverse transcriptase (Fermentas, St Leon-Roth, Germany) according to the manufacturer's instructions. Aliquots of cDNA were then real-time amplified using iQ SYBR Green mix (Bio-Rad, Hercules, CA) on a MyIQ thermocycler (Bio-Rad) and quantified using MyIQ software (Bio-Rad). Hypoxantine-guanine phosphoribosyl transferase (HPRT) expression was used to calculate relative expression levels. Sequences and conditions are available on request.
Retroviral vector construct and transduction of CB cells
For all transduction experiments, the MiGR1 retroviral expression vector was used, which contained murine stem cell virus long-terminal repeats and an internal ribosomal entry site (IRES2) upstream of the enhanced green fluorescent protein as marker gene. The BMI1 gene was cloned from cDNA of CB CD34+ cells, and a hemagglutinin tag was added to the N-terminus using the following primers: forward 5′-ATGTACCCATACGATGTTCCAGATTACGCTCATCGAACAACGAGAATCAA-3′ and reverse 5′-TCAACCAGAAGAAGT TGCTGATG-3′. The gene was cloned into the pCR4 cloning vector (Invitrogen, Carlsbad, CA), cut using Xho I-MunI sites and subcloned into Xho I-EcoRI sites of the MiGR1 vector. The construct was verified by sequencing.
Stable PG13 virus producer cell lines were generated by first transiently transfecting 293T cells with 2 μg MiGR1 or MiGR1-BMI1 plasmid DNA and 2 μg pCL-Eco ecotropic packaging plasmid using Fugene 6 (Roche Diagnostics, Mannheim, Germany). Supernatants from the 293T cells were used to transduce PG13 cells in the presence of 8 μg polybrene (Sigma). Retroviral supernatants from the PG13 cells were used to transduce CB CD34+ cells prestimulated for 48 to 72 hours in serum-free HPG medium (Lonza, Walkersville, MD) supplemented with 100 ng/mL SCF, TPO, and Flt3L. Three consecutive transduction rounds of 8 to 12 hours were performed on retronectin-coated plates (Takara, Tokyo, Japan) before the start of different assays.
Colony-forming cell, secondary colony-forming cell, long-term culture-initiating cell, and secondary cobblestone area-forming cell assay
Colony-forming cell (CFC) and LTC-IC assays on MS-5 stromal cells were performed as previously described.26 For the CFC assays, 1000 GFP+-sorted cells were plated directly after transduction and 10 000 GFP+ cells were used at later time points. For the colony replating experiments, 2 weeks after the primary plating, the colonies from one plate were collected, washed 3 times with phosphate-buffered saline (PBS), and the cells were plated in new methylcellulose for an additional 2 weeks. For the LTC-IC assays, transduced GFP+ cells were sorted on MS5 stromal cells in limiting dilutions from 10 to 810 cells per well in 96-well plates. Cultures were weekly fed with new medium. After 5 weeks of culture, the wells containing cobblestone areas were scored after which the medium from the wells was aspirated and replaced with methylcellulose containing cytokines. After an additional 2 weeks of culture, wells were scored as positive or negative to yield the LTC-IC frequency. For the secondary cobblestone area-forming cell (CAFC) assay, day 35 cells were harvested by trypsinization of the adherent cell population, replated on new MS5 stromal cells, and maintained for additional 5 weeks.
Primary and secondary transplantations into NOD-SCID mice
Eight- to 10-week-old female NOD/SCID mice (NOD.CB17-Prkdcscid/J) were purchased from Charles River Laboratory (Maastricht, Netherlands) and were maintained in specific pathogen-free conditions. Before transplantations mice were sublethally irradiated with 3 Gy. One group of mice was injected with 6 × 105 nonsorted CD34+ cells into the retro-orbital vein immediately after transduction and 6 weeks later the bone marrow (BM) cells from the recipients was used to assess the presence of GFP+ human CD45+ cells. Another cohort of mice received 3.8 × 106 cells cultured for 7 days after transduction in presence of: SCF (100 ng/mL), TPO (100 ng/mL), Flt3 ligand (100 ng/mL), IL-3 (10 ng/mL), and IL-6 (10 ng/mL). BM cells from these NOD/SCID mice were used to flow cytometrically analyze human cell engraftment after 8 weeks of transplantation. A third group of mice was injected with 5.1 × 105 CD34+ transduced cells, analyzed for presence of human GFP+ cells 8 weeks later, and BM from an individual chimeric primary recipient was injected into an individual secondary NOD/SCID recipient without repurification of human cells. Before the secondary transplantation, mice were treated with single intraperitoneal injection (200 μg/mouse) of anti-CD122 TM-β1 (BD Biosciences PharMingen, San Diego, CA) within 4 hours after 3 Gy of total body irradiation to eliminate residual murine natural killer cell activity. Mouse BM was analyzed for the presence of GFP+ CD45+ cells 8 weeks after transplantation.
Flow-cytometric analysis and sorting procedures
All antibodies were obtained from BD Biosciences (Alphen aan den Rijn, The Netherlands). Staining of the cells was performed for 45 minutes at 4°C. Mouse BM cells were blocked with anti-Fcγ antibody for 15 minutes at 4°C to avoid nonspecific binding. Sorting of the CB cells into stem and progenitor fractions was performed on the basis of the combinatorial expression of cell surface antigens as previously reported.27 HSCs were defined as CD34+CD38−, common myeloid progenitors (CMP) as CD34+CD38+IL3Rα+CD45RA−, megakaryocyte-erythroid progenitors (MEP) as CD34+CD38+IL3Rα−CD45RA− and granulocyte-macrophage progenitors (GM) as CD34+CD38+IL3Rα+CD45RA+. The fluorescence-activated cell-sorting analyses were performed on a FACS Calibur (BD Biosciences) and sorting of the cells was performed on MoFlo (Dako California, Carpinteria, CA). Data were analyzed using WinList 3D (Topsham, ME) and FlowJo (Tree Star, Ashland, OR) software.
Results
Retroviral introduction of BMI1 in CD34+ cells
To determine the normal expression of BMI1 in immature human hematopoietic cells, we sorted CB cells into different progenitor and stem cell fractions (Figure 1A). We observed that BMI1 is highly expressed in the CD34+CD38− fraction (HSCs) compared with the committed progenitor populations (Figure 1B). There was no significant difference in the expression levels of BMI1 in the different sorts for CMP, MEP, or GM progenitors. We next designed control MiGR1 or BMI1 retroviral vectors (Figure 1C), which were used to transduce CB CD34+ cells (hereafter referred to as MiGR1 or BMI1-expressing cells, respectively). Transduction efficiencies were determined by fluorescence-activated cell sorting (FACS) analysis on the basis of the GFP expression in the cells and ranged from 50% to 65% in the MiGR1 and 45% to 70% in BMI1-transduced cells (Figure 1D). Expression of BMI1 protein was confirmed by Western blotting in the PG-13 stable virus producers and in the transduced CB CD34+ cells (Figure 1E). Cord blood cells express endogenous BMI1, but upon retroviral transduction with BMI1 vector an approximately 4-fold increase in expression was observed. These data were further confirmed by quantitative polymerase chain reaction analysis (qPCR)(Figure 1F). Using analysis, we further checked whether overexpression of BMI1 affected expression of known downstream target genes, and we indeed observed that p16 and p19 were down-modulated (Figure 1F).
Retroviral introduction of BMI1 in human cord blood (CB) CD34+ cells. (A) Sorting strategies of nontransduced CB stem and progenitor fractions. (B) mRNA expression of BMI1 in human CB cells. Cells analyzed are: HSCs (CD34+CD38−), CMPs (CD34+CD38+IL3Rα+CD45RA−), MEPs (CD34+CD38+IL3Rα−CD45RA−), and GMs (CD34+CD38+IL3Rα+CD45RA+). (C) Schematic representation of the MiGR1 (control) and BMI1 retroviral vectors used in this study. (D) CB CD34+ cells were prestimulated for 48 hours in HPGM supplemented with KL, Flt3L, and TPO followed by 3 transduction rounds in the next 48 hours with MiGR1 or BMI1 retroviruses, and transduction efficiencies were determined on the basis of GFP expression by FACS. (E) Western blot analysis of total cell extracts from PG13 virus producer cells or CB CD34+ transduced cells. Membranes were probed with anti-BMI1 or anti-GFP antibodies. (F) Quantitative RT-PCR analysis of transduced cells for known downstream target genes of BMI1.
Retroviral introduction of BMI1 in human cord blood (CB) CD34+ cells. (A) Sorting strategies of nontransduced CB stem and progenitor fractions. (B) mRNA expression of BMI1 in human CB cells. Cells analyzed are: HSCs (CD34+CD38−), CMPs (CD34+CD38+IL3Rα+CD45RA−), MEPs (CD34+CD38+IL3Rα−CD45RA−), and GMs (CD34+CD38+IL3Rα+CD45RA+). (C) Schematic representation of the MiGR1 (control) and BMI1 retroviral vectors used in this study. (D) CB CD34+ cells were prestimulated for 48 hours in HPGM supplemented with KL, Flt3L, and TPO followed by 3 transduction rounds in the next 48 hours with MiGR1 or BMI1 retroviruses, and transduction efficiencies were determined on the basis of GFP expression by FACS. (E) Western blot analysis of total cell extracts from PG13 virus producer cells or CB CD34+ transduced cells. Membranes were probed with anti-BMI1 or anti-GFP antibodies. (F) Quantitative RT-PCR analysis of transduced cells for known downstream target genes of BMI1.
Long-term maintenance of human HSCs and progenitor cells by expression of BMI1
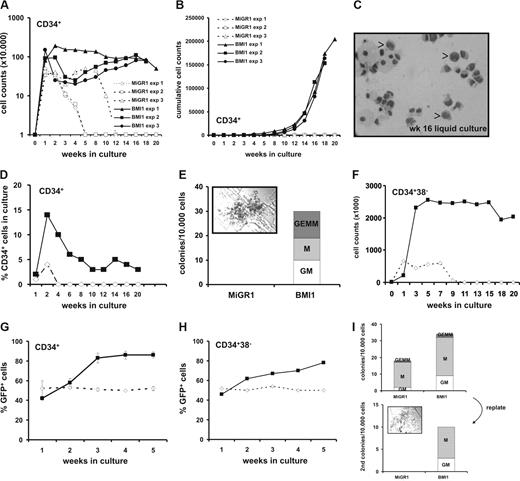
Transduced control MIGR1 or BMI1 cells were GFP-sorted and plated in cytokine-driven stroma-free cultures for weekly analysis. In Figure 2A, weekly cell counts of demi-depopulated cultures are shown. Whereas MiGR1 cells typically expanded transiently over a 3- to 5-week period, BMI1 cells maintained to proliferate over 20 weeks, resulting in a strong expansion of more than 2 × 106 fold, shown in Figure 2B as cumulative cell expansion. Even after 16 weeks, some of the cells retained a primitive morphology resembling blast-like cells (Figure 2C), whereas the control cells became terminally differentiated myeloid cells at earlier time points (data not shown). Phenotypical FACS analysis revealed that 1% to 5% of the cultured BMI1 cells remained CD34+ over a period of 20 weeks (Figure 2D), whereas the other differentiation markers for myeloid (CD11, CD14, CD15) and erythroid (CD71 and GPA) lineages did not reveal any differences between the 2 groups (data not shown). We used the cells from the culture (without further resorting) to perform weekly CFC assays and, as depicted in Figure 2E, overexpression of BMI1 resulted in colony formation even after 16 weeks of culture, whereas the MiGR1 cells lost their capacity to form colonies between 3 and 5 weeks (data not shown). Remarkably, one third of the BMI1 colonies at this time point were CFU-GEMMs (Figure 2E inset), which represent the most primitive type of progenitors, arguing that immature progenitors have been maintained for 16 weeks. We further wanted to test whether BMI1 would have the same effect on the more primitive cells, and we sorted CD34+CD38− cells and analyzed their growth in cytokine-driven liquid cultures as described in “Methods.” BMI1 cells were again maintained to proliferate up to 20 weeks, whereas the MiGR1 cells were exhausted after 7 to 9 weeks (Figure 2F).
BMI1 promotes long-term in vitro expansion of human CD34+ progenitor cells. (A) Cord blood CD34+ cells were transduced with either control (open symbols) or BMI1 (closed symbols) vectors and grown in stroma-free liquid cultures using a mix of cytokines as described in “Methods.” Cells were counted weekly and 3 independent experiments are shown. Week 0 represents the number of transduced cells that were plated. (B) Cumulative expansion of the same cultures is shown. (C) The May-Grünwald Giemsa stained picture shows a cytospin of 16-week cultured cells where monocytes, macrophages, granulocytes and some blast-like cells can be observed, indicated by the arrows. (D) A representative experiment where the percentage of CD34+ cells was maintained at approximately 4% over a period of 20 weeks in BMI1 overexpressing cells is shown. (E) After 16 weeks, cultures were analyzed for progenitor content in CFC assays in methylcellulose by plating 10 000 cells from the culture without further resorting. Progenitors were only detected in BMI1-expressing cells. A CFU-GEMM colony from a representative CFC experiment is shown in the inset. (F) Transduced and GFP+ sorted CB CD34+CD38− cells were propagated in the same cytokine-driven liquid culture conditions as in panel A for 20 weeks. (G) After transduction unsorted CD34+ cells were grown in cocultures on MS5 stromal cells. The cultures were weekly demidepopulated and analyzed for GFP expression by FACS. (H) Experiment as in panel G, but now transduced CD34+CD38− cells were plated on MS5 stroma. (I) Nonadherent cells from week 5 cocultures were used to perform CFC assays (top panel). After 2 weeks, CFCs were harvested and replated into new methylcellulose assays (bottom panel). Only the BMI1-expressing cells contained replating capacity in secondary CFC assays, and a colony is shown in the inset. A representative experiment of 4 performed experiments is shown.
BMI1 promotes long-term in vitro expansion of human CD34+ progenitor cells. (A) Cord blood CD34+ cells were transduced with either control (open symbols) or BMI1 (closed symbols) vectors and grown in stroma-free liquid cultures using a mix of cytokines as described in “Methods.” Cells were counted weekly and 3 independent experiments are shown. Week 0 represents the number of transduced cells that were plated. (B) Cumulative expansion of the same cultures is shown. (C) The May-Grünwald Giemsa stained picture shows a cytospin of 16-week cultured cells where monocytes, macrophages, granulocytes and some blast-like cells can be observed, indicated by the arrows. (D) A representative experiment where the percentage of CD34+ cells was maintained at approximately 4% over a period of 20 weeks in BMI1 overexpressing cells is shown. (E) After 16 weeks, cultures were analyzed for progenitor content in CFC assays in methylcellulose by plating 10 000 cells from the culture without further resorting. Progenitors were only detected in BMI1-expressing cells. A CFU-GEMM colony from a representative CFC experiment is shown in the inset. (F) Transduced and GFP+ sorted CB CD34+CD38− cells were propagated in the same cytokine-driven liquid culture conditions as in panel A for 20 weeks. (G) After transduction unsorted CD34+ cells were grown in cocultures on MS5 stromal cells. The cultures were weekly demidepopulated and analyzed for GFP expression by FACS. (H) Experiment as in panel G, but now transduced CD34+CD38− cells were plated on MS5 stroma. (I) Nonadherent cells from week 5 cocultures were used to perform CFC assays (top panel). After 2 weeks, CFCs were harvested and replated into new methylcellulose assays (bottom panel). Only the BMI1-expressing cells contained replating capacity in secondary CFC assays, and a colony is shown in the inset. A representative experiment of 4 performed experiments is shown.
Thus, we can conclude that introduction of BMI1 in human CD34+ and CD34+CD38− cells allows their continued and extensive proliferation in vitro in cytokine-driven stroma-free cultures conditions for more than 20 weeks while retaining their progenitor activity throughout this long-term culture period.
BMI1 provides a proliferative advantage of CB cells on stroma
To further asses the effects of BMI1 overexpression on the self-renewal and differentiation potential of HSCs, transduced CB CD34+ as well as the most primitive CD34+CD38− cells were cultured on MS5 murine stromal cells, and cocultures were demi-depopulated weekly for analysis. Plating of nonsorted cells on MS5 allowed analysis of relative contributions of GFP+ and GFP− cells to the culture. As indicated in Figure 2G, overexpression of BMI1 provided a proliferative advantage of the cells as the GFP+ population of the nonadherent cells in the CD34+ cultures increased from 41% to 80%, whereas the MiGR1 population remained constant between 50% and 55%. The same trend was observed in the cultures when CD34+CD38− cells were used (Figure 2H). Phenotypical FACS analysis revealed marginal differences of the differentiation markers between the 2 groups, whereas no morphologic differences were observed in the cytospin preparations (data not shown). We have not yet propagated BMI1 cells on MS5 until week 20 to determine whether blast-like CD34+ cells persist under these conditions, as observed in our cytokine-driven liquid-culture conditions. The number of progenitors in the MS5 cocultures was evaluated in colony assays. Transduced CB CD34+ cells were either plated directly for CFC assays or after expansion and weekly demi-depopulation (weeks 1-5) of cocultures on MS5 cells. As depicted in Figure 2I (top panel), where nonadherent cells from the week 5 coculture were used, overexpression of BMI1 resulted in production of more colony forming units (CFUs) than control MiGR1 cells (36 ± 9 vs 19 ± 4 colonies per 10 000 cultured cells, respectively). Similar results were obtained using nonadherent suspension cells from weeks 1 to 4 (data not shown). Two weeks after the primary plating of week 5 cells, the colonies were collected and the cells were plated in new methylcellulose for an additional 2 weeks to address self-renewal properties of progenitors. Whereas MiGR1 control cells did not give rise to any secondary colonies, BMI1 overexpressing cells did contain replating capacity (Figure 2I bottom panel).
Taken together, these data indicate that BMI1 provides a proliferative advantage of human CD34+ and CD34+CD38− cells in stromal cocultures with increased numbers of progenitors that contain self-renewal properties.
BMI1 promotes self-renewal of progenitors
In the coculture studies, we noted that the CFUs from the BMI1 expressing cells have replating capacity. However, in these experiments, bulk CD34+ transduced cells were used. To further discriminate which fraction of the cells may be responsible for these effects, CB CD34+ cells were sorted 48 hours after the start of the transduction period on the basis of cell-surface marker expression into HSCs, CMPs, MEPs, and GMs as described in Figure 1A. The cells were plated in methylcellulose, and colonies were scored on the basis of their morphology. Figure 3A demonstrates that the sorting procedure yielded relatively pure populations in the MEP and GM fractions in MiGR1 control cells, whereas, as expected, HSC and CMP populations contained both erythroid and myeloid progenitors. Notably, regardless of the cell subset, in all of the groups where BMI1 was overexpressed, a higher plating efficiency was observed. Secondary replating was performed in new methylcellulose medium, and the cells were cultured for an additional 2 weeks, after which colonies were scored again. Remarkably, overexpression of BMI1 in the stem and all progenitor cell subsets resulted in CFUs at a frequency of 22 to 50 colonies per 1000 initial cells. The control cells had replating capacity with a frequency of 4 to 7 colonies per 1000 initial cells, but only in the most primitive cell subsets of HSCs and CMPs. In conclusion, overexpression of BMI1 promotes self-renewal of both immature as well as more committed progenitor subtypes.
BMI1 promotes self-renewal of progenitors. (A) After transduction of CB CD34+ cells with either control or BMI1 vectors, the cells were sorted into HSC, CMP, MEP, and GM fractions and analyzed for CFC content. Two weeks after the primary plating, colonies were scored on the basis of morphology as CFU-GEMM, BFU-E, CFU-G, CFU-M, or CFU-GM, and secondary replating was performed in new methylcellulose assays. The cells were cultured for additional 2 weeks and colonies were scored.
BMI1 promotes self-renewal of progenitors. (A) After transduction of CB CD34+ cells with either control or BMI1 vectors, the cells were sorted into HSC, CMP, MEP, and GM fractions and analyzed for CFC content. Two weeks after the primary plating, colonies were scored on the basis of morphology as CFU-GEMM, BFU-E, CFU-G, CFU-M, or CFU-GM, and secondary replating was performed in new methylcellulose assays. The cells were cultured for additional 2 weeks and colonies were scored.
BMI1 overexpression results in enhanced stem-cell frequencies and elevates their self-renewal potential
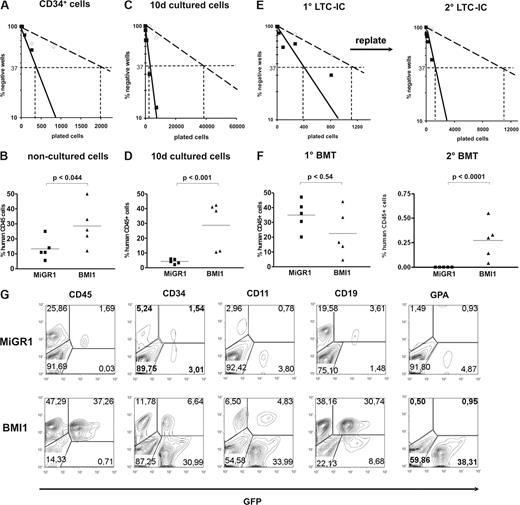
To address the effects of BMI1 on HSC frequency and self-renewal, we performed in vitro LTC-IC assays and in vivo transplantation studies in NOD-SCID mice. CD34+ cells were transduced with either MiGR1 or BMI1 vectors, GFP sorted, and plated in limiting dilutions in 96-well plates onto MS5 BM stroma. CAFCs were scored at week 5, and methylcellulose was added to the wells. After 2 additional weeks, the wells containing progenitors were scored as positive. These experiments revealed that the LTC-IC frequencies were 5.7-fold higher by overexpression of BMI1 (1 of 2077 and 1 of 361 in the control vs BMI1, respectively) (Figure 4A). It has been previously reported that the LTC-IC frequency in nonmanipulated CB CD34+ is 0.2 to 1 of 100.26,28,–30 In our experiments, during the manipulation (ie, transduction of the cells), the stem-cell frequencies dropped approximately 3.5-fold in the BMI-1 and 20-fold in the MiGR1 group. These data suggest that the overexpression of BMI1 did not impose a net expansion of HSCs compared with nonmanipulated cells but rather maintained a proportion of the manipulated cells in a primitive state. To further test the in vivo repopulating capacity of these cells, 6 × 105 CD34+ cells were injected directly after transduction into sublethally irradiated NOD-SCID recipients. In the mice transplanted with BMI1-expressing cells, 3.1-fold higher engraftment levels were achieved compared with the control mice (Figure 4B). Hence, BMI1 expression increases the number of cells with repopulating ability in NOD-SCID mice.
BMI1 overexpression results in enhanced stem-cell frequencies and elevates their self-renewal potential. Cord blood CD34+ cells transduced with MiGR1 (open symbols) and BMI1 (closed symbols) were sorted in limiting dilutions and used to enumerate LTC-IC frequencies (A) or were injected into sublethally irradiated NOD-SCID mice (n = 5 per group; B). Human CD45+ chimerism levels were determined 6 weeks after transplantation in the BM of the transplanted mice (B). (C) After transduction, GFP+ CD34+CD38− cells were sorted, cultured for 10 days in stroma-free cytokine-driven liquid culture conditions, and after which LTC-IC frequencies were determined on MS5 BM stroma in limiting dilution. (D) MiGR1 and BMI1-transduced CD34+ cells were cultured for 10 days in stroma-free conditions and 3.8 × 106 cells were injected into sublethally irradiated NOD-SCID mice (n = 5 per group). Eight weeks after transplantation, human BM engraftment was evaluated on the basis of human CD45+ expression. (E) Transduced and sorted cord blood CD34+CD38− cells were used to determine CAFC day 35 frequencies (left panel) and after 5 weeks the cultures were harvested and plated on new MS5 stroma to determine secondary LTC-IC frequencies (right panel). (F) Transduced CD34+ cells were used to perform transplantations into sublethally irradiated NOD-SCID mice (n = 5 per group) and engraftment levels after 8 weeks were determined (left panel). The BM from the primary recipients was used to perform secondary BM transplants and chimerism levels after an additional 8 weeks are shown in the right panel. (G) Multilineage engraftment of a representative mice transplanted with 10-day cultured cells is shown.
BMI1 overexpression results in enhanced stem-cell frequencies and elevates their self-renewal potential. Cord blood CD34+ cells transduced with MiGR1 (open symbols) and BMI1 (closed symbols) were sorted in limiting dilutions and used to enumerate LTC-IC frequencies (A) or were injected into sublethally irradiated NOD-SCID mice (n = 5 per group; B). Human CD45+ chimerism levels were determined 6 weeks after transplantation in the BM of the transplanted mice (B). (C) After transduction, GFP+ CD34+CD38− cells were sorted, cultured for 10 days in stroma-free cytokine-driven liquid culture conditions, and after which LTC-IC frequencies were determined on MS5 BM stroma in limiting dilution. (D) MiGR1 and BMI1-transduced CD34+ cells were cultured for 10 days in stroma-free conditions and 3.8 × 106 cells were injected into sublethally irradiated NOD-SCID mice (n = 5 per group). Eight weeks after transplantation, human BM engraftment was evaluated on the basis of human CD45+ expression. (E) Transduced and sorted cord blood CD34+CD38− cells were used to determine CAFC day 35 frequencies (left panel) and after 5 weeks the cultures were harvested and plated on new MS5 stroma to determine secondary LTC-IC frequencies (right panel). (F) Transduced CD34+ cells were used to perform transplantations into sublethally irradiated NOD-SCID mice (n = 5 per group) and engraftment levels after 8 weeks were determined (left panel). The BM from the primary recipients was used to perform secondary BM transplants and chimerism levels after an additional 8 weeks are shown in the right panel. (G) Multilineage engraftment of a representative mice transplanted with 10-day cultured cells is shown.
To test the effect of BMI1 expression on the most immature stem-cell fraction, MiGR1 and BMI1-transduced cells were sorted into the immature CD34+CD38− HSC population, and cells were cultured for 10 days in stroma-free cytokine-driven cultures. LTC-IC assays were performed using the cultured cells, and frequencies were scored 7 weeks later. Whereas only few positive wells could be scored in the MiGR1 control group, indicating that few stem cells were maintained during the 10-day culture period, expression of BMI1 did result in stem-cell maintenance as LTC-IC frequencies were significantly higher (1 of 41 111 vs 1 of 2491 for MiGR1 and BMI1 cells, respectively) (Figure 4C). These results tempted us to test whether constitutive expression of BMI1 could also maintain the most primitive stem cell fraction under prolonged ex vivo culture conditions as assessed in the in vivo NOD-SCID model. Lethally irradiated NOD-SCID recipients (n = 5/group) were injected with 3.8 × 106 transduced 10-day ex vivo cultured cells as described in “Methods,” and chimerism levels were analyzed in the BM of recipient mice 8 weeks later (Figure 4D). We observed that engraftment efficiencies ranged between 2.17% and 5.68% in the control group, whereas engraftment efficiencies in the BMI1 group were significantly higher, ranging between 10.61% and 42.36% (Figure 4D). Further analysis revealed that the donor-derived BMI1 GFP+ cells were CD34+, CD11b+, CD19+, and only few GPA+, indicating that multilineage engraftment was achieved with BMI1 ex vivo cultured cells (Figure 4G; Table 1).
Percent of human chimerism in each lineage in mice transplanted with cells cultured for 10 days in stoma-free cytokine– dependent conditions
| . | CD 45 . | CD34 . | CD11 . | CD19 . | GPA . |
|---|---|---|---|---|---|
| MiGR1 no. 1 | 2.7 | 1.03 | 0.46 | 3.63 | 0.54 |
| MiGR1 no. 2 | 2.14 | 0.47 | 0.92 | 1.89 | 1.51 |
| MiGR1 no.3 | 5.33 | 2.07 | 2.07 | 5.17 | 2.31 |
| MiGR1 no. 4 | 1.56 | 0.51 | 0.48 | 1.79 | 0.56 |
| MiGR1 no. 5 | 1.69 | 1.54 | 0.78 | 3.61 | 0.93 |
| BMI1 no. 1 | 11.67 | 1.65 | 0.92 | 9.88 | 0.55 |
| BMI1 no. 2 | 46.22 | 6.55 | 5.84 | 37.33 | 1.45 |
| BMI1 no. 3 | 47.14 | 10.36 | 6.01 | 35.3 | 1.03 |
| BMI1 no. 4 | 13.23 | 2.18 | 1.26 | 10.01 | 0.58 |
| BMI1 no. 5 | 37.26 | 6.64 | 4.83 | 30.74 | 0.95 |
| . | CD 45 . | CD34 . | CD11 . | CD19 . | GPA . |
|---|---|---|---|---|---|
| MiGR1 no. 1 | 2.7 | 1.03 | 0.46 | 3.63 | 0.54 |
| MiGR1 no. 2 | 2.14 | 0.47 | 0.92 | 1.89 | 1.51 |
| MiGR1 no.3 | 5.33 | 2.07 | 2.07 | 5.17 | 2.31 |
| MiGR1 no. 4 | 1.56 | 0.51 | 0.48 | 1.79 | 0.56 |
| MiGR1 no. 5 | 1.69 | 1.54 | 0.78 | 3.61 | 0.93 |
| BMI1 no. 1 | 11.67 | 1.65 | 0.92 | 9.88 | 0.55 |
| BMI1 no. 2 | 46.22 | 6.55 | 5.84 | 37.33 | 1.45 |
| BMI1 no. 3 | 47.14 | 10.36 | 6.01 | 35.3 | 1.03 |
| BMI1 no. 4 | 13.23 | 2.18 | 1.26 | 10.01 | 0.58 |
| BMI1 no. 5 | 37.26 | 6.64 | 4.83 | 30.74 | 0.95 |
Self-renewal of HSCs was addressed by the capacity of the transduced CD34+CD38−-sorted cells to form secondary CAFCs in serial replating experiments. MiGR1 and BMI1 transduced cells were plated in LTC-IC assays on MS5 cells. Week 5 CAFCs were scored (Figure 4E left panel), harvested, and replated onto new MS5 stroma. Cultures were maintained for another 5 weeks, after which methylcellulose was added and plates were scored after an additional 2 weeks of culture (Figure 4E right panel). Whereas control MiGR1 cells had a very low replating frequency (1 of 11 690), BMI1 overexpressing cells were capable of forming new CAFCs in the secondary cultures with much higher frequencies (1 of 1211), indicative for self-renewing capacity. Finally, a third cohort of sublethally irradiated NOD-SCID mice were transplanted with 5.1 × 105 cells transduced with MiGR1 or BMI1. Eight weeks after primary transplantation, the mice were killed and BM was collected for analysis and secondary transplantation experiments. In this experiment, both control and BMI1-transduced cells engrafted very efficiently without a significant difference in their engraftment levels (Figure 4F left panel; Table 2). It appears that, when initial engraftment levels are high, additional expression of BMI1 does not further increase the engraftment within a primary transplantation setting, although under more stringent conditions, such as secondary transplantation, BMI1-expressing cells did perform better than control mice. Chimerism of the secondary recipients was analyzed 8 weeks later when BMI1 engraftment levels ranged from 0.04% to 0.55%, whereas no secondary engraftment was observed with MiGR1 control cells (Figure 4F right panel; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Three of the 5 transplanted BMI1 mice showed multilineage engraftment (Table 2).
Percent of human chimerism in each lineage in secondary mice
| . | CD45 . | CD11 . | CD19 . | GPA . |
|---|---|---|---|---|
| BMI1 no. 1 | 0.56 | 0.06 | 0.61 | 0.04 |
| BMI1 no. 2 | 0.14 | 0 | 0.1 | 0 |
| BMI1 no. 3 | 0.04 | 0.01 | 0.01 | 0 |
| BMI1 no. 4 | 0.33 | 0.1 | 0.26 | 0.03 |
| BMI1 no. 5 | 0.3 | 0 | 0.3 | 0 |
| . | CD45 . | CD11 . | CD19 . | GPA . |
|---|---|---|---|---|
| BMI1 no. 1 | 0.56 | 0.06 | 0.61 | 0.04 |
| BMI1 no. 2 | 0.14 | 0 | 0.1 | 0 |
| BMI1 no. 3 | 0.04 | 0.01 | 0.01 | 0 |
| BMI1 no. 4 | 0.33 | 0.1 | 0.26 | 0.03 |
| BMI1 no. 5 | 0.3 | 0 | 0.3 | 0 |
In conclusion, these data indicate that overexpression of BMI1 promotes ex vivo maintenance of human stem/progenitor cells and promotes their self-renewal.
BMI-1 prevents apoptosis and maintains quiescence of CD34+CD38− cells
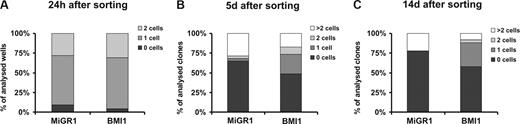
Because our in vivo and in vitro data suggested that BMI1 expression maintains stem-cell characteristics of CB CD34+ cells, we wished to study proliferation and survival at the single-cell level of the most immature CD34+CD38− population. After 48 hours of prestimulation, MoFlo-sorted CB CD34+CD38− cells were transduced with MiGR1 or BMI1 virus for another 48 hours. After transduction, single cells were deposited in 96-well plates and cultured in stroma-free conditions in medium supplemented with a cocktail of cytokines. The wells were scored for the presence of living cells at different time points (Figure 5). When a single-deposited cell was seen in the well after culture, it was referred to as a quiescent cell; and when 2 or more cells were counted in the well, they were considered to be proliferating cells. No differences were detected between MiGR1 and BMI1 groups within 24 hours after single-cell deposition (Figure 5A). Interestingly, under such stringent culture conditions for stem-cell growth where more than 75% of the control cells died after 5 days, BMI1-expressing cells were able to prevent apoptosis as approximately 50% of the cells remained alive after 5 and up to 14 days of culture (Figure 5B,C). However, the most striking difference was the observation that in the BMI1 group approximately 30% of the wells contained a single cell that remained quiescent over time, whereas the number of wells with proliferating cells was higher in the MiGR1 group. In conclusion, these data suggest that expression of BMI1 reduces apoptosis and increases survival of individual cells under stress conditions and maintains the CD34+CD38− population quiescent.
BMI1 prevent apoptosis and maintains quiescence of CD34+CD38− cells. After 48 hours of prestimulation, MoFlo-sorted CB CD34+CD38− cells were transduced with MiGR1 or BMI1 virus for another 48 hours. Following transduction, single cells were deposited in 96-well plates and cultured in stroma-free conditions in IMDM supplemented with 20% FCS and KL, Flt3L, TPO, IL-3, IL-6, and EPO. Wells were evaluated for presence of alive and/or proliferating cells at 24 hours (A), 5 days (B), and 14 days (C) after sorting. The data shown are the average of 3 independent experiments where 210 individual clones were analyzed per group.
BMI1 prevent apoptosis and maintains quiescence of CD34+CD38− cells. After 48 hours of prestimulation, MoFlo-sorted CB CD34+CD38− cells were transduced with MiGR1 or BMI1 virus for another 48 hours. Following transduction, single cells were deposited in 96-well plates and cultured in stroma-free conditions in IMDM supplemented with 20% FCS and KL, Flt3L, TPO, IL-3, IL-6, and EPO. Wells were evaluated for presence of alive and/or proliferating cells at 24 hours (A), 5 days (B), and 14 days (C) after sorting. The data shown are the average of 3 independent experiments where 210 individual clones were analyzed per group.
Discussion
For reasons unknown, several mouse stem-cell genes play no, or only a marginal, role in human HSCs. These include WNT3a,19,20 HOXB4,23,–25 and FGF-1 (A.R., unpublished data, July 2005). These observations suggest that self-renewal in human cells maybe intrinsically different than in mouse cells. Loss and gain-of-function analyses in mouse models have implicated BMI1 as one of the key regulators of stem-cell self-renewal. Severe hematopoietic defects attributed to impaired HSCs self-renewal are reported in bmi−/− mice,12,13 whereas overexpression studies have demonstrated increased self-renewal of mouse HSCs.14 However, to what extent BMI1 regulates human HSC self-renewal has remained elusive.
In this study, we show that persistent activation of BMI1 in human CB CD34+ cells results in long-term maintenance of hematopoietic stem and progenitor cells. An important component of the phenotypes observed by expression of BMI1 is the proliferative advantage over control MiGR1 cells in stroma-free conditions. In such ex vivo expansion cultures, normal CB CD34+ cells only transiently expand over a period of approximately 5 weeks, after which cultures are exhausted and have lost the ability to give rise to cells with colony-forming capacity. When BMI1 was overexpressed, the cultures could be maintained for over 20 weeks and retained colony forming capacity up to 16 weeks after culture. Interestingly, in our single-cell assays we observed that the MiGR1 CD34+CD38−-transduced cells displayed a higher proliferation rate compared with BMI1-expressing CD34+CD38− cells; thus, it is conceivable that cells expressing low levels of BMI1 exhaust faster. Because there is a limited reservoir of quiescent cells that would potentially sustain the formation of progeny at later time points, the control cultures collapse over time. In contrast, a “controlled proliferation” occurs in the BMI1 cultures where the presence of quiescent cells that maintain their primitive phenotype over a period of time is a source of relatively slow-cycling cells that retain the long-term capacity to generate progeny. In line with these considerations, our data indicate that a small population of BMI1-transduced cells indeed continued to express CD34 antigen and retained clonogenic ability for more than 16 weeks.
Cell-intrinsic properties are not the only determinants of stem-cell fate. HSCs are tightly regulated by their microenvironment. In our study, we report a moderate ex vivo proliferative advantage on BMI1 overexpression in coculture conditions with MS5 cells. In contrast to our previous studies in which overexpression of Flt3-ITDs31,32 or activating mutants of STAT526 induced a “hyperactive stem cell state,” a much more “controlled proliferation” phenotype was observed with BMI1 overexpression. Stem-cell frequencies were significantly increased by overexpression of BMI1, and these HSCs contained self-renewal potential. It is interesting to note that self-renewal phenotypes imposed on HSCs by the oncogenes Flt3-ITD and STAT5 required interaction with BM stromal cells, and no maintenance of HSCs was observed in liquid culture. In contrast, we showed that BMI1 overexpression was sufficient to maintain the multipotent characteristics of stem cells in liquid culture conditions, which even resulted in multilineage engraftment in NOD-SCID mice. Thus, BMI1 can be classified as a cell intrinsic regulator of human stem/progenitor cell self-renewal.
The mechanisms by which BMI1 alters the behavior of human stem and progenitor cells are currently unknown. In mouse models, the effects have so far been explained by BMI1-mediated suppression of the INK4a/ARF cell-cycle inhibitory proteins, p16 and p19.15,33 However, p16 and p19 do not account for all BMI1 actions, and a number of other possible downstream targets have recently been reported. E4F1, a transcriptional regulator, has been reported to mediate at least some of the effects of BMI1 in hematopoietic progenitors as a factor that directly binds to BMI1.34 Fasano et al recently provided functional evidence for the role of p21 downstream of BMI1 in neural stem cells,35 and up-regulation of p21 mRNA has been noted in bmi knockout mice.33 Our data indicate that, indeed, p16 and p19 are down-modulated by BMI1 in human CD34+ cells. Studies are under way to identify additional BMI1 target genes in human CD34+ cells, which should provide further insight into the mechanisms by which BMI1 exerts its effects on human HSCs.
Besides the functions that BMI1 has in normal hematopoiesis, constitutive activation of BMI1 has been observed in a variety of nonhematologic and hematologic malignancies. The first evidence for a possible role of BMI1 in the development of hematologic malignancies came from a provirus integration screen in which BMI1 was identified as cooperating factor of c-MYC in the induction of B-cell lymphomas.36 Later it was demonstrated that BMI1 not only determines the proliferative capacity of normal but also leukemic cells.12,36 We have observed in primary acute myeloid leukemia (AML) CD34+ cells that BMI1 is expressed at much higher levels compared with normal BM CD34+ cells in the majority of investigated cases.37 Others have also reported that BMI1 expression is elevated in many hematologic malignancies.38,,,–42 New data may provide some important insights into the possible role of BMI1 in the development of leukemic transformation. In a mouse model in which coexpression of the oncogenes HOXA9 and MEIS1 resulted in a quick onset of myeloid leukemia, no disease was observed in secondary bmi-1-deficient recipients,12 suggesting that BMI1 is essential for the maintenance of HOXA9-MEIS1 leukemic stem cells. Smith et al reported that the oncoprotein E2a-PBX1 enhanced the expression of BMI1 as a consequence of which the INK4a-ARF locus was repressed, and this condition was a requirement for hematopoietic transformation.43 Recently, it has been shown that SALL4, an oncogene that is expressed in AML and induces leukemia in transgenic mice,44 can also strongly up-regulate BMI1 expression.45 However, our data suggest that BMI1 is not sufficient to induce leukemia by itself. Self-renewal and repopulation activity are enhanced in mouse14 and human cells (our data), but it is plausible that secondary mutations are required to induce a full leukemic phenotype. Warner et al described a leukemia model in which the oncogene TLS-ERG was introduced into human hematopoietic CD34+ cells, and in a few cases the transduced cells underwent a stepwise transformation and immortalization whereby up-regulation of BMI1 was identified as cooperating hit.46 By bypassing senescence and maintaining the lifespan of stem cells as well as increasing their self-renewal by promoting symmetric cell divisions, the stem-cell pool may be more prone to acquire additional mutations that can ultimately result in leukemia, and this could be one of the possible mechanisms by which BMI1 may be involved in leukemogenesis.
In conclusion, our data characterize BMI1 as a potentially powerful mediator of maintenance and self-renewal of human hematopoietic stem cells and provide a platform to further elucidate the mechanisms of human hematopoietic stem cell fate decisions. These in vitro and in vivo models facilitate the study of the molecular mechanism underlying the BMI1-induced stem-cell maintenance in human cells and provide a valuable tool to build human leukemia models in which the relative contribution of BMI1 to induce disease can be evaluated. Finally, insight into BMI1 induced self-renewal of human hematopoietic stem cell may provide future possibilities to expand CB cells before clinical stem-cell transplantations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bertien Dethmers-Ausema and Ellen Weersing for technical assistance, Geert Mesander and Henk Moes for help with flow cytometry, Kirin Brewery for providing cytokines, and Dr Harm de Haan, Dr Peet Stienstra, Dr J. J. Erwich, Dr A. van Loon, and colleagues (Obstetrics Departments of Sophia Hospital in Zwolle, University Medical Center in Groningen, and Martini Hospital Groningen) for collecting cord blood.
This work was supported by grants from the European Community (Marie Curie RTN Eurythron, grant no. MRTN-CT-2004-005499) and NWO-VENI (2004).
Authorship
Contribution: A.R. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; B.D. performed animal research; E.V. designed research and interpreted data; G.d.H. designed research, interpreted data, and contributed to writing of the manuscript; and J.J.S. designed and performed research, analyzed and interpreted data, and drafted and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Jacob Schuringa, Department of Hematology, University Medical Center Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands; e-mail: j.schuringa@int.umcg.nl; or Gerald de Haan, Department of Cell Biology, Section Stem Cell Biology, University of Groningen, University Medical Center Groningen, Deusinglaan 1, 9717 AV Groningen, The Netherlands; e-mail: g.de.haan@med.umcg.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal