Recently we reported that intact apoptosis signaling is indicative of favorable outcome in childhood acute lymphoblastic leukemia. Here we addressed this issue in 45 pediatric acute myeloid leukemia patients analyzing 2 core apoptogenic events: cytochrome c release and caspase-3 activation. In patients with good prognosis cytochrome c release was clearly found to be caspasedependent and correlated with activated caspase-3, indicating that activation of initiator or amplifier caspases such as caspase-8 together with an intact apoptosome function are elementary for favorable outcome. The functional integrity of this apoptogenic checkpoint is reflected by the parameter caspase-dependent cytochrome c-related activation of caspase-3 (CRACdep). Patients with positive CRACdep values (intact signaling) exhibited superior survival compared with CRACdep negative patients (deficient signaling). Thus, the propensity to undergo apoptosis of leukemia cells is an important feature for favorable treatment outcome and may serve as an additional stratification tool for pediatric AML patients. This trial was registered at www.ClinicalTrials.gov as #NCT00111345.
Introduction
Successful treatment of children with acute myeloid leukemia (AML) has improved, reaching overall survival rates of 62%.1 A considerable number of patients still encounter relapse, however. Because apoptosis plays a key role in regulating tissue homeostasis, defects in apoptosis signaling have been considered to be responsible for treatment failure. Consequently, several apoptosis-regulating molecules have been evaluated for their prognostic impact. Imbalanced expression of Bax and Bcl-2 in favor of the proapoptotic molecule was found to be correlated to good prognosis in AML.2 On the contrary, studies assessing transcript levels of apoptosis signaling molecules showed inverse findings.3 However, expression of single factors may not reflect the efficiency of the cell death machinery. For this reason we previously developed a different approach analyzing the functional integrity of apoptosis signaling in individual cells by simultaneously investigating 2 key apoptogenic events: mitochondrial release of cytochrome c and activation of caspase-3.4 Using this method, a prognostic potential of intact apoptosis signaling in acute lymphoblastic leukemia (ALL) cells was described. A new parameter, cytochrome c–related activation of caspases (CRAC), was identified indicating intact or defective cytochrome c–related activation of caspases and implying prognostic impact.5 Based on these findings we analyzed 45 pediatric AML cell samples for the significance of intact apoptosis signaling for successful treatment.
Methods
The AML Berlin-Frankfurt-Münster (BFM) study was approved by the ethics commission of the board of physicians in Westfalen-Lippe, Germany, and the ethics review board of the faculty of medicine, University of Münster, Germany. Analysis of patient material was carried out in accordance with the guidelines of the AML BFM study.
Forty five diagnostic samples (frozen bone marrow or peripheral blood) obtained before treatment from pediatric de novo AML patients were analyzed. Patients were treated according to the AML BFM-98 or -2004 protocols after informed consent was obtained in accordance with the Declaration of Helsinki. Treatment and diagnosis of relapse were accomplished according to the AML BFM trial criteria. Treatment response or complete remission (CR) were examined by bone marrow morphology (BM) and detection of minimal residual disease (MRD) by flow cytometry (time point [tp] 1, day 15; tp2, days 21-28; tp3, days 42-56; and tp4, days 70-84 of treatment) as described earlier.1,6 Leukemia cells were analyzed for apoptosis signaling and correlated to clinical data as described previously.5 In brief, after induction of spontaneous apoptosis by factor deprivation in culture7 (with or without 100 μM zVADfmk), cells were analyzed by flow cytometry and evaluated in the caspase-3 versus cytochrome c plot. The parameters cell death, active caspase-3 (ac), total cytochrome c release (cctotal), caspase-dependent (ccdep) and -independent (ccindep) cytochrome c release were quantified as described earlier5 (Figure 1A).
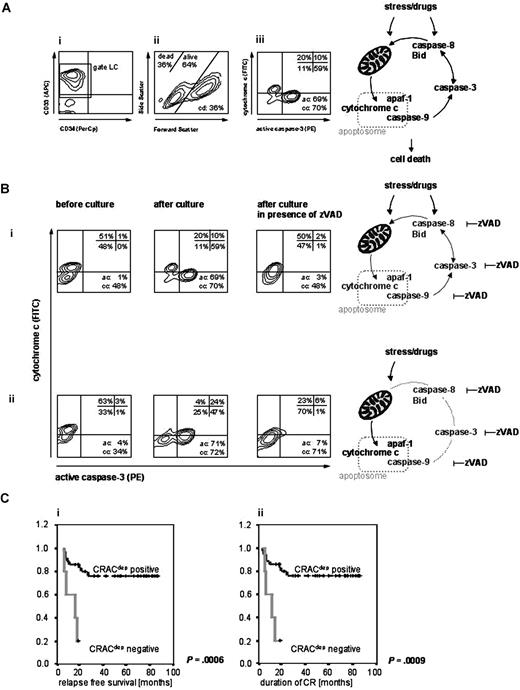
Quantification of apoptosis events in myeloid leukemia cells. Leukemia cells from pediatric AML patients were analyzed before and after culture with or without the caspase inhibitor zVADfmk by flow cytometry. (A) Identification of leukemia cells and analysis of cell death parameters. Leukemia cells were identified by surface markers (i: CD33 or CD34, gate LC) and further analysis was conducted gated on these cells. Cell death (cd) was estimated by forward/side scatter profile principally detecting apoptotic cells by changes of decreased volume and increased light scattering (ii). Activated caspase-3 (ac) and cytochrome c release (cc) were analyzed by simultaneous intracellular staining (phycoerythrin (PE)-conjugated anti–active caspase-3 and anti–cytochrome c 7H8.2C12 antibody, the latter followed by fluorescein-isothiocyanate (FITC)-conjugated goat anti–mouse IgG2b antibody) and quantified in the ac versus cc plot (iii). The staining was established using isotype-matched fluorochrome-conjugated unspecific antibodies. Cells with active caspase-3 were quantified as percentage of events in the upper and lower right quadrants (ac), cytochrome c release was estimated counting events in the lower left and right quadrants (cc). Because caspase-3 activation is completely caspase dependent, only the zVAD inhibitable caspase activation was considered (ac: difference of ac values after culture with and without zVAD); caspase dependent cytochrome c release (ccdep: difference of cc values after culture with and without zVAD) was discriminated from independent (ccindep: difference of cc values before and after culture with zVAD) and total cytochrome c release (cctotal, sum of ccdep and ccindep). (B) Distinct patterns of apoptosis signaling. Cytochrome c versus active caspase-3 plots for 2 patient samples showing cytochrome c release dependent (i) or independent (ii) on upstream or amplifier caspases such as caspase-8, resulting in positive (i) or negative (ii) CRACdep values. (C) Treatment outcome in different patient groups according to CRACdep. Superior relapse-free survival (i) and superior continuing remission (ii) of CRACdep-positive pediatric AML patients. Kaplan Meier analysis, P indicates significance.
Quantification of apoptosis events in myeloid leukemia cells. Leukemia cells from pediatric AML patients were analyzed before and after culture with or without the caspase inhibitor zVADfmk by flow cytometry. (A) Identification of leukemia cells and analysis of cell death parameters. Leukemia cells were identified by surface markers (i: CD33 or CD34, gate LC) and further analysis was conducted gated on these cells. Cell death (cd) was estimated by forward/side scatter profile principally detecting apoptotic cells by changes of decreased volume and increased light scattering (ii). Activated caspase-3 (ac) and cytochrome c release (cc) were analyzed by simultaneous intracellular staining (phycoerythrin (PE)-conjugated anti–active caspase-3 and anti–cytochrome c 7H8.2C12 antibody, the latter followed by fluorescein-isothiocyanate (FITC)-conjugated goat anti–mouse IgG2b antibody) and quantified in the ac versus cc plot (iii). The staining was established using isotype-matched fluorochrome-conjugated unspecific antibodies. Cells with active caspase-3 were quantified as percentage of events in the upper and lower right quadrants (ac), cytochrome c release was estimated counting events in the lower left and right quadrants (cc). Because caspase-3 activation is completely caspase dependent, only the zVAD inhibitable caspase activation was considered (ac: difference of ac values after culture with and without zVAD); caspase dependent cytochrome c release (ccdep: difference of cc values after culture with and without zVAD) was discriminated from independent (ccindep: difference of cc values before and after culture with zVAD) and total cytochrome c release (cctotal, sum of ccdep and ccindep). (B) Distinct patterns of apoptosis signaling. Cytochrome c versus active caspase-3 plots for 2 patient samples showing cytochrome c release dependent (i) or independent (ii) on upstream or amplifier caspases such as caspase-8, resulting in positive (i) or negative (ii) CRACdep values. (C) Treatment outcome in different patient groups according to CRACdep. Superior relapse-free survival (i) and superior continuing remission (ii) of CRACdep-positive pediatric AML patients. Kaplan Meier analysis, P indicates significance.
Results and discussion
Release of cytochrome c and consecutive apoptosome formation lead to activation of downstream effector caspases such as caspase-3, resulting in cells staining low for cytochrome c and positive for activated caspase-3 (Figure 1Bi,ii). Apoptosomal dysfunction results in impaired caspase-3 activation despite release of cytochrome c. Caspases other than caspase-3 acting upstream of mitochondria, such as caspase-8, are also involved in initiating or amplifying the formation or activity of this complex. By incubation in the presence or absence of the pan-caspase inhibitor zVADfmk, cytochrome c release (cc) was observed to be dependent (Figure 1Bi) or independent (Figure 1Bii) on upstream or amplifier caspases (ccdep, ccindep). Evaluating the differences in signaling pattern with respect to treatment outcome, the absolute values for cell death (cd), active caspase-3 (ac), and released cytochrome c (cctotal, ccdep and ccindep, respectively) were compared (independent samples t test) in patients grouped according to treatment response. No differences were found for the mean values of cd, cctotal and ccindep. However, differences in mean values for ccdep were detected comparing patients with good or poor response to treatment (BM; tp1: P = .036, tp3: P = .014 and CR/no CR: P = .016). Higher values for ac were observed in patients encountering relapse (overall relapse, trend P = .057; relapse within 12 months, P = .025 and within 24 months, P = .015) in contrast to not or late relapsing patients. According to the AML BFM protocol, patients are stratified into standard risk (SR) or high risk (HR) groups based on French-American-British (FAB) classification, cytogenetics, and response to induction therapy on day 15. Although in patient groups differentially responding on day 15 to induction therapy, no difference in ac values was found; HR patients showed higher ac values than SR patients (P = .002). Active caspase-3 was identified as an important factor for outcome in pediatric ALL and AML, however, with opposite features. While ALL patients with good response and no relapse revealed high levels of caspase activation, AML patients displayed an association of high caspase-3 activation to high risk and relapse. Likewise, in adult ALL high caspase-3 expression correlated with complete remission,8 whereas high levels of activated caspase-3 were associated with impaired apoptosis and decreased survival of adult AML patients.9 Together with conflicting reports on the prognostic relevance of caspase-3 in leukemia,10,–12 these findings strongly suggest that the complex regulated apoptosis signaling system is insufficiently represented if analyzed by one molecule alone. The findings also emphasise the importance to analyze checkpoints or molecules integrating diverse apoptosis signaling pathways, such as cytochrome c, caspase-3, and apoptosome formation.
We hypothesised that intact apoptosis signaling is characterized by concomitant release of cytochrome c and caspase-3 activation and therefore would be found in the patient groups with favorable outcome. Correlating these 2 events directly revealed that only cytochrome c release dependent on amplifying caspases (ccdep) significantly correlated with activated caspase-3 and was exclusively detected in patient groups with good response to initial treatment (BM blasts < 5%, tp1-4) or groups exhibiting MRD negativity (tp1-3, tp4 trend) (Table 1). The same finding was made in the groups achieving CR or retaining CR longer than 1 year and in the group without relapse. Remarkably, this correlation was completely absent in the group with poor response to initial treatment, groups not reaching CR or with CR less than 1 year as well as in the group of relapsing patients. This indicates that an intact apoptosome function and active amplifying caspases are elementary for favorable outcome in childhood AML.
Correlation of active caspase-3 to cytochrome c release, caspase dependent (ac to ccdep)
| . | n . | rs . | P . |
|---|---|---|---|
| Total | 45 | 0.360* | .015 |
| tp1, BM blast cells | |||
| Less than 5% | 28 | 0.568* | .002 |
| Greater than 5% | 9 | 0.548 | .127 |
| tp1, MRD (flow cytometry) | |||
| Negative | 13 | 0.642* | .018 |
| Positive | 23 | 0.358 | .094 |
| tp2, BM blast cells | |||
| Less than 5% | 14 | 0.646* | .043 |
| Greater than 5% | 21 | 0.287 | .124 |
| tp2, MRD (flow cytometry) | |||
| Negative | 14 | 0.528* | .014 |
| Positive | 21 | 0.055 | .851 |
| tp3, BM blast cells | |||
| Less than 5% | 26 | 0.619* | .006 |
| Greater than 5% | 14 | -0.094 | .750 |
| tp3, MRD (flow cytometry) | |||
| Negative | 8 | 0.764* | .027 |
| Positive | 12 | 0.267 | .402 |
| tp4, BM blast cells | |||
| Less than 5% | 36 | 0.497* | .002 |
| Greater than 5% | 4 | -0.258 | .742 |
| tp4, MRD (flow cytometry) | |||
| Negative | 23 | 0.386 | .069 |
| Positive | 7 | -0.204 | .661 |
| CR | |||
| Yes | 40 | 0.352* | .026 |
| No | 5 | 0.0 | .000 |
| Duration of CR, (solely CR patients) | |||
| More than 1 year | 32 | 0.460* | .008 |
| Less than 1year | 8 | -0.074 | .862 |
| Relapse | |||
| No | 28 | 0.519* | .005 |
| Yes | 12 | 0.134 | .679 |
| Relapse within 24 months after diagnosis | |||
| No | 29 | 0.516* | .004 |
| Yes | 11 | 0.070 | .838 |
| . | n . | rs . | P . |
|---|---|---|---|
| Total | 45 | 0.360* | .015 |
| tp1, BM blast cells | |||
| Less than 5% | 28 | 0.568* | .002 |
| Greater than 5% | 9 | 0.548 | .127 |
| tp1, MRD (flow cytometry) | |||
| Negative | 13 | 0.642* | .018 |
| Positive | 23 | 0.358 | .094 |
| tp2, BM blast cells | |||
| Less than 5% | 14 | 0.646* | .043 |
| Greater than 5% | 21 | 0.287 | .124 |
| tp2, MRD (flow cytometry) | |||
| Negative | 14 | 0.528* | .014 |
| Positive | 21 | 0.055 | .851 |
| tp3, BM blast cells | |||
| Less than 5% | 26 | 0.619* | .006 |
| Greater than 5% | 14 | -0.094 | .750 |
| tp3, MRD (flow cytometry) | |||
| Negative | 8 | 0.764* | .027 |
| Positive | 12 | 0.267 | .402 |
| tp4, BM blast cells | |||
| Less than 5% | 36 | 0.497* | .002 |
| Greater than 5% | 4 | -0.258 | .742 |
| tp4, MRD (flow cytometry) | |||
| Negative | 23 | 0.386 | .069 |
| Positive | 7 | -0.204 | .661 |
| CR | |||
| Yes | 40 | 0.352* | .026 |
| No | 5 | 0.0 | .000 |
| Duration of CR, (solely CR patients) | |||
| More than 1 year | 32 | 0.460* | .008 |
| Less than 1year | 8 | -0.074 | .862 |
| Relapse | |||
| No | 28 | 0.519* | .005 |
| Yes | 12 | 0.134 | .679 |
| Relapse within 24 months after diagnosis | |||
| No | 29 | 0.516* | .004 |
| Yes | 11 | 0.070 | .838 |
rs indicates correlation coefficient; and P, significance.
Correlation is significant (2-tailed).
The functional integrity of this important apoptogenic checkpoint is subsumed by the parameter “caspase dependent cytochrome c-related activation of caspase-3” (CRACdep), which was calculated as the difference of ac and ccdep in equivalence to the CRAC-value described for ALL.5 Proficient signaling results in positive CRACdep values in contrast to deficient signaling resulting in negative CRACdep values. Division of the patient cohort according to this new parameter resulted in 38 CRACdep-positive and 7 -negative patients. The distribution of CRACdep values was unrelated to features such as gender, FAB subtype, age or initial blast cell count (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Maintenance of CR longer than 1 year predisposes for favorable outcome in pediatric AML.13,14 Thirty of 32 patients sustaining CR for more than 1 year were CRACdep-positive (Fisher exact test, P = .015). Analysis of survival and remission duration revealed a superior outcome for CRACdep-positive patients overall but also specifically in the HR patient group. Notably, SR stratified patients merely displayed CRACdep-positivity (Figure 1C, Tables 2,3). Multivariate analysis revealed an increased risk for relapse and decreased probability of continuous CR for CRACdep negative patients in contrast to established risk factors like day 15 remission failure or hyperleucocytosis15 (Table 4).
Relapse-free survival in different risk groups according to CRACdep
| . | No. total . | No. censored . | No. of events (=relapse) . | Relapse-free survival, mo . | SE . | CI . | P . |
|---|---|---|---|---|---|---|---|
| All | |||||||
| CRACdep positive | 35 | 27 | 8 | 69.0 | 5.3 | 58.6-79.5 | <.001 |
| CRACdep negative | 5 | 1 | 4 | 13.3 | 2.2 | 8.9-17.6 | — |
| SR | |||||||
| CRACdep positive | 10 | 9 | 1 | 79.1 | 6.7 | 66.0-92.2 | — |
| CRACdep negative | — | — | — | — | — | — | — |
| HR | |||||||
| CRACdep positive | 24 | 17 | 7 | 63.4 | 6.9 | 49.7-77.0 | .010 |
| CRACdep negative | 5 | 1 | 4 | 13.3 | 2.2 | 8.9-17.6 | — |
| . | No. total . | No. censored . | No. of events (=relapse) . | Relapse-free survival, mo . | SE . | CI . | P . |
|---|---|---|---|---|---|---|---|
| All | |||||||
| CRACdep positive | 35 | 27 | 8 | 69.0 | 5.3 | 58.6-79.5 | <.001 |
| CRACdep negative | 5 | 1 | 4 | 13.3 | 2.2 | 8.9-17.6 | — |
| SR | |||||||
| CRACdep positive | 10 | 9 | 1 | 79.1 | 6.7 | 66.0-92.2 | — |
| CRACdep negative | — | — | — | — | — | — | — |
| HR | |||||||
| CRACdep positive | 24 | 17 | 7 | 63.4 | 6.9 | 49.7-77.0 | .010 |
| CRACdep negative | 5 | 1 | 4 | 13.3 | 2.2 | 8.9-17.6 | — |
CI indicates 95% confidence interval; and —, not applicable.
Duration of complete remission (CR) in different risk groups according to CRACdep
| Treatment groups . | No. total . | No. censored . | No. of events (=relapse) . | Duration of CR, mo . | SE . | CI . | P . |
|---|---|---|---|---|---|---|---|
| All | |||||||
| CRACdep positive | 35 | 27 | 8 | 68.5 | 5.4 | 58.8-79.1 | .001 |
| CRACdep negative | 5 | 1 | 4 | 11.4 | 2.2 | 7.1-15.7 | — |
| SR | |||||||
| CRACdep positive | 10 | 9 | 1 | 78.7 | 6.9 | 65.3-92.2 | — |
| CRACdep negative | — | — | — | — | — | — | — |
| HR | |||||||
| CRACdep positive | 24 | 17 | 7 | 62.6 | 7.1 | 48.7-76.6 | .014 |
| CRACdep negative | 5 | 1 | 4 | 11.4 | 2.2 | 7.1-15.7 | — |
| Treatment groups . | No. total . | No. censored . | No. of events (=relapse) . | Duration of CR, mo . | SE . | CI . | P . |
|---|---|---|---|---|---|---|---|
| All | |||||||
| CRACdep positive | 35 | 27 | 8 | 68.5 | 5.4 | 58.8-79.1 | .001 |
| CRACdep negative | 5 | 1 | 4 | 11.4 | 2.2 | 7.1-15.7 | — |
| SR | |||||||
| CRACdep positive | 10 | 9 | 1 | 78.7 | 6.9 | 65.3-92.2 | — |
| CRACdep negative | — | — | — | — | — | — | — |
| HR | |||||||
| CRACdep positive | 24 | 17 | 7 | 62.6 | 7.1 | 48.7-76.6 | .014 |
| CRACdep negative | 5 | 1 | 4 | 11.4 | 2.2 | 7.1-15.7 | — |
Risk group assignment was not available for one patient (CRACdep positive and no relapse) in these analyses.
P indicates significance by log rank test; and —, not applicable.
Multivariate analyses of risk ratio for relapse or continuous complete remission (CCR) using the Cox regression model
| . | Risk ratio . | CI . | P . | |
|---|---|---|---|---|
| Relapse . | CCR . | |||
| CRACdep negativity | 6.08 | — | 1.21-30.52 | .028 |
| Failure of remission on day 15 | 3.41 | — | 0.85-13.69 | .083 |
| Hyperleucocytosis (WBC> 100 × 109/L) | 2.09 | — | 0.53-8.21 | .293 |
| CRACdep negativity | — | .15 | 0.03-0.75 | .020 |
| Failure of remission on day 15 | — | .24 | 0.06-0.91 | .036 |
| Hyperleucocytosis (WBC> 100 × 109/L) | — | .45 | 0.11-1.84 | .264 |
| . | Risk ratio . | CI . | P . | |
|---|---|---|---|---|
| Relapse . | CCR . | |||
| CRACdep negativity | 6.08 | — | 1.21-30.52 | .028 |
| Failure of remission on day 15 | 3.41 | — | 0.85-13.69 | .083 |
| Hyperleucocytosis (WBC> 100 × 109/L) | 2.09 | — | 0.53-8.21 | .293 |
| CRACdep negativity | — | .15 | 0.03-0.75 | .020 |
| Failure of remission on day 15 | — | .24 | 0.06-0.91 | .036 |
| Hyperleucocytosis (WBC> 100 × 109/L) | — | .45 | 0.11-1.84 | .264 |
Number of patients was 35 (day 15 remission status was unknown for 5 of 40 patients who achieved remission).
WBC indicates white blood cell count; CCR, continuous complete remission; and —, not applicable.
Taken together, we clearly found that the propensity to undergo apoptosis of leukemia cells delineated by intact cytochrome c related caspase-3 activation (CRACdep) is required for successful treatment of pediatric AML. Analysis of the functional integrity of this apoptosis checkpoint can thus be used for future treatment stratification.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Johanna Pfeil for her excellent technical assistance.
This work was supported by a research fellowship of the European Hematology Association (L.H.M.) and a grant of the Deutsche Forschungsgemeinschaft (K.S. and K.-M. D.).
Authorship
Contribution: L.H.M. designed research, performed experiments, analyzed data, and wrote the manuscript; M.Q. performed experiments, analyzed data, prepared the figures, and critically read the manuscript; S.M.E. analyzed data and critically read the manuscript; U.C., D.R., and W.-D.L. collected and provided patient samples and clinical data, and critically read the manuscript; K.S. and L.K. designed research and critically read the manuscript; and K.-M.D. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Klaus-Michael Debatin, University of Ulm, Department of Paediatrics and Adolescent Medicine, Eythstrasse 24, 89075 Ulm, Germany; e-mail: klaus-michael.debatin@uniklinik-ulm.de.
References
Author notes
K.S. and K.-M.D. contributed equally to this paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal