Epstein-Barr virus (EBV)–specific cellular memory is not transferred from mother to child. Therefore, EBV-induced B-cell proliferation in in vitro–infected cord blood mononuclear cell cultures is not inhibited. However, by addition of immunomodulators, polysaccharide K (PSK) or truncated thioredoxin (Trx80) that activate monocytes, EBV-specific T-cell response could be generated in such cultures. Presently, we demonstrate that leukotriene B4 (LTB4) is involved in the effect of the immunomodulators. LTB4 was detected in the medium, and T-cell activation was compromised by addition of leukotriene biosynthesis inhibitors. Moreover, we found that LTB4 added to infected cultures, which did not receive the immunomodulators, induced functional activation of the T cells. LTB4 activated the monocytes and acted directly on the T cells. In consequence, addition of LTB4 inhibited the EBV-induced proliferation of B lymphocytes. Specific cytotoxicity could be generated by restimulation of the T cells. The experiments showed successive stages of T-cell activation in acquisition of their immunologic effector function. This is orchestrated by complex cellular interactions, and autocrine loops mediated by soluble factors—here interferon (IFN)-γ, interleukin (IL)-15, IL-12, and LTB4. Importantly, the results indicate that endogenous LTB4 can induce T-cell activation that inhibits the EBV-induced proliferation of B lymphocytes.
Introduction
Leukotriene B4 (LTB4) is a potent, fast-acting lipid mediator with a wide range of biologic effects.1 It is produced mainly by granulocytes, monocytes, and B lymphocytes1,–3 ; LTB4 binds to BLT1, a high-affinity cell membrane receptor.4 Several studies have been directed to the function of LTB4 and indicated its regulatory role in both natural and adaptive specific and nonspecific immune responses.1,5,–7
Epstein-Barr virus (EBV) is carried by the majority of human adults. Primary infection of adolescents occurs either without characteristic symptoms or leads to a benign disease of variable severity, infectious mononucleosis.8 Regardless of the clinical picture, EBV infection is followed by a lifelong carrier state that can be easily revealed by EBV-specific humoral and cellular immunity. B lymphocyte is the main target of EBV. In vitro–infected B lymphocytes are induced to proliferate.9,10 However the virus carrier state is harmless due to the finely tuned immune response against virally encoded proteins that leads to the recognition and elimination of EBV-transformed B cells. Several of the EBV-encoded proteins that function in transformation are immunogenic. The costimulatory proteins expressed by the transformed cells secure their recognition by immune effector cells.11,–13 Survival and proliferation of EBV-infected B cells is controlled by innate immunity, IFN-γ, and natural killer (NK) cells, and also by EBV-specific cytotoxic T lymphocytes (CTLs).14 The details of these immune responses have been extensively studied in the in vitro B-cell transformation system. Specific immune memory can be detected by inhibition of B-cell transformation.13,–15
EBV-specific cellular immunity is not transferred from mother to child. Therefore B lymphocytes in cord blood mononuclear cell (CBMC) cultures infected in vitro are efficiently transformed. We have established an experimental system that permits the study of relevant cellular interactions leading to EBV-specific cell-mediated immune responses without the need for substantial cell separation steps.16,17
In this system of in vitro–infected CBMC cultures, we found that addition of the immunomodulators (PSK and Trx80) can induce inhibition of EBV-transformed B-cell growth.17 We showed that the T cells were activated in such cultures by the EBV-infected B cells. For proper function, however, the T and NK cells required the assistance of activated monocytes. The monocytes were activated by the added PSK or Trx80, and in the presence of activated T cells they produced IL-15 and IL-12, respectively. In their turn, the activated T cells could respond to the lymphokines. They became functionally active and inhibited the EBV-induced B-lymphocyte proliferation.
We show now that LTB4 can act as immunomodulator in EBV-infected cord blood lymphocyte cultures. It activates the monocytes and also the T cells that, by the encounter of EBV-infected B lymphocytes, enter in a responsive state. The activated T cells then compromise the EBV-induced B-lymphocyte proliferation.
Methods
Reagents and antibodies
Ficoll-paque was purchased from Pharmacia Biotec (Uppsala, Sweden); PSK, from Kureha Chemical (Tokyo, Japan). Purified recombinant human Trx80 was the kind gift of A. Holmgren (Department of Medical Biochemistry and Biophysics, Karolinska Institutet).18 Synthetic LTB4, LTC4, LTD4, and 5(S),12(S)-DiHETE were obtained from Biomol (Plymouth, PA); BWA4C was a kind gift from L. Garland (Wellcome Research Laboratories, Beckenham, United Kingdom) and MK-886, from Merck Research Laboratories (Rahway, NJ). Anti-CD19 Ab-conjugated beads (Dynabeads M-450) and Dynabeads goat anti–mouse IgG were from Dynal (Oslo, Norway). 3H-thymidine was from Amersham Pharmacia Biotech (Uppsala, Sweden). Monoclonal mouse anti–human CD3; mouse monoclonal fluorescein isothiocyanate (FITC)–conjugated anti-CD3 and anti-CD19; and phycoerythrin (PE)–conjugated anti-CD4 and anti-CD8 antibodies were from Dako (Glostrup, Denmark). PE-Cy5–conjugated anti-CD56; peridinin chlorophyll protein (PerCP)–conjugated anti-CD3; and neutralization mAb of antihuman IL12 (p40/p70) (C8.6) were from BD PharMingen (San Diego, CA). PE-conjugated anti-CD69 was from Immuno Tools (Friesoythe, Germany). Anti-BLT1 antibody (7B1 FITC) was raised in-house.19 Monoclonal mouse anti-CCR5 was from Diaclone (Besançon, France). FITC-conjugated anti-CXCR4; monoclonal mouse anti-CXCR3; IL15, IL12, and IFN-γ enzyme-linked immunosorbent assay (ELISA) antibodies; IL18 ELISA kit; and neutralization mAb of mouse anti–human IL15 (MAB647) were from R&D Systems (Minneapolis, MN). Neutralization mAb of mouse anti–human IL18 (125–2H) was from MBL (Nagoya, Japan). The mAb anti–HLA class I (mAb W6/32) or II (mAb CR3/43) was from Dako. Affinity-purified rabbit anti-SAP antiserum was the kind gift of J. Sümegi (Cincinnati Medical Center, OH). HRP-conjugated donkey anti–rabbit Ig antibody; ECL+Plus detection reagent; and Hyperfilm-ECL film were from Amersham (Arlington Heights, IL).
Preparation and culture of cells
Cord blood samples were obtained from the Department of Obstetrics and Gynecology, Karolinska University Hospital. The study was approved by the Ethics Committee of Karolinska Institutet and the Karolinska Hospital.
Mononuclear cells were isolated from heparinized cord blood by Ficoll-paque density centrifugation.
For isolation of B cells, cord blood mononuclear cells (CBMCs) were incubated with anti-CD19 Ab-conjugated beads. The attached cells were recovered from the beads using DETACHaBEADs (Dynal).
Monocytes were isolated on gelatin/plasma-coated dishes as described earlier.17
For T-cell depletion, CBMCs were incubated with mouse antihuman CD3 mAb, followed by goat anti–mouse IgG-conjugated beads. The fluent cell suspension was collected.
The cells were cultured at a density of 106/mL in RPMI 1640 supplemented with 10% FCS, 2mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin at 37°C, 5% CO2.
EBV infection
CBMCs or separated B cells were exposed to B95-8 virus containing supernatant for 1.5 hours in a humidified 37°C, 5% CO2 incubator. The cells were washed and resuspended in complete RPMI at 106/mL concentration.
Proliferation of EBV-infected B cells
Proliferation of EBV-infected B cells was analyzed on the 6th day of culture by 3H-thymidine incorporation assay. The infected CBMC cells were seeded in 2 × 105 per well/200 μL, in 96-well plates. Each sample was represented by triplicate cultures. On the 6th day, 1 μCi (0.037 MBq) 3H-thymidine was added and 14 hours later the cells were harvested onto glass fiber filters. Radioactivity was measured in a liquid scintillation counter. At this time, the results reflect the number of B cells induced to proliferate by EBV.16,20
EBV-infected B cells were seeded in 105 per well/200 μL in 96-well plates. Each sample was represented by triplicates. 3H-thymidine (1 μCi [0.037 MBq]) was present during the final 14 hours.
Flow cytometric analysis
The subset-specific cell-surface markers CD3, CD4, CD8, CD14, CD19, CD56; the lymphocyte activation marker CD69; LTB4 receptor BLT1; and the chemokine receptor CXCR4 were detected with FITC-, PE-, PE-Cy5–, or PerCP-conjugated mouse antihuman monoclonal antibodies. CXCR3 and CCR5 were detected by indirect immunofluorescence. The cells were washed in cold PBS/1% FCS and then exposed to the relevant antibodies, washed, and thereafter incubated with the FITC-conjugated secondary antibody. All incubations were performed for 30 minutes at 4°C. Cells were then washed once with PBS/1% FCS and resuspended in 500 μL PBS. Ten thousand events were collected on a FACScan flow cytometer, and the results were analyzed using Cell Quest software (Becton Dickinson, Lincoln Park, NJ).
Detection of leukotriene B4
The cell populations were cultured in 24-well plates at a density of 106/mL. Supernatants of 24-hour-old cultures were collected and diluted in EIA-buffer, then added to an antibody-coated 96-well plate. The enzyme immunoassay (EIA) was performed according to manufacturer's protocol. The detection limit was 4 pg/mL.
Detection of cytokines in the culture medium
The cell populations were cultured in 24-well plates. Supernatants of the cultures were collected 3 days later, and analyzed in duplicate wells using sandwich ELISA for IL-15, IL-12, IL-18, and IFN-γ according to the manufacturer's instructions. The sandwich ELISA for IL-12 detected the IL-12 p70 heterodimer. The detection limit of the IL-15, IL-12, and IL-18 was 10 pg/mL; that of IFN-γ was 30 pg/mL.
SAP detected by immunoblotting
The cells were lysed directly in SDS gel-loading buffer. Aliquots corresponding to 1.5 × 105 cells were electrophoresed on 12% SDS–polyacrylamide gel electrophoresis (PAGE) gel and transferred to PVDF membranes at 75 V for 2 hours. After blocking for 1 hour with 5% nonfat dried milk in PBS-Tween 20, the membranes were incubated with the anti-SAP antibody overnight at 4°C. The blots were then incubated with HRP-conjugated donkey anti–rabbit Ig antibody and developed with ECL+Plus detection reagent. Hyperfilm-ECL film was exposed to the developed membranes for visualization.
Cytotoxic function generated in the cultures
EBV-infected cultures were stimulated on the 7th and 14th days, at 10:1 ratio, with irradiated (50 Gy) autologous EBV-infected B cells. At each stimulation, half of the medium was replaced with fresh medium. From the ninth day, 20 U/mL IL-2 was added every third day. On the 20th day, an aliquot of cells from cultures was used for analysis of cell composition by flow cytometry. B cells were depleted from the remaining cells using anti-CD19 Ab-conjugated beads and the residual cells were used as effectors in 51Cr-release cytotoxicity assay. Autologous LCLs (autologous EBV-infected B cells were cultured for 3 weeks), activated autologous B cells (autologous B cells were cultured with irradiated [50 Gy] CD40L-L cells and IL-4 [5 ng/mL] for 7 days), allogeneic LCLs, and K562 were used as targets.
Autologous target cells were used also after 30-minute incubation with the mAb W6/32 (anti–HLA class I) or with the mAb CR3/43 (anti–HLA class II).
The specific lysis was calculated as follows: specific release = [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100.
Statistical analysis
Statistical significance was determined using paired 2-tailed Student t test. Significance is presented for individual experiments (*P < .05, **P < .01).
Results
Involvement of LTB4 in T- and NK-cell activation and B-cell growth inhibition in EBV-infected cord blood cell cultures
We have shown earlier that T and NK cells were activated upon addition of the immunomodulators PSK or Trx80 to EBV-infected CBMC cultures and that they could inhibit B-cell proliferation.16,17 Activation of the T and NK cells was detected in the cell mixture by an increase of SAP protein seen in immunoblots. The SAP protein of the cell population was contributed by T and NK cells, and the level of SAP reflected their activation status. The blots were performed with lysates prepared from a fixed number of cells. Unless inhibited, the B lymphocytes are induced to proliferate in the infected cultures and they do not express SAP. Thus, the relative increase of B cells lowers the SAP content in the samples representing a fixed number of cells.
SAP expression.
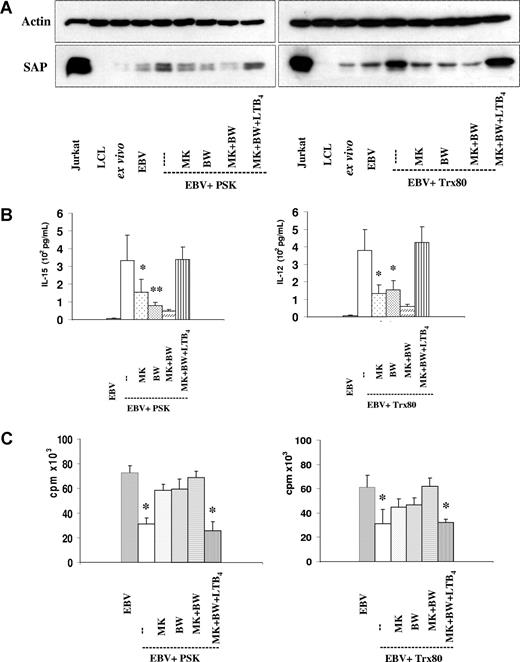
In accordance with our earlier results, the samples of the EBV-infected cultures containing PSK or Trx80 showed an increase of the SAP protein (Figure 1A).17 Lysates prepared from LCL (lymphoblastoid cell line) were included as controls. The lack of SAP band in the LCL samples confirms that EBV-infected B cells do not express SAP.
The involvement of LTB4 in T- and NK-cell activation in the EBV-infected PSK- and Trx80-containing cultures. (A) SAP expression. Immunoblot of lysates corresponding to 1.5 × 105 cells, harvested on the 6th day of culture. EBV-infected CBMCs were cultured with PSK (25 μg/mL) or Trx80 (100 nM). MK886 (1 μM), BWA4C (100 nM), and LTB4 (100 nM) were added as indicated. Jurkat cells and LCLs were used as positive and negative controls, respectively. The results show 1 representative of 3 experiments. (B) IL-15 and IL-12 in the supernatant of 3-day-old cultures were tested by ELISA. The results represent the means plus or minus SD of 3 independent experiments. *P < .05 and **P < .01 compared with PSK- or Trx80-treated cultures. (C) Inhibition of B-cell proliferation induced by EBV. 3H-thymidine incorporation was tested on the 6th day of culture. 3H-thymidine was present during the final 14 hours. The cultures were initiated with 2 × 105 cells. Means plus or minus SD of 3 independent experiments are shown. * indicates Pless than .05 compared with untreated cultures.
The involvement of LTB4 in T- and NK-cell activation in the EBV-infected PSK- and Trx80-containing cultures. (A) SAP expression. Immunoblot of lysates corresponding to 1.5 × 105 cells, harvested on the 6th day of culture. EBV-infected CBMCs were cultured with PSK (25 μg/mL) or Trx80 (100 nM). MK886 (1 μM), BWA4C (100 nM), and LTB4 (100 nM) were added as indicated. Jurkat cells and LCLs were used as positive and negative controls, respectively. The results show 1 representative of 3 experiments. (B) IL-15 and IL-12 in the supernatant of 3-day-old cultures were tested by ELISA. The results represent the means plus or minus SD of 3 independent experiments. *P < .05 and **P < .01 compared with PSK- or Trx80-treated cultures. (C) Inhibition of B-cell proliferation induced by EBV. 3H-thymidine incorporation was tested on the 6th day of culture. 3H-thymidine was present during the final 14 hours. The cultures were initiated with 2 × 105 cells. Means plus or minus SD of 3 independent experiments are shown. * indicates Pless than .05 compared with untreated cultures.
The SAP level increase induced by the immunomodulators was prevented by the leukotriene biosynthesis inhibitors MK886 or BWA4C. Addition of LTB4 (100 nM) to such cultures restored the intensity of the SAP bands. Inhibition of the endogenous LTB4 production could thus be counteracted by introduction of the leukotriene from the outside, confirming the contribution of LTB4 to the activation of T and NK cells.
Production of IL-15 and IL-12.
In accordance with our earlier findings, the supernatants of the PSK- and Trx80-containing cultures contained IL-15 and IL-12, respectively. Addition of the leukotriene biosynthesis inhibitors MK886 or BWA4C reduced the production of these cytokines (Figure 1B). MK886 and BWA4C reduced the IL-15 content from 334 (± 142) pg/mL to 156 (± 72) pg/mL and to 79 (± 16) pg/mL (inhibition: 54% and 74%), respectively. The corresponding values for IL-12 in the Trx80-containing cultures were from 378 (± 120) pg/mL to 132 (± 51) pg/mL and 155 (± 50) pg/mL (inhibition: 66% and 58%). Cytokine production was lower in presence of both inhibitors. Similarly to the re-establishment of SAP expression in the cells, the cytokine levels were restored to the control values when LTB4 was added to the cultures.
B-cell proliferation.
EBV-induced B-cell proliferation was inhibited in the cultures containing the immunomodulators. The inhibition was shown by the composition of the cell populations and by thymidine incorporation measured on day 6 of culture (Table 1; Figure 1C).16 The mean inhibition in 3 experiments was 57% (± 10%) and 50% (± 11%) for PSK and Trx80, respectively. The leukotriene biosynthesis inhibitors MK886 or BWA4C reduced the growth inhibitory effects of PSK to 21% (± 5%) and 19% (± 7%), respectively, and to 27% (± 4%) and 23% (± 6%), respectively, in Trx80-containing cultures. In the presence of both inhibitors, thymidine incorporation was similar to that of the control; thus the inhibition was completely eliminated.
Composition of the cell populations and expression of CD69, BLT1, CXCR4, CXCR3, and CCR5 in ex vivo CBMCs and 6-day-old cultures
| Cells . | Ex vivo . | CBMCs . | CBMCs + EBV . | CBMCs + EBV + PSK . | CBMCs + EBV + Trx80 . |
|---|---|---|---|---|---|
| CD3 T | 66 ± 9 | 59 ± 11 | 44 ± 7 | 61 ± 6 | 59 ± 6 |
| CD56 NK | 12 ± 7 | 13 ± 5 | 15 ± 2 | 19 ± 4 | 13 ± 3 |
| CD19 B | 14 ± 5 | 15 ± 4 | 41 ± 6 | 19 ± 7 | 22 ± 4 |
| CD69+CD3+/CD3 | 3 ± 2 | 8 ± 4 | 26 ± 5 | 71 ± 18 | 68 ± 16 |
| CD69+CD56+/CD56 | 8 ± 2 | 16 ± 3 | 44 ± 10 | 69 ± 8 | 71 ± 14 |
| BLT1+CD3+/CD3 | 0 | 1 | 33 ± 8 | 37 ± 6 | 41 ± 10 |
| BLT1+CD56+/CD56 | 0 | 0 | 0 | 0 | 0 |
| BLT1+CD19/CD19 | 51 ± 9 | 11 ± 4 | 7 ± 2 | 8 ± 6 | 9 ± 4 |
| CXCR4+CD3+/CD3 | 86 ± 6 | 16 ± 9 | 5 ± 2 | 3 ± 1 | 3 ± 3 |
| CXCR3+CD3+/CD3 | 3 ± 1 | 2 ± 2 | 51 ± 11 | 57 ± 14 | 56 ± 6 |
| CCR5+CD3+/CD3 | 4 ± 3 | 3 ± 2 | 54 ± 5 | 56 ± 7 | 61 ± 11 |
| Cells . | Ex vivo . | CBMCs . | CBMCs + EBV . | CBMCs + EBV + PSK . | CBMCs + EBV + Trx80 . |
|---|---|---|---|---|---|
| CD3 T | 66 ± 9 | 59 ± 11 | 44 ± 7 | 61 ± 6 | 59 ± 6 |
| CD56 NK | 12 ± 7 | 13 ± 5 | 15 ± 2 | 19 ± 4 | 13 ± 3 |
| CD19 B | 14 ± 5 | 15 ± 4 | 41 ± 6 | 19 ± 7 | 22 ± 4 |
| CD69+CD3+/CD3 | 3 ± 2 | 8 ± 4 | 26 ± 5 | 71 ± 18 | 68 ± 16 |
| CD69+CD56+/CD56 | 8 ± 2 | 16 ± 3 | 44 ± 10 | 69 ± 8 | 71 ± 14 |
| BLT1+CD3+/CD3 | 0 | 1 | 33 ± 8 | 37 ± 6 | 41 ± 10 |
| BLT1+CD56+/CD56 | 0 | 0 | 0 | 0 | 0 |
| BLT1+CD19/CD19 | 51 ± 9 | 11 ± 4 | 7 ± 2 | 8 ± 6 | 9 ± 4 |
| CXCR4+CD3+/CD3 | 86 ± 6 | 16 ± 9 | 5 ± 2 | 3 ± 1 | 3 ± 3 |
| CXCR3+CD3+/CD3 | 3 ± 1 | 2 ± 2 | 51 ± 11 | 57 ± 14 | 56 ± 6 |
| CCR5+CD3+/CD3 | 4 ± 3 | 3 ± 2 | 54 ± 5 | 56 ± 7 | 61 ± 11 |
Uninfected CBMCs, EBV-infected CBMCs without and with PSK (25 μg/mL), and EBV-infected CBMCs with Trx80 (100 nM) were cultured for 6 days. Expression of CD3, CD56, CD19, CD69, BLT1, CXCR4, CXCR3, and CCR5 was registered by flow cytometry and double staining. Results represent means plus or minus SD of 4 experiments.
These results confirm our earlier findings that activation of T and NK cells in the EBV-infected CBMC cultures can be detected by SAP expression, by the production of cytokines, and by the inhibition of B-cell proliferation. The new aspect that emerges from the present experiments is the essential role of LTB4 in the activation of the effector cells.
LTB4 and IFN-γ production in the EBV-infected cultures
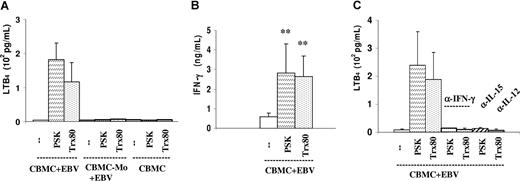
In line with the reduction of the immunomodulator-mediated effects by LTB4 synthesis inhibitors, LTB4 was detected in the EBV-infected cultures that received the immunomodulators and it required the presence of monocytes (Figure 2A). In 5 experiments, the mean concentrations were 182 (± 108) pg/mL (range: 63-353 pg/mL) in the PSK-containing cultures and 117 (± 105) pg/mL (range: 13-329 pg/mL) in the Trx80-containing cultures. This is consistent with the identification of monocytes as the main LTB4-producing cells under our culture conditions. Granulocytes are strong LTB4 producers, but they were not present in our experiments. The results showed that activation of monocytes by the immunomodulators was essential for LTB4 production, as shown earlier for the production of IL-15 and IL-12.
Production of LTB4 and IFN-γ in the EBV-infected PSK- and Trx80-containing cultures. (A) LTB4 in the supernatant of 24-hour-old cultures was detected by EIA. Cultures were initiated with 106/mL EBV-infected CBMCs, total and monocyte (Mo) depleted, and uninfected CBMCs. The results represent the means plus or minus SEM of 5 independent experiments. (B) IFN-γ in the supernatant of 3-day-old cultures was tested by ELISA. The results represent the means plus or minus SD of 4 independent experiments. **P < .01 compared with untreated cultures. (C) LTB4 production in cultures containing the indicated antibodies added at the initiation of cultures. The results represent the means plus or minus SEM of 3 independent experiments.
Production of LTB4 and IFN-γ in the EBV-infected PSK- and Trx80-containing cultures. (A) LTB4 in the supernatant of 24-hour-old cultures was detected by EIA. Cultures were initiated with 106/mL EBV-infected CBMCs, total and monocyte (Mo) depleted, and uninfected CBMCs. The results represent the means plus or minus SEM of 5 independent experiments. (B) IFN-γ in the supernatant of 3-day-old cultures was tested by ELISA. The results represent the means plus or minus SD of 4 independent experiments. **P < .01 compared with untreated cultures. (C) LTB4 production in cultures containing the indicated antibodies added at the initiation of cultures. The results represent the means plus or minus SEM of 3 independent experiments.
IFN-γ was produced in the infected cell population and its level increased in the cultures containing the immunomodulators (Figure 2B). Figure 2C shows that LTB4 production was considerably reduced when any one of the cytokines IFN-γ, IL-15, or IL-12 was neutralized by specific antibodies.
These results show the activation circuit between mono-cytes, NK cells, and T cells. The latter cells assist in the activation of monocytes for LTB4 production with provision of IFN-γ, but they require IL-15 or IL-12 produced by the monocytes.
Expression of the LTB4 and chemokine receptors in the cell subsets of EBV-infected cultures
The cellular composition of the ex vivo cells and cultures at day 6 is shown in Table 1. In the PSK- or Trx80-containing cultures, the enrichment of B cells was lower (19% or 22%, respectively, vs 41% in the absence of the immunomodulators), reflecting the inhibition of EBV-induced B-lymphocyte proliferation.
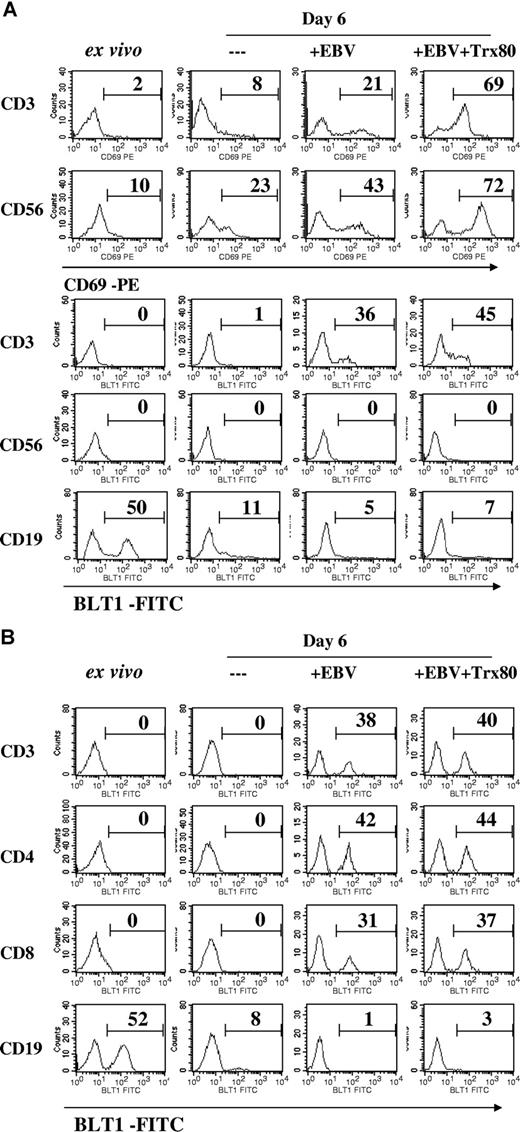
T and NK cells were activated in the infected cultures. A low proportion of T (CD3+) and NK (CD56+) cells expressed CD69 (activation marker) in the ex vivo samples. Their frequency increased in the virus infected, and it increased further in the immunomodulator-containing cultures. Although the frequencies of positive cells were lower, expression of BLT1 on the T cells showed a similar increase in these 2 cultures, including both subsets CD4 and CD8 (Figure 3A,B).
Expression of CD69 and BLT1 (LTB4 receptor) on T and NK cells and BLT1 on B cells. Expression of CD69 and BLT1 was registered by flow cytometry, using double staining. The numbers denote the percentage of positive cells in the indicated cell categories. Uninfected CBMCs and EBV-infected CBMCs were cultured for 6 days. (A) CD69 expression on CD3+ T cells and CD56+ NK cells. BLT1 expression on CD3+ T cells, CD56+ NK cells, and CD19+ B cells. The results show 1 representative of 4 independent experiments. The cell composition of the cultures is given in Table 1. (B) BLT1 expression on CD3+, CD4+, and CD8+ T cells.
Expression of CD69 and BLT1 (LTB4 receptor) on T and NK cells and BLT1 on B cells. Expression of CD69 and BLT1 was registered by flow cytometry, using double staining. The numbers denote the percentage of positive cells in the indicated cell categories. Uninfected CBMCs and EBV-infected CBMCs were cultured for 6 days. (A) CD69 expression on CD3+ T cells and CD56+ NK cells. BLT1 expression on CD3+ T cells, CD56+ NK cells, and CD19+ B cells. The results show 1 representative of 4 independent experiments. The cell composition of the cultures is given in Table 1. (B) BLT1 expression on CD3+, CD4+, and CD8+ T cells.
In accordance with earlier reports, ex vivo T cells did not express BLT1.4,21 Interestingly, the activated NK cells did not express BLT1.
BLT1 is known to be expressed on B lymphocytes.21 We detected 50% BLT1-positive cells in the CD19 subset of the ex vivo population, while in the cultures this subset did not express the receptor.
The majority of ex vivo T cells expressed the chemokine receptor CXCR4; it was down-regulated in the infected cultures (Table 1). Rare ex vivo T cells expressed CXCR3 and CCR5; they increased to 51% and 54%, respectively, in the infected cultures.
LTB4 mediated potentiation of T-cell activation in the EBV-infected cultures
The production of LTB4 in the immunomodulator-containing cultures and the restoration of lymphocyte activation by addition of exogenous LTB4 following inhibition of its endogenous synthesis prompted us to test the effect of LTB4 added to the cultures, in the absence of PSK and Trx80.
SAP expression.
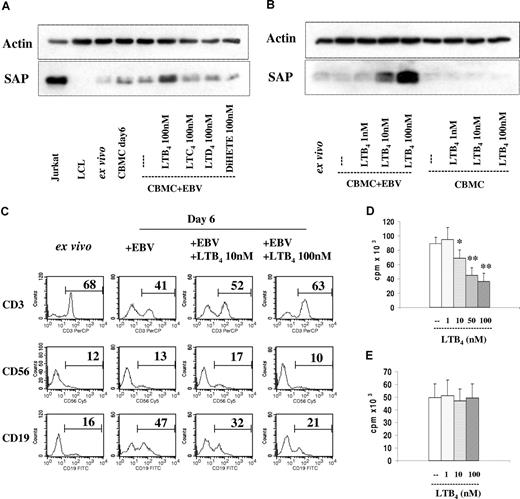
The experiment shown in Figure 4A was designed in the same way as the previous ones, except that the immunomodulators were omitted and LTB4 was added to the EBV-infected cultures. The results in Figure 4A,B show a dose-dependent increase of SAP expression. Specificity controls included addition of LTC4, LTD4, and 5(S),12(S)-DiHETE, which were inactive at a final concentration of 100 nM. LTB4 did not increase SAP expression in the uninfected cultures. Thus, LTB4 has a similar effect as the immunomodulators PSK and Trx80.
Added LTB4 induced SAP expression and inhibition of B-cell proliferation in the EBV-infected cord blood cell cultures. (A) SAP expression. Immunoblot as in Figure 1A. EBV-infected CBMCs were cultured for 6 days with LTB4, LTC4, LTD4, or 5(S),12(S)-DiHETE at 100 nM concentration. The results show 1 of 3 experiments. (B) Dose response of the added LTB4; 1 representative experiment of 4 performed. (C) Cell composition in EBV-infected CBMCs cultured with LTB4 for 6 days. The numbers denote the percentage of CD3+, CD56+, and CD19+ cells. (D) EBV-induced B-cell proliferation in the LTB4-containing cultures. 3H-thymidine incorporation was measured on the 6th day of culture, 3H-thymidine was present during the final 14 hours. The cultures were initiated with 2 × 105 cells. The results represent the means plus or minus SD of 4 independent experiments. * indicates P < .05, and **P < .01 compared with untreated cultures. (E) Isolated B cells were infected with EBV and cultured with LTB4. The cultures were initiated with 105 cells. Thymidine incorporation was measured on the 6th day of culture. The results represent the means plus or minus SD of 4 independent experiments.
Added LTB4 induced SAP expression and inhibition of B-cell proliferation in the EBV-infected cord blood cell cultures. (A) SAP expression. Immunoblot as in Figure 1A. EBV-infected CBMCs were cultured for 6 days with LTB4, LTC4, LTD4, or 5(S),12(S)-DiHETE at 100 nM concentration. The results show 1 of 3 experiments. (B) Dose response of the added LTB4; 1 representative experiment of 4 performed. (C) Cell composition in EBV-infected CBMCs cultured with LTB4 for 6 days. The numbers denote the percentage of CD3+, CD56+, and CD19+ cells. (D) EBV-induced B-cell proliferation in the LTB4-containing cultures. 3H-thymidine incorporation was measured on the 6th day of culture, 3H-thymidine was present during the final 14 hours. The cultures were initiated with 2 × 105 cells. The results represent the means plus or minus SD of 4 independent experiments. * indicates P < .05, and **P < .01 compared with untreated cultures. (E) Isolated B cells were infected with EBV and cultured with LTB4. The cultures were initiated with 105 cells. Thymidine incorporation was measured on the 6th day of culture. The results represent the means plus or minus SD of 4 independent experiments.
B-cell proliferation.
The B cells increased and the T cells decreased proportionally in the EBV-infected cultures. In the presence of LTB4, however, the B-cell enrichment was impaired (Figure 4C). The proportion of NK cells remained unchanged.
Proliferation of the B lymphocytes was in line with the composition of the cell populations in that the values were lower in the cultures containing LTB4 (Figure 4D). Inhibition of B-cell proliferation mediated by 10 nM, 50 nM, and 100 nM LTB4 was 23% (± 9%), 50% (± 7%), and 61% (± 12%), respectively.
The growth inhibition in the cultures could be attributed to the activated effector cells, because LTB4 did not influence the proliferation of separated B lymphocytes infected by the virus (Figure 4E).
Taken together, these data suggest that addition of LTB4 activated the T cells in the cultures and they inhibited EBV-induced B-cell proliferation.
Lymphokine production.
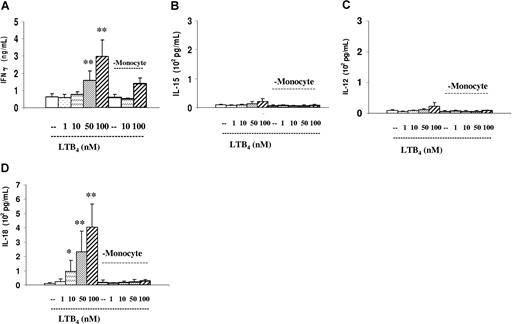
Similarly to the PSK- and Trx80-treated cultures, lymphokine production was induced by LTB4. It elevated the production of IFN-γ. Monocyte-depleted cultures contained less IFN-γ (Figure 5A). Interestingly, the assortment of T- and NK-cell–activating lymphokines IL-15, IL-12, and IL-18 differed from that induced by PSK and Trx80, in that IL-18 dominated (Figure 5B-D). These also required the presence of monocytes.
Cytokine production in the LTB4-containing EBV-infected cultures. IFN-γ, IL-15, IL-12, and IL-18 in the supernatants of 3-day-old cultures were tested by ELISA. Dose response of the added LTB4 in total and in monocyte-depleted cultures. (A) IFN-γ. (B) IL-15. (C) IL-12. (D) IL-18. The results represent means plus or minus SD of 4 independent experiments. * indicates P < .05, and **P < .01 compared with untreated cultures.
Cytokine production in the LTB4-containing EBV-infected cultures. IFN-γ, IL-15, IL-12, and IL-18 in the supernatants of 3-day-old cultures were tested by ELISA. Dose response of the added LTB4 in total and in monocyte-depleted cultures. (A) IFN-γ. (B) IL-15. (C) IL-12. (D) IL-18. The results represent means plus or minus SD of 4 independent experiments. * indicates P < .05, and **P < .01 compared with untreated cultures.
LTB4 added to the culture stimulated monocytes and activated T cells
A fraction of T cells expressed BLT1 in the infected cultures (Figure 3). Therefore, the added LTB4 could directly activate the T cells, and induced elevation of SAP expression even in the absence of monocytes (Figure 6A). Comparison of the dose responses indicated, however, that the SAP-inducing capacity of LTB4 was approximately 10-fold in the presence of monocytes. This effect was, in part, due to the autocrine effect of LTB4 for monocytes, which has been reported earlier.22
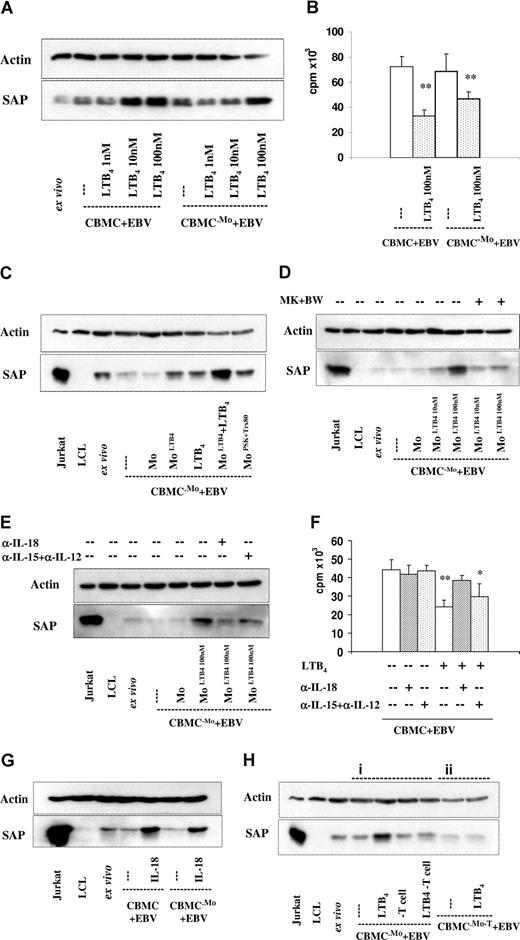
Contribution of monocytes (Mo's) and T lymphocytes to the SAP expression induced by LTB4 in the EBV-infected cultures. SAP expression was tested on the 6th day in all cultures. (A) EBV-infected CBMCs, total, and monocyte (Mo)–depleted populations were cultured with 1 nM, 10 nM, and 100 nM LTB4. One representative experiment of 4 performed. (B) B-cell proliferation. Total and monocyte (Mo)–depleted populations infected with EBV, and cultured without or with 100 nM LTB4. 3H-thymidine incorporation was measured on the 6th day of culture. The results represent the means plus or minus SD of 3 independent experiments. ** indicates P < .01 compared with untreated cultures. (C-E) Monocyte-depleted populations were infected with EBV. The separated monocytes were exposed to LTB4 for 20 hours, and thereafter reintroduced to the depleted population. The cultures were kept for further 5 days. (C) SAP expression. The separated monocytes were treated with LTB4 (100 nM), PSK (25 μg/mL), and Trx80 (100 nM). LTB4 was added to the cultures as indicated. The results show 1 representative of 3 experiments. (D) LTB4 (10 nM or 100 nM)–treated monocytes were reintroduced to the cultures. MK886 (1 μM) and BWA4C (100 nM) were added as indicated. (E) LTB4 (100 nM)–treated monocytes were reintroduced to the cultures. Anti–IL-18 (2 μg/mL), anti–IL-15 (10 μg/mL), and anti–IL-12 (10 μg/mL) reagents were added as indicated. (F) Cell proliferation. EBV-infected CBMC cultures without and with LTB4. To parallel samples, antibodies anti–IL-18 (2 μg/mL), anti–IL-15 (10 μg/mL), and anti–IL-12 (10 μg/mL) were added as indicated. 3H-thymidine incorporation was measured on the 6th day of culture. The results represent the means plus or minus SD of 3 independent experiments. * indicates P < .05, and **P < .01 compared with untreated EBV-infected cultures. (G) SAP expression. EBV-infected CBMCs, total, and monocyte (Mo)–depleted populations were cultured with 10 ng/mL IL-18. The results show 1 representative of 3 experiments. (H) SAP expression in monocyte and T-cell–depleted cultures. (i) Monocyte depleted cultures were infected with EBV and cultured without or with LTB4 (100 nM). On the sixth day of culture the cells were collected and the T cells were depleted before preparation of the lysates, which were then tested for SAP expression. (ii) Cell population depleted of Mo and T cells were infected with EBV and cultured without or with LTB4. The results show 1 representative of 3 experiments.
Contribution of monocytes (Mo's) and T lymphocytes to the SAP expression induced by LTB4 in the EBV-infected cultures. SAP expression was tested on the 6th day in all cultures. (A) EBV-infected CBMCs, total, and monocyte (Mo)–depleted populations were cultured with 1 nM, 10 nM, and 100 nM LTB4. One representative experiment of 4 performed. (B) B-cell proliferation. Total and monocyte (Mo)–depleted populations infected with EBV, and cultured without or with 100 nM LTB4. 3H-thymidine incorporation was measured on the 6th day of culture. The results represent the means plus or minus SD of 3 independent experiments. ** indicates P < .01 compared with untreated cultures. (C-E) Monocyte-depleted populations were infected with EBV. The separated monocytes were exposed to LTB4 for 20 hours, and thereafter reintroduced to the depleted population. The cultures were kept for further 5 days. (C) SAP expression. The separated monocytes were treated with LTB4 (100 nM), PSK (25 μg/mL), and Trx80 (100 nM). LTB4 was added to the cultures as indicated. The results show 1 representative of 3 experiments. (D) LTB4 (10 nM or 100 nM)–treated monocytes were reintroduced to the cultures. MK886 (1 μM) and BWA4C (100 nM) were added as indicated. (E) LTB4 (100 nM)–treated monocytes were reintroduced to the cultures. Anti–IL-18 (2 μg/mL), anti–IL-15 (10 μg/mL), and anti–IL-12 (10 μg/mL) reagents were added as indicated. (F) Cell proliferation. EBV-infected CBMC cultures without and with LTB4. To parallel samples, antibodies anti–IL-18 (2 μg/mL), anti–IL-15 (10 μg/mL), and anti–IL-12 (10 μg/mL) were added as indicated. 3H-thymidine incorporation was measured on the 6th day of culture. The results represent the means plus or minus SD of 3 independent experiments. * indicates P < .05, and **P < .01 compared with untreated EBV-infected cultures. (G) SAP expression. EBV-infected CBMCs, total, and monocyte (Mo)–depleted populations were cultured with 10 ng/mL IL-18. The results show 1 representative of 3 experiments. (H) SAP expression in monocyte and T-cell–depleted cultures. (i) Monocyte depleted cultures were infected with EBV and cultured without or with LTB4 (100 nM). On the sixth day of culture the cells were collected and the T cells were depleted before preparation of the lysates, which were then tested for SAP expression. (ii) Cell population depleted of Mo and T cells were infected with EBV and cultured without or with LTB4. The results show 1 representative of 3 experiments.
Inhibition of B-lymphocyte proliferation showed the functional aspect of the LTB4 treatment in that the cell proliferation was also inhibited in this culture (Figure 6B).
Separated monocytes could be activated by LTB4. When such cells were returned to the infected, monocyte-depleted residual cell population their SAP expression increased. Added LTB4 to these cultures further increased SAP expression (Figure 6C). Inhibition of LTB4 biosynthesis with MK886 or BWA4C to these reconstituted cultures abolished the effect on SAP expression (Figure 6D).
The LTB4-induced activation of monocytes can produce the T-cell– and NK-cell–activating lymphokines IL-18, IL-15, and IL-12. These lymphokines contributed to the effect of LTB4-treated monocytes as shown by the reduction of the intensity of SAP band in the immunoblot when they were neutralized by antibodies (Figure 6E). Neutralization of the lymphokines decreased the function of the T cells, in that the inhibition of B-cell proliferation was abolished by anti–IL-18 and decreased by the mixture of anti–IL-15 and anti–IL-12 reagents (Figure 6F). This was in line with the detection of these lymphokines in the supernatants and showed that LTB4 induced relatively higher amounts of IL-18 (Figure 5).
Addition of IL-18 to the infected cultures elevated SAP expression even if the cultures did not contain monocytes (Figure 6G).
We can conclude thus that LTB4 could activate monocytes and these produced LTB4, IL-18, IL-15, and IL-12, which all could contribute to the functional activation of T cells and the lymphokines could act on NK cells.
Activated T but not NK cells respond to LTB4
We have shown earlier that SAP can be expressed by both activated T and NK cells. Next, we posed the question whether both cell types contribute to the increase of SAP. To this end, LTB4 was added to monocyte-depleted cultures and this induced SAP expression; however, the lysates prepared after T-cell removal did not show the increase of SAP band intensity, indicating that T cells were responsible for the increase of SAP in the treated culture (Figure 6Hi). Apparently, in the remaining NK cells LTB4 did not elevate SAP expression. This is in line with the absence of the LTB4 receptor on the NK cells.
When monocytes and T cells were removed prior to infection and LTB4 administration, the SAP bands in the lysates of the remaining B and NK cells did not show the increased intensity (Figure 6Hii).
In conclusion, LTB4 added to the EBV-infected CBMC culture could activate both the monocytes and the T cells. Thus, in these cultures T cells can be activated both by the added LTB4 and by the products of the activated monocytes.
Generation of EBV-specific cytotoxic cells
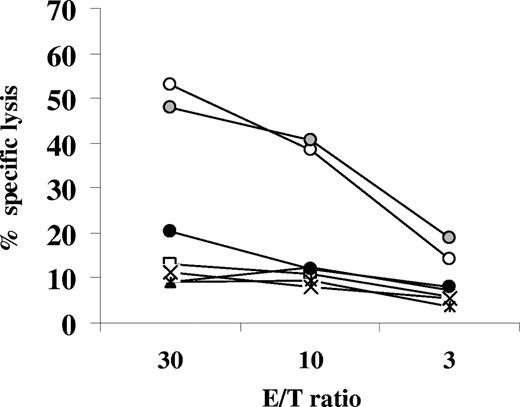
We have shown earlier that the immunomodulator-treated cultures could be restimulated by autologous EBV-infected B cells to yield specific cytotoxicity.16 Next, we tested whether this was also possible when LTB4 was used as immunomodulator. The cultures were stimulated twice with autologous EBV-infected B cells, on the seventh and 14th days. The composition of the cell population in the 20-day-old culture is shown in Table 2. CD19+ B cells dominated the culture that did not receive LTB4. In contrast, the LTB4-treated culture contained mainly CD4 T cells. After removal of B lymphocytes by anti-CD19 Ab-conjugated beads, the residual cells in the culture exhibited appreciable cytotoxicity affecting autologous EBV-infected B cells, 52% at 30:1 E/T ratio (Figure 7). In accordance with the preponderance of CD4 T cells in the culture, the lytic effect was reduced by HLA class II mAbs CR3/43 (to 20% at 30:1 E/T ratio). The effect seemed to be specific, because activated autologous B cells, K562, or allogeneic LCLs (CBM1 and LCL2996) were not damaged.17,23
Composition of the cell populations in 20-day-old cultures initiated with EBV-infected CBMCs (%)
| Cells . | CBMCs plus EBV plus LTB4* . | CBMCs plus EBV . |
|---|---|---|
| CD19 B | 11 ± 6 | 89 ± 7 |
| CD4 T | 71 ± 7 | 3 ± 3 |
| CD8 T | 6 ± 5 | 2 ± 1 |
| CD56 NK | 6 ± 3 | 4 ± 2 |
| CD3 T | 79 ± 8 | 5 ± 3 |
| Cells . | CBMCs plus EBV plus LTB4* . | CBMCs plus EBV . |
|---|---|---|
| CD19 B | 11 ± 6 | 89 ± 7 |
| CD4 T | 71 ± 7 | 3 ± 3 |
| CD8 T | 6 ± 5 | 2 ± 1 |
| CD56 NK | 6 ± 3 | 4 ± 2 |
| CD3 T | 79 ± 8 | 5 ± 3 |
The cultures were restimulated twice, on the 7th and 14th days with irradiated (50 Gy) autologous EBV-infected B cells. Half of the culture medium was replaced with fresh medium. From the 9th day, 20 U/mL IL2 was added every 3rd day. Results represent means plus or minus SD of 3 experiments.
LTB4 was added at the initiation of the culture.
Cytotoxic cells could be generated from the EBV-infected LTB4-containing cultures. EBV-infected CBMCs were cultured with LTB4 (100 nM) and restimulated twice (on seventh and 14th day of culture) with irradiated autologous EBV-infected B cells at a ratio of 10:1. Starting on the ninth day of culture, IL-2 (20 U/mL) was added every third day. The 20-day-old cultures were depleted of B cells. The cytotoxic effect of the residual cells was tested against autologous EBV-infected B cells (○), preincubated with mAb W6/32 ( ) and preincubated with the mAb CR3/43 (●); and with autologous B cells activated with CD40L and IL4 (□), K562 cells (▲), and allogeneic LCLs CBM1 (×) and LCL2996 (◇).
) and preincubated with the mAb CR3/43 (●); and with autologous B cells activated with CD40L and IL4 (□), K562 cells (▲), and allogeneic LCLs CBM1 (×) and LCL2996 (◇).
Cytotoxic cells could be generated from the EBV-infected LTB4-containing cultures. EBV-infected CBMCs were cultured with LTB4 (100 nM) and restimulated twice (on seventh and 14th day of culture) with irradiated autologous EBV-infected B cells at a ratio of 10:1. Starting on the ninth day of culture, IL-2 (20 U/mL) was added every third day. The 20-day-old cultures were depleted of B cells. The cytotoxic effect of the residual cells was tested against autologous EBV-infected B cells (○), preincubated with mAb W6/32 ( ) and preincubated with the mAb CR3/43 (●); and with autologous B cells activated with CD40L and IL4 (□), K562 cells (▲), and allogeneic LCLs CBM1 (×) and LCL2996 (◇).
) and preincubated with the mAb CR3/43 (●); and with autologous B cells activated with CD40L and IL4 (□), K562 cells (▲), and allogeneic LCLs CBM1 (×) and LCL2996 (◇).
Discussion
We have reported earlier that the immunomodulators PSK and Trx80 initiate a stimulatory circuit between various cell subsets in EBV-infected CMBC cultures. T and NK cells were recruited into the activation pathway by encounter of EBV-infected B lymphocytes and monocytes were activated by the immunomodulators.17 The present study showed that the fast-acting lipid mediator LTB4, produced by the activated monocytes, is an essential part of this circuit. LTB4 was detected in the supernatants of the cultures in which T-cell activation was induced and inhibition of LTB4 synthesis substantially reduced the T-cell activation.
Transformation of B lymphocytes by EBV is the first step that initiates the events in the virus-infected cultures by activation of T and NK cells. Our results did not reveal activation of monocytes by EBV, which was reported to occur under certain conditions.24,25
We found that LTB4 could initiate the response against the EBV-infected B lymphocytes. The events were similar to what was seen in the PSK- or Trx80-treated cultures, with the difference that monocytes were required for the effect of PSK or Trx80, but LTB4 could stimulate the T cells even in absence of monocytes. This was due to the expression of BLT1, the specific LTB4 receptor on the activated T cells. When the culture contained monocytes, however, the added LTB4 was more efficient because it also activated the monocytes and these provided additional LTB4 and other lymphokines.
The 3 monocyte-activating compounds used probably differ in their signals. PSK may activate a Toll-like receptor (Dr T. Ando, Kureha Chemical, Tokyo, Japan, written personal communication, August 2007). The receptor of Trx80 is unknown, while the specific receptor of LTB4 is well defined.4 Using these activators, the lymphokine profiles differed in these short-term experiments. PSK-treated monocytes produced predominantly IL-15, Trx80 induced IL-12, while LTB4 induced relatively higher amount of IL-18. Whether these differences are related to the differences in the activation signals remains to be seen. These lymphokines can activate T and NK cells.26,–28 It is certain that additional monocyte-derived factors contribute as well, however this experimental strategy could show that innate immunologic mechanisms operate in the mononuclear cell population and LTB4 could influence the outcome of EBV infection in vitro.29,30
The results obtained in the primary infection of mononuclear cell cultures are consistent with the immunologic analysis of acute infectious mononucleosis, such as activation of B and T lymphocytes, NK cells, and monocytes, and the presence of lymphokines (eg, IFN-γ and IL-18) in the serum.31,32
The CD4 T-cell response occurs in the in vivo primary infection as well. CD4 T cells with specificity for lytic and latent EBV-encoded proteins were detected in the initial phase of the disease and the early CD8 T cells were found to recognize lytic proteins.33 The BLT1 expression on the T cells in the 6-day-old cultures is in good accordance with the findings of Islam et al.34 In parallel with the intensity of blast transformation, BLT1 expression peaked on the sixth day on T cells stimulated by allogeneic dendritic cells.
It is noteworthy that activated, CD69+ NK cells in the infected cultures did not acquire BLT1. Consequently, NK cells were not influenced by LTB4 directly, but they could be activated by the lymphokines IL-18, IL-15, and IL-12 contributed by the activated monocytes. The difference between T and NK cells with regard to BLT1 expression is noteworthy and is one of the questions that emerges from this experiment system.
The expression of 3 chemokine receptors in the cells of the culture is in line with the finding on T cells in the blood of mononucleosis patients. The frequency of CXCR3- and CCR5-positive T cells increased in parallel with the degree of T-cell activation, while the frequency of CXCR4-positive cells decreased.34
In accordance with earlier reports, we detected BLT1-positive B lymphocytes in the ex vivo population, but not in the infected cultures.21 The loss of BLT1 on the CD19 B cells is in line with earlier functional results on B lymphocytes, in that LTB4 enhanced the effect of growth- and differentiation-inducing factors in resting, high density B cells, but only marginally or not at all in activated, low-density B cells.35,36 The lack of response by activated cells was confirmed with SAC-activated B cells. BLT1 has yet to be characterized at the time of these reports. Our findings suggest that the cells could not be activated by LTB4 because, concomitant with activation, BLT1 was down-regulated in these early experiments.
NK cells are required in the initiation of the events in the cultures when PSK and Trx80 were used as activators. Provision of IFN-γ was found to be the main function of the NK cells as shown by administration of IFN-γ that corrected their depletion (A.L., Arne Holmgren, G.K., E.K., manuscript submitted, October 2007).
In the EBV-infected mononuclear cultures derived from cord blood, T cells that lysed EBV-infected autologous B cells could be generated. The first step of this event can be ascribed to mobilization of innate immunity. Once the T lymphocytes were activated, selection could be applied for cells that recognize autologous EBV-infected B cells. The functional cells were CD4 T cells. We found that the initial activation of the effector T cells was essential, since EBV-specific cytotoxicity was obtained also without restimulation in the enlarged population (A.L., Arne Holmgren, G.K., E.K., manuscript submitted, October 2007).
Several studies have shown that LTB4 is involved in the host defense against bacterial and viral infections.37,–39 EBV infection can trigger innate immunity and LTB4 seems to be an essential contributor. EBV has unique characteristics, because its main target, the B lymphocyte, is activated by the virus, and activated B cells initiate cell interactions within the immune system.
Thus, LTB4 acted as immunoactivator in our short-term experiments. The immune parameters studied corresponded well to those observed in mononucleosis. Keeping in mind that the cord blood leukocyte population differs from the peripheral blood leukocyte population in children and adults, the model is still useful for analysis of LTB4-induced modification of the interaction between EBV-infected B cells and the innate and adaptive immune system.
LTB4 is a well-known physiological effector with multiple actions. The detailed knowledge and the availability of drugs that inhibit its synthesis are already exploited in therapy.2,40
In the in vitro experiments, the stimulatory circle between monocytes and T cells could be interrupted by the inhibition of LTB4 synthesis of the monocytes, neutralization of their lymphokine products, and neutralization of NK cell– and T cell–derived IFN-γ.
We anticipate that the experimental strategy used with the cells derived from cord blood can be used for study of the kinetics and the specificities of T cells as they emerge during the primary infection. Various aspects of the response to EBV infection have been extensively studied using mononuclear cell populations collected from seronegative and seropositive individuals. The EBV-encoded proteins that serve as targets for cytotoxic CD8 T cells selected from seropositive individuals have been identified in several studies.14,41,42 In cultures with mononuclear cells from seronegative individuals and from cord blood, the effector cells were CD4 cells.43,44 Further studies are needed for identification of the EBV-encoded proteins that serve as targets in the cord blood system.
Primary EBV infection may be silent or it may induce the symptoms of mononucleosis.8,9 The severity of the symptoms is highly variable. Sustained immunity is a regular consequence of both the silent infection and the overt disease. Therefore the development of lifelong immunity does not seem to require dramatic activation of the immune system. Conceivably, severe cases could be mollified by reducing the extensive immune activation.
LTB4 may be used to strengthen the immune response to avoid EBV-induced B-cell proliferation. Transplant patients receiving immunosuppressive treatment often have elevated EBV load and high risk of the developing EBV-positive B-cell malignancies. Administration of in vitro–educated T cells for the recognition of EBV-positive B lymphocytes showed good therapeutic effects in such cases. In experiments with cytomegalovirus (CMV)–infected mice, administration of LTB4 reduced the viral load but it did not compromise the efficiency of T-cell therapy.39
These considerations motivate detailed analysis of the role of LTB4 in the development of cellular immune responses and the possibility for its modification by LTB4-directed measures.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Department of Obstetrics and Gynecology, Karolinska University Hospital, for the provision of cord blood samples. We thank Dr Arne Holmgren (Medical Nobel Institute for Biochemistry, Karolinska Institutet) for continuing collaboration and providing the Trx80 preparations for this study. We thank Dr Peter Krammer (Deutsches Krebsforschungszentrum) for critical reading of the paper and valuable suggestions.
This work was supported by the Swedish Cancer Society and by the Cancer Research Institute (New York)/Concern Foundation (Los Angeles).
Authorship
Contribution: A.L. designed research, performed experiments, analyzed the results, and wrote the text; H.-E.C. designed research and analyzed the results; Y.M. performed the LT determinations; G.K. discussed the results and wrote the text; E.K. designed research, analyzed the results, and wrote the text.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anquan Liu, Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Nobels väg 16, 171 77 Stockholm, Sweden; e-mail: anquan.liu@ki.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal