Abstract
Hematopoietic stem cells (HSCs) are the basis of bone marrow transplantation and are attractive target cells for hematopoietic gene therapy, but these important clinical applications have been severely hampered by difficulties in ex vivo expansion of HSCs. In particular, the use of cord blood for adult transplantation is greatly limited by the number of HSCs. Previously we identified angiopoietin-like proteins and IGF-binding protein 2 (IGFBP2) as new hormones that, together with other factors, can expand mouse bone marrow HSCs in culture. Here, we measure the activity of multipotent human severe combined immunodeficient (SCID)–repopulating cells (SRCs) by transplantation into the nonobese diabetic SCID (NOD/SCID) mice; secondary transplantation was performed to evaluate the self-renewal potential of SRCs. A serum-free medium containing SCF, TPO, and FGF-1 or Flt3-L cannot significantly support expansion of the SRCs present in human cord blood CD133+ cells. Addition of either angiopoietin-like 5 or IGF-binding protein 2 to the cultures led to a sizable expansion of HSC numbers, as assayed by NOD/SCID transplantation. A serum-free culture containing SCF, TPO, FGF-1, angiopoietin-like 5, and IGFBP2 supports an approximately 20-fold net expansion of repopulating human cord blood HSCs, a number potentially applicable to several clinical processes including HSC transplantation.
Introduction
The hematopoietic stem cell (HSC), through proliferation and differentiation, gives rise to all lymphoid, myeloid, and erythroid cells. Many clinical applications of HSCs would become feasible if there were a culture system that expanded HSC numbers while maintaining stem-cell pluripotency.1-3 In particular, the ex vivo expansion of human cord blood HSCs would make this important source of cells useful for adult applications.4 During the last 2 decades, numerous attempts have been made to expand human cord blood HSCs in culture.3,5 Several types of primitive cord blood cells including those positive for CD34 or CD133 have been used as the starting populations.5 Most ex vivo culture of human cord blood HSCs used cytokine mixtures that included SCF, TPO, and Flt3-L. For example, Ueda et al showed that addition of a complex of IL-6 and soluble IL-6 receptor (IL-6/sIL-6R) to SCF, TPO, and Flt3-L supported expansion of human severe combined immunodeficient (SCID)–repopulating cells (SRCs).6 The Piacibello group reported a striking increase of SRC number of cord blood cells after 16 weeks of culture (Gammaitoni et al7 ). Summers et al reported a very high incidence of long-term culture-initiating cell (LTC-IC) and colony-forming cell (CFC) expansion in CD133+ cell cultures.8 Araki et al demonstrated that the treatment of cord blood CD34+ cells with chromatin-modifying agents 5-aza-2′-deoxycytidine and trichostatin A resulted in a 9.6-fold expansion of SRC numbers following 9 days of culture.9 Using serum-free medium supplemented with SCF, TPO, Flt3-L, IL-3, IL-6/sIL-6R, and Delta 1, Suzuki et al reported an approximate 6-fold increase of SRC numbers.10 In addition, a cell-permeable fusion protein containing the HOX B4 transcription factor, when expressed by stromal cells, stimulated ex vivo expansion of human SRCs.11 So far, the proper mixture of growth factors and cytokines used in culture conditions has not yet been determined, and new growth factors for HSCs are needed
We identified a novel cell population that supports mouse HSC expansion ex vivo—CD3+Ter119− cells isolated from embryonic day 15 (E15) mouse fetal livers. From these we identified IGF-212,13 and a group of angiopoietin-like proteins (Angptls) as new growth factors for mouse HSCs.14 We subsequently developed a simple serum-free culture system for mouse bone marrow HSCs; these optimized cultures use low but saturating levels of SCF, TPO, IGF-2, FGF-1, and Angptls. As measured by competitive repopulation analyses, there was a 24- to 30-fold increase in numbers of long-term repopulating HSCs (LT-HSCs) after 10 days of culture of highly enriched mouse HSCs.14 We recently identified IGF-binding protein 2 (IGFBP2) as another secreted protein that supports mouse HSC expansion (H.H., S.I., M.K., O. Kirak, H.F.L., and C.C.Z., unpublished data, September 2007). Based on these results, we sought to use a serum-free medium supplemented with IGFBP2 and Angptl protein, together with other growth factors, to culture and expand human HSCs.
Methods
Mice
Nonobese diabetic SCID (NOD/SCID; NOD.CB17-Prkdcscid/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were maintained at the Whitehead Institute or University of Texas Southwestern Medical Center animal facilities. All animal experiments were performed with the approval of MIT or University of Texas Southwestern Committee on Animal Care.
Culture medium
STIF medium is StemSpan serum-free medium (StemCell Technologies,Vancouver, BC) supplemented with 10 μg/mL heparin (Sigma-Aldrich, St Louis, MO), 10 ng/mL mouse SCF, 20 ng/mL mouse TPO, 20 ng/mL mouse IGF-2 (all from R&D Systems, Minneapolis, MN), and 10 ng/mL human FGF-1 (Invitrogen, Frederick, MD). STF medium is the same medium without IGF-2. When medium contained Flt3-L, we used 50 ng/mL human SCF, 10 ng/mL human TPO, and 50 ng/mL human Flt3-L. Indicated amounts of human Angptl5 (Abnova, Taipei City, Taiwan) or human IGFBP2 (R&D Systems) were added.
Human cell culture
Fresh and cryopreserved human cord blood cells were purchased from Cambrex (East Rutherford, NJ), StemCell Technologies, and AllCells (Berkeley, CA). All of cells were from pooled donors. CD34+ or CD133+ purity checked by flow cytometry was higher than 90%. After thawing, the cell viability tested by trypan blue exclusion was higher than 72%. The thawed cells were centrifuged and resuspended with StemSpan medium before being aliquoted for immediate transplantation or culture. In Figure 1, total human cord blood mononuclear cells were plated at 106 cells/mL STIF medium, with 100 ng/mL Angptl3 or Angptl5. Medium volume was increased by adding fresh medium at days 5, 8, 12, 15, and 18 to maintain cell densities at 5 × 105 to 1.5 × 106 cells/mL. Cells were cultured at 37°C in 5% CO2 and normal O2. Fresh human cord blood CD34+ cells and cryopreserved CD133+ cells used in the experiments in Figures 2 through 5 were plated at 104 cells/well in one well of a U-bottom 96-well plate (3799; Corning, Corning, NY) with 200 μL of the indicated medium for 2 days. On day 3, cells were pooled from individual wells and transferred to 6-well plates at 5 × 104 cells/mL. Fresh medium was added at days 4 and 7 to keep the cell density at 2 × 105 cells/mL (day 4) or 7 × 105/mL (day 7). Cells were cultured at 37°C in 5% CO2, and normal O2 or 5% O2 (low O2) levels. For transplantation, we pooled cells from all the culture wells before the indicated numbers of cells were transplanted into each mouse.
NOD/SCID transplantation
Uncultured or cultured progenies of human total cord blood mononuclear cells or CD133+ or CD34+ cells at indicated days were pooled together and the indicated portions were injected intravenously via the retro-orbital route into sublethally irradiated (3.5 Gy) 8- to 10-week-old NOD/SCID mice. Eight weeks after transplantation, bone marrow nucleated cells from animals that underwent transplantation were analyzed by flow cytometry for the presence of human cells. For secondary transplantations, bone marrow aspirates from one hind leg of a primary recipient were transplanted into 2 secondary recipients, as described.15 Calculation of competitive repopulating units (CRUs) in limiting-dilution experiments was conducted using L-Calc software (StemCell Technologies).13,14,16 For limiting-dilution analysis, mice were considered positive for human HSC engraftment when at least 1% (for primary transplantation) or 0.1% (for secondary transplantation) CD45/71+ human cells were detected among the mouse bone marrow cells, unless otherwise indicated.
Flow cytometry
For analyzing human hematopoietic engraftment in NOD/SCID mice, we followed a published protocol.17 Briefly, bone marrow cells from recipient NOD/SCID mice were stained with antihuman CD45-PE, CD71-PE, CD15-FITC, and CD66b-FITC to quantify the total human hematopoietic (CD45/71+) cell population as well as the subset of exclusively granulopoietic (CD15/66b+) cells within this population. Cells were stained with anti–human CD34-FITC and anti–human CD19-PE and CD20-PE to quantify human progenitor (CD34+) and B-lineage (CD34− CD19/20+) populations. In the experiment of Figure 1, only total human hematopoietic (CD45/71+) engraftment was measured. Antihuman CD34-FITC was used to quantitate CD34+ cells in culture. All antihuman antibodies were purchased from Becton Dickinson (Mountain View, CA).
Results
Culture of total human cord blood cells in serum-free medium containing Angptl5 stimulates ex vivo expansion of SRCs
Recently we showed that culture of mouse bone marrow side population (SP) CD45+Sca-1+ cells in serum-free medium containing 10 ng/mL SCF, 20 ng/mL TPO, 20 ng/mL IGF-2, and 10 ng/mL FGF-1 (STIF medium) supplemented with one of several Angptls—Angptl2, Angptl3, Angptl5, Angptl7, and Mfap4—stimulated a dramatic expansion of LT-HSC numbers and activities.14 We sought to test whether this culture could also expand human cord blood HSCs. To this end, total human cord blood cells (Figure 1A) were cultured in STIF medium containing Angptl5.18 We seeded 2.5 × 107 total cord blood cells at a density of 106 cells/mL medium. After 23 days of culture, the number of total cells in the presence of Angptl5 increased to 2.2 (± 0.3) × 108 cells (Figure 1A). The cultured cells were mostly in suspension with a minor adherent subpopulation.
Culture of total human cord blood cells in the presence of Angptl5 stimulates ex vivo expansion of HSCs. (A) Culture of 2.5 × 107 total human cord blood cells was initiated in serum-free STIF medium or the same STIF medium containing 100 ng/mL Angptl5, and total cell numbers were counted at the indicated time. (B) Amount of human chimerism in the bone marrow of NOD/SCID mice that received a transplant of 106 uncultured human mononuclear cord blood cells or the cultured progeny of 106 initial human cord blood cells. Each symbol represents the engraftment of a single mouse that underwent transplantation assayed at 2 months after transplantation. *Significantly different from lane 1 value. Student t test, P < .001.
Culture of total human cord blood cells in the presence of Angptl5 stimulates ex vivo expansion of HSCs. (A) Culture of 2.5 × 107 total human cord blood cells was initiated in serum-free STIF medium or the same STIF medium containing 100 ng/mL Angptl5, and total cell numbers were counted at the indicated time. (B) Amount of human chimerism in the bone marrow of NOD/SCID mice that received a transplant of 106 uncultured human mononuclear cord blood cells or the cultured progeny of 106 initial human cord blood cells. Each symbol represents the engraftment of a single mouse that underwent transplantation assayed at 2 months after transplantation. *Significantly different from lane 1 value. Student t test, P < .001.
We performed NOD/SCID repopulation assays19 to test whether ex vivo–expanded cells were capable of engraftment. Thus, 106 uncultured cells or their cultured progenies were injected into sublethally irradiated NOD/SCID recipients (Figure 1B). When 9.2 × 106 cells cultured with Angtpl5 for 19 days, that is, the progeny of 106 initially plated total cord blood cells, were transplanted, an average human hematopoietic chimerism of 8.8% was observed 2 months after transplantation (Figure 1B lane 2). This is much greater than the 0.6% engraftment shown by the equivalent 106 uncultured cells (Figure 1B lane 1; P < .001, Student t test). Since the cells cultured in STIF medium with Angptl5 exhibited significantly higher engraftment than uncultured cells, we opted to use Angptl5 in our further experiments.
Angptl5 and IGFBP2 individually stimulate ex vivo expansion of cultured human cord blood CD34+ and CD133+ cells
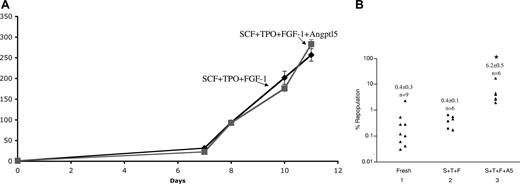
We next sought to test whether we could expand freshly isolated human cord blood CD34+ cells, an enriched HSC population routinely used in clinical applications. We seeded 104 CD34+ cells; as shown in Figure 2A, after 11 days of culture, the number of cells cultured in the presence of SCF + TPO + FGF-1 (STF medium) increased more than 250-fold. Addition of Angptl5 did not significantly change the overall extent of cell proliferation. We noticed that, at the end of the culture, most cells were in suspension but there was a minor adherent subpopulation.
Culture of human cord blood CD34+ cells in the presence of Angptl5 stimulates ex vivo expansion of SRCs. (A) Culture of 104 fresh human cord blood CD34+ cells was initiated in serum-free STF medium or STF medium supplemented with 500 ng/mL human Angptl5. Total cell numbers were counted. (B) Extent of human chimerism in the bone marrow of NOD/SCID mice that received a transplant of 10 000 fresh human cord blood CD34+ cells, or the progenies of initial 5000 cells cultured for 10 days in STF medium or STF medium supplemented with Angptl5. Each symbol represents the engraftment of a single mouse that underwent transplantation assayed at 2 months after transplantation. *Significantly different from lanes 1 and 2. Student t test, P < .05.
Culture of human cord blood CD34+ cells in the presence of Angptl5 stimulates ex vivo expansion of SRCs. (A) Culture of 104 fresh human cord blood CD34+ cells was initiated in serum-free STF medium or STF medium supplemented with 500 ng/mL human Angptl5. Total cell numbers were counted. (B) Extent of human chimerism in the bone marrow of NOD/SCID mice that received a transplant of 10 000 fresh human cord blood CD34+ cells, or the progenies of initial 5000 cells cultured for 10 days in STF medium or STF medium supplemented with Angptl5. Each symbol represents the engraftment of a single mouse that underwent transplantation assayed at 2 months after transplantation. *Significantly different from lanes 1 and 2. Student t test, P < .05.
Next, fresh CD34+ cells and their cultured progenies were transplanted into sublethally irradiated NOD/SCID mice. Ten thousand fresh CD34+ cells generated an average of chimerism of 0.4% at 2 months after transplantation (Figure 2B lane 1). The progenies of 5000 initially plated cells that were cultured in STF medium had a modest engraftment of 0.4% (± 0.1%; Figure 2B lane 2). Importantly, addition of Angplt5 to the culture medium significantly increased the engraftment to 6.2% (± 0.5%; Figure 2B compare lane 3 vs lanes 1 and 2, P < .05). This demonstrates that Angptl5 supports ex vivo expansion of human SRCs.
In a recent study, we showed that the secreted protein IGF-binding protein 2 (IGFBP2) is able to support ex vivo expansion of mouse HSCs. IGFBP2 can replace IGF-2 in HSC cultures; a serum-free medium containing SCF, TPO, FGF-1, Angptl3, and IGFBP2 supports a dramatic increase in numbers of long-term repopulating mouse HSCs, as measured by competitive repopulation analyses (H.H., S.I., M.K., O. Kirak, H.F.L., and C.C.Z., unpublished data, September 2007).
To test the role of IGFBP2 on human HSCs, and to confirm the effect of Angptl5, we used a different serum-free medium containing human SCF, TPO, and Flt3-L, a commonly used cocktail for human cell culture, as the basal medium to culture cryopreserved CD133+ cord blood cells. The total number of cells increased more than 210-, 162-, and 220-fold in the basal medium, basal medium supplemented with IGFBP2, and basal medium supplemented with Angptl5, respectively, over the 10-day culture (Figure 3A). Next, 8000 uncultured CD133+ cells, or their cultured progenies, were transplanted into NOD/SCID mice. Both IGFBP2 and Angptl5 significantly enhanced ex vivo expansion of SRCs (Figure 3B). Transplantation of cells cultured in the presence of SCF, TPO, and Flt3-L generated mice that had an average of 0.8% human cells in the bone marrow. Addition of IGFBP2 or Angptl5 to the culture increased the chimerism to 11.3% and 17.3%, respectively (Figure 3B compare lanes 1, 5, and 9). Both IGFBP2 (Figure 3B lanes 6-8) and Angptl5 (Figure 3B lanes 10-12) led to increases in the formation of human myeloid cells (CD15/66b+), B-lymphoid cells (CD34−CD19/20+), and primitive (CD34+) human cells in the bone marrow (Figure 3B). Thus either IGFBP2 or Angptl5, when added together with SCF, TPO, and Flt3-L, stimulated expansion of human cord blood SRCs.
Culture of human cord blood CD133+ cells in the presence of IGFBP2 or Angptl5 stimulates ex vivo expansion of SRCs. (A) Culture of 104 cryopreserved human cord blood CD133+ cells was initiated in serum-free StemSpan medium supplemented with 50 ng/mL human SCF, 10 ng/mL human TPO, and 50 ng/mL human Flt3-L, together with 100 ng/mL human IGFBP2 or 500 ng/mL human Angptl5. Total cell numbers were counted during the 10 days of culture period. (B) Multilineage engraftment in NOD/SCID recipients that received a transplant of cultured progenies from 8000 initial CD133+ cells (n = 6-9). Lanes 1-4 show cells cultured in SCF, TPO, and Flt3-L; lanes 5-8, cells cultured in SCF, TPO, Flt3-L, and IGFBP2; lanes 9-12, cells cultured in SCF, TPO, Flt3-L, and Angptl5. *Value is significantly different from the value of the lane 1 cells. Student t test, P < .05.
Culture of human cord blood CD133+ cells in the presence of IGFBP2 or Angptl5 stimulates ex vivo expansion of SRCs. (A) Culture of 104 cryopreserved human cord blood CD133+ cells was initiated in serum-free StemSpan medium supplemented with 50 ng/mL human SCF, 10 ng/mL human TPO, and 50 ng/mL human Flt3-L, together with 100 ng/mL human IGFBP2 or 500 ng/mL human Angptl5. Total cell numbers were counted during the 10 days of culture period. (B) Multilineage engraftment in NOD/SCID recipients that received a transplant of cultured progenies from 8000 initial CD133+ cells (n = 6-9). Lanes 1-4 show cells cultured in SCF, TPO, and Flt3-L; lanes 5-8, cells cultured in SCF, TPO, Flt3-L, and IGFBP2; lanes 9-12, cells cultured in SCF, TPO, Flt3-L, and Angptl5. *Value is significantly different from the value of the lane 1 cells. Student t test, P < .05.
IGFBP2 and Angptl5 together stimulate extensive ex vivo expansion of SRCs of cultured human cord blood CD133+ cells
In the experiment described in Figure 4, a representative of 3 independent experiments, we added IGFBP2 and Angptl5, together with SCF, TPO, and FGF-1 to our serum-free culture. A recent report suggested that hypoxia improves expansion of human SRCs20 and thus in parallel we sought to use a low O2 pressure (5% O2) to expand human HSCs. As shown in Figure 4A, we plated 104 cryopreserved human cord blood CD133+ cells; after 11 days of culture in 5% O2, the total numbers of cells increased greater than 200-fold either in STF medium or STF medium containing Angptl5 and IGFBP2.
Culture of human cord blood CD133+ cells in the presence of Angptl5 and IGFBP2 stimulates ex vivo expansion of SRCs. (A) Culture of 104 cryopreserved human cord blood CD133+ cells was initiated in serum-free STF medium (containing SCF, TPO, and FGF-1), or in STF medium supplemented with 500 ng/mL human Angptl5 and 500 ng/mL human IGFBP2, at 5% O2. Total cell numbers were counted during the 11 days of culture period. (B) Extent of human chimerism in the bone marrow of NOD/SCID mice that received a transplant of 8000 or 15 000 uncultured human cord blood CD133+ cells, or the progenies of 8000 initial CD133+ cells cultured in STF medium with or without Angptl5 and IGFBP2 for 11 days. Each symbol represents the engraftment of a single mouse that underwent transplantation assayed at 2 months after transplantation (n = 7-8). *Significantly different from lanes 1-3. Student t test, P < .05. (C) Representative fluorescence-activated cell sorting (FACS) plots of bone marrow cells from one mouse at the condition represented by lane 1 of panel B (after thaw), or at the condition represented by lane 4 of panel B (cultured in STF medium containing IGFBP2 and Angptl5) at 2 months after transplantation. Percentages of cells in each quadrant are listed. (D) Summary of multilineage reconstitution from mice in lanes 2 and 4 of panel B (n = 8, respectively). Some mice that received a transplant of uncultured cells had 0% donor repopulation and these data points are not plotted. *Values are significantly different from values of the uncultured cells. Student t test, P < .05. (E) Bone marrow cells collected from mice represented by lane 4 of panel B were transplanted into secondary recipients; bone marrow aspirate from one hind leg from a primary recipient was transplanted into 2 secondary recipients. Multilineage engraftment in secondary NOD/SCID recipients was assayed at 5 to 8 weeks after transplantation (n = 12 mice underwent transplantation).
Culture of human cord blood CD133+ cells in the presence of Angptl5 and IGFBP2 stimulates ex vivo expansion of SRCs. (A) Culture of 104 cryopreserved human cord blood CD133+ cells was initiated in serum-free STF medium (containing SCF, TPO, and FGF-1), or in STF medium supplemented with 500 ng/mL human Angptl5 and 500 ng/mL human IGFBP2, at 5% O2. Total cell numbers were counted during the 11 days of culture period. (B) Extent of human chimerism in the bone marrow of NOD/SCID mice that received a transplant of 8000 or 15 000 uncultured human cord blood CD133+ cells, or the progenies of 8000 initial CD133+ cells cultured in STF medium with or without Angptl5 and IGFBP2 for 11 days. Each symbol represents the engraftment of a single mouse that underwent transplantation assayed at 2 months after transplantation (n = 7-8). *Significantly different from lanes 1-3. Student t test, P < .05. (C) Representative fluorescence-activated cell sorting (FACS) plots of bone marrow cells from one mouse at the condition represented by lane 1 of panel B (after thaw), or at the condition represented by lane 4 of panel B (cultured in STF medium containing IGFBP2 and Angptl5) at 2 months after transplantation. Percentages of cells in each quadrant are listed. (D) Summary of multilineage reconstitution from mice in lanes 2 and 4 of panel B (n = 8, respectively). Some mice that received a transplant of uncultured cells had 0% donor repopulation and these data points are not plotted. *Values are significantly different from values of the uncultured cells. Student t test, P < .05. (E) Bone marrow cells collected from mice represented by lane 4 of panel B were transplanted into secondary recipients; bone marrow aspirate from one hind leg from a primary recipient was transplanted into 2 secondary recipients. Multilineage engraftment in secondary NOD/SCID recipients was assayed at 5 to 8 weeks after transplantation (n = 12 mice underwent transplantation).
We transplanted cells before and after culture into sublethally irradiated NOD/SCID mice. Eight thousand uncultured CD133+ cells had an average chimerism of 0.2% at 2 months after transplantation (Figure 4B lane1) and 15 000 uncultured CD133+ cells showed an increased but still modest engraftment—an average chimerism of 2.0% (Figure 4B lane 2). In striking contrast, 2.1 × 106 cells after 11 days of culture with SCF, TPO, FGF-1, Angptl5, and IGFBP2—that is, the progenies of 8000 initial cells—engrafted all recipient mice, and showed significantly increased chimerism relative to that from 8000 or 15 000 uncultured cells (average 39.5%; Figure 4B lane 4; P < .05, Student t test). In contrast, the cultured progenies of the same 8000 initial cells cultured in STF medium without Angptl5 and IGFBP2 (now 1.6 × 106 cells) exhibited poor engraftment, similar to that of their uncultured counterparts (Figure 4B lane 3).
Figure 4C shows human hematopoietic engraftment at 2 months in representative mice that received a transplant of uncultured or cultured human cord blood CD133+ cells. A mouse that received a transplant of cells cultured in STF medium containing both Angptl5 and IGFBP2 (Figure 4B lane 4) displayed a much higher engraftment of total hematopoietic (CD45/71+ 58%), myeloid (CD15/66b+ 8.1%), B-lymphoid (CD34−CD19/20+ 37%), and primitive (CD34+ 4%) human cells than the mouse that received the transplant of uncultured cells (Figure 3B lane 1: 0.19%, 0.03%, 0.1%, and 0.02%, respectively). A summary of multilineage engraftment of mice that received a transplant of uncultured cells (Figure 4B lane 2) and cells cultured in STF medium containing Angptl5 and IGFBP2 (Figure 4B lane 4) is shown in Figure 4D. The progenies of 8000 cells, after culture, repopulated myeloid and lymphoid lineages 2 months after transplantation, attesting to the expansion of human stem cell activity.
To measure the self-renewal potential of SRCs after culture in medium containing IGFBP2 and Angptl5, we collected bone marrow from the primary mice that received a transplant of uncultured cells (Figure 4B lane 2) or cells cultured in STF medium containing Angptl5 and IGFBP2 (Figure 4B lane 4) and transplanted the marrow into sublethally irradiated secondary recipients.15,21 While uncultured cells could not engraft secondary recipients (not shown), the cultured cells showed positive engraftment of myeloid, B-lymphoid, and primitive cells after the secondary transplantation (Figure 4E). These data indicate a net expansion of HSCs during the initial culture period, and we thus conclude that Angptl5 and IGFBP2 together support extensive ex vivo expansion of human SRCs.
We confirmed that we were able to dramatically expand human SRCs in our culture system in 2 additional independent experiments that also directly tested the response of HSCs to culture in low and ambient oxygen. In a representative study, we cultured 2 × 105 cryopreserved human cord blood CD133+ cells in STF medium containing Angptl5 and IGFBP2 under normal or low O2 conditions. After 10 days of culture, the number of total cells in both cultures had increased more than 200-fold (Figure 5A). We did observe a slightly higher number of CD34+ primitive cells after 5 days of culture at normal versus low O2 (Figure 5B). As part of the limiting-dilution assay to quantitate the SRC frequencies before and after culture, we measured the engraftment by 5000, 10 000, and 20 000 uncultured CD133+ cells, and the progenies of 1667, 5000, and 10 000 cells after culture. Figure 5C shows that all mice that received a transplant of the cultured progenies of 1667 CD133+ cells engrafted at a level greater than 0.1%. In a limiting-dilution assay, recipient mice are often considered positive for human HSC engraftment when at least 0.1% of human cells were detected among the mouse bone marrow cells.11 If we use this standard, then the CRU for cultured cells cannot be measured, as at the lowest dose all recipient mice are positive (Figure 5C).
Limiting-dilution analysis of human cord blood CD133+ cells transplanted into NOD/SCID mice after culture at normal or low oxygen levels. (A,B) Culture of 2 × 105 cryopreserved human cord blood CD133+ cells was initiated in serum-free STF medium supplemented with 500 ng/mL human Angptl5 and human 100 ng/mL IGFBP2. The numbers of total cells (A) and CD34+ cells (B) were counted. (C) Amount of human chimerism in the bone marrow of NOD/SCID mice that received a transplant of the indicated numbers (5000, 10 000, 20 000) of postthaw uncultured human cord blood CD133+ cells, or the progenies of 1667, 5000, or 10 000 CD133+ cells cultured in STF medium with Angptl5 and IGFBP2 in ambient oxygen (lanes 4-6) or 5% oxygen (lanes 7-9) for 10 days. Each symbol represents the engraftment of a single mouse that underwent transplantation assayed at 2 months after transplantation. Horizontal dotted lines represent arbitrary cutoffs of 0.1% and 1% reconstitution. (D,E) Limiting-dilution analysis of the repopulating ability of cells before culture (D) and after culture for 10 days in serum-free STF medium containing 500 ng/mL Angptl5 and 100 ng/mL IGFBP2 at normal or low O2 levels (E). Plotted is the percentage of recipient mice containing less than 1% human hematopoietic populations in recipient mouse bone marrow 6 to 8 weeks after transplantation versus the number of input or input-equivalent cells injected. The progenies of 10 000 input cells cultured at normal or low O2 repopulated all recipients and these data points (0% negative mice) are not plotted. (F) Multilineage engraftment in NOD/SCID recipients that received a transplant of 20 000 postthaw uncultured CD133+ cells (n = 8) or cultured progenies of 5000 initial CD133+ cells at normal O2 (n = 10). Some mice that received a transplant of uncultured cells had 0% donor repopulation and these data points are not plotted.
Limiting-dilution analysis of human cord blood CD133+ cells transplanted into NOD/SCID mice after culture at normal or low oxygen levels. (A,B) Culture of 2 × 105 cryopreserved human cord blood CD133+ cells was initiated in serum-free STF medium supplemented with 500 ng/mL human Angptl5 and human 100 ng/mL IGFBP2. The numbers of total cells (A) and CD34+ cells (B) were counted. (C) Amount of human chimerism in the bone marrow of NOD/SCID mice that received a transplant of the indicated numbers (5000, 10 000, 20 000) of postthaw uncultured human cord blood CD133+ cells, or the progenies of 1667, 5000, or 10 000 CD133+ cells cultured in STF medium with Angptl5 and IGFBP2 in ambient oxygen (lanes 4-6) or 5% oxygen (lanes 7-9) for 10 days. Each symbol represents the engraftment of a single mouse that underwent transplantation assayed at 2 months after transplantation. Horizontal dotted lines represent arbitrary cutoffs of 0.1% and 1% reconstitution. (D,E) Limiting-dilution analysis of the repopulating ability of cells before culture (D) and after culture for 10 days in serum-free STF medium containing 500 ng/mL Angptl5 and 100 ng/mL IGFBP2 at normal or low O2 levels (E). Plotted is the percentage of recipient mice containing less than 1% human hematopoietic populations in recipient mouse bone marrow 6 to 8 weeks after transplantation versus the number of input or input-equivalent cells injected. The progenies of 10 000 input cells cultured at normal or low O2 repopulated all recipients and these data points (0% negative mice) are not plotted. (F) Multilineage engraftment in NOD/SCID recipients that received a transplant of 20 000 postthaw uncultured CD133+ cells (n = 8) or cultured progenies of 5000 initial CD133+ cells at normal O2 (n = 10). Some mice that received a transplant of uncultured cells had 0% donor repopulation and these data points are not plotted.
To make a comparison possible between cultured and uncultured cells, we arbitrarily chose to use 1% chimerism as the cutoff for positive engraftment. Based on this standard, Figure 5D shows that the frequency of repopulating cells (CRUs) for this particular sample of uncultured CD133+ cells is 1 per 64 075 cells (95% confidence interval for mean: 1/23 919 to 1/171 643, n = 25). That is, as calculated from Poisson statistics, injection of an average of 64 075 cells from this lot of uncultured human CD133+ cells is sufficient to repopulate 63% (= 1 − 1/e) of mice that underwent transplantation. Figure 5E shows there was an 8- or 20-fold increase in the number of SRCs after cultured in STF medium containing Angptl5 and IGFBP2 at low or normal O2, respectively. Specifically, when cells were cultured in STF medium containing Angptl5 and IGFBP2 at low O2, the CRU frequency was 1/7871 normalized to the number of input cells (95% confidence interval for mean: 1/3460 to 1/17 903, n = 16), approximately 8-fold greater than that of the uncultured cells. Strikingly, when we cultured the cells at normal O2, the CRU frequency increased to 1/3235 input equivalent cells (95% confidence interval for mean: 1/1908 to 1/5486, n = 27). These cultured cells had much greater levels of multilineage engraftment than uncultured cells (Figure 5F).
To derive another estimation of the extent of ex vivo expansion of human HSCs, we combined data from Figures 4 and 5, and calculated CRUs using 0.1% and 1% chimerism as the cutoff, respectively (Table 1). As expected, the CRU values calculated based on the different cutoffs for positive engraftment are different. Nevertheless, and importantly, the increase in the CRU value of cells cultured at 5% O2 was again approximately 8-fold (= 43 992/5034), and at normal O2 was approximately 14-fold (= 43 992/3235). All these calculations indicate that the total number of functional human SRCs increased approximately 14- to 20-fold following culture in medium containing IGFBP2 and Angptl5 at normal O2. Table 2 summarizes the culture conditions and some of the key data presented in Figures 1 through 5.
| Input equivalent cell no. . | 0.1% engraftment as cutoff . | 1% engraftment as cutoff . | ||
|---|---|---|---|---|
| Positive mice vs total mice . | 1/CRU . | Positive mice vs total mice . | 1/CRU . | |
| Uncultured CD133+ cells | 10256 | 43992 | ||
| 5000 | 6/9 | — | 1/9 | — |
| 8000 | 2/7 | — | 1/7 | — |
| 10000 | 7/8 | — | 1/8 | — |
| 15000 | 4/8 | — | 4/8 | — |
| 20000 | 7/8 | — | 2/8 | — |
| Cultured CD133+ cells at normal O2 | <1667 | 3235 | ||
| 1667 | 7/7 | — | 3/7 | — |
| 5000 | 10/10 | — | 7/10 | — |
| 10000 | 10/10 | — | 10/10 | — |
| Cultured CD133+ cells at 5% O2 | <1667 | 5034 | ||
| 1667 | 6/6 | — | 2/6 | — |
| 5000 | 10/10 | — | 4/10 | — |
| 8000 | 8/8 | — | 8/8 | — |
| Input equivalent cell no. . | 0.1% engraftment as cutoff . | 1% engraftment as cutoff . | ||
|---|---|---|---|---|
| Positive mice vs total mice . | 1/CRU . | Positive mice vs total mice . | 1/CRU . | |
| Uncultured CD133+ cells | 10256 | 43992 | ||
| 5000 | 6/9 | — | 1/9 | — |
| 8000 | 2/7 | — | 1/7 | — |
| 10000 | 7/8 | — | 1/8 | — |
| 15000 | 4/8 | — | 4/8 | — |
| 20000 | 7/8 | — | 2/8 | — |
| Cultured CD133+ cells at normal O2 | <1667 | 3235 | ||
| 1667 | 7/7 | — | 3/7 | — |
| 5000 | 10/10 | — | 7/10 | — |
| 10000 | 10/10 | — | 10/10 | — |
| Cultured CD133+ cells at 5% O2 | <1667 | 5034 | ||
| 1667 | 6/6 | — | 2/6 | — |
| 5000 | 10/10 | — | 4/10 | — |
| 8000 | 8/8 | — | 8/8 | — |
— indicates not applicable.
| Figure no. . | CB cells . | Cytokines added and culture duration . | O2 . | Average fold increase in nucleated cells . | SRC expansion . |
|---|---|---|---|---|---|
| 1 | Total nucleated cells | SCF+TPO+FGF-1+IGF-2+Angptl5 19d | Normal | 19 | + |
| 2 | CD34+ (fresh) | SCF+TPO+Flt3L 11d | Normal | 257 | − |
| SCF+TPO+Flt3L+Angptl5 11d | Normal | 283 | + | ||
| 3 | CD133+ | SCF+TPO+Flt3L 10d | Normal | 210 | − |
| SCF+TPO+Flt3L+Angptl5 10d | Normal | 220 | ++ | ||
| SCF+TPO+Flt3L+IGFBP2 10d | Normal | 162 | ++ | ||
| 4 | CD133+ | SCF+TPO+FGF-1 11d | Low | 203 | − |
| SCF+TPO+FGF-1+Angptl5+IGFBP2 11d | Low | 266 | ++ | ||
| 5 | CD133+ | SCF+TPO+FGF-1+Angptl5+IGFBP2 10d | Normal | 231 | ++ |
| Low | 258 | ++ |
| Figure no. . | CB cells . | Cytokines added and culture duration . | O2 . | Average fold increase in nucleated cells . | SRC expansion . |
|---|---|---|---|---|---|
| 1 | Total nucleated cells | SCF+TPO+FGF-1+IGF-2+Angptl5 19d | Normal | 19 | + |
| 2 | CD34+ (fresh) | SCF+TPO+Flt3L 11d | Normal | 257 | − |
| SCF+TPO+Flt3L+Angptl5 11d | Normal | 283 | + | ||
| 3 | CD133+ | SCF+TPO+Flt3L 10d | Normal | 210 | − |
| SCF+TPO+Flt3L+Angptl5 10d | Normal | 220 | ++ | ||
| SCF+TPO+Flt3L+IGFBP2 10d | Normal | 162 | ++ | ||
| 4 | CD133+ | SCF+TPO+FGF-1 11d | Low | 203 | − |
| SCF+TPO+FGF-1+Angptl5+IGFBP2 11d | Low | 266 | ++ | ||
| 5 | CD133+ | SCF+TPO+FGF-1+Angptl5+IGFBP2 10d | Normal | 231 | ++ |
| Low | 258 | ++ |
The symbols denote relative, not absolute, values. ++ represents a greater expansion than +. We used freshly isolated CD34+ cells in the experiment depicted in Figure 2, and used cryopreserved cells in other experiments.
Discussion
Here, we describe a simple and robust culture system for ex vivo expansion of human cord blood HSCs. Our defined serum-free medium contains 3 well-studied hematopoietic growth factors, SCF, TPO, and FGF-1, together with 2 novel hematopoietic growth factors that we recently identified: Angptl5 and IGFBP2. As measured by transplantation in NOD/SCID mice, this culture supported a 20-fold increase of human SRCs.
Angptls are a family of secreted glycoproteins that share limited sequence homology with angiopoietins.22 However, given the paucity of publications on these Angptls, we presume that most of their physiological activities remain to be discovered. Recently, we showed that mouse fetal liver CD3+ cells, which support the ex vivo expansion of mouse HSCs, specifically expressed Angptl2 and Angptl3. A 24- or 30-fold net expansion of long-term mouse bone marrow HSCs was observed by reconstitution analysis when highly enriched HSCs were cultured for 10 days in serum-free medium in the presence of Angptl2 or Angptl3 together with saturating levels of SCF, TPO, IGF-2, and FGF-1. Among other Angptls tested, Angptl5 and Angptl7 also stimulated expansion of mouse HSCs.14 Interestingly, we showed here that Angptl5 stimulated ex vivo expansion of human HSCs. It is likely that other Angptls will also increase expansion of human SRCs and we are currently testing several.
IGF-binding proteins (IGFBPs) are a family of circulating proteins that bind IGF-1 and IGF-2 with an affinity equal to or greater than that of the IGF receptors. IGFBPs modulate the biologic effects of IGFs by controlling the distribution, function, and activity of IGFs.23 Human IGFBP2 is a secreted protein that preferentially binds IGF-2 relative to IGF-1.24,25 In addition to modulating the biologic activities of IGF-1 and IGF-2, IGFBP2 may also have intrinsic bioactivities that are independent of IGFs.26 To our knowledge, this is the first report that demonstrates an effect of IGFBP2 on human HSCs.
It is intriguing that 5 factors are needed for maximal expansion of both mouse and human HSCs, though the specific factors may be different in the 2 species. As is well known, HSCs have several cell fates including self-renewal, differentiation, apoptosis, and quiescence. SCF and FGF-1 activate different receptor protein-tyrosine kinases,12,27,28 while TPO signals through a member of the cytokine receptor superfamily that requires a Janus kinase to activate several intracellular signal transduction pathways.29 We previously suggested that the Angptls activate signal transduction pathways that cannot be activated by SCF, TPO, or FGF-1; however, until we clone and characterize the receptors for the Angptl proteins as well as the presumed IGFBP2 receptor(s) we will be unable to understand how these proteins synergize with the other growth factors to stimulate HSC expansion.30 It is possible that these individual protein factors affect different fates of HSCs, and it will be important to study how these factors synergize to stimulate a net expansion of HSC numbers. Some, for instance, could stimulate cell division, while others might prevent differentiation of the divided cells, and still others might prevent apoptosis.
Our ex vivo expansion system works for both freshly isolated and cryopreserved cord blood cells; the cryopreserved samples we used in our studies were purchased from commercial vendors. It is likely that multiple factors, including the cell-cycle status of the cells, storage medium, the procedures for centrifuging the cells, and the components of the resuspension medium, affect the ability of the stem cells to engraft a recipient mouse. Our limiting-dilution experiments strongly suggest that, under our culture conditions where there is a considerable enhancement of the engraftment of cultured hematopoietic precursor cells, there is a time- and cell division–dependent increase in the actual numbers of stem/precursor cells. Alternatively, we cannot exclude the possibility that one or more of our factors enhanced (eg, through induction of a specific set of HSC genes) the homing, survival, efficiency of engraftment, or some other properties of HSCs.
One report suggests that hypoxia improves expansion of human SRCs.20 We did observe a significant expansion of cord blood HSCs in a hypoxic culture, but unexpectedly SRC expansion was even greater under normoxic pressure. Whether our observation is related to the particular culture conditions we used needs to be clarified in future studies.
Numerous attempts have been made to improve the efficiency of ex vivo expansion of cord blood HSCs. These include the development of liquid culture systems, HSC–mesenchymal stromal cell cocultures, and continuous perfusion systems, and the use of transcription inhibitors, copper chelators, and glycogen synthase kinase-3 inhibitors (see review in Brunstein and Wagner4 ; Robinson et al5 ; and Hofmeister et al31 ). However, all previous clinical trials using ex vivo–expanded cells in human bone marrow transplantation failed to show significantly improved hematopoietic recovery.5,32 Our use of Angptls and IGFBP2 for the ex vivo expansion of human SRCs may translate directly into increased clinical applications of cord blood for bone marrow transplantation. Our technology also likely will be useful for the development of novel strategies of cell and gene therapies that use HSCs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Catherine J. Wu of the Dana-Farber Cancer Institute for advice in experiments, and Drs Oktay Kirak, Rudolf Jaenisch, and Robert Weinberg of the Whitehead Institute for providing the low O2 incubator. We are grateful to Dr Ai Kotani Yoshida for critically reading the paper.
This work was supported by National Institutes of Health (NIH) grant K01 CA 120099-01 and the Michael L. Rosenberg Endowed Scholar Fund from University of Texas Southwestern (C.C.Z.). Support to H.F.L. is from NIH grant R01 DK 067356.
National Institutes of Health
Authorship
Contribution: C.C.Z. contributed to design, experimental performance, interpretation, and writing; M.K., S.I., and H.H. contributed to experimental performance; and H.F.L. contributed to design, interpretation, and writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cheng Cheng Zhang, UT Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390-9133; e-mail: alec.zhang@utsouthwestern.edu; or Harvey F. Lodish, Nine Cambridge Center, Cambridge, MA 02142; e-mail: lodish@wi.mit.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal