We transduced chronic lymphocytic leukemia (CLL) cells lacking ZAP-70 with vectors encoding ZAP-70 or various mutant forms of ZAP-70 and monitored the response of transduced CLL cells to treatment with F(ab)2 anti-IgM (anti-μ). CLL cells made to express ZAP-70, a kinase-defective ZAP-70 (ZAP-70-KA369), or a ZAP-70 unable to bind c-Cbl (ZAP-YF292) experienced greater intracellular calcium flux and had greater increases in the levels of phosphorylated p72Syk, B-cell linker protein (BLNK), and phospholipase C-γ, and greater activation of the Ig accessory molecule CD79b in response to treatment with anti-μ than did mock-transfected CLL cells lacking ZAP-70. Transfection of CLL cells with vectors encoding truncated forms of ZAP-70 revealed that the SH2 domain, but not the SH1 domain, was necessary to enhance intracellular calcium flux in response to treatment with anti-μ. We conclude that ZAP-70 most likely acts as an adapter protein that facilitates B-cell receptor (BCR) signaling in CLL cells independent of its tyrosine kinase activity or its ability to interact with c-Cbl.
Introduction
ZAP-70 is a 70-kDa T-cell antigen receptor (TCR) z-chain–associated cytoplasmic protein tyrosine kinase (PTK) that initially was identified in T lymphocytes.1,2 Following ligation of the TCR, tyrosine-containing immunoreceptor tyrosine–based activation motifs (ITAMs) within the cytoplasmic tails of the CD3 molecules and the TCR zeta chain (CD247) are phosphorylated by the Src kinase, Lck.3 ZAP-70 is recruited to the phosphorylated ITAMs and becomes activated via tyrosine phosphorylation. The activated ZAP-70 in turn can induce activation of downstream signaling pathways, such as the phospholipase Cg/Ca2+ (PLC-γ) signaling pathway and the Ras/mitogen-activated protein kinase (MAPK) pathway.4 B cells generally lack ZAP-70, but instead use a related PTK, called p72Syk, to mediate signaling via the B-cell receptor (BCR) complex.5 Similar to ZAP-70, p72Syk is recruited to the phosphorylated ITAMs of the accessory molecules of the BCR complex, namely CD79a and CD79b, whereupon p72Syk becomes activated.6,7 As such, ZAP-70 and p72Syk play similar roles in antigen-receptor signaling pathways.
Previous studies demonstrated that chronic lymphocytic leukemia (CLL) B cells that express unmutated immunoglobulin heavy-chain variable region genes (IGHV) generally express ZAP-70, in contrast to normal B cells or most patients with CLL that use mutated IGHV,8,9 allowing ZAP-70 to be used as a surrogate marker for expression of unmutated IGHV.10 Patients with CLL cells that use unmutated IGHV and/or express ZAP-70 have a relatively short median time from diagnosis to initial treatment than patients with leukemia cells that use mutated IGHV. Recent studies have found that leukemia-cell expression of ZAP-70 actually might be a stronger risk factor for aggressive disease than use of unmutated IGHV genes by CLL cells.11,12
Conceivably, ZAP-70 contributes to the relatively aggressive clinical behavior of CLL cells that express unmutated IGHV genes. Indeed, the repertoire of Ig expressed in CLL is highly restricted, suggesting that leukemia cells are selected based upon their capacity to interact with some unknown antigen(s).13,14 More recent studies have found that expression of ZAP-70 in CLL is associated with enhanced Ig receptor signaling.8,15,16 This is despite the fact that most CLL cells also express p72Syk at levels similar to that of normal B cells, which do not require ZAP-70 for efficient BCR signaling. Moreover, the introduction of ZAP-70 into CLL cells that previously lacked this tyrosine kinase could enhance their capacity to undergo phosphorylation of P72Syk, B-cell linker protein (BLNK), and PLC-γ, and to experience intracellular calcium flux in response to surface IgM ligation.17 These studies demonstrated that ZAP-70 could enhance BCR signaling in CLL B cells. However, they did not establish whether this effect was dependent upon the kinase activity of ZAP-70. This would be important to resolve prior to the development of specific inhibitors of the ZAP-70 kinase for the treatment of patients with ZAP-70+ CLL.
Prior studies demonstrated that the functional catalytic domains of ZAP-70 are required for reconstituting BCR signaling in p72Syk-deficient B cells.18 However, ZAP-70+ CLL B cells generally express p72Syk at levels similar to that of ZAP-70− CLL cells or nonneoplastic B cells.8,19,20 Although p72Syk and ZAP-70 play similar roles in receptor signaling, p72Syk has approximately 100-fold greater kinase activity in vitro than does ZAP-70.21,22 As such, it is conceivable that the capacity of ZAP-70 to enhance BCR signaling is not dependent upon its kinase activity. For example, phosphorylated ZAP-70 could compete with phosphorylated p72Syk for binding to the E3 ubiquitin ligase, c-Cbl, which might then target these phosphorylated PTKs for proteosomal degradation.23,,–26 Alternatively, ZAP-70 could enhance recruitment of p72Syk to the BCR complex by promoting phosphorylation of the ITAMs of the Ig accessory molecules, such as CD79b, or by recruitment of other substrates or adaptor molecules. Indeed, prior studies found that ZAP-70 could promote phosphorylation of TCR-z ITAMs in thymocytes independent of its kinase activity, serving instead as an adapter protein that could facilitate recruitment of lck to the TCR complex.27,28
We investigated whether the kinase activity of ZAP-70 or its ability to interact with c-Cbl was required for it to enhance Ig signaling in CLL cells. For this, we transfected ZAP-70− CLL cells with adenovirus vectors encoding ZAP-70 or well-defined mutant forms of ZAP-70 (eg, ZAP-70-KA369 or ZAP-70-YF292).18,29,30 This allowed us to examine changes in the Ig signaling of such cells before and after expression of defined types of ZAP-70.
Methods
This study was approved by the University of California, San Diego, Human Research Protections Program (La Jolla, CA).
Cells and sample preparation
Blood samples were collected from consenting patients who satisfied diagnostic and immunophenotypic criteria for common B-cell CLL. These patients had not received prior therapy. The patients selected for this study had CLL cells that lacked expression of ZAP-70, expressed mutated Ig, and generally lacked expression of CD38 (data not shown). Blood mononuclear cells were isolated by density-gradient centrifugation using Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). We performed flow cytometry on the blood mononuclear cells to evaluate for expression of ZAP-70, as described.11 Patients were considered to have CLL cells that were positive for ZAP-70 if more than 20% of the leukemia cells had a fluorescence intensity that exceeded a defined threshold when labeled with a fluorescent anti–ZAP-70 antibody, as described.11 Leukemia cells were stimulated with biotinylated goat F(ab)2 anti–human IgM (anti-μ), as described.8 We prepared cell pellets before and after treatment with anti-μ and then lysed these in ice-cold 1% NP-40 lysis buffer for 20 minutes on ice. We clarified the cell lysates by centrifugation at 20 000g for 15 minutes and then determined the protein concentration of each using the Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Adenovirus transduction of CLL B cells
The cDNA encoding the various types of ZAP-70 was introduced into CLL B cells using adenovirus expression vectors. ZAP-70-KA369 has a substitution of alanine for the lysine at position 369 in the ATP binding site,18,29 and ZAP-70-YF292 has a substitution of phenylalanine for the tyrosine at position 292 that binds to c-Cbl.30 We subcloned these ZAP-70 cDNA into the multiple cloning sites of the shuttle plasmid MCS(SK)pXCX2 containing an expression cassette, which drives expression of the transgene via the cytomegalovirus (CMV) promoter/enhancer. The ZAP-70 pXCX2 plasmid was cotransfected with pJM17 into 293 cells. The titer of each adenovirus preparation was determined by infecting 293 cells with serial dilutions of the purified adenovirus. Viral titers ranged from 1010 to 2 × 1010 plaque-forming units (PFUs)/mL. We transduced 2 × 106 CLL B cells in 100 μL RPMI-1640 medium supplemented with 10% fetal bovine serum containing approximately 109 adenovirus PFUs. The cells were cultured for 2 days at 37°C prior to analysis.
Transfection of CLL cells
For some experiments, ZAP-70 or mutant forms of ZAP-70 were subcloned into pcDNA3 for transfection into CLL cells via electroporation using the Nucleofector system (Amaxa Biosystems, Cologne, Germany). For this, 107 CLL B cells were suspended in 100 μL of nucleofector solution and mixed with 16 μg of pcDNA3 encoding wild-type ZAP-70 (pZAP-70), ZAP-70-KA369 (pZAP-70-KA369), a truncated ZAP-70 possessing only the 2 SH2 domains (pZAP-70-SH2), or a truncated ZAP-70 with a dysfunctional SH2 domain (pZAP-70-SH2*).31 Nucleofections were done using the U-15 program (Amaxa Biosystems). The cells were cultured for 2 days at 37°C prior to flow cytometric analysis for expression of the transgene product and for changes in [Ca2+]I upon treatment with anti-μ.
Multiplexed bead assay
We used a BD cytometric bead array (CBA) assay (BD Biosciences, San Diego, CA) to measure the levels of tyrosine-phosphorylated p72Syk, BLNK, and PLC-γ, as described.17 The CBA assay allowed for simultaneous, quantitative assessment of tyrosine-phosphorylated p72Syk, BLNK, PLC-γ, or CD79b in small amounts of cell lysate. Each cell lysate was incubated with a mixture of CBA beads, each coated with antibodies specific for p72Syk, BLNK, PLC-γ, or CD79b, as described.17 After 3 hours at room temperature, the beads were washed, phycoerythrin (PE)–conjugated antiphosphotyrosine detector antibody was added, and the beads were then incubated at room temperature for another hour. We assessed the fluorescence intensities of the bead-bound antiphosphotyrosine detector antibody using a dual-laser FACSCalibur (Becton Dickinson, San Jose, CA). To report the change in phosphorylated protein following stimulation, we subtracted 1 from the ratio of the signal for each anti-μ–stimulated sample divided by the signal of the same CLL sample prior to anti-μ stimulation and multiplied this number by 100. This formula provided the percentage of change in protein tyrosine phosphorylation.
Measurement of intracellular calcium flux
We incubated 2 × 106 CLL cells for 30 minutes at 37°C in 2 μM Fluo-4AM in Hanks balanced salt solution lacking Ca++ and Mg++ (HBSS). The cells were washed twice with HBSS and then suspended in 1 mL of RPMI lacking Ca++ and Mg++. Fluorescence of the cellular suspension was observed with a flow cytometer (Becton Dickinson, San Jose, CA). Cells were kept at 37°C for IgM stimulation. To report the change in calcium influx following stimulation, we calculated the fluorescence intensity peak increase (FIPI) by subtracting the fluorescence intensity value before stimulation from the peak fluorescence intensity value after stimulation, as described.17
Immune precipitation and immunoblot analyses
We performed immunoblot analyses as described.8 Cell lysate was boiled in sodium dodecyl sulfate (SDS) sample buffer prior to polyacrylamide gel electrophoresis. Size-separated proteins were transferred to nitrocellulose membranes and then probed with primary antibody and secondary antibodies that were conjugated with horseradish peroxidase. The blots were prepared for enhanced chemiluminescence and subsequent autoradiography.
Statistical analysis
All studies were performed in duplicate or triplicate. Differences between 2 groups of CLL samples were determined by unpaired or paired t tests, with a P level less than .05 considered significant.
Results
BCR signaling in CLL B cells transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369
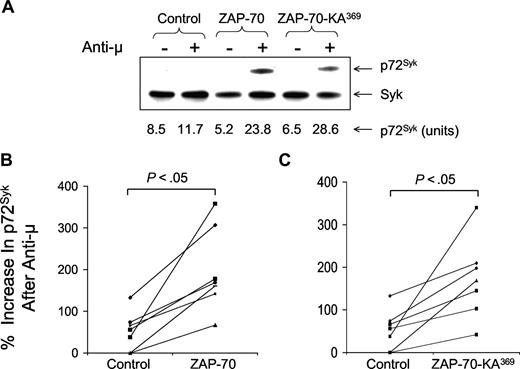
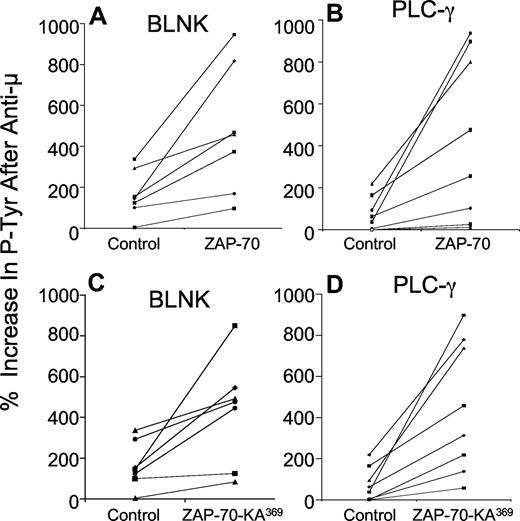
Following transduction with either Ad-ZAP-70 or Ad-ZAP-70-KA369, the previously ZAP-70− CLL cells had expression levels of wild-type ZAP-70 (Figure 1C) or ZAP-70-KA369 (Figure 1D) similar to that of CLL samples that were classified as positive for ZAP-70 using a flow-based assay and standardized gating strategy, as described11 (Figure 1A). In contrast, CLL cells transduced with a control adenovirus vector encoding b-galactosidase (Ad-lacZ) continued to register as ZAP-70− by these criteria (Figure 1B). We examined the effects following ligation of the surface IgM on the CLL cells of 7 unrelated patients. For this, we used immunoblot and immunoprecipitation analyses to examine for phosphorylation of p72Syk and a CBA assay to measure the levels of phosphorylated BLNK and phosphorylated PLC-γ, in addition to phosphorylated p72Syk. This and prior studies demonstrated that the latter assay provided results comparable with those obtained using immunoblot and immunoprecipitation analyses, but had improved range and sensitivity, and was more amenable to quantitative comparative analyses in small amounts of patient samples17 (Figure 2A). Treatment of control Ad-lacZ–transduced or mock-infected ZAP-70− CLL cells with anti-μ resulted in low-level increases in phosphorylation of these cytosolic proteins, with mean increases in phosphorylated p72Syk (Figure 2B,C), BLNK (Figure 3A,C), or PLC-γ (Figure 3B,D) of 46% plus or minus 47% (SD), 149% plus or minus 95%, or 73% plus or minus 76%, respectively (n = 7). In contrast, CLL cells engineered to express wild-type ZAP-70 exhibited significantly higher levels of tyrosine-phosphorylated protein following anti-μ treatment, with mean increases in phosphorylated p72Syk, BLNK, or PLC-γ of 199% plus or minus 93%, 516% plus or minus 311%, or 578% plus or minus 560%, respectively (Figures 2B,3A,B), as had been noted in earlier studies.17 Surprisingly, CLL cells made to express the kinase-inactive mutant of ZAP-70, namely ZAP-70-KA369, had increases in these phosphorylated proteins following treatment with anti-μ that were similar to those observed in anti-μ–treated CLL cells that were transduced to express wild-type ZAP-70 (Figures 2B,C,3). Following treatment with anti-μ, CLL cells transduced with Ad-ZAP-70-KA369 had mean increases in phosphorylated p72Syk, BLNK, or PLC-γ of 172% plus or minus 94%, 431% plus or minus 261%, or 450% plus or minus 320%, respectively (Figures 2C,3C,D). As such, the levels of protein tyrosine phosphorylation induced by anti-μ in CLL cells transduced with either vector were significantly greater than that noted for Ad-lacZ or mock-infected CLL cells (P < .05, Student t test; n = 7).
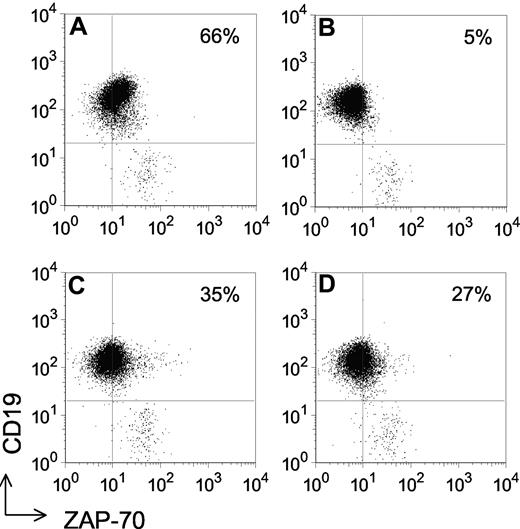
Detection of ZAP-70 in ZAP-70− CLL following transduction with Ad-ZAP-70 or Ad-ZAP-70-KA369. Dot plots depicting the log fluorescence of blood lymphocytes stained with Alexa-488–conjugated anti–ZAP-70 (x-axis) and PE-conjugated anti-CD19 (y-axis). The horizontal line depicts the threshold fluorescence exceeded by CD19+ CLL B cells, whereas the vertical line indicates the threshold fluorescence exceeded by cells scored as ZAP-70+, using a defined gating strategy, as described.11 The number provided in the top right-hand corner of each panel provides the percentage of the CD19+ CLL B cells with fluorescence exceeding the ZAP-70 threshold. CLL cell samples that have more than 20% ZAP-70+ cells are scored as being ZAP-70+. (A) ZAP-70+ CLL cell sample. (B) a ZAP-70− CLL cell sample transduced with a control adenovirus vector. (C) The same CLL sample as in panel B transduced with Ad-ZAP-70. (D) The same CLL sample as in panel B transduced with Ad-ZAP-70-KA369.
Detection of ZAP-70 in ZAP-70− CLL following transduction with Ad-ZAP-70 or Ad-ZAP-70-KA369. Dot plots depicting the log fluorescence of blood lymphocytes stained with Alexa-488–conjugated anti–ZAP-70 (x-axis) and PE-conjugated anti-CD19 (y-axis). The horizontal line depicts the threshold fluorescence exceeded by CD19+ CLL B cells, whereas the vertical line indicates the threshold fluorescence exceeded by cells scored as ZAP-70+, using a defined gating strategy, as described.11 The number provided in the top right-hand corner of each panel provides the percentage of the CD19+ CLL B cells with fluorescence exceeding the ZAP-70 threshold. CLL cell samples that have more than 20% ZAP-70+ cells are scored as being ZAP-70+. (A) ZAP-70+ CLL cell sample. (B) a ZAP-70− CLL cell sample transduced with a control adenovirus vector. (C) The same CLL sample as in panel B transduced with Ad-ZAP-70. (D) The same CLL sample as in panel B transduced with Ad-ZAP-70-KA369.
Increases in phosphorylated p72Syk induced by anti-μ in ZAP-70− CLL transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. (A) Comparison of CBA assay with immunoblot analysis to detect phosphorylated p72Syk. Phosphorylated p72Syk was determined by immunoblot analysis and CBA assay for ZAP-70− CLL transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. The band intensity for the anti-μ–stimulated CLL samples transfected with wild-type ZAP-70 was similar to that noted for the anti-μ–stimulated samples transfected with ZAP-70-KA369, providing a ratio of signal intensity that was similar to that using the CBA assay. The immunoblot results are a representative one of 3 experiments. (B,C) Percentage increase in phosphorylated p72Syk induced by treatment with anti-μ, as indicated on the y-axis of each panel. Each symbol represents the percentage increase in phosphorylated protein detected in an individual CLL cell sample. The lines connect the percentage increase in detected phosphorylated protein of anti-μ–treated CLL cell samples transduced with Ad-lacZ (“Control” in each panel) versus that measured in the same CLL cells that had been transduced with Ad-ZAP-70 (B) or Ad-ZAP-70-KA369 (C). The mean anti-μ–induced increase in each phosphoprotein detected in the Ad-ZAP-70– or Ad-ZAP-70-KA369–transduced CLL cells was significantly greater than that noted for the same phosphoprotein in the Ad-lacZ–transduced CLL cells following treatment with anti-μ (P < .05), which was not significantly different from that of anti-μ–treated mock-transfected CLL cells.
Increases in phosphorylated p72Syk induced by anti-μ in ZAP-70− CLL transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. (A) Comparison of CBA assay with immunoblot analysis to detect phosphorylated p72Syk. Phosphorylated p72Syk was determined by immunoblot analysis and CBA assay for ZAP-70− CLL transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. The band intensity for the anti-μ–stimulated CLL samples transfected with wild-type ZAP-70 was similar to that noted for the anti-μ–stimulated samples transfected with ZAP-70-KA369, providing a ratio of signal intensity that was similar to that using the CBA assay. The immunoblot results are a representative one of 3 experiments. (B,C) Percentage increase in phosphorylated p72Syk induced by treatment with anti-μ, as indicated on the y-axis of each panel. Each symbol represents the percentage increase in phosphorylated protein detected in an individual CLL cell sample. The lines connect the percentage increase in detected phosphorylated protein of anti-μ–treated CLL cell samples transduced with Ad-lacZ (“Control” in each panel) versus that measured in the same CLL cells that had been transduced with Ad-ZAP-70 (B) or Ad-ZAP-70-KA369 (C). The mean anti-μ–induced increase in each phosphoprotein detected in the Ad-ZAP-70– or Ad-ZAP-70-KA369–transduced CLL cells was significantly greater than that noted for the same phosphoprotein in the Ad-lacZ–transduced CLL cells following treatment with anti-μ (P < .05), which was not significantly different from that of anti-μ–treated mock-transfected CLL cells.
Increases in phosphorylated BLNK and PLC-γ induced by anti-μ in ZAP-70− CLL transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. The panels show the percentage increase in phosphorylated BLNK (A,C) and PLC-γ (B,D) induced by treatment with anti-μ, as indicated on the y-axis of each panel. Each symbol represents the percentage increase in phosphorylated protein detected in an individual CLL cell sample. The lines connect the percentage increase in detected phosphorylated protein of anti-μ–treated CLL cell samples transduced with Ad-lacZ (“Control” in each panel) versus that measured in the same CLL cells that had been transduced with Ad-ZAP-70 (A,B) or Ad-ZAP-70-KA369 (C,D). The mean anti-μ–induced increase in each phosphoprotein detected in the Ad-ZAP-70– or Ad-ZAP-70-KA369–transduced CLL cells was significantly greater than that noted for the same phosphoprotein in the Ad-lacZ–transduced CLL cells following treatment with anti-μ (P < .05), which was not significantly different from that of anti-μ–treated mock-transfected CLL cells.
Increases in phosphorylated BLNK and PLC-γ induced by anti-μ in ZAP-70− CLL transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. The panels show the percentage increase in phosphorylated BLNK (A,C) and PLC-γ (B,D) induced by treatment with anti-μ, as indicated on the y-axis of each panel. Each symbol represents the percentage increase in phosphorylated protein detected in an individual CLL cell sample. The lines connect the percentage increase in detected phosphorylated protein of anti-μ–treated CLL cell samples transduced with Ad-lacZ (“Control” in each panel) versus that measured in the same CLL cells that had been transduced with Ad-ZAP-70 (A,B) or Ad-ZAP-70-KA369 (C,D). The mean anti-μ–induced increase in each phosphoprotein detected in the Ad-ZAP-70– or Ad-ZAP-70-KA369–transduced CLL cells was significantly greater than that noted for the same phosphoprotein in the Ad-lacZ–transduced CLL cells following treatment with anti-μ (P < .05), which was not significantly different from that of anti-μ–treated mock-transfected CLL cells.
To use an independent means with which to monitor the magnitude of BCR signaling, we examined the relative magnitude of [Ca2+]I induced in each sample following treatment with anti-μ (Figure 4A). Whereas anti-μ treatment of Ad-lacZ or mock-infected CLL cells resulted in negligible to minimal increases in [Ca2+]I of 0.25 plus or minus 0.19 U (n = 6), anti-μ treatment of CLL cells transduced with either Ad-ZAP-70 or Ad-ZAP-70-KA369 induced increases in [Ca2+]I of 1.05 plus or minus 0.53 U or 0.95 plus or minus 0.46 U, respectively (n = 6). These values each were significantly higher than that noted for mock-infected CLL cells (P < .05, Student t test; Figure 4B).
IgM-induced calcium mobilization in ZAP-70− CLL B cells transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. (A) The fluorescence of labeled CLL cells induced by treatment with anti-μ is indicated on the y-axis over time (in seconds, as indicated on the x-axis). The horizontal dotted line in each panel provides the basal fluorescence of labeled cells prior to treatment. The arrow indicates the time when the cells were treated with anti-μ (IgM). The top panel shows the changes in calcium flux ([Ca2+]i) detected in ZAP-70− CLL cells transduced with Ad-lacZ (Control); the middle panel shows the [Ca2+]i observed in the same CLL sample transduced with Ad-ZAP-70; and the bottom panel shows the [Ca2+]i observed in the same CLL sample transduced with Ad-ZAP-70-KA369. (B) Peak increases in fluorescence intensity over the basal level in ZAP-70− CLL cells transduced with Ad-lacZ (Control) versus that of CLL cells transduced with Ad-ZAP-70 (top) or Ad-ZAP-70-KA369 (bottom). The lines connect the data points obtained from the same CLL sample, but transduced with Ad-lacZ (control) or Ad-ZAP-70 or Ad-ZAP-70-KA369, as indicated at the bottom of the panels.
IgM-induced calcium mobilization in ZAP-70− CLL B cells transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. (A) The fluorescence of labeled CLL cells induced by treatment with anti-μ is indicated on the y-axis over time (in seconds, as indicated on the x-axis). The horizontal dotted line in each panel provides the basal fluorescence of labeled cells prior to treatment. The arrow indicates the time when the cells were treated with anti-μ (IgM). The top panel shows the changes in calcium flux ([Ca2+]i) detected in ZAP-70− CLL cells transduced with Ad-lacZ (Control); the middle panel shows the [Ca2+]i observed in the same CLL sample transduced with Ad-ZAP-70; and the bottom panel shows the [Ca2+]i observed in the same CLL sample transduced with Ad-ZAP-70-KA369. (B) Peak increases in fluorescence intensity over the basal level in ZAP-70− CLL cells transduced with Ad-lacZ (Control) versus that of CLL cells transduced with Ad-ZAP-70 (top) or Ad-ZAP-70-KA369 (bottom). The lines connect the data points obtained from the same CLL sample, but transduced with Ad-lacZ (control) or Ad-ZAP-70 or Ad-ZAP-70-KA369, as indicated at the bottom of the panels.
BCR signaling in CLL B cells transduced with Ad-ZAP-70-YF292
Because the kinase-defective mutant of ZAP-70 could enhance BCR signaling in CLL, we hypothesized that ZAP-70 might facilitate Ig receptor signaling indirectly by competing with activated p72Syk for binding to the E3 ubiquitin ligase, c-Cbl. The c-Cbl ligase targets phosphorylated PTK for proteosomal degradation, thereby helping to limit the signaling induced by Ig-receptor ligation. Moreover, interaction with C-Cbl can shorten the half-life of the activated PTK, thereby limiting the induction of signaling events that occur following antigen-receptor ligation. Because ZAP-70 undergoes phosphorylation following surface IgM ligation in CLL cells,8 it potentially could compete with phosphorylated p72Syk for binding c-Cbl and thereby enhance the half-life of activated p72Syk, allowing it greater time to phosphorylate downstream adaptor proteins and signaling molecules. To test this hypothesis, we generated an adenovirus, designated Ad-ZAP-70-YF292, that encodes a mutant form of ZAP-70 that possesses a mutation at amino acid residue 292, which precludes even its phosphorylated form from binding c-Cbl.32 ZAP-70− CLL cells were transduced with Ad-ZAP-70, Ad-ZAP-70-YF292, or Ad-lacZ, and then stimulated 48 hours later with anti-μ. Both Ad-ZAP-70– and Ad-ZAP-70-YF292–transduced CLL cells expressed similar amounts of ZAP-70, as assessed by immunoblot analysis (Figure 5A). Following treatment with anti-μ, CLL B cells that expressed ZAP-70-YF292 had significantly greater levels of phosphorylated p72Syk (188% ± 62%) than did CLL B cells that were mock-infected (87% ± 33%; P < .05, Student t test; n = 4). Moreover, we did not observe any significant differences in the anti-μ–induced levels of phosphorylated p72Syk in CLL cells transduced with adenovirus encoding either wild-type ZAP-70 (200% ± 72%) or ZAP-70-YF292 (Figure 5B; P > .05, Student t test; n = 4). Similarly, the increased levels of phosphorylated BLNK (539% ± 285%) and PLC-γ (595% ± 245%) were significantly greater in cells transduced with ZAP-70-YF292 than that experienced in mock-infected cells, namely 204% plus or minus 94% and 135% plus or minus 70%, respectively (P < .05, Student t test; n = 4). No significant differences in the anti-μ–induced levels of phosphorylated BLNK and PLC-γ were observed in CLL cells transduced to express either wild-type ZAP-70 or ZAP-70-YF292 (Figure 5C,D; P > .05, Student t test; n = 4).
Transduction of ZAP-70− CLL cells with Ad-ZAP-70 versus Ad-ZAP-70-YF292 and measurement of anti-μ–induced increases in phosphorylated p72Syk, BLNK, or PLC-γ. (A) Immunoblot ana-lysis for ZAP-70 (top row) in lysates of CLL cells before or after transduction with Ad-ZAP-70 or Ad-ZAP-70-YF,292 as indicated at the top of each lane. The blots were stripped and then reprobed with anti–β-actin to monitor for protein loading (bottom row), as indicated on the left of each blot. (B-D) Anti-μ–induced percentage increases in phosphorylated p72Syk (B), BLNK (C), or PLC-γ (D) detected in ZAP-70− CLL B cells that were transduced with the control vector or Ad-ZAP-70-YF292 (left panel) or with Ad-ZAP-70 or Ad-ZAP-70-YF292 (right panel). Each symbol represents the percentage increase in phosphorylated protein detected in an individual CLL cell sample. The lines connect the percentage increase in detected phosphorylated protein of anti-μ–treated CLL cells of the same sample transduced with each of the different vectors. The differences in the mean percentage increase of phosphorylated protein upon anti-μ treatment in control vector–transduced CLL cells versus that of Ad-ZAP-70-YF292–transduced CLL cells was significant (P < .05). However, there was not a significant difference in the mean percentage increase of phosphorylated protein upon anti-μ treatment of Ad-ZAP-70–transduced CLL cells versus that of Ad-ZAP-70-YF292–transduced CLL cells.
Transduction of ZAP-70− CLL cells with Ad-ZAP-70 versus Ad-ZAP-70-YF292 and measurement of anti-μ–induced increases in phosphorylated p72Syk, BLNK, or PLC-γ. (A) Immunoblot ana-lysis for ZAP-70 (top row) in lysates of CLL cells before or after transduction with Ad-ZAP-70 or Ad-ZAP-70-YF,292 as indicated at the top of each lane. The blots were stripped and then reprobed with anti–β-actin to monitor for protein loading (bottom row), as indicated on the left of each blot. (B-D) Anti-μ–induced percentage increases in phosphorylated p72Syk (B), BLNK (C), or PLC-γ (D) detected in ZAP-70− CLL B cells that were transduced with the control vector or Ad-ZAP-70-YF292 (left panel) or with Ad-ZAP-70 or Ad-ZAP-70-YF292 (right panel). Each symbol represents the percentage increase in phosphorylated protein detected in an individual CLL cell sample. The lines connect the percentage increase in detected phosphorylated protein of anti-μ–treated CLL cells of the same sample transduced with each of the different vectors. The differences in the mean percentage increase of phosphorylated protein upon anti-μ treatment in control vector–transduced CLL cells versus that of Ad-ZAP-70-YF292–transduced CLL cells was significant (P < .05). However, there was not a significant difference in the mean percentage increase of phosphorylated protein upon anti-μ treatment of Ad-ZAP-70–transduced CLL cells versus that of Ad-ZAP-70-YF292–transduced CLL cells.
BCR signaling leading to intracellular calcium flux in CLL cells transfected with plasmid DNA encoding ZAP-70 or mutant forms of ZAP-70
We generated plasmid expression vectors encoding ZAP-70 (designated pZAP-70), ZAP-70-KA369 (pZAP-70-KA369), a truncated ZAP-70 possessing the 2 SH2 domains but lacking the SH1 domain (pZAP-70-SH2), or a truncated ZAP-70 lacking the SH1 domain and one functional SH2 domain (pZAP-70-SH*; Figure 6B-E). These plasmids each were transfected into ZAP-70− CLL cells via electroporation. After 2 days of culture, the transfected cells were examined for expression for protein and changes in intracellular calcium upon treatment with anti-μ. The previously ZAP-70− CLL cells transfected with each of these vectors were made to express ZAP-70, as assessed via flow cytometry (Figure 6B-E). We found that anti-μ treatment of CLL cells transfected with either pZAP-70-KA369 or pZAP-70-SH2 induced increases in [Ca2+]I that were comparable with those of CLL cells transfected with pZAP-70 (Figure 6B-D). On the other hand, anti-μ treatment of CLL cells transfected with pZAP-70-SH* failed to induce changes in [Ca2+]I that were greater than that of ZAP-70− CLL cells transfected with the control plasmid vector (Figure 6A,E).
Transfection of ZAP-70− CLL cells with plasmid DNA encoding ZAP-70 or various mutant forms of ZAP-70. Left column shows schematics of ZAP-70 and each of the various ZAP-70 mutants used in these studies. Middle column shows the histogram depicting the autofluorescence of the control vector–transfected CLL cells (shaded histogram) and fluorescence of the cells stained for ZAP-70 (open histogram). Right column shows changes in calcium flux ([Ca2+]i) of each CLL sample that is observed upon treatment with anti-μ, as in Figure 4A. (A) CLL cells transfected with the empty control vector pcDNA3. (B) CLL cells transfected with pZAP-70. (C) CLL cells transfected with pZAP-70-KA369. (D) CLL cells transfected with pZAP-70-SH2. (E) CLL cells transfected with pZAP-70-SH*.
Transfection of ZAP-70− CLL cells with plasmid DNA encoding ZAP-70 or various mutant forms of ZAP-70. Left column shows schematics of ZAP-70 and each of the various ZAP-70 mutants used in these studies. Middle column shows the histogram depicting the autofluorescence of the control vector–transfected CLL cells (shaded histogram) and fluorescence of the cells stained for ZAP-70 (open histogram). Right column shows changes in calcium flux ([Ca2+]i) of each CLL sample that is observed upon treatment with anti-μ, as in Figure 4A. (A) CLL cells transfected with the empty control vector pcDNA3. (B) CLL cells transfected with pZAP-70. (C) CLL cells transfected with pZAP-70-KA369. (D) CLL cells transfected with pZAP-70-SH2. (E) CLL cells transfected with pZAP-70-SH*.
Anti-μ–induced phosphorylation of CD79b
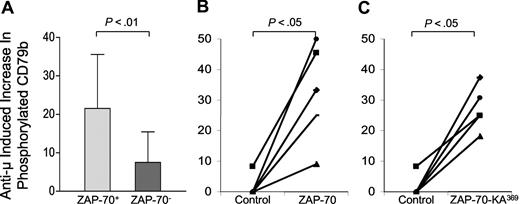
We looked for phosphorylation of CD79b in CLL B cells that did or did not express ZAP-70 using the CBA assay. Following treatment with anti-μ, CLL cells that expressed ZAP-70 had a mean increase in the levels of phosphorylated CD79b of 22% (± 14.0% SD; n = 28), which were significantly greater than the 8% increase (± 8% SD; n = 19) noted for CLL cells that lacked expression of ZAP-70 (P < .01, Student t test; Figure 7A). We transduced CLL cells that lacked ZAP-70 with Ad-ZAP-70, Ad-ZAP-70-KA369, or Ad-lacZ and looked for increases in levels of phosphorylated CD79b that were induced by treatment with anti-μ. Following such BCR ligation, the increases in the mean levels of phosphorylated CD79b (33% ± 16%; n = 5) were significantly greater in CLL B cells transduced with Ad-ZAP-70 than in Ad-lacZ–transduced CLL B cells (4% ± 2%; n = 5; P < .05, Student t test; Figure 7B). CLL cells transduced with Ad-ZAP-70-KA369 had similar increases in the mean level of phosphorylated CD79b (27% ± 7%; n = 5) following treatment with anti-μ. These increases were significantly greater than those noted for CLL B cells transduced with Ad-lacZ (P < .05, Student t test; Figure 7C).
Anti-μ–induced increases in phosphorylated CD79b in ZAP-70+ versus ZAP-70− CLL cells or ZAP-70− CLL B cells made to express ZAP-70-Wt or ZAP-70-KA369. (A) Each bar represents the mean percentage increase in phosphorylated CD79b observed following treatment with anti-μ of CLL B cells that were ZAP-70+ (N = 28) or ZAP-70− (N = 19), as indicated at the bottom of the figure. The error bars indicate the standard deviation of the mean. The difference in the mean percentage increase in ZAP-70+ CLL cells versus ZAP-70− CLL cells was significant, as indicated by the P value at the top of the figure. (B,C) Anti-μ–induced increases in the level of phosphorylated CD79b detected in ZAP-70− CLL cells transduced with a control adenovirus or Ad-ZAP-70 (B), or control adenovirus or Ad-ZAP-70-KA369 (C). The lines connect the percentage increase in detected phosphorylated CD79b of anti-μ–treated CLL cell samples transduced with Ad-lacZ (“Control” in each panel) versus that measured in the same CLL cells that had been transduced with Ad-ZAP-70 (B) or Ad-ZAP-70-KA369 (C).
Anti-μ–induced increases in phosphorylated CD79b in ZAP-70+ versus ZAP-70− CLL cells or ZAP-70− CLL B cells made to express ZAP-70-Wt or ZAP-70-KA369. (A) Each bar represents the mean percentage increase in phosphorylated CD79b observed following treatment with anti-μ of CLL B cells that were ZAP-70+ (N = 28) or ZAP-70− (N = 19), as indicated at the bottom of the figure. The error bars indicate the standard deviation of the mean. The difference in the mean percentage increase in ZAP-70+ CLL cells versus ZAP-70− CLL cells was significant, as indicated by the P value at the top of the figure. (B,C) Anti-μ–induced increases in the level of phosphorylated CD79b detected in ZAP-70− CLL cells transduced with a control adenovirus or Ad-ZAP-70 (B), or control adenovirus or Ad-ZAP-70-KA369 (C). The lines connect the percentage increase in detected phosphorylated CD79b of anti-μ–treated CLL cell samples transduced with Ad-lacZ (“Control” in each panel) versus that measured in the same CLL cells that had been transduced with Ad-ZAP-70 (B) or Ad-ZAP-70-KA369 (C).
Discussion
In this study, we found that CLL cells transduced to express ZAP-70-KA369 responded to anti-μ treatment in a manner similar to that of most CLL cells that already expressed ZAP-70 or that were transduced to express the wild-type ZAP-70 protein. Prior studies demonstrated that ZAP-70-KA369 lacks kinase activity.29,31 Nevertheless, our studies revealed that ZAP-70− CLL cells made to express either wild-type ZAP-70 or ZAP-70-KA369 exhibited significantly greater tyrosine phosphorylation of downstream adapter proteins and increases in intracellular calcium following treatment with anti-μ than did mock-transfected CLL cells or CLL cells transduced with a control adenovirus vector. The latter indicates that the improved signaling in response to ligation of surface IgM in Ad-ZAP-70–transduced CLL cells was not secondary to transduction with adenovirus per se, but rather secondary to the expression of the ZAP-70, including a mutant form of ZAP-70 lacking a functional SH1 kinase domain.
We considered the possibility that ZAP-70 instead may enhance BCR signaling indirectly by enhancing the stability of activated p72Syk following treatment with anti-μ. Activation of either PTK targets it for binding by the E3 ubiquitin ligase, c-Cbl,23,33 which in turn directs polyubiquitination and proteosomal degradation of the activated PTK.24,34 In prior studies, we found that both ZAP-70 and p72Syk undergo tyrosine phosphorylation in CLL cells following treatment of anti-μ,8,17 and that CLL cells that express only p72Syk have significantly lower levels of phosphorylated p72Syk following anti-μ treatment. Conceivably, the activated ZAP-70 might compete with activated p72Syk for binding c-Cbl and thereby prolong the half-life of activated p72Syk. However, transduction of CLL cells with an adenovirus encoding a mutant form of ZAP-70 carrying a mutation at position 292, which abrogates the ability of the mutant form to interact with c-Cbl, also enhanced BCR signaling of ZAP-70− CLL cells. Moreover, the levels of phosphorylated p72Syk observed following anti-μ treatment of CLL cells transduced with Ad-ZAP-70292 were comparable to that of CLL cells transduced with the adenovirus vector encoding the wild-type ZAP-70 protein. We conclude that competition for c-Cbl–directed proteolytic degradation is unlikely to account for the capacity of ZAP-70 to enhance BCR signaling in CLL B cells.
Because expression of ZAP-70-KA369 or ZAP-70292 could enhance BCR signaling, we examined additional constructs encoding mutant forms of ZAP-70 that lacked the entire SH1, one of which also lacked a functional SH2 domain. We found that ZAP-70− CLL cells transfected with pZAP-70-KA369 or pZAP-70-SH2 had an increased capacity to respond to treatment with anti-μ that was similar to that of CLL cells transfected with pZAP-70 encoding the wild-type PTK. On the other hand, CLL cells transfected with a plasmid expression vector encoding a ZAP-70 mutant also lacking a functional SH2 domain did not experience enhanced calcium flux following treatment with anti-μ, suggesting that docking at the ITAM may be necessary for ZAP-70 to have an effect on CLL Ig receptor signaling. Collectively, these results suggest that the 2 functional SH2 domains of ZAP-70 are required for enhanced IgM signaling in CLL B cells, and that the SH1 kinase domain of ZAP-70 is dispensable.
The finding that the kinase inactive ZAP-70-KA369 could reconstitute BCR signaling in CLL B cells is in apparent disagreement with a prior study demonstrating that reconstitution of BCR-signaling in p72Syk-deficient B cells by ZAP-70 required it to have functional kinase activity.18 However, in contrast to this prior study, we examined CLL B cells that were not deficient in p72Syk, but instead had levels of p72Syk that were similar to that of normal adult blood B lymphocytes8,20 (and data not shown). As noted, p72Syk plays a pivotal role in coupling the BCR to downstream signaling events,6 and has an approximately 100-fold greater intrinsic PTK activity than does ZAP-70 in vitro.21 Moreover, prior studies indicated that the expression of ZAP-70 in CLL cells allowed for significantly greater tyrosine phosphorylation of p72Syk in response to surface IgM ligation than that noted in CLL cells lacking expression of ZAP-70.8,17 As such, it is conceivable that the kinase function of p72Syk still plays the dominant role in the phosphorylation of adapter proteins and molecules that are responsible for the downstream signaling events that occur following surface Ig ligation.
We found that CLL cells that expressed ZAP-70, or that were made to express either wild-type ZAP-70 or ZAP-70-KA369, had significantly greater levels of phosphorylated CD79b following anti-μ treatment than comparably stimulated CLL cells that were mock-infected or transduced with Ad-lacZ. Prior studies demonstrated that CD79b is expressed at lower levels on CLL cells than on normal B lymphocytes.35,36 The low levels of CD79b could be due to expression of alternative transcripts of CD79b37 or mutation.38 In any case, the limiting amounts of CD79b could preclude high-level surface Ig expression and limit the number of ITAMs that can be phosphorylated following BCR ligation by Src kinases, such as Lyn. Conceivably, ZAP-70 could facilitate recruitment of Lyn and/or other Src kinases or adaptors to the BCR complex following surface Ig ligation in a manner analogous to the role it plays in double-positive thymocytes, which have limiting amounts of lck.27 In such cells, a kinase-defective ZAP-70 still could enhance phosphorylation of the TCR-z chain following TCR ligation, indicating that ZAP-70 could facilitate recruitment of lck to the TCR complex independent of its kinase activity. A recent study demonstrated that surface IgM ligation induced translocation of the BCR to lipid rafts on CLL cells that expressed unmutated IGHV genes (and hence were likely ZAP-70+), whereas the BCR did not translocate to lipid rafts following anti-μ treatment on CLL cells that expressed mutated IGHV genes (and hence were likely to lack ZAP-70 expression).39 In addition, BCR internalization was decreased in BJAB B cells transfected with ZAP-70.16 Conceivably, ZAP-70 can facilitate entry of the BCR into lipid rafts more effectively than p72Syk, thereby being better able to recruit Src kinases to the Ig receptor complex. In this regard, it is noteworthy that ZAP-70 is more effective than p72Syk in promoting phosphorylation of TCR-z by src kinases following TCR ligation.28
The association of ZAP-70 with Ig receptor signaling has been challenged in studies by Deglesne and colleagues, who used immobilized anti-μ to stimulate CLL cells in vitro and then monitored for CLL cell survival 72 hours later. They segregated patients into 2 groups, one of which had enhanced CLL cell survival with anti-μ (group A), and the other, which had CLL cell survival that apparently was unaffected by treatment with anti-μ (group B). Whereas all of the 20 nonresponder patients comprising group B had CLL cells that used mutated IGHV, 2 of the 10 CLL samples tested for ZAP-70 in this group were found to express this PTK. On the other hand, only 50% of the CLL samples in the responder group A expressed IGHV without somatic mutations, and only 56% of the 32 samples tested for ZAP-70 in this group were found to express ZAP-70. As such, the association between responsiveness to anti-μ in this assay and the expression of unmutated IGHV or ZAP-70 was not absolute. Because a higher proportion of patients in responder group A had advanced and/or progressive disease than those in nonresponder group B, the investigators of this study suggested that the survival induced by immobilized anti-μ in this assay was associated with more aggressive and/or later-stage disease. In contrast, in our current study, we examined for phosphorylation events and changes in calcium flux that occurred within minutes of treatment with nonimmobilized F(ab)2 anti-μ. These early events are less likely to be influenced by factors that can influence the relative survival of CLL cells cultured for days in vitro, such as the relative dependency of CLL cells on accessory cells (eg, nurselike cells or dendritic cells) or the peculiar culture conditions.15,40 A strong dependency on such factors for CLL cell survival could influence the relative capacity of anti-μ to influence CLL cell survival after 72 hours in culture. Conceivably, such factors could account for the noted discrepancies between expression of unmutated IGHV and/or ZAP-70 and the responsiveness of the leukemia cells to stimulation by anti-μ.
It also should be noted that expression of ZAP-70 is not sufficient to allow for proficient BCR signaling in all patients examined. Although patients with CLL who express ZAP-70 as a whole have more robust BCR signaling than CLL cells lacking ZAP-70, we8,17 and others41 have found individual patients with CLL who have levels of BCR signaling following treatment with anti-μ that were comparable with that of CLL cells that lack expression of ZAP-70. This indicates that expression of ZAP-70 alone is not sufficient to allow for proficient Ig signaling. Conversely, some cells from patients with CLL that lack expression of ZAP-70 apparently could respond well to surface IgM ligation,8,17 indicating that in certain circumstances the leukemia B-cell signaling capacity is independent of expression of ZAP-70, as is the case with normal blood B cells. However, in our current studies, we examined the signaling potential of CLL cells before and after transduced expression of ZAP-70 or its various mutant forms. These studies demonstrated that the de novo expression of ZAP-70 could at least enhance the signaling capacity of these cells to respond to surface IgM ligation. Moreover, our studies indicate that the ability of ZAP-70 to facilitate signaling appears independent of its kinase activity, indicating that this protein more likely functions as an adaptor protein to facilitate BCR signaling in CLL.
These studies have implications for development of new therapies for patients with CLL that target ZAP-70. Indeed, recent studies have discerned a highly significant association between leukemia cell expression of ZAP-70 and risk for early disease progression requiring therapy.11,42 Because ZAP-70 possesses catalytic activity, it could be assumed that the kinase function of this protein contributes to the relatively aggressive nature of ZAP-70+ CLL. However, based on the studies presented here, it is necessary also to consider that the kinase-independent capacity of ZAP-70 to enhance Ig signaling in CLL cells could be responsible for this association. As such, agents that reduce the levels of ZAP-70 protein in CLL cells43 or that interfere with its capacity to facilitate phosphorylation of ITAMs or other downstream substrates might be required to affect a beneficial therapeutic outcome in patients with CLL cells that express this PTK.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was funded in part by National Institutes of Health grant R37-CA49870 to T.J.K. and 2 PO1-CA081534 for the Chronic Lymphocytic Leukemia (CLL) Research Consortium, and a Specialized Center of Research (SCOR) grant of the Leukemia and Lymphoma Society.
National Institutes of Health
Authorship
Contribution: L.C. designed and performed research, analyzed data, and wrote the manuscript; L.H. and L.T. performed research and analyzed data; L.R. contributed patient samples and data; A.W. supplied the ZAP-70 construct and provided helpful discussion and advice; J.A. performed research; and T.J.K. designed the research, contributed patient samples, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no conflicting financial interests.
Correspondence: Thomas J. Kipps, Moores UCSD Cancer Center, 3855 Health Sciences Dr, No. 0820, La Jolla, CA 92093-0820; e-mail: tkipps@ucsd.edu.

![Figure 4. IgM-induced calcium mobilization in ZAP-70− CLL B cells transduced with Ad-ZAP-70 or Ad-ZAP-70-KA369. (A) The fluorescence of labeled CLL cells induced by treatment with anti-μ is indicated on the y-axis over time (in seconds, as indicated on the x-axis). The horizontal dotted line in each panel provides the basal fluorescence of labeled cells prior to treatment. The arrow indicates the time when the cells were treated with anti-μ (IgM). The top panel shows the changes in calcium flux ([Ca2+]i) detected in ZAP-70− CLL cells transduced with Ad-lacZ (Control); the middle panel shows the [Ca2+]i observed in the same CLL sample transduced with Ad-ZAP-70; and the bottom panel shows the [Ca2+]i observed in the same CLL sample transduced with Ad-ZAP-70-KA369. (B) Peak increases in fluorescence intensity over the basal level in ZAP-70− CLL cells transduced with Ad-lacZ (Control) versus that of CLL cells transduced with Ad-ZAP-70 (top) or Ad-ZAP-70-KA369 (bottom). The lines connect the data points obtained from the same CLL sample, but transduced with Ad-lacZ (control) or Ad-ZAP-70 or Ad-ZAP-70-KA369, as indicated at the bottom of the panels.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/5/10.1182_blood-2006-12-062265/2/m_zh80040814770004.jpeg?Expires=1767702143&Signature=M5~Zsn8O6F~j5d8gADFSalycWYKxWzMEYlt~8avM101mqu4stra35kb8g09rm504sGGJ05Ydk3d4Qd~l7HGOYf-KBKRXR~h6CLskMYSPb9YhOqawR-xrkNYsQ-QYtHkflve226Heysw5qefOR-fN1b1eR546Zqk9fFfvw390IG0xJW6Ew~JPgkc~EEnB6YH9Y~-3m5SlOf0phVOpOkhJLJm5NfxBggyJGaITSGmnGrfSOmh7Z78-sHNnJ0s5QZDT659NsAxxeNMdyfxpa2j8esnIjOevWNRlYxbCUrOK5oE12Vty1kL7T3tAM062ePn3jK~R5qHBzdhNAwUODHQ0Vw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Transfection of ZAP-70− CLL cells with plasmid DNA encoding ZAP-70 or various mutant forms of ZAP-70. Left column shows schematics of ZAP-70 and each of the various ZAP-70 mutants used in these studies. Middle column shows the histogram depicting the autofluorescence of the control vector–transfected CLL cells (shaded histogram) and fluorescence of the cells stained for ZAP-70 (open histogram). Right column shows changes in calcium flux ([Ca2+]i) of each CLL sample that is observed upon treatment with anti-μ, as in Figure 4A. (A) CLL cells transfected with the empty control vector pcDNA3. (B) CLL cells transfected with pZAP-70. (C) CLL cells transfected with pZAP-70-KA369. (D) CLL cells transfected with pZAP-70-SH2. (E) CLL cells transfected with pZAP-70-SH*.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/5/10.1182_blood-2006-12-062265/2/m_zh80040814770006.jpeg?Expires=1767702143&Signature=pgqJeZmjAosETzAETk49NakhLlHEA~JAGI85nwC1SuPlfuneVGrmbHCa0FgY6hg5NCwtQuodWRzZad3ROpDj2R2pBq1rwMIeJFZwa7tcBC1J7jTul4D1z4ZrjsuiOiX4YbDOhxrIFIrxQBmVMRZELAu2YDxLa8L2AEV2Mxxhwj2kRmZWLK1Gn7Yf0rMMhvu9qbgYrMC-hYzv61ACrifHp6BnP6EVi-gOt-issjgzwQRUDN6OMNGbNfpYn~kuGF2nux1ObtlbkEgSDIbhvgEW0mFcfFVsP6l0J2JM5r99TK9AEDGVFw4Ou2tkek9I6Yf1YUPQlK5N3MgtVSGAwSasBQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal