Abstract
This review highlights major scientific developments over the past 50 years or so in concepts related to stem-cell ecology and to stem cells in motion. Many thorough and eloquent reviews have been presented in the last 5 years updating progress in these issues. Some paradigms have been challenged, others validated, or new ones brought to light. In the present review, we will confine our remarks to the historical development of progress. In doing so, we will refrain from a detailed analysis of controversial data, emphasizing instead widely accepted views and some challenging novel ones.
Introduction
The bone marrow (BM) with its specialized vascular anatomy and the bone-shielded environment provides an ideal habitat for hematopoietic stem-cell maintenance and for the development of mature cells to be deployed to peripheral tissues where they exercise their function. As the BM is the unique site for hematopoietic development and provides quick and appropriate responses to peripheral needs, a dynamic interaction between hematopoietic cells and their BM environmental cells must be enforced at all times. The best manifestation that hematopoietic cells, especially stem and progenitor cells, mutually interact or “talk” to their environment is embodied in the concepts of stem-cell niches and stem-cell mobilization. As is often the case with scientific evolution, these concepts developed much earlier, well before there was any available experimental evidence for their existence. With the availability of evermore refined analytical tools at the protein and gene levels, these concepts were recently explored or challenged using many model systems. Despite a surge of new and insightful experimental observations, significant gaps in knowledge remain, especially in definition of structural components and molecular networks controlling the fate of stem cells at the niche and their trafficking.
The stem-cell niche: the concept, the update
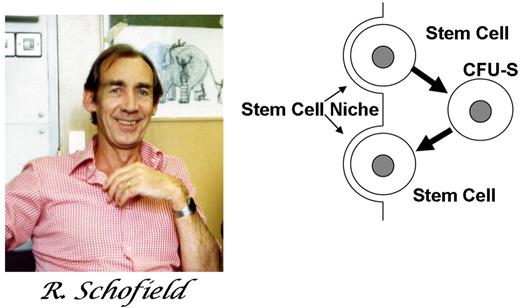
Following the breakthrough demonstration of the hematopoietic stem cell by Till and McCulloch in 1961,1 a complementary concept emerged, the concept of the stem-cell niche. A specialized place for the localization and regulation of stem cells seems a logical extension of the stem-cell hypothesis, but details regarding it and implications of it remain controversial today and are of practical importance for hematologists and their patients. Here we describe the early formulation of the niche hypothesis and its experimental examination. Since microscopic examination of the bone marrow began, it was recognized that the bone marrow had a special relationship to blood and was soon appreciated as the place where blood cells were made. That it was the place of residence for blood stem cells was not a great intellectual leap. What was striking about the first proposal of the niche, however, was its prescient capturing of central features of stem-cell niches broadly in biology. The concept of a stem-cell niche was first proposed by Schofield in 1978.2 He was wrestling with the apparent paradox that bone marrow cells derived from young or old wild-type mice transplanted into W/Wv mice (mutation in kit) continued to be able to reconstitute hematopoiesis indefinitely. Yet, cells that formed colonies in the spleen upon transplantation (colony-forming units– spleen cells [CFU-Ss]), the cells Till and McCulloch had defined as stem cells, had a limited serial passage capability. He concluded that the CFU-S was not the ultimate stem cell. Rather, stem cells needed to be resident in the marrow as essentially fixed tissue cells. If they left the “niche,” they could become CFU-Ss, but in doing so, killed their “immortality” (Figure 1). His hypothesis was likely driven both by his own experimental experience and by his fellow scientists Lord and Dexter. Dexter et al had by then published landmark work on ex vivo hematopoietic cultures able to sustain primitive cells only if in the presence of a bone marrow stromal feeder layer.3 Lord et al defined the marrow space as not uniform in its localization of primitive cells.4 From the rich intellectual environment of those assessing cellular microenvironments came a seminal hypothesis about the specialized locale of stem cells.
The hypothetical view of a stem-cell niche. The stem-cell daughter is a CFU-S. However, if it can find and occupy a niche, it will itself become a stem cell. Figure from Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7-25. Reprinted with kind permission of Springer Science and Business Media.
The hypothetical view of a stem-cell niche. The stem-cell daughter is a CFU-S. However, if it can find and occupy a niche, it will itself become a stem cell. Figure from Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7-25. Reprinted with kind permission of Springer Science and Business Media.
Schofield's proposal laid out the basic concepts of a stem-cell niche: (1) a defined anatomic site, (2) a location where stem cells could be sustained and reproduce, (3) a place where differentiation was inhibited, (4) a limited space that also limited the numbers of stem cells, and (5) most controversial, a place where reversion to a stem-cell phenotype could be induced in a slightly more mature cell type. He had no direct evidence of any of these concepts and was met by a highly skeptical audience of authorities on hematopoiesis, but he was absolutely correct.
The concept of a niche remained a hypothesis from 1978 until the pioneering work of Xie and Spradling in 1998.5 Using an entirely different model (Drosophila melanogaster) and a different tissue (ovary), they empirically validated each of the Schofield concepts about a stem-cell niche. Their work has shown that, indeed, the female germ stem cell resides adjacent to a heterologous cell type in a highly specialized location. This location is required for preservation of the stem-cell phenotype and provides specific signals, including bone morphogenic protein (BMP) signals, capable of inhibiting differentiation. In the movement away from the immediate contact with the niche cells, the stem cell loses this inhibitory constraint and begins an inexorable differentiation process. A very similar validation of the niche model quickly followed in the testes of Drosophila by Fuller (Kiger et al6 ) and in C elegans by Kimble (Crittenden et al7 ). Interestingly, even the most controversial of the concepts of Schofield, that a niche could impose a stem-cell phenotype on a cell no longer a stem cell, has been validated in these invertebrate models. Cells ectopically occupying a vacant niche were shown to acquire stem-cell features, a concept of particular interest in considering how the niche may participate in neoplasia.
Stem-cell niches in hematopoiesis
With the validation of the niche concept in invertebrates, the issue was of course intensely interesting to mammalian biologists, particularly in hematology. The lengthy history of microscopic examination of blood and bone marrow and the clear demonstration of stem cells in bone marrow was the solid footing by which to examine the issue. That bone marrow cells might be geographically localized to specific places in the marrow space was evident to all who examined bone marrows under the microscope where it is easy to identify nests of erythroid and lymphoid cells. In 1968, Maloney and Patt8 provided evidence for the localization of marrow regenerating cells. When hematopoietic cells were essentially eliminated from the marrow space by several different approaches, the researchers found that hematopoiesis regenerated in the marrow of the mechanically depopulated femur with equal kinetics whether the body had been irradiated or not. Their conclusion that “the most plausible explanation for these results is that cells in the bone crevices or the bone substance itself initiate the process of marrow regeneration” (page 72) focused the work of several laboratories on bone as a critical contributor to bone marrow.
Further analysis of where primitive cells might reside within the marrow space took 2 interesting lines of investigation. First was the anatomic partitioning of cells in relation to the central axis of a long bone and evaluating the function of the cells. These studies were largely conducted in the laboratory of Lord (Lord et al4 ; Lord and Hendry9 ). The results indicated that more mature granulocytic cells increased in representation as one moved from the radial margin of bone in toward the central arteriole. Conversely, more immature CFU-Ss increased in relative concentration toward the peripheral margin. Second, Taichman et al provided the first indications that the bone-forming cells themselves, the osteoblasts, might be relevant in influencing stem-cell function. A series of ex vivo studies with osteosarcoma cell lines or primary osteoblasts showed that the latter were capable of providing support of primitive human hematopoietic cells in long-term ex vivo culture.10 These studies further focused the subsequent efforts to define exactly what it is about bone that rendered it a preferential place for bone marrow.
Combined, the concepts that the endosteal surface is a place for marrow regeneration and that osteoblasts provide support for primitive hematopoietic cells poised the field for the formal demonstration of a hematopoietic stem-cell niche in vivo. It came simultaneously from 2 laboratory groups working with very different animal models. Each engineered mouse model modified the osteoblast and induced changes in both the architectural organization of the marrow and the hematopoietic stem-cell population within it. One of the models conditionally deleted the BMP1a receptor causing alteration in multiple tissues,11 while the other specifically targeted the osteoblast using an osteoblast-restricted 2.6-kb promoter of collagen 1a to drive expression of a constitutively active parathyroid hormone/parathyroid hormone–related peptide receptor.12 In both cases the number of osteoblasts increased, the trabecular bone increased, and the number of hematopoietic stem cells increased. Each attempted to define further what might be providing the molecular interaction between osteoblasts and stem cells as the molecules targeted in the mouse models were not expressed on the stem cells. Zhang et al11 demonstrated that there was N-cadherin present at the contact between label retaining hematopoietic cells representing potential stem cells and spindle-shaped osteoblasts on the endosteal surface. Calvi et al12 showed that the activated osteoblasts in their model overexpressed the Notch ligand, Jagged 1; that the stem cells in the transgenic animals had excess activation of Notch; and that in vitro, with a γ-secretase inhibitor of Notch activation, the primitive cell increase was blocked. Together these studies indicated that the osteoblast is a participant in the microenvironment that regulates the size of the hematopoietic stem-cell compartment and is therefore a niche component. These studies were thereby the first formal definition of heterologous cell types interacting in a special anatomic place to modulate how stem cells are maintained or can reproduce in a mammal, meeting the core criteria of Schofield's stem-cell niche hypothesis.
A number of studies have since emerged that enrich the concept of the bone as a regulatory niche, including evidence for other bone constituents contributing to hematopoietic stem-cell regulation. Among these are Tie2 on osteoblasts interacting with angiopoietin-1 in stem cells to regulate stem-cell quiescence,13 selective depletion of osteoblasts disrupting hematopoiesis,14 the extracellular matrix protein abundant in bone, osteopontin, negatively regulating stem- cell number,15,16 and the requirement for the calcium-sensing receptor on stem cells to be present for the stem cells to localize to the endosteal surface and engraft in bone marrow.17 Each of these builds a case for the interplay between bone and bone marrow biology. What they not do is exclude the potential for other nonbone elements participating in a hematopoietic stem-cell niche, nor do they demonstrate the requirement for cell-cell contact between stem cells and osteoblasts.
Beyond bone
It is clear from the developmental history of hematopoiesis in mammals that there must be other, non–bone-related stem-cell niches. The bone forms only in the second trimester of gestation and blood has clearly been produced, stem cells generated and expanded, and a spectrum of differentiation outcomes accomplished well before that time. The details of the microenvironments in settings such as the aorta-gonad-mesonephros or fetal liver are beyond the scope of this review, but each continues to be actively explored. Further, the potential for extramedullary sites to support hematopoiesis in the adult is well known to every practicing hematologist. Bone does not play a role in locations such as the spleen or lymph node, and yet stem-cell niches are created or, in the case of spleen, perhaps reactivated there with stress or disease. So the idea that other bone elements contribute to stem-cell niches is neither surprising nor should it be controversial. What remains somewhat controversial is exactly the nature of these additional niches. The controversy is indeed a healthy one for the field and has arisen because of new technical capabilities. First, a series of new antigens of the SLAM family were described by the laboratory of Morrison that enabled for the first time detailed histologic examination of where stem cells resided in standard histologic analysis. These studies indicated that cells immunophenotypically marking for stem-cell antigens were in far greater abundance at sites surrounding marrow blood vessels than on the endosteal surface (Kiel et al18 ). This was very compelling evidence for the anatomic localization criteria for the Schofield definition of a niche. However, caveats exist. Stem cells are known to traffic into and out of the circulation and therefore a static image of cells near vessels cannot exclude the potential for these cells collecting there the way cars collect at a physical constraint to their movement, a toll booth for example. In the absence of the cells having some regulation of the replication or differentiation at the site, it is difficult to conclude that the site meets the criteria for a niche. In addition, the imaging used was 2-dimensional. In areas of spongelike bony trabeculae, it is difficult to exclude immediately proximate bone surface on a plane different from the one examined.
Sequential evaluation of primitive cells in the perivascular space provided some functional information. Using in situ video confocal imaging, primitive hematopoietic cells labeled ex vivo and intravenously injected could be seen at SDF-1–rich subdomains of microvessels in the calvarial bone marrow of live mice.19 These cells persisted locally and increased in number 70 days after transplantation. However, the cells were not highly purified stem cells. Therefore, the data in support of the perivascular region as a niche include the anatomic dimension, but are not yet conclusive in the required functional dimension needed for niche definition. Is it likely that the perivascular space is a stem-cell niche? Absolutely. Developmental relationships of blood vessels to blood are simply too strong to not encourage such a hypothesis. While it is simple to view the marrow space as endosteal or perivascular and examine these as niche sites, the intercalating mesenchyme that provides the tissue cohesion within which vessels and differentiating hematopoietic cells mingle may also function as a niche. Data have emerged that a subset of mesenchymal cells abundantly expressing SDF-1 associate with and support stem cells and yet they do not reside in a highly concentrated marrow locale.20 Surely many features of the marrow including relative hypoxia, mechanical forces imposed by stromal elements, and metabolic activity of surrounding elements will likely contribute to the fine-tuned regulation of the hematopoietic stem cell.21
A historic travelogue for circulating stem cells
“…[U]nder the influence of stimulation, they [hemocytoblasts, aka stem cells] can be mobilized and become transformed into free, wandering…elements….”
A. Maximow, 1924,22 pages 541-542.
At homeostasis, most hematopoietic stem/progenitor cells remain enclaved within BM spaces; however, a small proportion of them constantly escapes the confines of BM and circulates in peripheral blood. The concept of circulating stem cells was first presented as a vision at the dawn of the 20th century by Maximow (Figure 2).22 Their presence in circulation was experimentally proven much later in transplantation experiments using whole blood or leukocytes in lethally irradiated recipients.23,24 (and references therein) Shortly after the CFU-S stem-cell assay became available,1 a plethora of studies revealed perturbations in the number of circulating stem/progenitor cells (CFU-Ss) following several empiric treatments (endotoxin,25,26 proteases,25,27 vaccines,28 Epo crude preparations,29 polymethacrylic acid [PMAA],25 poly I-C,29,30 etc). Some of the treatments were interpreted to induce complement activation31 or to release colony-stimulating factor,30,32 or were seen as an insult to sinusoidal architecture of BM (ie, endotoxin, enzymes, neutral polysaccharides25,30,33 ), whereas the mechanisms of other treatments (ie, pertussis vaccine,28 polyanions, PMAA,25 or dextran sulfate,34,35 or after chemotherapy36,37 ) remained totally obscure. Thus a variety of molecules with diverse cellular targets and of diverse biologic activities elicited increases in the circulating BM-derived stem/progenitor cells (ie, they triggered the egress or mobilization of stem/progenitor cells from BM). Although the efficiency of mobilization was quite modest in these early studies, the latter not only demonstrated that stem/progenitor cells can be forced out of BM spaces, but they should also be credited with several important conclusions regarding the trafficking of stem/progenitor cells. The studies demonstrated that the mobilizable pool from BM is much larger than the circulating pool at steady state,38 and provided important insights regarding the destiny and function of stem cells after their mobilization; it was concluded that their half-time in circulation (best estimate of T½ = 1-2 hours)39 is very short compared with all other leukocytes and that circulating stem cells may be at equilibrium with stem-cell pools in BM40 ; the studies demonstrated that mobilized cells were fully capable for hematopoietic repopulation and they did so in a more enhanced fashion than cells from BM. Only fetal liver cells had a replicating power that exceeded that of BM and blood-derived stem cells; finally, because of differences in kinetics of mobilization (ie, rapid or delayed25 ), the studies also concluded that different mechanisms may be at play.
Alexander Maximow, a great visionary in hematopoiesis. He introduced the “unitarian theory of hematopoiesis”22, page 538 (ie, “common stem cell for all blood elements”22, page 545) and pioneered other concepts about stem cells and their microecological niches within BM stroma. Photograph reprinted from Konstantinov IE, In Search of Alexander A. Maximow: The Man Behind the Unitarian Theory of Hematopoiesis, Perspectives in Biology and Medicine. Vol. 43. Winter 2000:269-276, with permission of Special Collections Research Center, University of Chicago Library.
Alexander Maximow, a great visionary in hematopoiesis. He introduced the “unitarian theory of hematopoiesis”22, page 538 (ie, “common stem cell for all blood elements”22, page 545) and pioneered other concepts about stem cells and their microecological niches within BM stroma. Photograph reprinted from Konstantinov IE, In Search of Alexander A. Maximow: The Man Behind the Unitarian Theory of Hematopoiesis, Perspectives in Biology and Medicine. Vol. 43. Winter 2000:269-276, with permission of Special Collections Research Center, University of Chicago Library.
The discovery in the 1980s to 1990s of many biologically active molecules influencing hematopoietic cell growth in vitro and in vivo (ie, the hematopoietic cytokines) ushered a new era in mobilization experiments. Many of the interleukins, the chemokines, or the hematopoietic growth factors influencing early or late differentiation stages of hematopoietic cells (G, GM, KL, Epo, Tpo, FLT3) were shown to bring about mobilization with various efficiencies and kinetics.41-43 As in vitro stem-cell assays became more refined, the detailed spectrum of mobilized stem/progenitor cells was defined and shown to represent all types of lineage-committed or noncommitted stem cells. By combining one cytokine with another cytokine,44 or adding a chemokine to a cytokine,45-47 cooperativity was uncovered among them in a synergistic or additive fashion, since they mobilized cells with better efficiency. These studies suggested that many different pathways participate in bringing about mobilization. However, despite numerous studies, the precise mechanism of mobilization with growth factors proved quite recalcitrant to pinpoint. Complex effects or a cascade of effects was suspected, since a wide range of target cells (hematopoietic mature or immature, or nonhematopoietic) could be affected by these cytokines; multiple doses were required for full effects and these could encompass both primary and secondary effects to bring about mobilization. Nevertheless, because of the high efficiency of mobilization with cytokines, especially the well-tolerated granulocyte colony-stimulating factor (G-CSF), not only were multiple studies on mobilized cells feasible, but their clinical application took off well ahead of the uncovering of any mechanistic insights.48,49 The use of mobilized stem cells is currently surpassing the use of BM-derived cells as a source of cells for transplantation, and the former rather than the latter might be the preferred source in the future for genetic engineering of autologous cells. Using mobilized blood, many of the conclusions drawn previously about faster recovery after transplantation of mobilized cells48 or about the fate of mobilized cells held true under a new experimental light.50,51
The road to unveiling mobilization mechanisms: many twists and turns
Because of the plethora of studies invested in uncovering mechanisms, our understanding of the biology of mobilization has been enhanced in the last 15 years. Despite the historical precedence and the wealth of studies with G-CSF–mobilized cells, progress occurred in several incremental steps and insightful cues were generated using other sources for mobilization. Thus, as detailed in several recent reviews41,42,52-54 extensive experimental evidence has stressed the importance of 2 pathways, one dependent on α4β1 integrin, the other on CXCR4/CXCL12 signaling, for the retention of stem/progenitor cells within BM at homeostasis, although additional pathways likely participate in a cooperative or synergistic fashion. α4β1 integrin is widely expressed in hematopoietic and a variety of nonhematopoietic cells, and its counterreceptors (VCAM-1, fibronectin, osteopontin, etc) are constitutively expressed on BM stromal and endothelial cells, thus facilitating adhesive interactions of hematopoietic cells with their BM stroma. When antifunctional Abs were used in vivo, they elicited the release of stem/progenitor cells in several species55 during steady-state hematopoiesis. In contrast to this effect, in vivo studies of α4β1 inhibitors (antibodies or inhibitory small molecules) abolished the recruitment of leukocytes (lymphocytes) from circulation to inflammatory sites,56 (and references therein) corroborating many elegant in vitro studies describing the classical leukocyte transmigration cascade. Paradoxically, the movement of stem/progenitor cells from extramedullary sites of BM to the circulation (through sinusoidal endothelium) was facilitated by blocking α4β1 function.55 The validity of this conclusion was later reaffirmed by studies in mice with conditional ablation of α4β1 integrin in adult life57 and in transplantation experiments with α4β1-deficient donor cells.58 It is important to emphasize that the effects through the α4β1/VCAM-1 pathway were partial and a cooperative contribution was unmasked by blocking other classes of adhesion molecules (E-selectin, β2 integrins), which had no demonstrable effect on their own in mobilization of stem/progenitor cells,59 although they did have a distinct functional role in the mature leukocyte recruitment cascade. In addition to the α4β1-dependent pathway, it was later shown that inhibition of CXCR4/CXCL12 pathway by a variety of biologically active molecules also led to mobilization of stem/progenitor cells.42,52 This realization was introduced with the use of Met-SDF-1 (an SDF-1 analog leading to prolonged desensitization of CXCR4)60 and was followed by several other inhibitor molecules blocking CXCR4 receptor binding46 or its signaling (Ptx),61 or displacing62 or inactivating SDF-1 by proteolytic cleavage from BM microenvironment.63 The end result of all these treatments was the inhibition of CXCR4/SDF1 signaling. Perhaps the most convincing in vivo experiments were the ones with transplantation of CXCR4−/− fetal liver cells that showed the importance of CXCR4/SDF-1 pathway in retention of stem/progenitor cells in BM during homeostasis.64-66 In contrast to the aforementioned exit of stem/progenitor cells that was initiated by the loss of CXCR4/SDF-1 signaling, active SDF-1–dependent signaling is needed for migration of mature leukocytes. Thus, the loss of CXCR4/SDF-1, like that of α4β1 signaling and their downstream partners (ie, Rac1/Rac2),67 led to mobilization of stem/progenitor cells, reinforcing the concept that ongoing signaling is needed for their BM retention. Nevertheless, when the latter were studied in vitro, there was a lack of fidelity between in vitro and in vivo results, so that the in vitro results (ie, inhibition of migration by Ptx61 or with Rac1/Rac2 null cells67 ) could not predict the in vivo outcome (ie, mobilization). Moreover, it is unclear at present how perturbation in chemokine/cytokine gradients (ie, adeno-mediated increase in SDF-1, VEGF, PlGF, or Ang-154 ) or the use of CXCR4 agonistic peptides68 or other CXC chemokines (Gro-β, IL-8) influence mobilization and from what anatomic locations within BM. Thus the delineation of molecular forces navigating the exit of stem/progenitor cells from BM using these modalities needs to be further defined.
Progress in uncovering specific mechanisms in G-CSF–induced mobilization was slower, despite a data-ridden landscape. Several putative mechanistic pathways were proposed, but the picture proved more complex than originally thought. Successive clues were generated by studying G-CSFR−/− mice and by detailed evaluation of post–G-CSF effects within BM environment and on BM cells compared with PB. It was shown that G-CSF mobilization required the presence of functional neutrophils and it could work in trans by diffusible factors once an adequate number of normal neutrophils are present.69 The diffusible molecules were granule-enclaved proteases, which were liberated within the BM environment.63 These omnivorous proteases (elastase, MMPs, cathepsins, etc) could attack several targets within BM, including proteins with perceived roles in mobilization (ie, VCAM-1, Kit/KL, CXCR4/SDF-1, etc). Even this attractive hypothesis did not survive the scrutiny of subsequent work, as protease-deficient models displayed unimpaired responses to G-CSF.70 Then it was subsequently shown that G-CSF through an indirect mechanism can attack osteoblasts and decrease available sources of SDF-1, either by a protease or nonprotease-dependent mechanism,71 or through sensing of peripheral adrenergic changes provoked by G-CSF72 or by influencing osteoclastic activity.73 Combination of G-CSF with molecules inhibiting CXCR4/SDF1 function had at least an additive or synergistic effect and it could accommodate hard to mobilize healthy volunteers or especially patients with compromised bone marrow.74 Other approaches to combine the mobilizing power of G-CSF at the tails of a more prolonged treatment (with rhGH75 or rhPTH76 ) increasing the number of stem cells were recently presented. Thus, creative combinations of G-CSF with chemokine inhibitors displaying fast kinetics or with treatments increasing the stem/progenitor pool have enhanced mobilization efficiency. The mechanism of mobilization, when other growth factors (ie, KL, FLT3, Tpo) are used, has not been delineated, although certain causative associations have been proposed.43,54 One could speculate that in addition to pathways, the disruption of which compromises stem-cell retention within BM, alterations in BM architecture either by affecting the metabolic state of certain structural components (ie, osteoblasts, osteoclasts) or causing hyperproliferation of hematopoietic elements within the confined spaces of BM could offset the balance of existing milieu and the vascular permeability forcing the exit of hematopoietic stem cells (HSCs) from BM. For example, genetic alterations in microenvironmental cells (ie, RARγ77 or CaR17 deficiency) were shown to have an impact on stem-cell behavior and their mobilization, the latter initiated entirely by extrinsic cues. Furthermore, new paradigms recently came to light, suggesting that in addition to either intrinsic (ie, kit, myb, p21, Gfi1, HOXB4, etc21 ) or extrinsic (ie, CXCL12, Tie2, Opn, RARγ, or CaR) cues, synergistic interaction of altered stem cells with altered microenvironmental cells78 can upset the retaining forces within BM and promote mobilization when only one altered partner (ie, Rb−/− cells or Rb−/− stroma) fails to do so.
Leukemic cells hijack normal migratory pathways while hiding their own secrets
Neoplastic cells develop in their primary sites because of favorable interactions with supporting microenvironmental cells or matrix. Two concepts have recently given new impetus to the biology of leukemia research: First, the realization that leukemic stem cells with self-renewal capacity exist and second, that such cells through dynamic and reciprocal interactions with a nurturing microenvironment maintain and expand the disease process.79,80 Once these interactions are weakened, leukemic/tumor cells tend to leave the primary site and migrate to other tissues. It has been increasingly recognized that metastatic invasiveness of leukemic/tumor cells is greatly dependent on cell-surface adhesion molecule expression. Numerous examples can be cited uncovering the fact that leukemic cells interact with their intimate microenvironment using the same pathways that normal cells do. Thus, α4β1, α5β1, αLβ2, CD44, or CXCR4/CXCL12 have been implicated in their adhesive interactions with their environment, or in influencing their survival and attenuating chemotherapy-induced death.81-87 High expression levels of α4β1 in acute myeloid leukemia (AML)82 or myeloma,88 or CXCR4 in AML,83,89 acute lymphoblastic leukemia (ALL),84 or chronic lymphoblastic leukemia (CLL),85,90 appeared to confer a more aggressive phenotype associated with decreased sensitivity to chemotherapy. Yet other phenotypes (ie, with loss of α4β1 integrin or β1 integrin) correlated with increased cell motility and dissemination.91,92 Thus, depending on cell context, either high expression or loss of expression of α4β1 has been implicated to facilitate the migratory phenotype and invasiveness at early or later stages of the disease. Certain mutations in leukemic cells (AML1/ETO, or hERG1 expression) confer a promigratory phenotype either by affecting indirectly cytoadhesion molecules (α4β1) expressed in leukemic cells93 or by forming signaling complexes with them.94 That signaling may be important in sensitization of AML cells to chemotherapy is further exemplified by the fact that integrins and CXCR4 have been frequent targets of aberrant signaling in leukemic cells yielding the dependency of cytotoxic chemotherapy to the activation or loss of integrin/CXCR4-mediated signaling.83,85,89,95 These examples buttress the concept that the aggressiveness or invasive potential of a tumor cell could be altered through aberrations in signaling in which a given gene is forced to participate.
In addition to common pathways used by normal and leukemic stem cells, the latter may either depend more on certain pathways or recruit novel pathways. For example, chronic myelogenous leukemia (CML) cells or AML stem cells, in contrast to normal cells, preferentially use the CD44/HA pathway for modulating their differentiation86 or their retention within BM.87 Even then, depending on cell contextual cues, either the inhibition (in CML) or activation of CD44/HA pathway (in AML) led to amelioration of disease. Further, CLL cells, in contrast to normal B cells, fail to activate αLβ2 integrin for their chemokine-induced migration unless VEGF and α4β1 are engaged.96 Apart from aforementioned pathways, the motility and transmigration of leukemic cells to extramedullary sites are regulated by angiogenic factors or other molecules (VEGFR-1,94,97 TGFβ, proteases). It is of interest that deployment of BM-derived angiogenic progenitors or other cells are important in setting up distal premetastatic niches for tumor cells.98,99 Thus it is becoming increasingly apparent that adhesion/signaling molecules modulate the interaction of tumor cells with their microenvironment in a reciprocal manner and by several diverse ways: by affecting their migratory phenotype and invasiveness of other tissues; by influencing the expression of apoptosis related proteins; or by altering through signaling their sensitivity to chemotherapy. The availability of newer information on how normal or leukemic/tumor cells interact with their microenvironment not only will amplify the biologic interest in the field, but also will enrich the therapeutic arsenal for treating leukemia/tumors by providing a switch in the current therapeutic paradigm in which increased emphasis is placed on target cells rather than the microenvironment or the blocking of premetastatic niches.
Epilogue/unanswered questions
Niche
The conceptual framework provided by Schofield 3 decades ago remains accurate, indeed prescient. Its fundamental message is that local tissue constituents can alter the behavior of the base unit of tissue maintenance and repair, the stem cell. Unraveling how these constituents interact is critical if we are to understand how to better use the stem cell therapeutically and how to intervene in settings of bone marrow dysfunction. These are the 2 issues of greatest importance in niche biology. They are beginning to be addressed with ongoing clinical trials to modify the niche pharmacologically as a means of improving stem-cell transplantation therapies.76 In addition, recent studies indicate that alterations in niche function can participate in provoking hematologic disorders in mouse models.78,100 These are only the first forays into what will be areas of more extensive study.
A central issue that remains unaddressed is whether the niche for malignant or dysplastic cells is the same as that for normal stem cells. It is almost certainly the case that malignant cells do not have the same niche dependence as normal stem cells, but they also are not without some dependence or leukemia would be an extravascular, extramedullary disease—fortunately a rare occurrence. A corollary of such studies is how do abnormal cells in the marrow influence the support of normal stem cells? Do abnormal or normal cells change the capacity of the niche? Can niche properties be modulated with drugs to preferentially support normal over malignant or dysplastic cells? Can understanding the niche provide novel strategies for more effectively delivering and engrafting transplanted stem cells? Can it lead to more efficient means of mobilization from the niche to the blood for harvest? Indeed, might the participation of bone in the stem-cell niche be the reason stem cells circulate throughout adulthood? Is the ever-remodeling bone providing ever-remodeling niches that need to be interrogated for vacancy by stem cells in motion to prevent ectopic cell occupancy of a microenvironment that may foster self-renewal and impair differentiation?
Mobilization
Mobilization, like BM homing, is a complex biologic process requiring the orchestrated participation of several upstream regulators and downstream effectors. Copresentation of immobilized chemokines/adhesion molecules and their downstream partners present a roadmap to HSCs within BM by guiding their directed migration (at times independently of a soluble chemokine gradient). Weakened interactions of adhesion molecules and their crosstalk with receptor protein kinases, GPCRs (G-protein–coupled receptors), and signaling molecules, initiated either upstream or downstream in the mobilization cascade, facilitate the exit of stem/progenitor cells from BM. Vascular (sinusoidal) endothelial cells in BM could serve as “gatekeepers” in the interface between BM and the peripheral circulation. As such, they must assert a final control or facilitate the traffic of HSCs in and out of the BM. Given the complexity of the available data, it has not been clear whether the same molecular pathways are in motion when HSCs have been triggered to exit, compared with the cells seeking an active entry through the endothelial cells. In other words, do the “blood-traveled” cells entering BM use the same molecular pathways used by BM-resident cells triggered to exit? To some extent, the recruited pathways may be redundant, but details about prevailing signaling networks in each case are missing. Additional outstanding questions remain: Is there an immediately mobilizable pool in BM, and if so where is it located and what are its characteristics? Can mobilization schemes be found that can preferentially mobilize long-term repopulating cells and from what anatomic locations and under what molecular pathways? Are prevailing pathways of mobilization altered after stress, or do the homing profiles of mobilized cells differ when different mobilization schemes are used? Knowledge in these and other aspects of the biology of cell mobilization is expected to pay high clinical dividends in the future.
Authorship
Contribution: T.P. and D.T.S. cowrote the paper.
Conflict-of-interest disclosure: T.P. declares no competing financial interests. D.T.S. declares the following: founder of Zhealis Pharmaceuticals; consultant/advisor to Assymetrix, Bessemer Trust, Osiris Therapeutics, Cell Science Therapeutics, Genzyme, Hollis-Eden Pharmaceuticals, JSB Partners, LEK Consulting, Phylogix, Pure Tech, Shaklee Corp, Select Therapeutics, Cytometrix, and Guava Technologies.
Correspondence: Thalia Papayannopoulou, University of Washington, Health Sciences Building, Room K243, Box 357710, Seattle, WA 98195; e-mail: thalp@u.washington.edu.
References
National Institutes of Health


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal