Abstract
An acquired somatic mutation in the JAK2 gene (JAK2-V617F) is present in the majority of patients with myeloproliferative disorders (MPDs). Several phenotypic manifestations (polycythemia vera [PV], essential thrombocythemia [ET], and primary myelofibrosis) can be associated with the same mutation. We generated JAK2-V617F transgenic mice using a human JAK2 gene with the sequences encoding the kinase domain placed in the inverse orientation and flanked by antiparallel loxP sites. Crossing mice of one transgenic line (FF1) with transgenic mice expressing Cre-recombinase under the control of the hematopoiesis specific Vav promoter led to expression of JAK2-V617F that was lower than the endogenous wild-type Jak2. These mice developed a phenotype resembling ET with strongly elevated platelet counts and moderate neutrophilia. Induction of the JAK2-V617F transgene with the interferon-inducible MxCre resulted in expression of JAK2-V617F approximately equal to wild-type Jak2 and a PV-like phenotype with increased hemoglobin, thrombocytosis, and neutrophilia. Higher levels of JAK2-V617F in mouse bone marrow by retroviral transduction caused a PV-like phenotype without thrombocytosis. These data are consistent with the hypothesis that the ratio of mutant to wild-type JAK2 is critical for the phenotypic manifestation. A similar correlation was also found in patients with MPD.
Introduction
An acquired somatic mutation in the JAK2 gene resulting in a valine to phenylalanine substitution at position 617 (JAK2-V617F) is present in the majority of patients with myeloproliferative disorders (MPDs).1-4 This discovery suggested that the presence of the JAK2-V617F mutation could represent the primary causative lesion in MPD. While the JAK2-V617F mutation is found in approximately 95% of patients with polycythemia vera (PV), it is also detectable in about 50% of patients with primary myelofibrosis (PMF) and essential thrombocythemia (ET).2,5 It remains unclear how the identical JAK2-V617F mutation can cause 3 distinct clinical entities. In patients with PV and PMF, but only rarely in ET, the JAK2-V617F mutation progresses from the heterozygous state to homozygosity through mitotic recombination of the distal part of chromosome 9p.4,6 Retroviral transduction of mouse bone marrow cells followed by transplantation into lethally irradiated mice demonstrated that the expression of Jak2-V617F is sufficient to induce a phenotype resembling PV.1,7-10 These mice showed massive increase in hematocrit and hemoglobin concentration and a variable degree of neutrophilia. In contrast to patients with PV, the platelet numbers in these mice remained normal or were even decreased. After several months some of the mice also developed myelofibrosis. The phenotype was not affected when bone marrow from donor mice deficient for the Src family kinases Lyn, Hck and Fgr were used, but was dependent on the presence of Stat5.10,11
To establish a mouse model for MPD we generated bacterial artificial chromosome (BAC) transgenic mice that express the human JAK2-V617F driven by the JAK2 promoter. A constitutively active version and an inducible version of the BAC transgene were constructed. Transgenic mice that display ET or PV phenotypes have been obtained in the C57BL/6 background.
Methods
Transgenic mice
The human JAK2-V617F transgene was constructed using the BAC CTD2025A15 (CalTech D human BAC library; obtained from Open Biosystems, Huntsville, AL), which is approximately 190-kb long and contains part of the JAK2 gene reaching from 96 kb upstream of exon 1 to the first 1 kb of intron 12. A construct was assembled from the rest of intron 12 with 100-bp overlap, cDNA encoding JAK2 exons 13-25, a polyadenylation signal from SV40,12 an ampicillin resistance cassette flanked by Frt sites (from the plasmid phCre.myc.nuc.FRT.AMP.FRT, kindly provided by Dr Günther Schütz, DKFZ, Heidelberg, Germany), and 100 bp of sequence homologous to the BAC vector pBeloBAC11. This construct was inserted into the BAC by homologous recombination in the bacterial strain E350 (kindly provided by Dr Neal Copeland, Institute of Molecular and Cell Biology, Singapore), and the ampicillin cassette was subsequently removed by induction of Flpe recombinase.13 For the inducible Flip-Flop transgene, the construct was modified by inserting a lox66 site into intron 12 and a lox71 site after the polyadenylation signal.14 The segment between the loxP sites was then inverted by restriction digest, and the new construct was inserted into the BAC as before.
For oocyte injection, BAC DNA was digested with NotI to remove the vector and purified over a Sepharose CL4b column (GE Healthcare, Little Chalfont, United Kingdom). Genotyping of transgenic Flip-Flop mice was performed using the human JAK2-specific primers GAGCAAGCTTTCTCACAAGC and AATTCTGCCCACTTTGGTGC that amplify a 530-bp fragment. The number of integrated transgene copies was determined by real-time polymerase chain reaction (PCR) with the primers GTGGCAGCAACAGAGCCTATC and GGAGCTTCAGCACCTCGAGAT for human JAK2 and TGGCAGCAGCAGAACCTACA and GGAGCTTCAGCCCCACG for mouse Jak2. MxCre mice were genotyped using the primers AGGTGTAGAGAAGGCACTTAGC and CTAATCGCCATCTTCCAGCAGG that amplify a 300-bp fragment. Cre expression was induced in MxCre;FF1 double transgenic mice by intraperitoneal injection of 300 μg polyinosine-polycytosine (pIpC) 3 times every second day. VavCre mice were kindly provided by Dr Dimitris Kioussis (National Institute for Medical Research, London, United Kingdom) and genotyped using the primers CTCTGACAGATGCCAGGACA and TGATTTCAGGGATGGACACA (500-bp fragment). The integration site of the transgene in the FF1 strain was localized by fluorescence in situ hybridization using the BAC construct as a probe and by spectral karyotyping (Van Andel Institute, Grand Rapids, MI). Whole genome single nucleotide polymorphism (SNP) genotyping was performed by Drs Jennifer Moran and David Beier (Mutation Mapping and Developmental Analysis Project, and The Broad Institute Center for Genotyping and Analysis, Boston, MA). All mice used in this study were kept under specific pathogen-free conditions and in accordance to Swiss federal regulations.
Retroviral transduction and bone marrow transplantation
Human JAK2 cDNA (kindly provided by Dr Jan Cools, Flanders Interuniversity Institute for Biotechnology, Leuven, Belgium) or human JAK2-V617F cDNA was cloned into the retroviral vector pMSCV-IRES GFP. An equivalent plasmid containing mouse Jak2-V617F was kindly provided by Dr D. Gary Gilliland (Brigham and Women's Hospital, Boston, MA). Bone marrow transplantation was performed as previously described.15
Blood and tissue analysis
Blood counts were determined on an Advia 120 Hematology Analyzer using the Multispecies Software (Bayer, Leverkusen, Germany). Plasma erythropoietin and thrombopoietin levels were measured with the Quantikine Mouse/Rat Epo or Tpo Immunoassay kit (R&D Systems, Abingdon, United Kingdom). For histopathological analysis, tissue specimen were fixed in 4% neutral buffered formalin and embedded in paraffin. Paraffin sections (4 μm) were stained with hematoxylin and eosin (H&E) or Gömöri (reticulin fibers). Images were taken using an Axio Imager A1 microscope equipped with a Plan Apochromat objective (63×/1.4 NA, oil) and a mounted Axio Cam HR digital camera (all Zeiss, Jena, Germany). The software for image acquisition was AxioVision Rel. 4.6.
Hematopoietic progenitor assays and flow cytometry
Methylcellulose media were purchased from Stem-Alpha (St Genis L'Argentière, France), and 2 × 104 bone marrow or spleen cells were plated in duplicate in STEMα-mIE with cytokines, or for detection of endogenous colonies, 105 cells were plated in STEMα-mI without cytokines. Colony-forming unit-megakaryocyte (CFU-MK) cultures were grown in chamber slides using MegaCult-C media (StemCell Technologies, Vancouver, BC). For flow cytometric analysis, bone marrow and spleen cells were stained with fluoroisothiocyanate (FITC)–, phycoerythrine (PE)– or allophycocyanin (APC)–conjugated monoclonal antibodies against TER-119, CD71, Mac-1, Gr-1, B220, CD3 or isotype controls (Becton Dickinson, Franklin Lakes, NJ) and analyzed on a FACSCalibur (Becton Dickinson).
Real-time PCR, allelic ratio, and Southern blot analysis
Expression analysis in mice was performed with Power SYBR Green PCR Master mix on a 7500 Fast machine (Applied Biosystems, Foster City, CA) using the following primers: TCACCAACATTACAGAGGCCTACTC and GCCAAGGCTTTCATTAAATATCAAA (human JAK2), CCACGGCCCAATATCAATG and CCCGCCTTCTTTAGTTTGCTA (mouse Jak2). Results were confirmed with the Taqman Gene Expression assays Hs01078117_m1 for human JAK2 and Mm01208495_m1 for mouse Jak2 (Applied Biosystems). Ratios of human and mouse Jak2 were assessed in the “Absolute Quantification” setup with standard curves made from linearized pMSCV-IRES GFP plasmids containing either human JAK2 or mouse Jak2. Total JAK2 expression in patients was assessed using the Taqman Gene Expression assays Hs01078124_m1 (JAK2) and Hs02338565_gH (human RPL19). Primers and probes for the housekeeping genes GUSB and B2M were described elsewhere.16 The primers for mouse Gusb were ATAAGACGCATCAGAAGCCG and ACTCCTCACTGAACATGCGA. Expression of mutant and wild-type JAK2 and quantification of allelic ratios in granulocyte DNA from patients was done as previously described.17,18 For Southern blot analysis, 5 μg genomic DNA was digested with XbaI and fragments blotted onto a Hybond N + membrane (GE Healthcare) were hybridized with a radiolabeled probe generated by PCR with the primers TTCTCGTCTCCACAGACACA and ATTCTGCCTCTGCAGACCAC. Radioactive bands were quantified on a Molecular Imager FX (BioRad, Hercules, CA). Transgene copy numbers were measured by real-time PCR with the primers GTGGCAGCAACAGAGCCTATC and GGAGCTTCAGCACCTCGAGAT for human JAK2 and TGGCAGCAGCAGAACCTACA and GGAGCTTCAGCCCCACG for mouse Jak2. To determine the number of transgene copies in the native orientation, the primers GCTGCAGCACAGAGATTAAATAGC and TGGATCGACATAACTTCGTATAATGTATG were used, which are located 5′ and 3′ of the lox71 site (Figure 1). Real-time PCR efficiency with these primers was very similar to the efficiency of copy number primers that were used to determine total human JAK2. Therefore, the number of actively rearranged transgene copies could be calculated and results were comparable to Southern blot analysis.
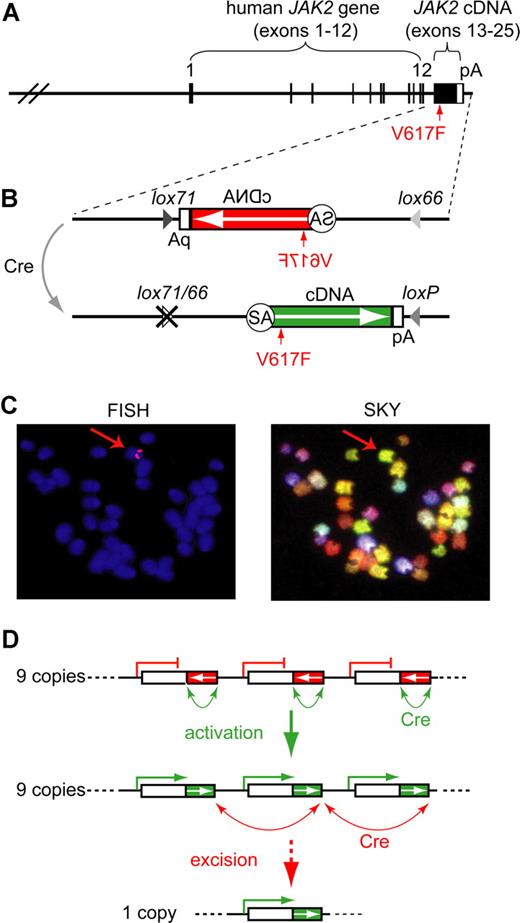
The human JAK2-V617F (Flip-Flop) transgene. (A) Transgenic construct. A human BAC containing JAK2 exons 1-12 was combined with a partial cDNA encoding JAK2 exons 13-25 (black box; not to scale) and a polyadenylation signal (white box). (B) Inducible transgenic construct. The cDNA is placed in the inverse orientation (white arrow) and is flanked with mutant loxP sites (magnified insert). Cre-mediated recombination flips the orientation of the cDNA and places the intron 12 splice acceptor (SA) into the correct position to allow proper splicing of the transgenic pre-mRNA. Recombination generates one wild-type loxP and one double mutant lox66/77 site, which is no longer substrate for the Cre-recombinase. The position of the V617F mutation is indicated by a red arrow. (C) Localization of the transgene in the transgenic strain FF1. Fluorescent in situ hybridization (FISH) shows that the transgene integrated into a single locus, which appears as 2 red signals in metaphase chromosomes (red arrow in left panel). Spectral karyotyping (SKY) identified chromosome 8, band A1 as the transgene integration site (red arrow in right panel). (D) Model of Cre-mediated rearrangements in the transgenic integration site. A perfect head-to-tail orientation of all transgenic copies is assumed. In the FF1 strain we found that 9 copies of the transgene have integrated, of which only 3 are shown. The native configuration, in which all transgenic copies are in the inactive orientation (red boxes), is shown on top. Cre-recombination between adjacent loxP sites (green arrows) leads to reversal of the orientation and activation of the transgene (green boxes). A maximum of 9 active transgene copies can be generated (middle panel). Cre-recombination between distant loxP sites that are in a parallel orientation (red arrows) results in the excision of one ore more copies of the transgene (bottom panel).
The human JAK2-V617F (Flip-Flop) transgene. (A) Transgenic construct. A human BAC containing JAK2 exons 1-12 was combined with a partial cDNA encoding JAK2 exons 13-25 (black box; not to scale) and a polyadenylation signal (white box). (B) Inducible transgenic construct. The cDNA is placed in the inverse orientation (white arrow) and is flanked with mutant loxP sites (magnified insert). Cre-mediated recombination flips the orientation of the cDNA and places the intron 12 splice acceptor (SA) into the correct position to allow proper splicing of the transgenic pre-mRNA. Recombination generates one wild-type loxP and one double mutant lox66/77 site, which is no longer substrate for the Cre-recombinase. The position of the V617F mutation is indicated by a red arrow. (C) Localization of the transgene in the transgenic strain FF1. Fluorescent in situ hybridization (FISH) shows that the transgene integrated into a single locus, which appears as 2 red signals in metaphase chromosomes (red arrow in left panel). Spectral karyotyping (SKY) identified chromosome 8, band A1 as the transgene integration site (red arrow in right panel). (D) Model of Cre-mediated rearrangements in the transgenic integration site. A perfect head-to-tail orientation of all transgenic copies is assumed. In the FF1 strain we found that 9 copies of the transgene have integrated, of which only 3 are shown. The native configuration, in which all transgenic copies are in the inactive orientation (red boxes), is shown on top. Cre-recombination between adjacent loxP sites (green arrows) leads to reversal of the orientation and activation of the transgene (green boxes). A maximum of 9 active transgene copies can be generated (middle panel). Cre-recombination between distant loxP sites that are in a parallel orientation (red arrows) results in the excision of one ore more copies of the transgene (bottom panel).
Patients
JAK2 expression was analyzed in 88 patients with JAK2-V617F–positive MPDs (25 ET, 55 PV, 8 PMF; diagnosed according to World Health Organization criteria) and in 17 healthy individuals.19 The study was approved by the ethics committee of Basel (Ethikkommission beider Basel), Switzerland. Informed consent was obtained in accordance with the Declaration of Helsinki.
Results
Generation of JAK2-V617 transgenic mice
To study the in vivo role of JAK2-V617F in the pathogenesis of MPD, we made BAC-transgenic mice that express the human JAK2-V617F driven by the endogenous human JAK2 promoter. The 190-kb BAC clone contained 96 kb of JAK2 5′-upstream region that does not include other known genes, as well as exons 1-12 and a part of intron 12 of the human JAK2 gene (Figure 1A). Using homologous recombination in bacteria, we added the missing part of intron 12 including the splice acceptor and a cDNA fragment consisting of exons 13 to 25 followed by an SV40-derived polyadenylation signal.13 The construct was prepared in 2 versions, one containing the V617F mutation (VF) and a control construct with wild-type sequence (WT). Microinjection was performed into oocytes from inbred C57BL/6 mice. Three lines carrying the WT transgene were established and found to have no alterations in blood counts (not shown). Two VF founders with MPD phenotype died before transgenic lines could be established. We therefore generated an inducible version of the transgenic construct. To this end, we added antiparallel loxP sites to both ends of the intron12-cDNA-polyA fragment and inserted this construct into the BAC clone in an inverted orientation (Figure 1B). In this configuration, no full-length Jak2 protein can be made, since the mRNA is truncated after exon 12. The kinase domain of human Jak2 is encoded by exons 19-25. Recombination of antiparallel loxP sites by Cre results in flipping the orientation of the cDNA segment, restoring a functionally active transgene configuration (Figure 1B bottom part). To make the recombination unidirectional and irreversible, we made use of previously described mutant versions of the loxP site named lox66 and lox71.14 Recombination between antiparallel lox66 and lox71 sites creates one wild-type loxP site and one double mutant site (lox66/71) with greatly reduced affinity for Cre (Figure 1B). Oocyte injection of this modified BAC construct, named Flip-Flop (FF), yielded 2 transgenic lines in the inbred C57BL/6 background, named FF1 and FF2. The FF1 strain carries 9 copies of the transgene integrated in a single chromosomal locus, which was mapped by fluorescent in situ hybridization and spectral karyotyping to mouse chromosome 8, band A1 (Figure 1C). We determined the integration site by restriction digest and circular ligation followed by PCR and sequencing, and found that the integration occurred at a locus near the centromere that does not contain any known genes (data not shown). A combination of transgene activation and copy number reduction can result when Cre-recombinase is expressed (Figure 1D). Ultimately, a single copy of the transgene in the active orientation will result from this process, provided that all transgene copies were in the head to tail orientation and that the Cre-recombinase reaction was allowed to proceed to completion.
Polycythemia vera- or essential thrombocythemia-like phenotype in JAK2-V617F transgenic mice
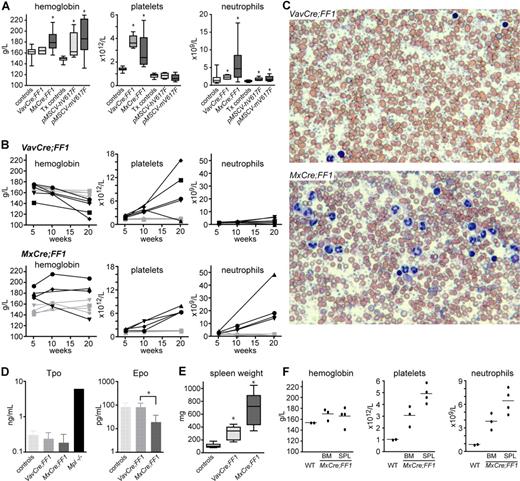
To induce recombination and activation of the FF transgene we crossed the FF mice with the VavCre and MxCre transgenic mice.20,21 The Vav promoter was previously shown to direct constitutive Cre expression to all hematopoietic cells.20 In MxCre mice, the Cre cDNA is under the control of the interferon inducible Mx1 promoter, which can be activated by injection of polyinosine-polycytosine (pIpC). The MxCre inducible mouse has been widely used in studies of hematopoiesis and showed high efficiency of recombination in bone marrow.21,22 The genetic background of the Mx-Cre and VavCre mice was determined by SNP array analysis containing 748 informative SNPs and found to be more than 97% and more than 82% C57BL/6, respectively (data not shown). Analysis of blood from double transgenic VavCre;FF1 and MxCre;FF1 mice is shown in Figure 2 and Table S1 (available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article). At 10 weeks, VavCre;FF1 mice showed normal hemoglobin, slight increase in neutrophils, and marked thrombocytosis, resembling ET (Figure 2A). At 10 weeks after pIpC induction, MxCre;FF1 displayed a PV-like phenotype with elevated hemoglobin, thrombocytosis, and neutrophilia (Figure 2A). A considerable phenotypic variability, in particular in respect to platelets and neutrophils, was observed. The increase in erythrocyte numbers was accompanied by a slight decrease in mean corpuscular volume (Table S1). In comparison, C57BL/6 mice transplanted with bone marrow transduced with retrovirus carrying the human or mouse JAK2-V617F cDNA showed increased hemoglobin, but normal platelets and only mildly elevated neutrophils (Figure 2A). The time course of alteration in blood parameters in transgenic mice is shown in Figure 2B. VavCre;FF1 mice (top row) continued to show a ET phenotype at 20 weeks with a massive increase in platelets in all but one mouse. A slight decrease in hemoglobin, compatible with the presence of myelofibrosis, and mild increase in neutrophils also were observed. MxCre;FF1 mice (bottom row) retained high hemoglobin in 3/4 cases and developed pronounced neutrophilia at 20 weeks after pIpC injection. All showed marked thrombocytosis that was also evident in blood smears (Figure 2C). Despite massive thrombocytosis, we did not observe thrombotic events. The second Flip-Flop strain, FF2, was also crossed with MxCre to obtain MxCre;FF2 mice. We did not observe any significant abnormalities in blood counts after pIpC induction and found that the expression levels of the transgene were low (data not shown). We therefore focused our analysis on the FF1 strain. Tpo plasma concentrations at 20 weeks in both MxCre;FF1 and VavCre;FF1 mice showed no significant differences from normal controls, although a trend to lower values was noted, particularly in MxCre;FF1 mice (Figure 2D). Epo plasma levels were slightly suppressed in MxCre;FF1 mice, as frequently observed in PV patients (Figure 2D).23,24 Double transgenics of both genotypes showed splenomegaly, which was more pronounced in MxCre;FF1 mice (Figure 2E). To test whether the phenotype of MxCre;FF1 mice is cell autonomous, we transplanted bone marrow or spleen cells from an MxCre;FF1 mouse 20 weeks after pIpC injection into lethally irradiated C57BL/6 recipients. Elevated hematocrit accompanied by neutrophilia and thrombocytosis was found in recipients 10 weeks after transplantation (Figure 2F). Thus, the phenotypes of MxCre;FF1 mice is not dependent on expression of the transgene in nonhematopoietic cells. In summary, VavCre;FF1 mice developed a phenotype that resembles ET while MxCre;FF1 displayed a PV-like phenotype.
Peripheral blood parameters in transgenic mice and controls. (A) Hemoglobin, platelets, and neutrophils were determined in controls and double transgenic mice (left half of diagrams) or in mice transplanted with retrovirally transduced bone marrow (right half of diagrams). Controls, wild-type, or single transgenic mice (n = 19); VavCre;FF1 double transgenic mice (age 10-12 weeks, n = 6); MxCre;FF1 double transgenic mice (10-12 weeks after 3 × pIpC injection, n = 11); Tx controls, mice transplanted with bone marrow cells transduced with empty vector or wild-type Jak2 (8 weeks after transplantation, n = 12); pMSCV-hV617F, mice transplanted with bone marrow transduced with human JAK2-V617F (8 weeks after transplantation, n = 9); pMSCV-mV617F, mice transplanted with bone marrow transduced with mouse Jak2-V617F (8 weeks after transplantation, n = 9). Boxes represent the interquartile range that contains 50% of the values, the horizontal line in the box marks the median, and bars indicate the range of values. Asterisks indicate significant differences compared with controls (Mann-Whitney test, P < .05). (B) Time course of blood parameters in VavCre;FF1, MxCre;FF1, and control mice. Blood counts of individual mice at 5-6 weeks, 10-12 weeks, and 20 weeks of age (VavCre;FF1) or after 3 × pIpC injection (MxCre;FF1) are shown. Each mouse is represented by the same symbol in all 3 graphs, and values of individual mice are connected by solid lines. Black symbols, transgenic mice; gray symbols, control mice. (C) Blood films of mice of the indicated genotypes stained with May-Grünwald-Giemsa. VavCre;FF1 mice (20 weeks) and MxCre;FF1 (20 weeks after 3 × pIpC). Note massive thrombocytosis in both mice and prominent neutrophilia in the MxCre;FF1 mouse. (D) Plasma levels of thrombopoietin (Tpo) and erythropoietin (Epo) determined by ELISA. Tpo levels were not significantly different between controls (n = 5), VavCre;FF1 mice (n = 5) and MxCre;FF1 mice (n = 3). Plasma from Mpl-deficient mice (Mpl-/-) served as a control for elevated Tpo levels. Epo plasma concentrations were determined in controls (n = 4), VavCre;FF1 mice (n = 7) and MxCre;FF1 mice (n = 4). Epo was found to be significantly lower in MxCre;FF1 mice than in VavCre;FF1 mice (Mann-Whitney test, P = .012) (E) Spleen weight in VavCre;FF1 mice (20 weeks) and in MxCre;FF1 (20 weeks after 3 × pIpC). (F) The MPD phenotype of MxCre;FF1 mice is transplantable. Bone marrow (BM) or spleen cells (SPL) derived from an MxCre;FF1 mouse (20 weeks after 3 × pIpC) or bone marrow from a wild-type (WT) mouse were transplanted into lethally irradiated C57BL/6 recipients. Blood counts were performed 10 weeks after transplantation. Dots represent the values of individual mice and horizontal lines, the mean.
Peripheral blood parameters in transgenic mice and controls. (A) Hemoglobin, platelets, and neutrophils were determined in controls and double transgenic mice (left half of diagrams) or in mice transplanted with retrovirally transduced bone marrow (right half of diagrams). Controls, wild-type, or single transgenic mice (n = 19); VavCre;FF1 double transgenic mice (age 10-12 weeks, n = 6); MxCre;FF1 double transgenic mice (10-12 weeks after 3 × pIpC injection, n = 11); Tx controls, mice transplanted with bone marrow cells transduced with empty vector or wild-type Jak2 (8 weeks after transplantation, n = 12); pMSCV-hV617F, mice transplanted with bone marrow transduced with human JAK2-V617F (8 weeks after transplantation, n = 9); pMSCV-mV617F, mice transplanted with bone marrow transduced with mouse Jak2-V617F (8 weeks after transplantation, n = 9). Boxes represent the interquartile range that contains 50% of the values, the horizontal line in the box marks the median, and bars indicate the range of values. Asterisks indicate significant differences compared with controls (Mann-Whitney test, P < .05). (B) Time course of blood parameters in VavCre;FF1, MxCre;FF1, and control mice. Blood counts of individual mice at 5-6 weeks, 10-12 weeks, and 20 weeks of age (VavCre;FF1) or after 3 × pIpC injection (MxCre;FF1) are shown. Each mouse is represented by the same symbol in all 3 graphs, and values of individual mice are connected by solid lines. Black symbols, transgenic mice; gray symbols, control mice. (C) Blood films of mice of the indicated genotypes stained with May-Grünwald-Giemsa. VavCre;FF1 mice (20 weeks) and MxCre;FF1 (20 weeks after 3 × pIpC). Note massive thrombocytosis in both mice and prominent neutrophilia in the MxCre;FF1 mouse. (D) Plasma levels of thrombopoietin (Tpo) and erythropoietin (Epo) determined by ELISA. Tpo levels were not significantly different between controls (n = 5), VavCre;FF1 mice (n = 5) and MxCre;FF1 mice (n = 3). Plasma from Mpl-deficient mice (Mpl-/-) served as a control for elevated Tpo levels. Epo plasma concentrations were determined in controls (n = 4), VavCre;FF1 mice (n = 7) and MxCre;FF1 mice (n = 4). Epo was found to be significantly lower in MxCre;FF1 mice than in VavCre;FF1 mice (Mann-Whitney test, P = .012) (E) Spleen weight in VavCre;FF1 mice (20 weeks) and in MxCre;FF1 (20 weeks after 3 × pIpC). (F) The MPD phenotype of MxCre;FF1 mice is transplantable. Bone marrow (BM) or spleen cells (SPL) derived from an MxCre;FF1 mouse (20 weeks after 3 × pIpC) or bone marrow from a wild-type (WT) mouse were transplanted into lethally irradiated C57BL/6 recipients. Blood counts were performed 10 weeks after transplantation. Dots represent the values of individual mice and horizontal lines, the mean.
Megakaryocyte hyperplasia and bone marrow fibrosis accompanied by extramedullary hematopoiesis in JAK2-V617F transgenic mice
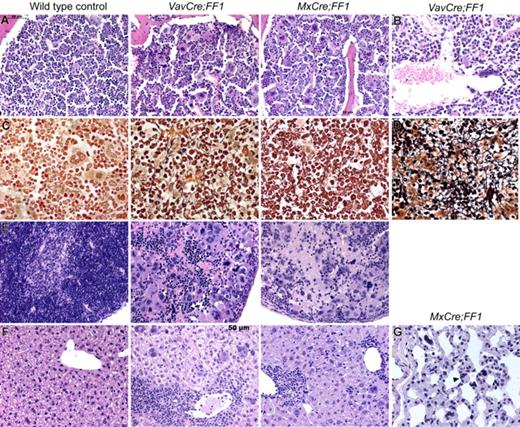
For more detailed analysis, we killed VavCre;FF1 mice at 20 weeks and MxCre;FF1 mice 20 weeks after pIpC injection and performed histopathological analysis (Figure 3). Bone marrow trephine sections of both strains showed hypercellularity with trilineage hyperplasia (Figure 3A). Markedly increased numbers of megakaryocytes were present, most of them with morphological abnormalities (hyperchromatic, hyperlobulated nuclei, and bizarre nuclear configuration) and often forming clusters. A particular finding in VavCre;FF1 mice, but not MxCre;FF1 mice, were dilated sinusoids with intrasinusoidal hematopoiesis (Figure 3B) that are characteristic of PMF in humans. A reticulin stain (Gömöri) highlights the fibrosis that was present in bone marrow and spleen of VavCre;FF1 and MxCre;FF1 mice (Figure 3C,D). Sections of the spleen demonstrate destruction of normal splenic architecture by atypical hematopoiesis (Figure 3E). Megakaryocytes were markedly increased in numbers and displayed the same atypical morphology as in the bone marrow in both VavCre;FF1 and MxCre;FF1 mice. The liver showed extramedullary hematopoiesis with highly atypical megakaryocytes, but islands with myelopoiesis and erythropoiesis could also be found (Figure 3F). Hematopoietic cells, including megakaryocytes, also were found in the lung (Figure 3G).
Histopathological analysis of VavCre;FF1 and MxCre;FF1 mice (20 weeks). H&E staining of bone marrow of both double transgenic mice (A) show trilineage hyperplasia with markedly increased numbers of megakaryocytes, most of which are morphologically abnormal (hyperchromatic, hyperlobulated nuclei, bizarre nuclear configuration) and present in clusters. (B) Dilated sinusoids were observed in 3/3 VavCre;FF1 mice in the bone marrow, but were absent in the 3 examined MxCre;FF1 mice. (C) Reticulin staining (Gömöri) demonstrates fibrosis in bone marrow. (D) Fibrosis also was seen in the spleen (only VavCre;FF1 is shown). (E) H&E staining of spleens demonstrate destruction of normal splenic architecture by atypical hematopoiesis in both MxCre;FF1 and VavCre;FF1 mice. In particular, megakaryocytes are markedly increased in numbers and display the same atypical morphology as in the bone marrow. (F) The liver shows extramedullary hematopoiesis indicated by the presence of megakaryocytes and clusters of granulopoiesis or erythropoiesis. (G) Megakaryocytes (arrowhead) were detected in the lung of MxCre;FF1 mice. VavCre;FF1 mice were not analyzed.
Histopathological analysis of VavCre;FF1 and MxCre;FF1 mice (20 weeks). H&E staining of bone marrow of both double transgenic mice (A) show trilineage hyperplasia with markedly increased numbers of megakaryocytes, most of which are morphologically abnormal (hyperchromatic, hyperlobulated nuclei, bizarre nuclear configuration) and present in clusters. (B) Dilated sinusoids were observed in 3/3 VavCre;FF1 mice in the bone marrow, but were absent in the 3 examined MxCre;FF1 mice. (C) Reticulin staining (Gömöri) demonstrates fibrosis in bone marrow. (D) Fibrosis also was seen in the spleen (only VavCre;FF1 is shown). (E) H&E staining of spleens demonstrate destruction of normal splenic architecture by atypical hematopoiesis in both MxCre;FF1 and VavCre;FF1 mice. In particular, megakaryocytes are markedly increased in numbers and display the same atypical morphology as in the bone marrow. (F) The liver shows extramedullary hematopoiesis indicated by the presence of megakaryocytes and clusters of granulopoiesis or erythropoiesis. (G) Megakaryocytes (arrowhead) were detected in the lung of MxCre;FF1 mice. VavCre;FF1 mice were not analyzed.
The bones were pale upon gross examination, suggesting decreased erythropoiesis. Flow cytometric analysis confirmed that erythroid TER-119–positive cells were reduced in bone marrow, and a compensatory increase was detected in the spleen of both double transgenic mice (Figure S1). Myeloid cells (Gr-1/Mac-1–positive) were the predominant cell population in bone marrow and also clearly increased in spleen. The relative amount of B and T cells in the spleen and bone marrow of both MxCre;FF1 and VavCre;FF1 mice was reduced, whereas the number of megakaryocytes was increased (Figure S1). Colony assays of progenitors in methylcellulose confirmed extramedullary hematopoiesis with markedly increased erythroid and myeloid progenitor numbers in spleen (Figure 4A). Megakaryopoiesis was assayed in collagen-based cultures and revealed a small increase in CFU-MK in bone marrow of VavCre;FF1 mice and a massive expansion of CFU-MK in the spleens of both double transgenic mice (Figure 4B). No growth of CFU-E on days 2 to 3 or BFU-E on days 8 to 10 was observed in methylcellulose media without cytokines, despite plating 5 times more cells (data not shown).
Colony assays for the quantification of hematopoietic progenitors. Bone marrow and spleen cells of transgenic VavCre;FF1 (n = 4) and MxCre;FF1 mice (n = 4) and controls (n = 6) were seeded in methylcellulose containing mouse interleukin-3 (IL-3), human IL-6 and IL-9, mouse stem cell factor, and human erythropoietin (A), or in collagen media containing thrombopoietin, mouse IL-3 and human IL-6 (B). Colonies were enumerated at day 8. Prior to counting, collagen cultures were fixed and stained for acetyl cholinesterase activity to visualize megakaryocytes. CFU-GEMM, colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte; CFU-GM, sum of colony forming unit-granulocyte, colony forming unit-macrophage and colony forming unit-granulocyte, macrophage; BFU-E, burst forming unit-erythroid. Spleens of double transgenic mice showed marked increase in hematopoietic progenitors. For statistical analysis, we performed pairwise Mann-Whitney tests. Asterisks indicate statistically significant differences (P < .05; in panel A for all colony types)
Colony assays for the quantification of hematopoietic progenitors. Bone marrow and spleen cells of transgenic VavCre;FF1 (n = 4) and MxCre;FF1 mice (n = 4) and controls (n = 6) were seeded in methylcellulose containing mouse interleukin-3 (IL-3), human IL-6 and IL-9, mouse stem cell factor, and human erythropoietin (A), or in collagen media containing thrombopoietin, mouse IL-3 and human IL-6 (B). Colonies were enumerated at day 8. Prior to counting, collagen cultures were fixed and stained for acetyl cholinesterase activity to visualize megakaryocytes. CFU-GEMM, colony forming unit-granulocyte, erythroid, macrophage, megakaryocyte; CFU-GM, sum of colony forming unit-granulocyte, colony forming unit-macrophage and colony forming unit-granulocyte, macrophage; BFU-E, burst forming unit-erythroid. Spleens of double transgenic mice showed marked increase in hematopoietic progenitors. For statistical analysis, we performed pairwise Mann-Whitney tests. Asterisks indicate statistically significant differences (P < .05; in panel A for all colony types)
The ratios between the expression levels of mutant JAK2-V617F and wild-type Jak2 correlate with disease phenotype
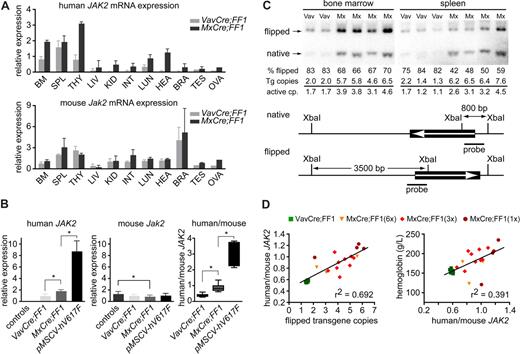
To determine whether the phenotypic differences between VavCre;FF1 and MxCre;FF1 mice might be due to differences in the expression levels of the human JAK2-V617F, we quantified the expression of the transgenic JAK2-V617F and endogenous Jak2 mRNA by real-time PCR (Figure 5A). To detect solely transcripts of the flipped FF1 transgene, oligonucleotides located in exons 12 and 13 of human JAK2 were used that do not prime in mouse Jak2. In addition, mouse-specific primers were used to assess expression of endogenous Jak2. The expression values were normalized to the mRNA for β-glucuronidase (Gusb). VavCre;FF1 mice showed JAK2-V617F expression in hematopoietic tissues (Figure 5A top panel). In addition, we also detected transgene expression in lung and at low levels in liver and testis. The MxCre;FF1 mice showed a wide distribution of transgene expression. The presence of hematopoietic cells might contribute to the relatively high expression levels observed in the lung (Figure 3G). The endogenous mouse Jak2 was expressed in all tissues examined and no major differences were found between VavCre;FF1 and MxCre;FF1 mice (Figure 5A bottom panel). We next compared the expression of human JAK2-V617F and mouse Jak2 in the bone marrow of double transgenic mice to mice transplanted with bone marrow retrovirally transduced with human JAK2-V617F (Figure 5B). VavCre;FF1 mice showed the lowest expression of JAK2-V617F, followed by MxCre;FF1, and the highest expression levels were found in retrovirally transduced bone marrow cells (Figure 5B left). Endogenous mouse Jak2 was slightly reduced in MxCre;FF1 mice compared with controls (Figure 5B middle). To determine the ratio of JAK2-V617F to wild-type Jak2, we developed a quantification assay that normalizes for differences in the amplification efficiencies between the primers used for human and mouse JAK2 (Figure 5B right). For this purpose, standard curves were generated from plasmids that contained either human JAK2 or mouse Jak2 but were otherwise identical. Transgene expression was less than half of endogenous Jak2 in bone marrow of VavCre;FF1 mice. In MxCre;FF1 mice the expression of mutant JAK2 was comparable to wild-type Jak2, whereas in retrovirally transduced bone marrow the mutant JAK2 was around 3-fold higher than wild-type Jak2 (Figure 5B right). A similar pattern, but slightly lower ratios, also was seen in spleen and in peripheral blood granulocytes (not shown). Since the expression levels of JAK2-V617F could depend on the number of functionally rearranged copies of the FF1 transgene, we performed Southern blot analysis to determine the ratio of flipped versus native form of the transgene (Figure 5C). In the same samples we measured the transgene copy number by real-time PCR to determine the rate of excision by Cre-recombinase. Cre activity is expected to generate both active rearrangement and copy number reduction by excision (Figure 1D). In bone marrow of VavCre;FF1 mice we found more than 80% of the transgene to be flipped into the active configuration, but at the same time the number of transgene copies was reduced to an average of 2 (Figure 5C). In contrast, MxCre;FF1 displayed approximately 70% active configuration in bone marrow, and an average of 5.7 transgene copies (Figure 5C). The number of active transgene copies in bone marrow cells was between 1 and 2 in VavCre;FF1 mice and between 3 and 4.5 in MxCre;FF1 mice. The higher rate of excision in VavCre;FF1 is not unexpected, since Cre expression in the bone marrow of these mice is constitutive, whereas the induction of Cre expression in MxCre;FF1 mice by pIpC is only transient. By altering the number of pIpC injections in MxCre;FF1 mice we obtained mice with variable number of active transgene copies (Figure S2). We confirmed the correlation between flipped transgene copies and ratio of human JAK2-V617F to mouse Jak2 (Figure 5D left panel). Furthermore, the hemoglobin concentration correlated with the ratio of human JAK2-V617F to mouse Jak2 (Figure 5D right panel). Two outliers showed unexpectedly low hemoglobin. These 2 mice showed very high platelet counts (12′144 × 109/L and 11′298 × 109/L, respectively). In summary, FF1 transgene expression in bone marrow, spleen, and peripheral blood granulocytes was higher when induced with MxCre than when induced with VavCre and correlated with the number of transgene copies in the active configuration and with the observed differences in phenotype: low expression in VavCre;FF1 appears to favor expansion of the megakaryocytic lineage; intermediate expression in MxCre;FF1 mice was accompanied by increased erythropoiesis, granulopoiesis, and thrombopoiesis; whereas high expression from a retroviral vector resulted in erythroid expansion, but normal granulopoiesis and normal megakaryopoiesis.
Transgene mRNA expression and Cre-loxP–mediated DNA recombination. (A) Expression of human JAK2-V617F mRNA and endogenous Jak2 in tissues of 2 VavCre;FF1 and 3 MxCre;FF1 mice. BM, bone marrow; SPL, spleen; THY, thymus; LIV, liver; KID, kidney; INT, intestine; LUN, lung; HEA, heart; BRA, brain; TES, testis; OVA, ovary. Real-time PCR was performed with primers specific for the activated configuration of the human JAK2 transgene (top panel) or specific for mouse Jak2 (bottom panel). The numbers represent relative expression values calculated by the ΔΔCT method after normalization to the mRNA of mouse Gusb and arbitrarily choosing one bone marrow sample from a VavCre;FF1 mouse as the calibrator. Separate calculations were carried out for human and mouse Jak2. (B) Expression of human JAK2-V617F (left panel), mouse Jak2 (middle panel), and ratio between human JAK2-V617F and mouse Jak2 (right panel) in total bone marrow. Expression was measured in control mice (n = 7), VavCre;FF1 (n = 7, age 20-30 weeks), MxCre;FF1 (n = 8, 15-20 weeks after 3 × pIpC injection), or in mice transplanted with retrovirally transduced bone marrow expressing JAK2-V617F (pMSCV-hV617F; n = 6, 20 weeks after transplantation; values corrected for the percentage of transduced cells based on green fluorescent protein (GFP) expression are shown, GFP-positive cells = 35% ± 24%). Expression in MxCre;FF1 was significantly higher than in VavCre;FF1 samples (Mann-Whitney test, P = .0012). The highest levels of JAK2-V617F were observed with retroviral transduction (P = .0016 vs MxCre;FF1). A slight decrease in expression of mouse Jak2 was noted in MxCre;FF1 compared with controls (middle panel, P = .04), whereas no significant difference was found between VavCre;FF1 and MxCre;FF1. The ratios between human JAK2-V617F and mouse Jak2 in bone marrow were calculated from the absolute expression values of human JAK2 and mouse Jak2 that were determined by comparison with standard curves set up from purified plasmids containing human JAK2 or mouse Jak2. Significant differences between VavCre;FF1 and MxCre;FF1 (P = .0003) and between MxCre;FF1 and pMSCV-hV617F (P = .0007) were noted. All significant differences (P < .05) are marked by asterisks. (C) Assessment of Cre-mediated recombination by Southern blot analysis and copy number determination by real-time PCR. DNA from bone marrow and spleen cells was digested with XbaI and the Southern blots of DNA fragments separated by electrophoresis were visualized with a 32P-labeled human JAK2 cDNA probe (thick solid line). The scheme below shows the position of XbaI restriction sites and of the probe. The expected fragments sizes are 800 bp for the native configuration and 3′500 bp for the flipped configuration. Vav, bone marrow or spleen from VavCre;FF1 mice; Mx, bone marrow or spleen from MxCre;FF1 mice. The percentage of flipped transgenes (% flipped) was determined by quantification of the flipped and native bands on a phosphorimager. Transgene (Tg) copies were determined by real-time PCR, and the numbers of active copies were calculated by multiplying with the percentage of flipped alleles. (D) Correlation of human/mouse JAK2 mRNA ratio with the number of flipped alleles (left) and correlation of hemoglobin with the human/mouse mRNA ratio (right). MxCre;FF1 mice received 1 ×, 3 ×, or 6 × injections of pIpC and were analyzed at 12 weeks. The number of flipped alleles was determined by real-time PCR, which yielded similar results as Southern blot analysis. VavCre;FF1 mice (age 12 weeks) were included for comparison.
Transgene mRNA expression and Cre-loxP–mediated DNA recombination. (A) Expression of human JAK2-V617F mRNA and endogenous Jak2 in tissues of 2 VavCre;FF1 and 3 MxCre;FF1 mice. BM, bone marrow; SPL, spleen; THY, thymus; LIV, liver; KID, kidney; INT, intestine; LUN, lung; HEA, heart; BRA, brain; TES, testis; OVA, ovary. Real-time PCR was performed with primers specific for the activated configuration of the human JAK2 transgene (top panel) or specific for mouse Jak2 (bottom panel). The numbers represent relative expression values calculated by the ΔΔCT method after normalization to the mRNA of mouse Gusb and arbitrarily choosing one bone marrow sample from a VavCre;FF1 mouse as the calibrator. Separate calculations were carried out for human and mouse Jak2. (B) Expression of human JAK2-V617F (left panel), mouse Jak2 (middle panel), and ratio between human JAK2-V617F and mouse Jak2 (right panel) in total bone marrow. Expression was measured in control mice (n = 7), VavCre;FF1 (n = 7, age 20-30 weeks), MxCre;FF1 (n = 8, 15-20 weeks after 3 × pIpC injection), or in mice transplanted with retrovirally transduced bone marrow expressing JAK2-V617F (pMSCV-hV617F; n = 6, 20 weeks after transplantation; values corrected for the percentage of transduced cells based on green fluorescent protein (GFP) expression are shown, GFP-positive cells = 35% ± 24%). Expression in MxCre;FF1 was significantly higher than in VavCre;FF1 samples (Mann-Whitney test, P = .0012). The highest levels of JAK2-V617F were observed with retroviral transduction (P = .0016 vs MxCre;FF1). A slight decrease in expression of mouse Jak2 was noted in MxCre;FF1 compared with controls (middle panel, P = .04), whereas no significant difference was found between VavCre;FF1 and MxCre;FF1. The ratios between human JAK2-V617F and mouse Jak2 in bone marrow were calculated from the absolute expression values of human JAK2 and mouse Jak2 that were determined by comparison with standard curves set up from purified plasmids containing human JAK2 or mouse Jak2. Significant differences between VavCre;FF1 and MxCre;FF1 (P = .0003) and between MxCre;FF1 and pMSCV-hV617F (P = .0007) were noted. All significant differences (P < .05) are marked by asterisks. (C) Assessment of Cre-mediated recombination by Southern blot analysis and copy number determination by real-time PCR. DNA from bone marrow and spleen cells was digested with XbaI and the Southern blots of DNA fragments separated by electrophoresis were visualized with a 32P-labeled human JAK2 cDNA probe (thick solid line). The scheme below shows the position of XbaI restriction sites and of the probe. The expected fragments sizes are 800 bp for the native configuration and 3′500 bp for the flipped configuration. Vav, bone marrow or spleen from VavCre;FF1 mice; Mx, bone marrow or spleen from MxCre;FF1 mice. The percentage of flipped transgenes (% flipped) was determined by quantification of the flipped and native bands on a phosphorimager. Transgene (Tg) copies were determined by real-time PCR, and the numbers of active copies were calculated by multiplying with the percentage of flipped alleles. (D) Correlation of human/mouse JAK2 mRNA ratio with the number of flipped alleles (left) and correlation of hemoglobin with the human/mouse mRNA ratio (right). MxCre;FF1 mice received 1 ×, 3 ×, or 6 × injections of pIpC and were analyzed at 12 weeks. The number of flipped alleles was determined by real-time PCR, which yielded similar results as Southern blot analysis. VavCre;FF1 mice (age 12 weeks) were included for comparison.
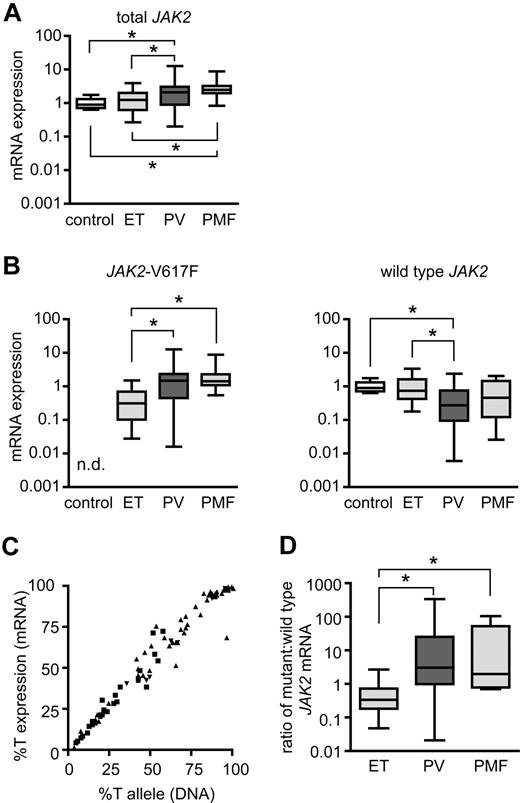
PV patients show higher mutant-to-wild-type JAK2 expression ratio than ET patients
To determine whether a similar correlation between expression levels of JAK2-V617F and the ET and PV phenotypes also exists in patients with MPD, we measured total JAK2, JAK2-V617F, and wild-type JAK2 in granulocyte RNA from 82 patients with JAK2-V617F–positive MPDs (25 ET, 49 PV, 8 PMF) and in 11 healthy individuals (Figure 6). Patients with PV and PMF showed slightly higher expression of total JAK2 (Figure 6A) and clearly higher expression of JAK2-V617F mRNA than ET patients (Figure 6B left). In PV patients we detected lower wild-type JAK2 mRNA levels than in ET patients or healthy controls (Figure 6B right). The cause of the interindividual differences in total JAK2 expression levels (Figure 6A) is currently unknown. The amplitudes of these differences, but not the overall conclusions, are influenced by the choice of gene for normalization (Figure S3). A strong linear correlation between the percentage of JAK2-V617F mRNA and the allelic ratio of JAK2-V617F in genomic DNA was noted (Figure 6C), similar to a previous study.17 Thus, despite the interindividual variations in the total expression levels, the relative expression of the mutant versus wild-type JAK2 mRNA in humans appears to depend primarily on the percentage of chromosomes carrying the JAK2-V617F mutation (%T). Since the %T in ET patients is on average lower than in PV and PMF, ET patients also displayed lower ratios of mutant/wild-type JAK2 mRNA than PV patients (Figure 6D), reminiscent of the pattern found in our transgenic mice (Figure 5B).
Expression levels of JAK2-V617F in granulocytes from human patients with MPD. (A) Expression of total JAK2 mRNA was determined by Taqman real-time PCR in purified granulocytes from healthy controls (n = 11) and patients with essential thrombocythemia (ET; n = 25), polycythemia vera (PV; n = 49), and primary myelofibrosis (PMF; n = 8). Values were normalized to the expression levels of β-glucuronidase (GUSB) mRNA. One control was chosen as the calibrator, and expression of JAK2 set to the value of 1 to calculate the fold expression in all other samples (ΔΔCT method). Boxes represent the interquartile range that contains 50% of the values, the horizontal line in the box marks the median and bars indicate the range of values. All significant differences with P < .05 (pairwise Mann-Whitney tests) are marked with asterisks (control vs PV: P = .0084, control vs PMF: P = .0003, ET vs PV: P = .026, ET vs PMF: P = .0197). Note that the expression values are shown on a logarithmic scale. (B) JAK2-V617F mRNA (left) and wild-type JAK2 mRNA (right) were quantified with allele-specific Taqman real-time assays, and the fold expression was calculated based on the values shown in panel A. JAK2-V617F expression was significantly higher in PV and PMF than in ET (ET vs PV: P < .0001, ET vs PMF: P = .0002, all other pairs: P > .05); n.d., not detectable. Wild-type JAK2 mRNA was significantly lower in PV than in ET or controls (control vs PV: P = .0026, ET vs PV: P = .0029). (C) Correlation between JAK2-V617F mRNA and JAK2-V617F DNA in patients with ET (■), PV (▴), and PMF (▾). The percentages of JAK2-V617F (%T = mutant JAK2 divided by total JAK2) in granulocyte RNA were plotted against the percentages of JAK2-V617F alleles in granulocyte genomic DNA for all patients studied. A strong linear correlation was noted (linear regression, r2 = 0.97). (D) Ratios of mutant to wild-type JAK2 expression in granulocyte RNA calculated from the values shown in B (ET vs PV: P < .0001, ET vs PMF: P = .001).
Expression levels of JAK2-V617F in granulocytes from human patients with MPD. (A) Expression of total JAK2 mRNA was determined by Taqman real-time PCR in purified granulocytes from healthy controls (n = 11) and patients with essential thrombocythemia (ET; n = 25), polycythemia vera (PV; n = 49), and primary myelofibrosis (PMF; n = 8). Values were normalized to the expression levels of β-glucuronidase (GUSB) mRNA. One control was chosen as the calibrator, and expression of JAK2 set to the value of 1 to calculate the fold expression in all other samples (ΔΔCT method). Boxes represent the interquartile range that contains 50% of the values, the horizontal line in the box marks the median and bars indicate the range of values. All significant differences with P < .05 (pairwise Mann-Whitney tests) are marked with asterisks (control vs PV: P = .0084, control vs PMF: P = .0003, ET vs PV: P = .026, ET vs PMF: P = .0197). Note that the expression values are shown on a logarithmic scale. (B) JAK2-V617F mRNA (left) and wild-type JAK2 mRNA (right) were quantified with allele-specific Taqman real-time assays, and the fold expression was calculated based on the values shown in panel A. JAK2-V617F expression was significantly higher in PV and PMF than in ET (ET vs PV: P < .0001, ET vs PMF: P = .0002, all other pairs: P > .05); n.d., not detectable. Wild-type JAK2 mRNA was significantly lower in PV than in ET or controls (control vs PV: P = .0026, ET vs PV: P = .0029). (C) Correlation between JAK2-V617F mRNA and JAK2-V617F DNA in patients with ET (■), PV (▴), and PMF (▾). The percentages of JAK2-V617F (%T = mutant JAK2 divided by total JAK2) in granulocyte RNA were plotted against the percentages of JAK2-V617F alleles in granulocyte genomic DNA for all patients studied. A strong linear correlation was noted (linear regression, r2 = 0.97). (D) Ratios of mutant to wild-type JAK2 expression in granulocyte RNA calculated from the values shown in B (ET vs PV: P < .0001, ET vs PMF: P = .001).
Discussion
Retroviral mouse models have demonstrated that expression of Jak2-V617F in bone marrow causes a PV-like phenotype, which can progress to myelofibrosis,1,7-10 but thrombocytosis was absent in these mice. The megakaryocytes were markedly smaller and displayed reduced ploidy, suggesting impaired megakaryocyte maturation.8,10 All published retroviral reports used a mouse Jak2 cDNA, in which the G > T mutation in codon 617 has been introduced. We show that the human JAK2-V617F also can cause an MPD phenotype in mice. Our transgenic mice developed marked thrombocytosis that has never been observed with the retroviral models. A variable phenotype ranging from thrombocytosis only (in one of the VF founders and some VavCre;FF1 mice), bilineage disease involving thrombopoiesis and granulopoiesis (in the majority of VavCre;FF1 mice), to full trilineage disease with increased erythropoiesis, thrombopoiesis, and granulopoiesis (in MxCre:FF1 mice) has been observed. These phenotypes correlated with the number of actively rearranged transgene copies and the ratio of expression levels of transgenic JAK2-V617F in respect to the endogenous mouse Jak2. The transgenic mice displayed clearly lower expression levels than bone marrow cells retrovirally transduced with JAK2-V617F. These results suggest that the hematopoietic lineages show differences in responsiveness to the presence of the mutated JAK2-V617F, with megakaryopoiesis being most sensitive, followed by granulopoiesis and erythropoiesis. High levels of JAK2-V617F appear to be inhibitory to megakaryopoiesis, as illustrated by normal or decreased platelet counts in the retroviral models. In this context it is noteworthy that in PV patients a negative correlation of JAK2-V617F expression level with platelet counts, but a positive correlation with hemoglobin and granulocyte counts, has been reported.17,25,26 In addition, ET patients on average display higher platelet levels than PV patients,17 which is reflected in our VavCre;FF1 mice that show higher platelet levels than MxCre;FF1 mice. Jak2 protein was found to function as a chaperone promoting cell surface localization of the thrombopoietin receptor Mpl,27 and reduced Mpl protein on platelets was observed in MPD patients.19,28-30 One study noted a reciprocal relationship between JAK2-V617F alleles in granulocytes and Mpl protein on platelets,31 suggesting that Mpl protein can be down-regulated by Jak2-V617F. However, we did not observe reduced Mpl protein levels on platelets of our VavCre;FF1 or MxCre;FF1 transgenic mice (data not shown).
Ubiquitous high level of JAK2-V617F transgene expression might be lethal, as we were unable to establish a VF transgenic line that exhibits a MPD phenotype. Two founder mice died before giving rise to offspring, both with a clearly enlarged spleen. The only transgenic line we obtained (VF1) showed low expression (ratio mutant/wild-type JAK2 mRNA = 0.32 ± 0.02 in bone marrow, n = 5) and appears to have been selected for minimal phenotype (Table S1). Consistently, no germ line mutations in JAK2 have been reported to date in human familial MPD. The inducible BAC construct allowed us to establish the transgenic line FF1 that exhibits either an ET or a PV phenotype, depending on the mode of transgene activation. Because 9 copies of the transgene are present in FF1 mice, Cre-mediated excision can result in activation and/or excision of the transgene (Figure 1D). The numbers of actively rearranged copies of the transgene correlated with JAK2-V617F expression levels. Dependence of expression on transgene copy numbers has been found in many BAC transgenes and is considered an advantage over the use of classical small transgenic constructs.32-34
The Vav promoter used in our VavCre mice has been shown to display variable expression efficiency in erythroid cells.20 However, the absence of an erythroid phenotype in VavCre;FF1 mice was not due to the absence of transgene activation/excision in erythropoietic cells, as rearrangement of the transgene was found in DNA from isolated erythroid colonies of VavCre;FF1 mice grown in methylcellulose (data not shown). In contrast to other Vav transgenic mice, transgene expression in our case depends on the activity of the JAK2 promoter. Active rearrangement of the transgene in hematopoietic stem cells is theoretically sufficient to maintain expression in all hematopoietic lineages that are derived from such a stem cell. The Vav promoter has been shown to be active in hematopoietic stem cells,35,36 but it remains to be established how efficient the excision occurs at the stem cell level in VavCre;FF1 mice. If excision in stem cells is only partial, lineage-specific differences in Cre expression levels between VavCre and MxCre cannot be excluded as the cause of phenotypic differences. However, the ET-like phenotype was not unique to the VavCre;FF1 mice, as MxCre;FF1 mice with a lower number of actively rearranged transgene copies (after receiving 6 doses of pIpC) also displayed a trend toward lower hemoglobin (Figure 5D and Table S1). Conversely MxCre;FF1 mice that received a single dose of pIpC showed the highest number of actively rearranged transgene copies and the highest hemoglobin levels. Although this data are based on a relatively small number of mice, it suggests that variations in phenotype primarily depend on the number of actively rearranged transgene copies, rather than the lineage specificity of the promoter driving Cre expression. The Vav promoter is already active in the fetal liver.35 VavCre;FF1 mice therefore could be expected to show thrombocytosis at a young age. However, we found that platelet levels at 5 weeks in these mice were only slightly elevated, and a marked increase was observed only at later time points (Figure 2B). The reason for the slow disease progression is currently unknown.
Quantification of JAK2-V617F expression using 2 different real-time PCR approaches showed a strong correlation with phenotype (Figure 5). The ΔΔCT method allows to reliably determining the relative expression of the human and mouse JAK2, but the absolute levels cannot be directly compared, because the amplification efficiency of the human and mouse primer pairs is likely to vary. We therefore established standard curves to derive the ratios of human/mouse JAK2 presented in Figure 5B, which confirmed the differences between the 2 transgenic strains and the retroviral model. A dependence of the platelet phenotype on Jak2-V617F expression levels also was noted in one of the retroviral mouse models, in which a subgroup of secondary recipients of bone marrow transplantation showed a 1.6-fold increase in platelet numbers 2 weeks after transplantation.7 At 3 and 4 weeks the platelet levels were equal to the controls. This subgroup of mice showed a lower ratio of mutant/wild-type Jak2 mRNA (average, 2.8-fold) than other mice that received transplants with normal platelet counts (average, 8.2-fold). These ratios are higher than those observed in our mice. Despite showing a ratio of mutant/wild-type mRNA of 3, our mice transplanted with retrovirally transduced bone marrow expressing JAK2-V617F showed normal platelet numbers (Figure 2A). Since the retroviral constructs as well as the methods used for quantification were different, we cannot directly compare our results with the study by Lacout and colleagues.7 Nevertheless, the same trend was observed, although at different mutant/wild-type ratios. Thus, in mouse models the presence of the JAK2-V617F is sufficient to generate the full range of MPD phenotypes. The phenotypic variation appears to depend on the ratio of mutant/wild-type JAK2 mRNA. A contribution of genetic background on the phenotypic expression is unlikely, since our MxCre;FF1 mice were more than 98% C57BL/6 and showed ET or PV phenotypes depending on the scheme of transgene activation, that is, number of pIpC injections (Figure 5D and Table S1). Clearly, the ratio of mutant/wild-type JAK2 mRNA is only a surrogate parameter, and the expression and phosphorylation status of the mutant and wild-type Jak2 proteins in hematopoietic progenitors need to be monitored. Furthermore, in some mice the blood counts did not correlate with transgene expression (Figure 5D), suggesting that additional unknown factors, possibly somatic mutations, may modify the phenotype.
Increased bone marrow fiber content was more pronounced in VavCre;FF1 mice than in MxCre;FF1 mice and appears to correlate better with the extent of thrombocytosis than with the mutant/wild-type mRNA ratio because VavCre;FF1 mice showed dilated sinusoids and more pronounced fibrosis despite a low mutant/wild-type mRNA ratio. In contrast, a high mutant/wild-type mRNA ratio was detected in PMF patients. In the retroviral models, megakaryocyte maturation defects have been observed that are thought to be responsible for the normal or low platelet counts possibly through an altered chaperone function of Jak2-V617F protein. Fibrosis in these mice is likely related to the elevated megakaryocyte mass in the bone marrow.
In ET patients we also observed lower expression levels of JAK2-V617F than in PV (Figure 6). However, a logarithmic scale was used to show the differences in human patients, whereas a linear scale was used for the differences in mice (Figure 5), reflecting a much wider range of ratios present in the patients. In contrast to our transgenic mice that display graded levels of JAK2-V617F with wild-type JAK2 being present in every cell, each individual blood cell from patients with MPD can only be homozygous or heterozygous for the mutation, or normal. Thus, the resemblance of the plots is due to the fact that a mixture of granulocytes with wild-type JAK2 and granulocytes heterozygous for JAK2-V617F are present in ET, whereas patients with PV and PMF frequently have additional cells homozygous for JAK2-V617F.4,37,38 Therefore, the molecular mechanism determining the phenotype in humans may be more complex than in our mouse model and appears to be linked to the transition of the JAK2-V617F mutation to homozygosity. A subset of PV patients that lack cells with homozygous JAK2-V617F was recently described, suggesting that alternative mechanisms to cause expansion of the erythroid lineage exist in PV patients.26 Similarly, patients with mutations in exon 12 of JAK2 generally do not progress to homozygosity.39,40
The FF1 transgenic mouse model offers several advantages over the previously reported retroviral models using bone marrow transplantation: our transgenic mice exhibit bi- or tri-lineage disease including thrombocytosis, as observed in human patients with ET and PV. The expression levels of the JAK2-V617F transgene are comparable to the endogenous Jak2, in contrast to the very high expression levels observed in most of the retroviral models. In addition, we show that the human JAK2-V617F causes MPD in mice, which will be important for testing Jak2 inhibitors as potential therapeutic agents.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Daniela Nebenius-Oosthuizen for oocyte microinjections, David Beier and Jennifer Moran for SNP-array analysis, and Robert Kralovics and Nico Ghilardi for helpful comments on the manuscript.
This work was supported by grant 310000-108006/1 from the Swiss National Science Foundation and grant OCS-01742-08-2005 from the Swiss Cancer League/Oncosuisse.
Authorship
Contribution: R.T. designed and performed research, analyzed data, and wrote the paper; H.H. and R.L. performed research; M.A.S., S.D., and J.S. performed research and analyzed data; R.C.S. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Radek C. Skoda, Department of Research, Experimental Hematology, University Hospital Basel, Hebelstrasse 20, 4031 Basel, Switzerland; e-mail: radek.skoda@unibas.ch.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal