Abstract
Flow cytometric immunophenotyping remains an indispensable tool for the diagnosis, classification, staging, and monitoring of hematologic neoplasms. The last 10 years have seen advances in flow cytometry instrumentation and availability of an expanded range of antibodies and fluorochromes that have improved our ability to identify different normal cell populations and recognize phenotypic aberrancies, even when present in a small proportion of the cells analyzed. Phenotypically abnormal populations have been documented in many hematologic neoplasms, including lymphoma, chronic lymphoid leukemias, plasma cell neoplasms, acute leukemia, paroxysmal nocturnal hemoglobinuria, mast cell disease, myelodysplastic syndromes, and myeloproliferative disorders. The past decade has also seen refinement of the criteria used to identify distinct disease entities with widespread adoption of the 2001 World Health Organization (WHO) classification. This classification endorses a multiparametric approach to diagnosis and outlines the morphologic, immunophenotypic, and genotypic features characteristic of each disease entity. When should flow cytometric immunophenotyping be applied? The recent Bethesda International Consensus Conference on flow cytometric immunophenotypic analysis of hematolymphoid neoplasms made recommendations on the medical indications for flow cytometric testing. This review discusses how flow cytometric testing is currently applied in these clinical situations and how the information obtained can be used to direct other testing.
Introduction
A decade has passed since the review “Recent advances in flow cytometry: application to the diagnosis of hematologic malignancy” was published in Blood.1 In the past 10 years, flow cytometric immunophenotyping has maintained its position as an indispensable diagnostic tool. Improvements in flow cytometry instrumentation and availability of an expanded range of antibodies and fluorochromes has led to more accurate phenotyping of cells, leading to enhanced identification of abnormal populations.2 The last 10 years have also seen refinement of the criteria used to identify distinct disease entities among the hematologic malignancies. The World Health Organization (WHO) classification for tumors of the hematopoietic and lymphoid tissues delineates many of these entities and has been widely adopted.3 This classification endorses a multiparametric approach to diagnosis with identification of morphologic, phenotypic, and genotypic features that are characteristic of each disease entity. However, it is neither necessary nor cost-effective to perform multiple studies on every specimen. When should flow cytometric testing be ordered?
In 2006, a group of international experts met in Bethesda, Maryland, to formulate consensus recommendations for flow cytometric testing.4 In contrast to previous consensus meetings that had considered the reagents required to evaluate a specific disease entity, the Bethesda group took a more practical approach and addressed the flow cytometric evaluation of specimens based on the clinical presentation.5 Consensus was reached that flow cytometric immunophenotyping is indicated in the following clinical situations: cytopenias, especially bicytopenia and pancytopenia; elevated leukocyte count, including lymphocytosis, monocytosis, and eosinophilia; the presence of atypical cells or blasts in the peripheral blood, bone marrow, or body fluids; plasmacytosis or monoclonal gammopathy; and organomegaly and tissue masses.5 In these clinical situations, flow cytometric immunophenotyping can provide a sensitive screen for the presence of hematologic malignancy and assist in demonstrating the absence of disease. In contrast, the Bethesda group agreed that flow cytometry was generally not indicated in the following situations: mature neutrophilia, polyclonal hypergammaglobulinemia, polycythemia, thrombocytosis, and basophilia.5 In addition, the consensus group agreed that flow cytometry is a useful tool for staging a previously diagnosed hematolymphoid neoplasm, monitoring response to treatment including detection of minimal residual disease (MRD), documenting relapse or progression, and diagnosing an intercurrent hematologic malignancy, such as a therapy-related myelodysplastic syndrome (MDS).
Taking a similar practical approach, this review discusses how flow cytometric immunophenotyping is currently applied in these clinical settings to establish the diagnosis of a hematologic malignancy, including how the information obtained can be used to direct other ancillary testing.
Flow cytometric immunophenotyping for the diagnosis and monitoring of hematologic neoplasms
Flow cytometric immunophenotyping evaluates individual cells in suspension for the presence and absence of specific antigens (phenotype). In the assessment for hematologic malignancies, several steps are taken in the application and interpretation of this immunophenotypic information: (1) identification of cells from different lineages and determination of whether they are mature or immature, such as detection of mature B-lymphoid cells and myeloblasts; (2) detection of abnormal cells through identification of antigen expression that differs significantly from normal; (3) detailed documentation of the phenotype of abnormal cell populations (ie, the presence or absence of antigens) and, in comparison to their normal cell counterpart, documentation of increased or decreased intensity of staining by fluorochrome labeled antibodies; (4) evaluation of whether the information available is diagnostic of a distinct disease entity and, if not, development of a list of possible entities with suggestion of additional studies that might be of diagnostic value such as immunohistochemistry, conventional cytogenetic, fluorescence in situ hybridization (FISH), and molecular diagnostic studies; and (5) provision of immunophenotypic information that might be of additional prognostic value, including the identification of targets for potential directed therapy.
When a specimen is received for flow cytometric testing, a decision is made regarding the cell lineages and antigens to be evaluated that is based on the type of specimen and other available information, such as the medical indication for testing listed on the requisition, clinical history, morphologic findings, history of prior flow cytometric testing, results of other laboratory testing, and possibly results of any preliminary screening testing performed in the flow cytometric laboratory. For the medical indications identified by the 2006 Bethesda group, consensus was reached on the cell lineages that should be evaluated and the antigens to include in a primary evaluation of each lineage.6 In addition, general recommendations were made on the approach used to evaluate these antigens by flow cytometry.6 Using this approach, flow cytometric immunophenotyping of clinical specimens can provide a rapid screen for hematologic neoplasms and play a key role in diagnosis and classification. The following sections address the application of flow cytometric immunophenotyping to the evaluation for fairly broad groups of hematologic neoplasms: mature lymphoid neoplasms, plasma cell neoplasms, blastic malignancies, maturing myeloid and monocytic malignancies, and for the detection of MRD.
Mature lymphoid neoplasms
Neoplasms of mature lymphoid cells include the chronic leukemia lymphoid neoplasms and non-Hodgkin lymphomas. This group of diseases is recognized by an immunophenotype that is similar to normal mature lymphoid cells (eg, surface immunoglobulin on mature B cells) and lack of antigenic features of immaturity, such as expression of TdT, CD34, or weak intensity staining for CD45. Through identification of lineage-associated antigens, neoplasms of mature lymphoid cells can be divided into those of B-, T- and natural killer (NK)–cell lineages.3 Hodgkin lymphoma will not be discussed in this review. Although a recent study demonstrated that using a multicolor flow cytometric approach abnormal cells with a characteristic phenotype could be identified in most patients with Hodgkin lymphoma, this technique has not yet been adopted by other laboratories.7
Mature B-cell lymphoid neoplasms
Flow cytometric immunophenotyping studies are indispensable for the diagnosis of mature B-cell lymphoid neoplasms through the identification of phenotypically abnormal cells belonging to the B-cell lineage and recognition of phenotypes characteristic of separate disease entities. In addition, flow cytometry can be used to identify expression of targets for potential antibody-directed therapy and provide some additional prognostic information such as CD38 and ZAP-70 expression in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).8 Following therapy, flow cytometry is becoming an established method for the evaluation of minimal residual disease.9,10 Table 1 outlines the normal pattern of staining and clinical utility of reagents recommended by the 2006 Bethesda consensus group for the evaluation of the B-lineage. Plasma cell neoplasms (PCNs) will be considered separately because their clinical presentation, morphologic appearance, and phenotype are usually distinct.
Reagents of clinical utility in the evaluation of mature B-cell lymphoid neoplasms
| Reagent . | Normal distribution of staining . | Clinical utility in mature B-cell lymphoid malignancy . | Comments . |
|---|---|---|---|
| CD5 | T cells and minor B-cell subset. | Expression on B cells: CLL, MCL. | — |
| CD10 | Immature T cells and B cells, subset of mature T cells and B cells, and neutrophils. | Germinal center–like phenotype: FL, DLBCL, BL. Frequently present in ALL. | |
| CD19 | All B cells, including lymphoblasts, mature B-lymphoid cells, and most plasma cells. | Indicates B-cell lineage. May demonstrate abnormal intensity in B-cell neoplasms. Usually absent in plasma cell neoplasms. | Aberrant expression on myeloid cells in AML or MDS. |
| CD20 | Acquired during maturation of precursor B cells (hematogones). Mature B-lymphoid cells positive. Absent on most BM plasma cells. Minor T-cell subset. | Supports B-cell lineage. Intensity often differs between subtypes: CLL/SLL dim, FL brighter. Aberrant expression on ALL or PCN. | Present on T-cell lymphoid neoplasms. |
| CD45 | All B cells (weaker intensity on precursors and plasma cells), all T cells (weaker intensity on precursors). | Useful in distinguishing mature lymphoid neoplasms (bright intensity) from ALL and PCN (weak intensity to negative). | — |
| Kappa and lambda, surface | Mature B cells. | Immunoglobulin light chain restriction. | — |
| CD9* | Precursor B cells, activated T cells, platelets. | Precursor B-cell ALL. | — |
| CD11c* | Some B cells, some T cells. | Hairy cell leukemia CD11c (+ br.). | Frequent weaker expression on CLL, MCL and others. |
| CD15* | Myeloid and monocytic cells. | May be aberrantly expressed in B-cell neoplasia. | More frequently seen in ALL than in mature neoplasm. |
| CD22* | Cytoplasmic expression in early B cells. Surface expression acquired during maturation of precursor B cells. | Indicates B-cell lineage in ALL and mature lymphoid neoplasms. Intensity often differs between subtypes of mature B-cell neoplasm: CLL/SLL dim. | Cross reactivity of some clones with monocytes and basophils. |
| CD23* | Weak intensity expression on resting B cells and increased with activation. | Distinguish CD5+B-cell lymphoid neoplasms: CLL/SLL (+ br). | — |
| CD25* | Activated B cells and T cells. | Hairy cell leukemia in combination with CD11c and CD103. | — |
| CD13* | Myeloid and monocytic cells. | May be aberrantly expressed in B-cell neoplasia. | More frequently seen in ALL than in mature neoplasm. |
| CD33* | Myeloid and monocytic cells. | May be aberrantly expressed in B-cell neoplasia. | More frequently seen in ALL than in mature neoplasm. |
| CD34* | B-cell and T-cell precursors and myeloblasts. | ALL. | Also AML. |
| CD38* | Precursor B cells (hematogones), normal follicle center B cells, immature and activated T cells, plasma cells (bright intensity), myeloid and monocytic cells, and erythroid precursors. | Bright intensity staining may indicate plasmacytic differentiation. Prognostic marker in CLL/SLL. | — |
| CD43* | T cells, myeloid, monocytes, small B-cell subset. | Aberrant expression in CLL, MCL, some MZL. | — |
| CD58* | Leukocytes including bright intensity staining of precursors and decreased intensity with maturation. | Distinction of ALL from normal precursor B-cell (hematogones) including detection of MRD. | — |
| CD79a and b* | Cytoplasmic staining in precursor B cells, plasma cells positive, variable expression mature B cells. | Indicates B-cell lineage in ALL and mature lymphoid neoplasms. Intensity often differs between subtypes of mature B-cell neoplasm: CLL/SLL dim CD79b. | CD79a staining has been reported in some T-ALL and rare mature T-cell lymphoid neoplasms. |
| CD103* | B-cell subset, intramucosal T cells. | Hairy cell leukemia and some MZL. | Also EATCL. |
| FMC-7* | B cells. | Distinguish CD5+ lymphoid neoplasm: CLL−, MCL often positive. Also HCL+. | |
| bcl-2* | T cells, some B cells; negative normal germinal center cells. | Distinguish CD10+ lymphoid neoplasms: FL+, BL−. | Variable staining in DLBCL. |
| Kappa and lambda, cytoplasmic* | Plasma cells. | Light chain restriction in cells with plasmacytic differentiation. | Most flow cytometric assays detect surface and cytoplasmic Ig. |
| Zap-70* | T cells, NK cells, precursor B cells. | Prognostic marker in CLL/SLL. | — |
| TdT* | B-cell and T-cell precursors. | ALL. | Also some AML. |
| cIgM* | First Ig component in precursor B cells. Expressed by subset of plasma cells and mature B cells. | IgM producing neoplasms that might be associated with Waldenstrom macroglobulinemia | — |
| Reagent . | Normal distribution of staining . | Clinical utility in mature B-cell lymphoid malignancy . | Comments . |
|---|---|---|---|
| CD5 | T cells and minor B-cell subset. | Expression on B cells: CLL, MCL. | — |
| CD10 | Immature T cells and B cells, subset of mature T cells and B cells, and neutrophils. | Germinal center–like phenotype: FL, DLBCL, BL. Frequently present in ALL. | |
| CD19 | All B cells, including lymphoblasts, mature B-lymphoid cells, and most plasma cells. | Indicates B-cell lineage. May demonstrate abnormal intensity in B-cell neoplasms. Usually absent in plasma cell neoplasms. | Aberrant expression on myeloid cells in AML or MDS. |
| CD20 | Acquired during maturation of precursor B cells (hematogones). Mature B-lymphoid cells positive. Absent on most BM plasma cells. Minor T-cell subset. | Supports B-cell lineage. Intensity often differs between subtypes: CLL/SLL dim, FL brighter. Aberrant expression on ALL or PCN. | Present on T-cell lymphoid neoplasms. |
| CD45 | All B cells (weaker intensity on precursors and plasma cells), all T cells (weaker intensity on precursors). | Useful in distinguishing mature lymphoid neoplasms (bright intensity) from ALL and PCN (weak intensity to negative). | — |
| Kappa and lambda, surface | Mature B cells. | Immunoglobulin light chain restriction. | — |
| CD9* | Precursor B cells, activated T cells, platelets. | Precursor B-cell ALL. | — |
| CD11c* | Some B cells, some T cells. | Hairy cell leukemia CD11c (+ br.). | Frequent weaker expression on CLL, MCL and others. |
| CD15* | Myeloid and monocytic cells. | May be aberrantly expressed in B-cell neoplasia. | More frequently seen in ALL than in mature neoplasm. |
| CD22* | Cytoplasmic expression in early B cells. Surface expression acquired during maturation of precursor B cells. | Indicates B-cell lineage in ALL and mature lymphoid neoplasms. Intensity often differs between subtypes of mature B-cell neoplasm: CLL/SLL dim. | Cross reactivity of some clones with monocytes and basophils. |
| CD23* | Weak intensity expression on resting B cells and increased with activation. | Distinguish CD5+B-cell lymphoid neoplasms: CLL/SLL (+ br). | — |
| CD25* | Activated B cells and T cells. | Hairy cell leukemia in combination with CD11c and CD103. | — |
| CD13* | Myeloid and monocytic cells. | May be aberrantly expressed in B-cell neoplasia. | More frequently seen in ALL than in mature neoplasm. |
| CD33* | Myeloid and monocytic cells. | May be aberrantly expressed in B-cell neoplasia. | More frequently seen in ALL than in mature neoplasm. |
| CD34* | B-cell and T-cell precursors and myeloblasts. | ALL. | Also AML. |
| CD38* | Precursor B cells (hematogones), normal follicle center B cells, immature and activated T cells, plasma cells (bright intensity), myeloid and monocytic cells, and erythroid precursors. | Bright intensity staining may indicate plasmacytic differentiation. Prognostic marker in CLL/SLL. | — |
| CD43* | T cells, myeloid, monocytes, small B-cell subset. | Aberrant expression in CLL, MCL, some MZL. | — |
| CD58* | Leukocytes including bright intensity staining of precursors and decreased intensity with maturation. | Distinction of ALL from normal precursor B-cell (hematogones) including detection of MRD. | — |
| CD79a and b* | Cytoplasmic staining in precursor B cells, plasma cells positive, variable expression mature B cells. | Indicates B-cell lineage in ALL and mature lymphoid neoplasms. Intensity often differs between subtypes of mature B-cell neoplasm: CLL/SLL dim CD79b. | CD79a staining has been reported in some T-ALL and rare mature T-cell lymphoid neoplasms. |
| CD103* | B-cell subset, intramucosal T cells. | Hairy cell leukemia and some MZL. | Also EATCL. |
| FMC-7* | B cells. | Distinguish CD5+ lymphoid neoplasm: CLL−, MCL often positive. Also HCL+. | |
| bcl-2* | T cells, some B cells; negative normal germinal center cells. | Distinguish CD10+ lymphoid neoplasms: FL+, BL−. | Variable staining in DLBCL. |
| Kappa and lambda, cytoplasmic* | Plasma cells. | Light chain restriction in cells with plasmacytic differentiation. | Most flow cytometric assays detect surface and cytoplasmic Ig. |
| Zap-70* | T cells, NK cells, precursor B cells. | Prognostic marker in CLL/SLL. | — |
| TdT* | B-cell and T-cell precursors. | ALL. | Also some AML. |
| cIgM* | First Ig component in precursor B cells. Expressed by subset of plasma cells and mature B cells. | IgM producing neoplasms that might be associated with Waldenstrom macroglobulinemia | — |
Reagents included in this table were recommended in the consensus guidelines.
+ indicates usually positive; −, usually negative; b, bright or strong intensity; Ig, immunoglobulin; TdT, terminal deoxynucleotidyl transferase; clg, cytoplastic immunoglobulin; and —, not applicable.
These reagents may be considered for secondary evaluation, after other reagents listed have been used in the initial evaluation.
Identification of abnormal mature B-lymphoid cells
Neoplastic mature B-lymphoid cells can be distinguished from normal cells by the identification of 2 main types of phenotypic abnormality: immunoglobulin light chain class restriction and aberrant antigen expression.
Immunoglobulin light chain class restriction
In contrast to most normal and reactive populations, neoplasms of mature B cells usually represent a single clone of cells that express only one class of immunoglobulin light chain (ie, kappa or lambda). Although often used as a surrogate marker of clonality, light chain class restriction has been reported in rare nonclonal reactive B-cell populations.11 For example, lambda immunoglobulin light chain class–restricted populations have been identified in nonclonal proliferations in tonsillar specimens during childhood,12 and in multicentric Castleman disease.13 Therefore, it should not be assumed that immunoglobulin light chain class restriction is synonymous with monoclonality or is by itself diagnostic of neoplasia. In addition, some light chain class–restricted populations that are truly monoclonal are not neoplastic.11 For example, clonal populations have been identified in florid follicular hyperplasia, including that seen in patients with HIV.14 Therefore, the results of flow cytometric immunophenotyping should be interpreted in conjunction with other clinical, morphologic, and sometimes genotypic data.
Identification of a large relatively pure population of light chain–restricted B cells is fairly straightforward using flow cytometric immunophenotyping, and is usually reflected in an abnormal kappa-lambda ratio. However, evaluation of the kappa-lambda ratio may fail to identify smaller clonal populations admixed with reactive polyclonal B cells. A more sensitive approach for the detection of light chain restriction is the separate evaluation of populations of cells that have a distinct phenotype and/or light scatter characteristic. This approach can be used for the diagnosis of lymphoid neoplasms that have a large number of accompanying reactive cells, as seen in marginal zone B-cell lymphoma (MZL). However, caution should be exercised when evaluating very small populations of cells for light chain restriction because reactive B cells may include small subsets of phenotypically identical cells. Therefore, detection of MRD following therapy usually involves identification of populations of cells with abnormal antigen ex-pression rather than the presence of immunoglobulin class restriction (see “Role of flow cytometric immunophenotyping in the detection of MRD”).
Interpretation of staining for kappa and lambda immunoglobulin light chains can be made more difficult by the presence of nonspecific staining. Nonspecific (cytophilic) binding of antibodies can occur through association with Fc receptors and adherence of antibody to “sticky” cells, including damaged or dying cells. Binding of antibodies to non–B cells can be excluded by evaluating only cells that express one or more B-lineage–associated antigens: for example, by gating on CD19+ or CD20+ cells. Nonspecific staining can also be minimized by incubation of cells with a blocking reagent such as immune sera prior to staining with anti–light chain antibodies. Blocking can be used if nonspecific staining is encountered using conventional staining techniques or in situations where nonspecific staining is frequently encountered, for instance in the evaluation for hairy cell leukemia (HCL).
Another issue encountered in the flow cytometry laboratory is apparent lack of staining for surface immunoglobulin. To avoid false-negative results due to soluble antibody interfering with the binding of detection antibody, it is important to include an initial wash step for flow cytometric tubes containing anti-immunoglobulin antibodies. In addition, some lymphoid neoplasms lack staining for immunoglobulin because the epitope recognized has been deleted or altered, such as with ongoing somatic hypermutation in follicular lymphoma (FL).15 This phenomenon occurs more frequently with monoclonal antibodies than polyclonal antibodies, and may be overcome by trying a number of different types and clones of detection antibody. If this strategy is not successful, normal and abnormal mature B-lymphoid cells can be distinguished by identifying other immunophenotypic abnormalities, such as coexpression of bcl-2 and CD10. Some B cells actually lack surface immunoglobulin, including lymphoblasts, plasma cells, thymic B cells, and their neoplastic counterparts: acute lymphoblastic leukemia (ALL), PCN, and primary mediastinal B-cell lymphoma, respectively. In addition, CLL typically demonstrates only low-intensity staining for immunoglobulin that is probably due to a low density of all components of the membrane B-cell–receptor complex that includes immunoglobulin, CD20, CD22, and CD79b.
Aberrant B-cell antigen expression.
Flow cytometric immunophenotyping can be used to identify deviations from the normal pattern of B-cell antigen expression. One type of phenotypic aberrancy is the presence of antigens not normally expressed by B cells (eg, myeloid antigens CD13 or CD33). Aberrant expression of myeloid antigens is found less frequently in mature B-cell lymphoid neoplasms than in ALL. Although it has been reported infrequently in many different subtypes of mature lymphoid neoplasm, aberrant myeloid antigen expression is perhaps most often found in lymphoplasmacytic lymphoma (LPL).16 Notably, CD5 expression on B cells is often referred to as an aberrant phenotype, but small populations of normal, mature CD5+ B cells exist. Nonneoplastic CD5+ B cells are found most often in the peripheral blood, but may also be seen in lymph node specimens, especially in patients with autoimmune disease.17 CD5 expression has also been reported in a subset of normal bone marrow B-cell precursors (hematogones).18 Therefore, interpretation of CD5 expression by B cells requires evaluation for other abnormalities, including immunoglobulin light chain restriction and altered intensity staining for CD20, CD22, and CD79b.
Another type of phenotypic aberrancy is abnormal expression of antigens not typically present in a subset of B cells belonging to a distinct biologic compartment (eg, detectable bcl-2 expression on CD10+ B cells). Normal germinal center B cells and hematogones are both CD10+ and bcl-2−, whereas bcl-2 is expressed by most other B-cell subsets. Abnormally increased bcl-2 expression can be found in most FL, some diffuse large B-cell lymphoma (DLBCL), and some B-lineage ALL.19,20 In contrast, Burkitt lymphoma (BL) is usually CD10+ and bcl-2−. More subtle phenotypic aberrancies include alteration in intensity of staining for B-lineage–associated antigens. For example, FL often demonstrates decreased intensity staining for CD19 and brighter intensity for CD10, which can help in the distinction from normal follicular germinal center cells.21
Significance of small populations of phenotypically abnormal B cells.
In the staging of patients with previously characterized lymphoid neoplasms, identification of a small population of phenotypically abnormal cells can be used to determine the presence of involvement by the neoplasm, particularly if the phenotype matches that of the original diagnostic specimen. However, in patients who do not have a previous diagnosis of a lymphoid malignancy, the significance of a small population of phenotypically abnormal B cells (less than 5% of the total cells analyzed) is less clear.22 The best documented example of this is identification of small clinically insignificant CLL-like populations in peripheral blood and bone marrow specimens from older patients.23 Small populations of B cells with other abnormal phenotypes have also been reported in peripheral blood and bone marrow specimens, and are not necessarily associated with a diagnosable neoplasm.22 Therefore, if a small population of phenotypically abnormal B cells is identified in a patient with no previous diagnosis of a lymphoid neoplasm, it should not be used to establish a new diagnosis of malignancy, but correlated with the morphology, clinical information, and other findings.
False-negative flow cytometric evaluation.
Occasionally, flow cytometric evaluation fails to detect an abnormal population of B cells in a specimen involved by a B-cell lymphoid neoplasm. There are several possible explanations.
Sampling error.
Allocation of appropriate material for flow cytometric studies is rarely an issue in liquid specimens, but becomes essential for tissue samples because the infiltrate of interest might not involve the entire specimen. Therefore, fresh tissue should be evaluated, such as with touch preparations, to identify representative areas to allocate for flow cytometric and other testing.24
Cell loss during processing.
The frequency of cell loss during processing for flow cytometric studies varies with the type of cells present and the procedure used to process the specimen. Large lymphoid cells and plasma cells appear to be more easily lost during processing, particularly following manual disaggregation of tissue specimens. Comparison of smears or touch imprints prepared from the fresh specimen with a cytospin prepared from the cell suspension after processing can help to confirm the presence of the cells of interest.24
Paucity of neoplastic cells.
Some tumors contain relatively few neoplastic cells, such as the T cell/histiocyte–rich variant of DLBCL, or include many admixed reactive B cells, such as MZL. Although, it is important to acquire enough events to detect small populations of abnormal cells, most clinical laboratories have not routinely acquired the 500 000 to 1 million events usually required for MRD detection, primarily because of time constraints. More frequently, clinical laboratories acquire 30 000 to 100 000 events with acknowledgment of the limitations of routine clinical flow cytometric testing.
Difficult-to-identify cell populations.
Populations of abnormal B cells may be present but not recognized on flow cytometric immunophenotypic studies. Examples of populations that are easily overlooked include B cells that are negative for CD20, such as may be seen following therapy with rituximab anti-CD20 monoclonal antibody therapy,25 and B cells lacking demonstrable surface immunoglobulin.15 The following strategies can be used to avoid overlooking elusive populations: perform a basic evaluation of all cell types present in the specimen, not just those that are CD20+; evaluate more than one B cell–associated antigen such as CD19, CD20, CD22, or CD79; and thoroughly assess all B-cell populations for phenotypic aberrancies, including cells lacking staining for surface immunoglobulin.
Role of flow cytometric immunophenotyping in the classification of mature B-cell lymphoid neoplasms
In contrast to the disease oriented approach taken in the 2001 WHO classification and previous Blood review, this section will discuss an approach to the classification of neoplasms of mature B cells that is based primarily on flow cytometric data.1,3 In the flow cytometric evaluation of mature B-cell lymphoid neoplasms, it is useful to consider 4 broad groups as determined by their expression of CD5 and CD10: CD5+/CD10−, CD5−/CD10+, CD5+/CD10+, and CD5−/CD10−. For each group, additional flow cytometric data in combination with the morphology can narrow down the diagnostic possibilities and direct the use of additional ancillary studies (Table 2).
Flow cytometric approach to the diagnosis and classification of B-cell lymphoid neoplasms
| Disease entities to consider . | Distinguishing phenotypic features . | Additional diagnostic information . |
|---|---|---|
| CD5+ CD10− | ||
| Chronic lymphocytic leukemia | Typical phenotype: CD20 (d), CD22 (d), sIg (d), CD23+, FMC-7− | Characteristic morphology |
| Mantle cell lymphoma | Variable phenotype not typical for CLL; often CD20 (i), sIg (i), CD23+/−, FMC-7+/− | Cyclin-D1 IHC, t(11;14)/CCND rearrangement |
| Prolymphocytic leukemia | Variable phenotype, may overlap with CLL and MCL CD20 (+i), sIg (+i), FMC-7+/−, CD5+/− | Large cells prominent nucleoli; exclude blastic MCL |
| Marginal zone B-cell lymphoma | Variable phenotype, not typical for CLL: CD23−; often CD11c+/−, CD103+/− but not typical for HCL, sometimes cIg only | Growth around and into follicles, may demonstrate plasmacytic differentiation, t(11;18), t(1;14), t(14;18)/MALT-1 rearrangement |
| Diffuse large B-cell lymphoma | Variable phenotype | Large cells, diffuse growth pattern; consider Richter transformation CLL and MCL |
| Lymphoplasmacytic lymphoma | Phenotype not typical for CLL, often CD23(-/d), sometimes sIg− but cIg+ | Small cells, subset with plasmacytic differentiation Primarily PB and BM |
| CD5− CD10+ | ||
| Follicular lymphoma | Usually bcl-2+, CD43− | Some follicular growth, t(14;18)/BCL-2 rearrangement |
| Diffuse large B-cell lymphoma | Variable phenotype, bcl-2+/−, CD43+/− | Large cells somewhat pleomorphic, diffuse growth |
| Burkitt lymphoma | Usually bcl-2−,CD10 (+b), CD43+ | Uniform intermediate size cells; MYC rearrangement, Ki-67 approximately 100% |
| Hairy cell leukemia | Typical phenotype: CD20 (b), CD22 (b), CD11c (b), CD25+, CD103+, sIg (i), CD123+ | Characteristic morphology; Annexin-A1+ |
| CD5+ CD10+ | ||
| Follicular lymphoma | Usually bcl-2+, CD43− | Some follicular growth, t(14;18)/BCL-2 rearrangement |
| Diffuse large B-cell lymphoma | Variable phenotype, bcl-2+/−, CD43+/− | Large cells, diffuse growth pattern |
| Mantle cell lymphoma | Variable phenotype not typical for CLL; often CD20 (i), sIg (i), CD23+/−, FMC-7+/− | Cyclin-D1 IHC, t(11;14)/CCND rearrangement |
| Burkitt lymphoma | Usually bcl-2−, CD10 (b), CD43+ | Uniform intermediate size cells; MYC rearrangement, Ki-67 approximately 100% |
| CD5− CD10− | ||
| Hairy cell leukemia | Typical pheotype: CD20 (b), CD22 (b), CD11c (b), CD25+, CD103+, sIg (i) | Confirm characteristic morphology |
| Marginal zone B-cell lymphoma | Often CD11c+/−, CD103+/− but not typical for HCL, sometimes sIg− but cIg+ | Growth around and into follicles, maybe plasmacytic t(11;18), t(1;14), t(14;18)/MALT-1 rearrangement |
| Diffuse large B-cell lymphoma | Variable phenotype | Large cells, diffuse growth pattern |
| Follicular lymphoma CD10− | Variable phenotype | Some follicular growth, t(14;18)/BCL-2 rearrangement |
| Mantle cell lymphoma CD5− | Variable phenotype | Cyclin-D1 IHC, t(11;14)/CCND rearrangement |
| Disease entities to consider . | Distinguishing phenotypic features . | Additional diagnostic information . |
|---|---|---|
| CD5+ CD10− | ||
| Chronic lymphocytic leukemia | Typical phenotype: CD20 (d), CD22 (d), sIg (d), CD23+, FMC-7− | Characteristic morphology |
| Mantle cell lymphoma | Variable phenotype not typical for CLL; often CD20 (i), sIg (i), CD23+/−, FMC-7+/− | Cyclin-D1 IHC, t(11;14)/CCND rearrangement |
| Prolymphocytic leukemia | Variable phenotype, may overlap with CLL and MCL CD20 (+i), sIg (+i), FMC-7+/−, CD5+/− | Large cells prominent nucleoli; exclude blastic MCL |
| Marginal zone B-cell lymphoma | Variable phenotype, not typical for CLL: CD23−; often CD11c+/−, CD103+/− but not typical for HCL, sometimes cIg only | Growth around and into follicles, may demonstrate plasmacytic differentiation, t(11;18), t(1;14), t(14;18)/MALT-1 rearrangement |
| Diffuse large B-cell lymphoma | Variable phenotype | Large cells, diffuse growth pattern; consider Richter transformation CLL and MCL |
| Lymphoplasmacytic lymphoma | Phenotype not typical for CLL, often CD23(-/d), sometimes sIg− but cIg+ | Small cells, subset with plasmacytic differentiation Primarily PB and BM |
| CD5− CD10+ | ||
| Follicular lymphoma | Usually bcl-2+, CD43− | Some follicular growth, t(14;18)/BCL-2 rearrangement |
| Diffuse large B-cell lymphoma | Variable phenotype, bcl-2+/−, CD43+/− | Large cells somewhat pleomorphic, diffuse growth |
| Burkitt lymphoma | Usually bcl-2−,CD10 (+b), CD43+ | Uniform intermediate size cells; MYC rearrangement, Ki-67 approximately 100% |
| Hairy cell leukemia | Typical phenotype: CD20 (b), CD22 (b), CD11c (b), CD25+, CD103+, sIg (i), CD123+ | Characteristic morphology; Annexin-A1+ |
| CD5+ CD10+ | ||
| Follicular lymphoma | Usually bcl-2+, CD43− | Some follicular growth, t(14;18)/BCL-2 rearrangement |
| Diffuse large B-cell lymphoma | Variable phenotype, bcl-2+/−, CD43+/− | Large cells, diffuse growth pattern |
| Mantle cell lymphoma | Variable phenotype not typical for CLL; often CD20 (i), sIg (i), CD23+/−, FMC-7+/− | Cyclin-D1 IHC, t(11;14)/CCND rearrangement |
| Burkitt lymphoma | Usually bcl-2−, CD10 (b), CD43+ | Uniform intermediate size cells; MYC rearrangement, Ki-67 approximately 100% |
| CD5− CD10− | ||
| Hairy cell leukemia | Typical pheotype: CD20 (b), CD22 (b), CD11c (b), CD25+, CD103+, sIg (i) | Confirm characteristic morphology |
| Marginal zone B-cell lymphoma | Often CD11c+/−, CD103+/− but not typical for HCL, sometimes sIg− but cIg+ | Growth around and into follicles, maybe plasmacytic t(11;18), t(1;14), t(14;18)/MALT-1 rearrangement |
| Diffuse large B-cell lymphoma | Variable phenotype | Large cells, diffuse growth pattern |
| Follicular lymphoma CD10− | Variable phenotype | Some follicular growth, t(14;18)/BCL-2 rearrangement |
| Mantle cell lymphoma CD5− | Variable phenotype | Cyclin-D1 IHC, t(11;14)/CCND rearrangement |
+ indicates usually positive; −, usually negative; +/−, may be positive or negative; d, dim or weak intensity; i, intermediate intensity; b, bright or strong intensity; sIg, surface immunoglobulin; and cIg, cytoplasmic immunoglobulin.
CD5+ CD10−.
B-cell lymphoid neoplasms positive for CD5 and negative for CD10 include most patients with CLL/SLL and mantle cell lymphoma (MCL), some patients with B-cell prolymphocytic leukemia (B-PLL), small percentages of MZL and DLBCL, and possibly some patients with LPL. In addition, it should be remembered that CD5 is expressed on a population of normal B cells and therefore should not be used in isolation to establish the presence of a neoplasm.17
Among the CD5+ CD10− mature B-cell lymphoid neoplasms, CLL/SLL has the most characteristic phenotype: CD20 weak intensity, CD22 weak intensity, CD79b weak intensity, CD23+ (often moderate to strong intensity), FMC-7−, and surface immunoglobulin weak intensity.23 However, this phenotype is not entirely specific and should be considered in conjunction with morphology to confirm a diagnosis of CLL/SLL and exclude DLBCL and B-PLL. If a diagnosis of CLL/SLL is being entertained, testing for the prognostic markers CD38 and ZAP-70 can then be considered (“Immunophenotypic information of additional prognostic value in mature B-cell lymphoid neoplasms”). Genotypic studies are not necessary for diagnosis but may provide prognostic information.26 B-cell lymphoid neoplasms with a CD5+, CD10− phenotype that differs from the typical phenotype of CLL/SLL are more difficult to classify using flow cytometric studies.27-30 Although variant phenotypes have been described in CLL/SLL (eg, brighter intensity CD20, brighter intensity surface immunoglobulin, weaker or absent CD23, and expression of FMC-7), additional work-up is generally required to exclude other CD5+ B-cell lymphoid neoplasms.30
MCL usually demonstrates a CD5+ phenotype that differs from typical CLL/SLL: CD20 moderate to bright intensity, surface immunoglobulin moderate to bright intensity, CD23 negative or only weak intensity, and FMC-7+. However, the phenotype of MCL is more variable than that of CLL/SLL and overlaps with that of other CD5+ mature B-cell lymphoid neoplasms. Therefore, additional studies are recommended for diagnosis, such as paraffin section immunohistochemistry for overexpression of cyclin-D1 protein, classical cytogenetics to identify the translocation t(11;14)(q13;q32), or fluorescence in situ hybridization for CCND1 gene rearrangement (Figure 1). Although several groups have attempted to develop a flow cytometric assay for cyclin-D1, most lack sensitivity, and probably the most sensitive method, which uses an enzymatic amplification step, appears to lack specificity in the distinction between CLL/SLL and MCL.31
Mantle cell lymphoma. (A) Histologic section from a submandibular gland biopsy specimen demonstrating an abnormal diffuse infiltrate of small to intermediate-size lymphoid cells. Several mitotic figures are present. Hematoxylin & eosin stain, magnification ×40. (B) Representative flow cytometric dot plots with population of interest highlighted in green: CD19 versus CD5 demonstrates CD5+ B-cell population with weak intensity staining for CD19; FMC-7 versus CD5 demonstrates positivity for FMC-7; CD20 versus kappa and CD20 versus lambda demonstrate moderate intensity staining for CD20 and kappa immunoglobulin light chain restriction. In addition, B cells were CD10− and CD23− (data not shown). The flow cytometric data was acquired using a BD FACS Calibur flow cytometer (BD Biosciences, San Jose, CA) and the dot plots were created using BD FACSDiva software v5.0.2 (BD Biosciences). (C) Cyclin-D1 paraffin section immunohistochemical stain, demonstrating many positive cells with characteristic nuclear staining; magnification ×40. (D) FISH studies demonstrating the IGH/CCND1 [t(11,14)(q13;q32)] rearrangement. Hybridization with the LSI IGH/CCND1-XT dual color, dual fusion DNA probe demonstrates one green signal from the unrearranged chromosome 14q32, one red signal from the unrearranged 11q13, and 3 fusion signals: one from the derivative chromosome 11, one from the derivative chromosome 14, and an extra signal suggesting the presence of an additional copy of all or part of one of the derivative chromosomes involved in the IGH/CCND1rearrangement. Courtesy of the Pittsburgh Cytogenetics Laboratory, Magee-Womens Hospital, Pittsburgh, PA. The images were taken through an Olympus BX40 microscope (Olympus, Tokyo, Japan) and acquired with a SPOT Insight 2 megapixel 3-shot color camera and SPOT Advanced imaging software (Diagnostic Instruments, Sterling Heights, MI).
Mantle cell lymphoma. (A) Histologic section from a submandibular gland biopsy specimen demonstrating an abnormal diffuse infiltrate of small to intermediate-size lymphoid cells. Several mitotic figures are present. Hematoxylin & eosin stain, magnification ×40. (B) Representative flow cytometric dot plots with population of interest highlighted in green: CD19 versus CD5 demonstrates CD5+ B-cell population with weak intensity staining for CD19; FMC-7 versus CD5 demonstrates positivity for FMC-7; CD20 versus kappa and CD20 versus lambda demonstrate moderate intensity staining for CD20 and kappa immunoglobulin light chain restriction. In addition, B cells were CD10− and CD23− (data not shown). The flow cytometric data was acquired using a BD FACS Calibur flow cytometer (BD Biosciences, San Jose, CA) and the dot plots were created using BD FACSDiva software v5.0.2 (BD Biosciences). (C) Cyclin-D1 paraffin section immunohistochemical stain, demonstrating many positive cells with characteristic nuclear staining; magnification ×40. (D) FISH studies demonstrating the IGH/CCND1 [t(11,14)(q13;q32)] rearrangement. Hybridization with the LSI IGH/CCND1-XT dual color, dual fusion DNA probe demonstrates one green signal from the unrearranged chromosome 14q32, one red signal from the unrearranged 11q13, and 3 fusion signals: one from the derivative chromosome 11, one from the derivative chromosome 14, and an extra signal suggesting the presence of an additional copy of all or part of one of the derivative chromosomes involved in the IGH/CCND1rearrangement. Courtesy of the Pittsburgh Cytogenetics Laboratory, Magee-Womens Hospital, Pittsburgh, PA. The images were taken through an Olympus BX40 microscope (Olympus, Tokyo, Japan) and acquired with a SPOT Insight 2 megapixel 3-shot color camera and SPOT Advanced imaging software (Diagnostic Instruments, Sterling Heights, MI).
MZL is CD5+ in approximately 5% of patients and can be difficult to distinguish from CLL/SLL with a variant phenotype and other CD5+ lymphoid neoplasms.32,33 Possible distinguishing features include lack of expression of CD23 in most MZL and presence of plasmacytic differentiation in a significant subset of MZL, as demonstrated by expression of CD138, bright expression of CD38, and cytoplasmic immunoglobulin light chain restriction in at least a subset of the neoplastic cells. However, although identification of plasmacytic differentiation can assist in the distinction of MZL from typical CLL/SLL, it raises the possibility of other subtypes of B-lymphoid neoplasms that may demonstrate plasmacytic differentiation, especially LPL. Morphologic evaluation of lymphoid tissues can sometimes assist in reaching a definitive diagnosis. MZL lacks the proliferation centers that are characteristic of CLL/SLL and often demonstrates a distinctive growth pattern with infiltration around and into residual benign follicular germinal centers. In addition, genotypic studies can sometimes assist in establishing a diagnosis of MZL. Although some genetic abnormalities can be seen in both CLL/SLL and MZL (eg, trisomy 18), the following abnormalities are more typical for MZL: t(11;18)(q21;q21), t(1;14)(p22;q32), t(14;18)(q32;q21) involving the MALT1 gene, and deletion 7q31 in splenic MZL.34 However, like CLL/SLL, many MZLs do not have a unique genotype and therefore, they cannot be reliably distinguished.
LPL is a less well-defined entity that can be difficult to distinguish from MZL and other B-cell lymphoid neoplasms demonstrating plasmacytic differentiation. Probably because of these uncertainties, it is difficult to determine from the literature the phenotypic features that are characteristic of this entity. Approximately 5% of LPLs are reported to be CD5+.35,36 CD23 is usually negative, and when positive often demonstrates weak, variable staining. Therefore, even if CD5 is expressed, LPL does not demonstrate a phenotype characteristic of CLL/SLL. The distinction of LPL from other CD5+ small B-cell lymphomas is more difficult and often requires a combination of morphologic and clinical features. Although the genotypic abnormality del 6q has been associated with bone marrow–based LPL, it is not entirely specific, and does not appear to be a marker of nodal LPL.37,38
A subset of DLBCL is CD5+ and can be distinguished from the small CD5+ B-cell lymphoid neoplasms by their morphologic size and sometimes by an associated high forward angle light scatter. Exclusion of the blastoid variant of MCL is recommended, through testing for cyclin-D1 overexpression, t(11;14)(q13;q32), or CCND1 gene rearrangement. CD5+ DLBCL may represent large cell transformation of a lower-grade CD5+ B-cell neoplasm such as CLL/SLL (Richter transformation) or de novo CD5+ DLBCL, including extremely rare instances of CD5+ intravascular large B-cell lymphoma.39,40 Although it is uncertain if de novo CD5+ DLBCL is a distinct entity, it appears to have some genotypic differences from other DLBCL and may be associated with a worse prognosis.40
There is some controversy over the phenotype of B-PLL. Although many patients with B-PLL are CD5−, a small subset appears to be CD5+. However, some patients previously diagnosed as CD5+ B-PLL were subsequently shown to represent the blastoid variant of MCL through testing for the translocation t(11;14)(q13;q32) or CCND1 gene rearrangement.28,41 In addition, it is difficult to reliably distinguish between CLL with increased prolymphocytes (CLL/PL), prolymphocytoid transformation of CLL, and de novo B-PLL. In comparison to CLL/SLL, prolymphocytoid transformation has been described as having decreased staining for CD5, increased intensity staining for CD20 and surface immunoglobulin, and acquisition of FMC-7. However, some patients with prolymphocytoid transformation of CLL/SLL demonstrate a phenotype identical to that of the preceding CLL/SLL and can only be recognized by morphologic review.42 The phenotype of de novo B-PLL is also quite variable but appears to include some CD5+ patients that, in contrast to CLL/SLL, usually lack staining for CD23.43,44
CD5− CD10+.
DLBCL and FL represent the most frequent CD10+, CD5− mature B-cell lymphoid neoplasms, followed by BL. CD10+ HCL is uncommon but can be easily overlooked if the appropriate antibody combinations are not included in an initial flow cytometric screening panel.45 CD10 expression in other types of lymphoma is unusual with only a few reports of CD10+ LPL,35 and very rare CD10+ MZL and MCL. It should also be remembered that CD10 is also expressed by normal follicular center B cells, precursor B-lymphoblastic leukemia/lymphoma, subsets of mature T cells, precursor T-cell lymphoblasts, neutrophils, and some nonhematolymphoid cells.
DLBCL is a heterogeneous category that includes a subset with a CD10+ germinal center–like phenotype, which represents approximately 20% to 40% of all DLBCL. Although phenotypic classification of DLBCL into germinal center–like and non–germinal center–like DLBCL has been proposed to be of prognostic significance, the currently proposed algorithms use paraffin section immunohistochemical staining.46 CD10+ DLBCL may be difficult to distinguish from BL (see discussion of BL in the next paragraph) and FL composed of many large cells (ie, higher-grade FL). When a mature CD10+ B-cell phenotype is identified by flow cytometry, distinction between these possibilities should be further evaluated by morphology. Although on histologic sections the diffuse growth pattern of DLBCL can readily be distinguished from the nodular growth pattern of FL, this distinction is often not possible in fine-needle aspirate, body fluid, peripheral blood, and bone marrow specimens. In addition, FL and DLBCL overlap in their genotype since the translocation t(14;18)(q32;q21) is identified in approximately 20% of DLBCL and 70% to 95% of FL.
Childhood BL is consistently CD10+ and CD5−.47 Although adult BL is also usually CD10+, the phenotype is more variable than that of childhood BL, and more difficult to reliably distinguish from DLBCL.47 In contrast to some patients with DLBCL, and most with FL, BL is usually bcl-2−. However, the reliable distinction of these 2 entities usually requires a multiparametric approach, including evaluation of the phenotype, morphologic appearance, proliferative index, and genotype. BL is usually composed of a uniform population of intermediate size cells with basophilic cytoplasm, often cytoplasmic vacuoles, a Ki-67 proliferative index approaching 100%, and an isolated MYC rearrangement. DLBCL is more heterogeneous but is usually composed of more pleomorphic large cells, a lower Ki-67 proliferative index, and a more variable genotype that may include one or more of the following rearrangements: MYC, BCL-2, or BCL-6.48
A small subset of HCL are CD10+ but are morphologically similar to CD10− HCL, and usually respond to typical HCL therapy.45 Therefore, HCL should be considered when a CD10+, CD5− phenotype is identified by flow cytometric evaluation, especially if there is relatively bright intensity staining for CD20, CD22, and surface immunoglobulin; lack of staining for CD38; and expression of FMC-7. A diagnosis of CD10+ HCL can usually be established by identification of other phenotypic features characteristic of HCL such as CD11c+ (bright intensity), CD25+, and CD103+.
CD5− CD10−.
Mature B-cell neoplasms lacking expression of CD5 and CD10 represent a diverse group that includes DLBCL, MZL, HCL, LPL, CD10− FL, and CD5− MCL. Further classification usually requires correlation with morphology and often additional ancillary studies.
HCL has a distinctive phenotype that permits diagnosis and detection of low levels of disease following therapy: CD20 bright intensity, CD22 bright intensity, CD11c bright intensity, CD25+, CD103+, sIg intermediate to bright intensity, FMC-7+, CD23−, CD5−, and CD10−. This phenotype is more sensitive and specific for the diagnosis of HCL than staining for tartrate-resistant acid phosphatase (TRAP).49 On occasion, classical HCL deviates from this characteristic phenotype.50,51 CD10+ HCL has already been discussed. Other immunophenotypic variations reported include lack of CD103, lack of CD25, and staining for CD23.51 These phenotypic variations should be distinguished from HCL variant (HCLv).50 The term HCLv has been used to describe patients with an unusual combination of morphologic, clinical, and phenotypic findings. HCLv presents with a higher white blood cell count, lacks accompanying monocytopenia, and is composed of cells that demonstrate prominent nucleoli, often lack staining for TRAP, and are negative for CD25, but otherwise phenotypically similar to classical HCL. However, the existence of HCLv is debated, and it has been questioned if some of these neoplasms represent splenic MZL.50
MZL usually has a CD5−, CD10− phenotype and is composed predominantly of small cells.52 A diagnosis of MZL is often established by identification of characteristic morphologic features and exclusion of other small lymphoid B-cell neoplasms: CD10− FL, CD5− MCL, and HCL. In peripheral blood and bone marrow aspirate specimens, the morphologic features of MZL are often less distinctive than those present in lymphoid tissues and may overlap those of HCL. In particular, circulating villous lymphocytes have been described in splenic MZL involving the peripheral blood. The distinction of MZL and HCL is made more difficult by overlapping phenotypes. MZL is often CD11c+, and may be positive for CD103.50,51 However, MZL usually demonstrates weaker, more variable staining for CD11c than HCL; lacks the combination of CD103, CD11c, and CD25; and lacks bright intensity staining for CD20 and CD22. Although a specific genotype has not been described in HCL, deletion 7q31 has been identified in some patients with splenic MZL.
Approximately 60% to 80% of patients with LPL are reported to have a CD5−, CD10−, CD23− phenotype.35,36 Many patients express CD11c and CD25, but in contrast to HCL are usually CD103−.35,36 Evidence of plasmacytic differentiation can be demonstrated in at least a subset of cells. However, as described in “CD5+CD10−,” distinction from MZL is often difficult.35,36
CD10− FL53,54 and CD5− MCL55 are both recognized and would fall in the CD5− CD10− group. Recognition of these unusual variants usually requires a combined morphologic and immunophenotypic approach, and may require additional testing for characteristic genotypic abnormalities. At least in part because of the lack of a specific genotypic marker for CLL/SLL, CD5− CLL/SLL is not currently recognized. In some studies, the proportion of CD10− FL is higher using flow cytometry than paraffin section immunohistochemistry. Given the sensitivity of flow cytometric immunophenotyping, this observation is surprising. One possible explanation is that many of these studies used fluorescein isothiocyanate (FITC)–conjugated antibodies that typically provide a relatively weak signal in comparison to that of phycoerythrin (PE) and related flurochromes.56 Another possible explanation is that manual disaggregation performed for flow cytometric studies preferentially selects interfollicular cells that may have down-regulated CD10 expression.57
CD5+ CD10+.
Mature B-cell lymphoid neoplasms expressing both CD5 and CD10 are uncommon.58-61 This group includes several different subtypes of lymphoma (in order of incidence): DLBCL, FL, MCL, CLL/SLL, BL, and rare individual reports of other mature B-cell malignancies. Morphologic evaluation can assist in the identification of lymphoid neoplasms composed of small cells (FL, MCL, CLL/SLL) from those composed of larger cells (DLBCL and BL) and blastic malignancies (ALL). Evaluation for BCL-2 gene rearrangement by molecular diagnostic or FISH studies may assist in establishing a diagnosis of FL. Evaluation for cyclin-D1 staining, the translocation t(11;14)(q13;q32), or CCND1 gene rearrangement is important for consideration of CD10+ MCL, and testing for MYC translocations would be necessary to establish a diagnosis of CD5+ BL.
Immunophenotypic information of additional prognostic value in mature B-cell lymphoid neoplasms
Expression of CD38 and ZAP-70, as determined by flow cytometric immunophenotyping, has been reported to have prognostic significance in CLL/SLL.62 Although CD38 expression was initially thought to correlate with unmutated status of the immunoglobulin heavy-chain variable region gene (IgVH), subsequent studies demonstrated a significant number of discordant results.63 Despite this, CD38 expression might be an independent marker of a poor prognosis in CLL/SLL.63 Although most studies use 30% positive cells as the cut-off for determining positivity for CD38 expression, some studies have demonstrated an adverse prognosis for patients with CD38 expression on greater than 20% of CLL/SLL cells, or even less. The following factors can make determination of the percentage of CD38+ cells difficult: a spectrum of intensity for CD38 staining without clear distinction between positive and negative populations, differences in intensity that derive from the fluorochrome (for example, PE usually gives a brighter signal than FITC and would therefore provide better separation of positive and negative cells), bimodal staining with the presence of positive and negative cells in the same sample, differences in staining between tissue sites such as peripheral blood and bone marrow, and changes in CD38 expression during the course of the disease and with therapy.62,63
ZAP-70 was identified in a search for genes that are differentially expressed in CLL/SLL with mutated and unmutated IgVH. Although the initial flow cytometric study of ZAP-70 expression in CLL/SLL using an indirect staining method demonstrated a strong association of ZAP-70 expression on greater than 20% of CLL/SLL cells with unmutated IgVH, subsequent studies have demonstrated a higher number of discordant patients.9 Some studies have suggested that in these discordant patients, ZAP-70 staining is the best indicator of prognosis in CLL/SLL.64 However, as summarized in a special issue of Cytometry Part B (Clinical Cytometry),65 there are technical difficulties that make reliable determination of ZAP-70 status a challenge.65,66
Weak intensity staining for ZAP-70.
The intensity of staining for ZAP-70 with many commercially available fluorochrome-conjugated antibodies is relatively weak, making it difficult to distinguish positive and negative cells. In addition, ZAP-70 is localized in the cytoplasm, and therefore detection requires cell permeabilization techniques that can lead to decreased intensity staining.
Nonspecific staining.
Many ZAP-70 procedures demonstrate nonspecific staining, as best demonstrated by the presence of staining of nonneoplastic B cells. Possible sources of nonspecific staining include antibody specificity and the use of permeabilization procedures.
Decrease in staining over time.
ZAP-70 expression appears to be labile over time and sensitive to different anticoagulants. EDTA anticoagulation and rapid delivery to the laboratory within 24 hours of specimen collection has been recommended.66
What to call positive.
Consensus has not been reached on the optimal method for determining which cells should be considered positive for ZAP-70. The original flow cytometric study of ZAP-70 staining in CLL/SLL used the normal staining of T cells within the specimen to determine the lower limit for positive ZAP-70 staining. However, T cells demonstrate some variability in staining intensity for ZAP-70 within a specimen and between samples, and this method does not take into account nonspecific staining. Because CLL/SLL cells often demonstrate a narrow range of staining for ZAP-70, small differences in the position of the cursor used to divide cells designated as positive and negative can make a large difference in the percent of ZAP-70+ CLL/SLL cells. More recently, several calculations involving mean and median ZAP-70 fluorescence intensity of the CLL/SLL cells, T and NK cells, and normal B cells have been proposed.65
Despite these difficulties and lack of consensus on an optimal method for the flow cytometric evaluation of ZAP-70 in CLL/SLL, many flow cytometric laboratories are attempting to perform the procedure, and report out difficult-to-interpret specimens as “indeterminate.”
Mature T- and NK-cell lymphoid neoplasms
Flow cytometric immunophenotypic studies may assist in the diagnosis and classification of mature T- and NK-cell lymphoid neoplasms. However, it is often more difficult to identify phenotypically abnormal T or NK cells than abnormal mature B cells. In addition, the classification of T- and NK-cell neoplasms is less well established than that of B-cell neoplasms, and often requires assimilation of information from multiple sources. Therefore, as described in the next 2 sections, flow cytometric studies usually represent only part of the work-up for T- and NK-cell neoplasms. Once a diagnosis of a T- or NK-cell neoplasm has been established, flow cytometric studies can also assist in the detection of potential targets for directed therapy, such as CD25 and CD52.8
Identification of abnormal mature T and NK lymphoid cells
Neoplasms of mature T and NK cells can often be identified by flow cytometric immunophenotyping through detection of aberrant antigen expression.67,68 Table 3 outlines the normal pattern of staining and clinical utility of reagents recommended by the 2006 Bethesda consensus group for the evaluation of the T-cell lineage. However, neoplastic T- and NK cells are often more difficult to identify than neoplastic B cells. Some of this difficulty relates to lack of a surrogate marker of T- and NK-cell clonality that is as effective as kappa and lambda expression by B cells. In addition, aberrant antigen expression by neoplastic T and NK cells must be distinguished from the normal phenotypic variation seen between the multiple subsets of nonneoplastic cells.69
Reagents of clinical utility in the evaluation of mature T- and NK-cell lymphoid neoplasms
| Reagent . | Normal distribution . | Clinical utility in mature T- and NK-cell lymphoid neoplasms . | Comments . |
|---|---|---|---|
| CD2 | T cells and NK cells. | Indicator of T- or NK-cell lineage. | May be aberrantly expressed in AML. |
| CD3, surface | Acquired during maturation of T cells. | Indicator of T-cell lineage. May be aberrantly lost or decreased in intensity. | — |
| CD4 | T-cell subset and monocytes/histiocytes. | Useful in classification of mature T-cell lymphoid neoplasms. | NOT indicator of clonality. Also may be positive in AML and HDN. |
| CD5 | T cells and minor B-cell subset. | Indicator of T-cell lineage. May be aberrantly lost or decreased in intensity. | May be aberrantly expressed on B cells. |
| CD7 | T cells and NK cells. | Indicator of T-cell lineage. May be aberrantly lost or decreased in intensity. | Some normal and reactive CD7− cells. May be aberrantly expressed in AML. |
| CD8 | T-cell subset and some NK cells. | Useful in classification of mature T-cell lymphoid neoplasms. | NOT indicator of clonality |
| CD45 | All B cells (weaker intensity on precursors and plasma cells), all T cells (weaker intensity on precursors). | Useful in distinguishing mature lymphoid neoplasms (bright intensity) from ALL and PCN (weak intensity to negative). | — |
| CD56 | NK cells and NK-like T cells. | Indicator of NK differentiation. | Aberrant expression in AML, PCN. Positive HDN. Small subset of regenerating myeloid cells demonstrates weak expression. |
| CD1a* | Immature T cells. | ALL. | Also positive on Langerhans cells. |
| CD3, cytoplasmic* | All T cells including lymphoblasts. | Indicator of T- or NK cell lineage. NK cells contain cCD3 epsilon. | — |
| CD10* | Immature T cells and B cells, subset of mature T cells and B cells, and neutrophils. | Frequently present in ALL. Found on some mature T-cell neoplasms, in particular AITCL. | Minor subset of normal T cells. |
| CD16* | NK cells, NK-like T cells, and maturing neutrophilic cells. | Indicator of NK differentiation. | Antibodies with differing specificity for lymphoid cells and neutrophilic cells.. |
| CD25* | Activated T cells. | Uniform strong positivity in ATLL. More variable expression in other subtypes. Assessment for anti-CD25 therapy, eg, Ontak. | — |
| CD26* | Immature T cells, NK cells, and activated T cells. Most CD4+ T cells also CD26+. | CTCL/Sézary syndrome often CD26 negative (> 30% CD4+ cells, CD26−). | Not specific for Sézary/CTCL. |
| CD30* | Activated T and B cells, and monocytes. | Strong uniform staining in ALCL. More variable staining in other mature T- and NK-cell neoplasms. Positive in Hodgkin lymphoma. | — |
| CD45RA* | B- and T-cell subsets, including mostly naive T cells. | May help to identify restricted population. | — |
| CD45RO* | B- and T-cell subsets, including mostly memory T cells. | May help to identify restricted population. | — |
| CD57* | NK cells, NK-like T cells. | Indicator of NK-differentiation. | — |
| TCR α/β* | Mature T cells in association with sCD3. | Classification mature T-cell lymphoid neoplasms. | — |
| TCR γ/δ* | Mature T cells in association with sCD3. | Classification mature T-cell lymphoid neoplasms. May help to identify restricted population. | — |
| TIA-1* | Cytotoxic T cells. | Classification mature T-cell lymphoid neoplasms. | — |
| T-β* chain isoforms | T cells. | Restricted expression associated with clonality. | — |
| Reagent . | Normal distribution . | Clinical utility in mature T- and NK-cell lymphoid neoplasms . | Comments . |
|---|---|---|---|
| CD2 | T cells and NK cells. | Indicator of T- or NK-cell lineage. | May be aberrantly expressed in AML. |
| CD3, surface | Acquired during maturation of T cells. | Indicator of T-cell lineage. May be aberrantly lost or decreased in intensity. | — |
| CD4 | T-cell subset and monocytes/histiocytes. | Useful in classification of mature T-cell lymphoid neoplasms. | NOT indicator of clonality. Also may be positive in AML and HDN. |
| CD5 | T cells and minor B-cell subset. | Indicator of T-cell lineage. May be aberrantly lost or decreased in intensity. | May be aberrantly expressed on B cells. |
| CD7 | T cells and NK cells. | Indicator of T-cell lineage. May be aberrantly lost or decreased in intensity. | Some normal and reactive CD7− cells. May be aberrantly expressed in AML. |
| CD8 | T-cell subset and some NK cells. | Useful in classification of mature T-cell lymphoid neoplasms. | NOT indicator of clonality |
| CD45 | All B cells (weaker intensity on precursors and plasma cells), all T cells (weaker intensity on precursors). | Useful in distinguishing mature lymphoid neoplasms (bright intensity) from ALL and PCN (weak intensity to negative). | — |
| CD56 | NK cells and NK-like T cells. | Indicator of NK differentiation. | Aberrant expression in AML, PCN. Positive HDN. Small subset of regenerating myeloid cells demonstrates weak expression. |
| CD1a* | Immature T cells. | ALL. | Also positive on Langerhans cells. |
| CD3, cytoplasmic* | All T cells including lymphoblasts. | Indicator of T- or NK cell lineage. NK cells contain cCD3 epsilon. | — |
| CD10* | Immature T cells and B cells, subset of mature T cells and B cells, and neutrophils. | Frequently present in ALL. Found on some mature T-cell neoplasms, in particular AITCL. | Minor subset of normal T cells. |
| CD16* | NK cells, NK-like T cells, and maturing neutrophilic cells. | Indicator of NK differentiation. | Antibodies with differing specificity for lymphoid cells and neutrophilic cells.. |
| CD25* | Activated T cells. | Uniform strong positivity in ATLL. More variable expression in other subtypes. Assessment for anti-CD25 therapy, eg, Ontak. | — |
| CD26* | Immature T cells, NK cells, and activated T cells. Most CD4+ T cells also CD26+. | CTCL/Sézary syndrome often CD26 negative (> 30% CD4+ cells, CD26−). | Not specific for Sézary/CTCL. |
| CD30* | Activated T and B cells, and monocytes. | Strong uniform staining in ALCL. More variable staining in other mature T- and NK-cell neoplasms. Positive in Hodgkin lymphoma. | — |
| CD45RA* | B- and T-cell subsets, including mostly naive T cells. | May help to identify restricted population. | — |
| CD45RO* | B- and T-cell subsets, including mostly memory T cells. | May help to identify restricted population. | — |
| CD57* | NK cells, NK-like T cells. | Indicator of NK-differentiation. | — |
| TCR α/β* | Mature T cells in association with sCD3. | Classification mature T-cell lymphoid neoplasms. | — |
| TCR γ/δ* | Mature T cells in association with sCD3. | Classification mature T-cell lymphoid neoplasms. May help to identify restricted population. | — |
| TIA-1* | Cytotoxic T cells. | Classification mature T-cell lymphoid neoplasms. | — |
| T-β* chain isoforms | T cells. | Restricted expression associated with clonality. | — |
Reagents included in this table were recommended in the consensus guidelines.
sCD3 indicates surface CD3.
These reagents may be considered for secondary evaluation, after other reagents listed have been used in the initial evaluation.
Aberrant T-cell antigen expression.
T- and NK-cell lymphoid neoplasms often demonstrate altered expression of T cell– and NK cell–associated antigens.68,70 On occasion, this is reflected in complete lack of staining for one or more pan–T-cell antigens. Indeed, lack of staining for multiple T cell–associated antigens may impart a “null” phenotype and raise questions about the lineage of the cells.71 CD5 and CD7 are the most frequently lost antigens in T-cell neoplasms. However, CD7− neoplastic cells must be distinguished from the small population of normal CD7− T cells that are well recognized in the skin and blood and may expand in benign dermatoses and other reactive conditions.72,73 In addition, it is important to recognize normal subsets of T cells, including T-cell receptor (TCR)-γ/δ+ cells, that may lack staining for CD5, CD4 and CD8, and normal NK cells usually lack staining for CD5 and demonstrate variable lack of staining for CD8.67
More frequently, rather than complete lack of staining, T and NK neoplasms demonstrate more subtle altered intensity of staining for antigens. Some of the more frequently encountered abnormalities include brighter or weaker staining of T cells for CD3 or CD5; weaker staining of NK cells for CD2, CD7, CD56, and CD57; and more uniform bright expression by NK cells of CD8 and CD16.68,70 However, these abnormalities must be distinguished from normal small subsets of T cells that may have a somewhat unusual phenotype, (eg, γ/δ+ T cells with low intensity or absence of staining for CD5 and possibly CD2 and brighter CD3; CD45RO+ primarily memory T cells with brighter CD2 staining than CD45RO− primarily naive T cells).67,74 In addition, it is important to recognize that NK cells normally demonstrate somewhat variable intensity staining for CD2, CD7, CD16, and CD56.68,70
Populations of abnormal T and NK cells can also be recognized by expression of antigens that are not normally expressed in these lineages. The myeloid antigens CD15, CD13, and CD33 have been described on some mature T-cell malignancies and may lead to confusion with acute myeloid leukemia.71,75,76 NK cells may gain staining for CD5.70 Expression of the B-cell antigen CD20 has been described in a small percentage of T-cell malignancies and can also be detected on a small subset of normal T cells using flow cytometric immunophenotyping.77,78
Identification of restricted populations of T cells.
T-cell neoplasms represent an expanded clone of cells that usually demonstrates more restricted expression of antigens than that of normal or reactive populations of T cells. However, identification of abnormally restricted T cells is often difficult because reactive stimuli may evoke a relatively restricted T-cell response and, in addition, T-cell neoplasms often contain admixed reactive T cells. Alteration in the ratio of CD4+ to CD8+ T cells is not a useful indicator of neoplasia because it varies considerably in normal and reactive populations of T cells. In addition, CD4 and CD8 expression is not a surrogate marker of clonality because the genes do not demonstrate allelic exclusion. However, deviation of the CD4/CD8 ratio from normal might raise concern for the presence of an abnormally restricted population, and lead to more thorough evaluation for an abnormal subset of T cells. For example, an increased CD4/CD8 ratio in peripheral blood T cells might be further evaluated for expression of CD26 and CD7. Normally in the peripheral blood CD4+ T cells are mostly CD26+ (more than 70% of CD4+ T cells are CD26+). In contrast, the neoplastic CD4+ cells of Sézary syndrome demonstrate decreased staining for CD26 and are usually CD7−.79,80 Therefore, evaluation of the combination CD7, CD26, CD3, and CD4 in one analysis tube could assist in identification of a restricted population of T cells. Again, this finding does not necessarily represent clonality and should be correlated with clinical, morphologic, and other findings to establish a diagnosis of malignancy.
Flow cytometric evaluation of the TCR V-β expression provides a more sensitive and specific assay for the detection of restricted populations of T cells.81 Normally, T-cell populations include a mixture of cells with variable expression of the V-β family subtypes. In T-cell neoplasia, there is expansion of a clone of cells with more restricted V-β expression. This flow cytometric assay is in many ways similar to PCR tests for TCR gene rearrangement and is subject to the same limitations, including false-positive results in some restricted T-cell responses and in specimens with very few T cells, and false-negative results when small clones of T cells are admixed with many reactive cells. In addition, most flow cytometric assays assess for only a subset of the total 25 functional V-β families and 91 subfamilies and allele members. Despite this limited analysis, V-β testing is still labor intensive and also requires analysis of viable samples, preferably within 24 hours of collection. In contrast, molecular diagnostic studies for clonal TCR gene rearrangement can be performed on fresh, frozen, or fixed specimens.
NK cells lack expression of the TCR and therefore cannot be assessed for clonality using V-β flow cytometric analysis or molecular diagnostic studies for clonal TCR gene rearrangement. Flow cytometric analysis of NK-receptor (NKR) expression, including killer cell immunoglobulin receptors (KIRs) and the CD94/NKG2 complex, has been developed primarily to identify evidence of NK-cell clonality, but can also be applied to the evaluation of memory cytotoxic T cells as seen in T-cell large granular lymphocyte leukemia (LGL).82,83 Normal and reactive populations of NK cells express a variety of NKRs, whereas neoplastic clones express a more restricted repertoire. Although several studies have demonstrated abnormal KIR expression in LGL, there has to date been only limited testing of reactive conditions that may mimic NK- or T-cell neoplasia.
Role of flow cytometric immunophenotyping in the classification of mature T- and NK-cell lymphoid neoplasms
Although progress has been made in the classification of mature T- and NK-cell lymphoid malignancies with the identification of some distinct disease entities, the boundaries of the recognized entities are still being established, and many neoplasms remain in the peripheral T-cell lymphoma, unspecified (PTCL, U) category. Classification using the current scheme does not follow a simple algorithm and often requires assimilation of multiple diverse pieces of information. Therefore, it is prudent to allocate fixed tissue for morphologic review, paraffin section immunohistochemical and in situ hybridization stains, fresh tissue for flow cytometric studies, fresh tissue for cytogenetic studies, and fresh or frozen tissue for molecular diagnostic studies.
Clinical information is perhaps even more important in the classification of T- and NK-cell neoplasms than for mature B-cell neoplasms. Does the neoplasm primarily involve the blood, lymph nodes, or extranodal sites? What is the distribution of nodal and extranodal disease? Is the disease behaving in an aggressive or indolent fashion? Are there clinical findings that might be associated with a particular subtype of T-or NK-cell neoplasm, such as widespread lymphadenopathy, constitutional symptoms, skin rash, and hypergammaglobulinemia with angioimmunoblastic T-cell lymphoma (AITCL), celiac disease with enteropathy associated T-cell lymphoma (EATCL), or neutropenia and rheumatoid arthritis with LGL? Morphologic assessment is essential in the classification of T and NK neoplasms and includes evaluation for features suggestive of a particular subtype of T- or NK-cell neoplasm, such as growth around residual lymph node structures, including expanded follicular dendritic meshwork structures in AITCL, an anaplastic morphologic appearance in anaplastic large cell lymphoma (ALCL), or large granular lymphocytes in LGL?
Immunophenotypic information also forms an important part of the classification of mature T- and NK-cell lymphoid neoplasms and can be performed either by flow cytometry or paraffin section immunohistochemistry (IHC). Therefore, it is then important to determine the questions to be addressed and select the optimal technique(s) to provide answers.
Are the neoplastic cells T cells or NK cells?
Flow cytometric studies are superior to IHC stains for the distinction between T cells and NK cells. The CD3 antibody used by flow cytometry usually detects the fully assembled TCR-CD3 complex, which is present on the surface of T cells and absent from NK cells. In contrast, the CD3 IHC stain usually detects only the epsilon component of CD3 and therefore cannot distinguish between T cells and NK cells. However, it is important to further evaluate cells lacking surface CD3 expression to distinguish between NK cells, abnormal T cells with aberrant loss of CD3, and immature T cells. Molecular diagnostic studies demonstrating clonal TCR gene rearrangement can also assist in confirming T-cell, rather than NK-cell, lineage.
Is there expression of CD4 or CD8?
Although expression of CD4 and CD8 can be detected by either flow cytometry or IHC, it is sometimes difficult to identify the neoplastic cells by IHC because of the presence of many admixed reactive T or NK cells. Multicolor flow cytometry has the advantage of more readily identifying the neoplastic cells through aberrant expression of other antigens, and isolating them for further characterization. As described in the next section focusing on mature T-cell neoplasms, the expression of CD4 and CD8 can assist in the further classification of T-cell lymphoid neoplasms.
Is there expression of the NK cell–associated antigens CD16, CD56, and CD57?
Although both IHC and flow cytometric studies can be used to identify NK-cell differentiation through the detection of CD56 and CD57, flow cytometry is considered to be a more sensitive technique. In addition, an IHC stain is not currently available for CD16. For the flow cytometric detection of CD16 on NK cells, antibodies against the CD16A isoform rather than the CD16B granulocyte associated isoform should be used.
Is there is expression of the components of the TCR, and if so, is it of the α/β or γ/δ type?
Flow cytometric studies are the optimal method for determining whether cells express the α/β or γ/δ form of the TCR. The IHC BF1 stain can detect the α/β form of the receptor, but a suitable γ/δ paraffin section IHC stain is not currently available. A positive BF1 stain provides definitive evidence of the α/β form of the TCR, but a negative stain cannot be assumed to represent γ/δ T cells. This distinction has increased in importance because, at least in some circumstances, γ/δ T-cell lymphoma behaves more aggressively.84
Do the cells express the cytotoxic granule–associated proteins TIA-1, granzyme-B, or perforin?
Staining can be performed either by flow cytometry or paraffin section immunohistochemistry. TIA-1 staining is identified in most cytotoxic T cells. Staining for granzyme-B and/or perforin indicates an activated cytotoxic phenotype.
Are there phenotypic features that are associated with ALCL including diffuse uniform staining for CD30, and staining for Alk-1 protein?
Is the lymphoma EBV associated, and if so is EBV present in the neoplastic or accompanying B cells?
Epstein-Barr virus (EBV) can be detected by either paraffin section IHC staining for the latent membrane protein (LMP1) or EBV-encoded RNA (EBER) in situ hybridization. The EBER in situ hybridization stain is probably one of the more sensitive widely available techniques for the detection of EBV but relies on adequate RNA preservation. The LMP-1 IHC stain detects protein expression and is therefore more robust than the EBER stain but LMP1 is not expressed in all EBV-infected cells. In extranodal T/NK-cell lymphoma-nasal type EBV is present in the neoplastic T or NK cells. In contrast, in AITCL, EBV is usually present in scattered large B cells.
Do the neoplastic T cells express CD103?
A subset of normal intramucosal T cells and EATCL are positive for CD103. This antigen currently cannot be detected by paraffin section IHC but, as described for the evaluation for HCL, is detectable by flow cytometry.
In contrast to the disease-oriented approach taken in the 2001 WHO classification and previous Blood review, the following two sections will discuss an approach to the classification of neoplasms of mature T and NK cells that is based primarily on flow cytometric data.1,3 When considering the results of flow cytometric immunophenotyping, it is often useful to first separate neoplasms with a T-cell phenotype from those with an NK-cell phenotype, as described in the previous section on the role of flow cytometric immunophenotyping in the classification of mature T and NK neoplasms.
Mature T-cell neoplasms
Among mature lymphoid neoplasms with a T-cell phenotype, expression of CD4 and CD8 can be used to formulate a list of diagnostic possibilities and determine what additional information is required for further classification (Table 4).
Flow cytometric approach to the diagnosis and classification of T- and NK-cell lymphoid neoplasms
| Disease entities to consider . | Distinguishing phenotypic features . | Additional diagnostic information . |
|---|---|---|
| CD4+ CD8− | ||
| CTCL/Sézary syndrome | Often CD7−, CD26−, CD25+/− (with heterogeneous staining intensity). | Confirm characteristic morphology and clinical presentation. HYLV-1− |
| T-PLL | Usually lacks significant phenotypic aberrancy. CD16−, CD56−, CD57−. | 80% t(14;14)(q11;q32) or inv(14)(q11;q32). TCL1 protein overexpression. |
| Adult T-cell leukemia/lymphoma | CD7−, CD25+ (uniform bright intensity). | HTLV-1+, endemic to Japan and Caribbean. |
| Anaplastic large cell lymphoma | Often loss of many pan–T-cell antigens. Strong uniform CD30+, Alk-1 protein+/−. CD56+/−, CD13+/−, CD15+/−, CD33+/−. Cytotoxic granule–associated protein (+). | Anaplastic morphology, except in small cell/monomorphic variant. ALK gene rearrangement. |
| Angioimmunoblastic TCL | Often aberrant phenotype (eg, decreased intensity CD7 and CD3). CD10+/−. | Characteristic morphology. Proliferative follicular dendritic meshwork, CXCL13+, scattered EBV+ B cells. |
| Peripheral TCL, NOS | Variable phenotype, often aberrant loss of CD5 and/or CD7. | Diagnosis by exclusion of other distinct disease entities. |
| CD4−/CD8+ | ||
| T-cell large granular lymphocyte leukemia | Frequent aberrant expression CD5 and/or CD7. CD16+/−, CD56+/−,CD57+. TIA-1+, granzyme-B+, perforin+. | Often LGL morphology. Usually indolent course, associated with rheumatoid arthritis and cytopenias (eg, neutropenia and anemia). EBV−. |
| Subcutaneous panniculitis-like TCL | Usually only focal CD56, EBV−, TCR α/β, TIA-1+, granzyme-B+, perforin+ | Infiltrate in subcutis with rimming fat droplets, beanbag histiocytes. Must distinguish from lupus profundus. |
| Hepatosplenic TCL | Usually CD5− and CD7+. CD16+/−, CD56+, CD57−, TIA-1+, granzyme-B−, perforin−. | Often TCR γ/δ but may be TCR α/β, EBV−. Frequent isochromosome 7q. Usually aggressive clinical course. |
| CD4+/CD8+ | ||
| T-PLL | Usually lacks significant phenotypic aberrancy. CD16−, CD56−, CD57−. | 80% t(14;14)(q11;q32). TCL1 protein overexpression. |
| Adult T-cell leukemia/lymphoma | CD7−, CD25+ (uniform bright). | HTLV-1+, endemic to Japan and Caribbean. |
| Peripheral TCL, NOS | Variable phenotype, often aberrant loss of CD5 and/or CD7. | Diagnosis by exclusion of other distinct disease entities. |
| CD4−/CD8− | ||
| Enteropathy-associated TCL | CD5−, CD3+, CD7+, CD103+, CD56+/−. TIA-1+, granzyme-B+, perforin+. | CD30+/−, EBV−. FISH for gains 9q33–34. History of celiac disease. |
| Hepatosplenic TCL | Usually CD5− and CD7+. CD16+/−, CD56+, CD57−. TIA-1+, granzyme-B−, perforin−. | Often TCR δ but may be TCR α/β, EBV−. Frequent isochromosome 7q. Usually aggressive clinical course. |
| Nonhepatosplenic γ/δ TCL | CD5−, CD56+, mostly CD57−, TCR γ/δ. TIA-1+, granzyme-B+, perforin+. | Skin, mucosal sites, and other extranodal locations. EBV often positive in mucosal and negative in cutaneous lymphoma. |
| Disease entities to consider . | Distinguishing phenotypic features . | Additional diagnostic information . |
|---|---|---|
| CD4+ CD8− | ||
| CTCL/Sézary syndrome | Often CD7−, CD26−, CD25+/− (with heterogeneous staining intensity). | Confirm characteristic morphology and clinical presentation. HYLV-1− |
| T-PLL | Usually lacks significant phenotypic aberrancy. CD16−, CD56−, CD57−. | 80% t(14;14)(q11;q32) or inv(14)(q11;q32). TCL1 protein overexpression. |
| Adult T-cell leukemia/lymphoma | CD7−, CD25+ (uniform bright intensity). | HTLV-1+, endemic to Japan and Caribbean. |
| Anaplastic large cell lymphoma | Often loss of many pan–T-cell antigens. Strong uniform CD30+, Alk-1 protein+/−. CD56+/−, CD13+/−, CD15+/−, CD33+/−. Cytotoxic granule–associated protein (+). | Anaplastic morphology, except in small cell/monomorphic variant. ALK gene rearrangement. |
| Angioimmunoblastic TCL | Often aberrant phenotype (eg, decreased intensity CD7 and CD3). CD10+/−. | Characteristic morphology. Proliferative follicular dendritic meshwork, CXCL13+, scattered EBV+ B cells. |
| Peripheral TCL, NOS | Variable phenotype, often aberrant loss of CD5 and/or CD7. | Diagnosis by exclusion of other distinct disease entities. |
| CD4−/CD8+ | ||
| T-cell large granular lymphocyte leukemia | Frequent aberrant expression CD5 and/or CD7. CD16+/−, CD56+/−,CD57+. TIA-1+, granzyme-B+, perforin+. | Often LGL morphology. Usually indolent course, associated with rheumatoid arthritis and cytopenias (eg, neutropenia and anemia). EBV−. |
| Subcutaneous panniculitis-like TCL | Usually only focal CD56, EBV−, TCR α/β, TIA-1+, granzyme-B+, perforin+ | Infiltrate in subcutis with rimming fat droplets, beanbag histiocytes. Must distinguish from lupus profundus. |
| Hepatosplenic TCL | Usually CD5− and CD7+. CD16+/−, CD56+, CD57−, TIA-1+, granzyme-B−, perforin−. | Often TCR γ/δ but may be TCR α/β, EBV−. Frequent isochromosome 7q. Usually aggressive clinical course. |
| CD4+/CD8+ | ||
| T-PLL | Usually lacks significant phenotypic aberrancy. CD16−, CD56−, CD57−. | 80% t(14;14)(q11;q32). TCL1 protein overexpression. |
| Adult T-cell leukemia/lymphoma | CD7−, CD25+ (uniform bright). | HTLV-1+, endemic to Japan and Caribbean. |
| Peripheral TCL, NOS | Variable phenotype, often aberrant loss of CD5 and/or CD7. | Diagnosis by exclusion of other distinct disease entities. |
| CD4−/CD8− | ||
| Enteropathy-associated TCL | CD5−, CD3+, CD7+, CD103+, CD56+/−. TIA-1+, granzyme-B+, perforin+. | CD30+/−, EBV−. FISH for gains 9q33–34. History of celiac disease. |
| Hepatosplenic TCL | Usually CD5− and CD7+. CD16+/−, CD56+, CD57−. TIA-1+, granzyme-B−, perforin−. | Often TCR δ but may be TCR α/β, EBV−. Frequent isochromosome 7q. Usually aggressive clinical course. |
| Nonhepatosplenic γ/δ TCL | CD5−, CD56+, mostly CD57−, TCR γ/δ. TIA-1+, granzyme-B+, perforin+. | Skin, mucosal sites, and other extranodal locations. EBV often positive in mucosal and negative in cutaneous lymphoma. |
+ indicates usually positive; −, usually negative; +/−, may be positive or negative; d, dim or weak intensity; I, intermediate intensity; b, bright or strong intensity.
CD4+/CD8−.
T-cell neoplasms positive for CD4 include Sézary syndrome/cutaneous T-cell lymphoma (CTCL); T-cell PLL (T-PLL); adult T-cell leukemia lymphoma (ATLL); ALCL; AITCL; PTCL, U; and rare instances of CD4+ LGL. Diseases primarily involving the peripheral blood will be considered first and include Sézary syndrome/CTCL, ATLL, and T-PLL. The more recently recognized entity CD4+CD56+ hematodermic neoplasm (HDN), previously referred to as blastic NK-cell lymphoma, is discussed in “Blastic neoplasms” because of its frequent blastoid appearance and usual lack of T-lineage–associated antigens.86
Sézary syndrome/CTCL usually has a CD4+, CD26−, CD7− T-cell phenotype, with variable presence and intensity of staining for CD25 (Figure 2).79,80 However, this phenotype is not specific for this disease grouping and therefore should be combined with the clinical presentation and morphologic appearance.
Sézary Syndrome. (A) Peripheral blood smear demonstrating an abnormal lymphoid cell with an irregular folded nucleus. Wright Giemsa stain, magnification ×100. Images were acquired as in Figure 1. (B) Representative flow cytometric dot plots with population of interest highlighted in green: CD3 versus CD7, demonstrating many CD7− CD3+ T cells; CD3 versus CD16 and/or CD57, demonstrating lack of NK-associated antigen expression; CD26 versus CD4, demonstrating many CD4+ cells, including more than 30% CD26− cells; and CD3 versus CD25, demonstrating only partial weak intensity staining of T cells for CD25.
Sézary Syndrome. (A) Peripheral blood smear demonstrating an abnormal lymphoid cell with an irregular folded nucleus. Wright Giemsa stain, magnification ×100. Images were acquired as in Figure 1. (B) Representative flow cytometric dot plots with population of interest highlighted in green: CD3 versus CD7, demonstrating many CD7− CD3+ T cells; CD3 versus CD16 and/or CD57, demonstrating lack of NK-associated antigen expression; CD26 versus CD4, demonstrating many CD4+ cells, including more than 30% CD26− cells; and CD3 versus CD25, demonstrating only partial weak intensity staining of T cells for CD25.
Adult T-cell leukemia lymphoma has a similar CD4+, CD7− phenotype to Sézary syndrome/CTCL, but demonstrates more uniform strong staining for CD25.80 However, because of the overlapping phenotypic features, patients who cannot be distinguished on morphologic and clinical grounds are often evaluated for human T-cell leukemia virus-1 (HTLV-1), the etiologic agent for ATLL.
T-PLL is most frequently CD4+, may show dual staining for CD4 and CD8, and is less frequently positive for CD8 only.68,80 Unlike CTCL and ATLL, T-PLL is usually positive for CD7, negative for CD25, and lacks aberrant loss or decreased expression of other T-cell antigens.68 The cells of T-PLL are usually negative for NK-associated antigens and cytotoxic granule–associated proteins; therefore, expression should lead to consideration of a rare CD4+ LGL. Correlation with morphologic and clinical findings remains important. Although the morphologic appearance of T-PLL is quite varied, the cells usually do not resemble large granular lymphocytes, and the clinical presentation is often characteristic with marked lymphocytosis, cytopenias, splenomegaly, and an aggressive clinical course. Cytogenetic studies can be performed for confirmation since more than 80% of patients with T-PLL have either inv(14)(q11;q32) or the translocation t(14;14)(q11;q32) involving the TCL1 gene and TCR α/δ locus. These genetic abnormalities lead to overexpression of TCL1 protein.
ALCL primarily involves lymph nodes and skin but, particularly in the small-cell variant, may demonstrate peripheral blood involvement.87 ALCL is usually CD4+, may express CD56, is most frequently CD2+ but often lacks many other T-cell antigens, including CD3, CD5, and CD7, and may express the myeloid associated antigens CD13, CD15, and CD33.76 Absence of T cell–associated antigens and expression of myeloid antigens may lead to confusion with acute myeloid leukemia (AML). The phenotype of ALCL does not distinguish it from other mature T-cell neoplasms, and the morphologic appearance is quite varied. IHC stains are often used for further characterization. ALCL demonstrates strong uniform expression of CD30 with a characteristic membrane and Golgi distribution and is cytotoxic granule–associated protein positive. A definitive diagnosis can be established in patients expressing Alk-1 protein or demonstrating ALK1 gene rearrangement by conventional cytogenetics or FISH studies. The existence of Alk− systemic ALCL is debated, but could probably be considered for patients with characteristic anaplastic morphologic features, strong uniform CD30 staining, and cytotoxic granule protein expression. Cutaneous ALCL is most frequently Alk-1− and may have overlapping morphologic and immunophenotypic features with systemic Alk− ALCL, lymphomatoid papulosis, borderline CD30+ lymphoproliferative disorders, and transformed CTCL.71 Probably the most important distinguishing features are the clinical presentation and course of the disease.
AITCL is a CD4+ neoplasm that is usually CD2+ and CD5+, often demonstrates decreased expression of CD7, and sometimes demonstrates decreased CD3.88 Although a subset of neoplastic cells in AITCL may be CD10+, T-cell expression of CD10 can also be seen in precursor T-ALL, rarely in other mature T-cell neoplasms, and in a subset of normal T cells. Recently, AITCL has also been demonstrated to be positive for CXCL13 by IHC.89 However, it remains important to evaluate for morphologic and clinical features characteristic of this disease.
As expected, the phenotype of the PTCL, U category is quite heterogeneous and overlaps that of other mature T-cell neoplasms. Neoplasms in this category are most frequently CD4+, and often demonstrate aberrant loss of CD7 and CD5 expression. By definition, other specified disease entities must be excluded before a diagnosis of PTCL, U can be made. The possibilities to consider can often be narrowed down by the specimen being evaluated and the clinical presentation. In the peripheral blood, PTCL, U is usually distinguished from Sézary syndrome/CTCL, ATLL, and T-PLL on clinical grounds. In addition, T-PLL usually demonstrates preservation of T-cell antigens and has the typical chromosome 14 abnormalities. In nodal tissue and skin it is important to rule out ALCL. Although PTCL, U can demonstrate staining for CD30, it is usually weaker and more heterogeneous than that seen in ALCL. PTCL, U is negative for Alk-1 protein expression and ALK gene rearrangement.
CD4−/CD8+.
The most frequent CD8+ T-cell neoplasm encountered by flow cytometry is T-LGL. In the peripheral blood the other considerations are CD8+ PTCL, U; a small proportion of PLL; and rarely CTCL/Sézary syndrome. In the spleen and other extranodal sites, the differential becomes broader with inclusion of subcutaneous panniculitis-like TCL (SPTCL), and rare CD8+ patients with hepatosplenic TCL (HSTCL) and nonhepatosplenic γ/δ T-cell lymphoma.
T-LGL demonstrates a CD8+ T-cell phenotype with frequent decreased intensity staining for CD5 or CD7 and expression of NK-associated antigens.68,90,91 Most patients with T-LGL express CD57, many express CD16, and a few are CD56+. In addition, there is usually expression of the cytotoxic granule–associated proteins TIA-1, granzyme-B, and perforin. Although most patients with T-LGL express TCR α/β, a few TCR γ/δ patients have been reported. A diagnosis of T-LGL is often established by combining the phenotype with morphologic and clinical features. T-LGL is a disease of adults with a median age of 55 years, is often associated with rheumatoid arthritis, including Felty syndrome (rheumatoid arthritis, splenomegaly, neutropenia), and usually presents with peripheral blood lymphocytosis, neutropenia, and anemia. The neoplastic cells typically have abundant pale-staining cytoplasm with azurophilic granules and small bland nuclei. Cytologic atypia or significant involvement of lymph nodes or extranodal lymphoid sites other than the spleen should raise the possibility of a more aggressive cytotoxic T-cell lymphoma. LGL with an NK phenotype may also demonstrate a CD8+, CD4− phenotype, but lacks expression of the surface CD3–TCR complex and therefore is not considered with the mature T-cell lymphoid neoplasms.
SPTCL is a rare cytotoxic T-cell lymphoma that is usually CD8+, demonstrates expression of one or more cytotoxic granule associate proteins, is only focally positive for CD56, and is usually EBV−.71,84 In addition, SPTCL demonstrates characteristic morphologic features with infiltration of subcutaneous fat, including rimming of fat droplets by neoplastic cells, fat cell necrosis, apoptosis, and prominent phagocytosis of cellular debris by histiocytes (beanbag cells). SPTCL is most frequently TCR α/β+. Indeed, it has been proposed that patients with features of SPTCL who are TCR γ/δ+ should not be included in this group, but classified with other cutaneous (nonhepatosplenic) γ/δ T-cell lymphomas.
Although HSTCL is more frequently negative for both CD4 and CD8, rare CD8+ patients have been described and may be difficult to distinguish from LGL.84 In common with T-LGL, HSTCL usually involves the spleen and can involve the peripheral blood, is frequently CD16+, and expresses cytotoxic granule–associated proteins. However, HSTCL differ from most T-LGLs in frequently expressing CD56 and lacking CD57, demonstrating complete lack of CD5 rather than decreased intensity staining, more often expressing TCR γ/δ, and frequently demonstrating the cytogenetic abnormality isochromosome 7q. Clinical features are also important in the distinction between T-LGL and HSTCL: HSTCL is typically an aggressive disease affecting younger adults that presents with massive hepatosplenomegaly, constitutional symptoms, and cytopenias. Rare instances of nonhepatosplenic γ/δ T-cell lymphoma are CD8+ but are not usually confused with LGL because of lack of peripheral blood and splenic involvement. A small proportion of CTCLs are CD8+ and should be distinguished from the more aggressive cutaneous, nonhepatosplenic, TCR γ/δ T-cell lymphomas.92 Although CTCL is usually negative for cytotoxic granule–associate proteins, they may be present in large-cell transformation of CTCL. Morphology, clinical presentation, and clinical course remain important distinguishing features.
CD4+/CD8+.
Dual expression of CD4 and CD8 is unusual in mature T-cell neoplasms and should lead to consideration of precursor T-ALL. Probably the most characteristic CD4+, CD8+ mature T-cell lymphoid neoplasm is T-PLL.80,93 T-PLL has a mature T-cell phenotype with expression of surface CD3 and a full compliment of T-cell antigens such as CD2, CD5, and CD7, and lacks markers of immaturity such as CD34, CD10, and TdT. A diagnosis of T-PLL can be confirmed by evaluation of the morphologic and clinical features and demonstration of inv(14)(q11;q32) or translocation t(14;14)(q11;q32). Rare patients with ATLL and LGL coexpress CD4 and CD8 and require identification of other characteristic features. If other defined categories can be excluded, the possibility of PTCL, U could be considered, particularly in patients with primarily nodal disease.
CD4−/CD8−.
The CD4−, CD8− category includes EATCL; HSTCL; nonhepatosplenic γ/δ T-cell lymphoma; PTCL, U; and rare instances of NK/T-cell lymphoma nasal type with a T-cell rather than NK-cell phenotype.
EATCL is usually negative for CD4, CD8, and CD5, expresses CD3 and CD7, is positive for cytotoxic granule–associated proteins TIA-1, granzyme-B, and perforin, and is CD103+.94 EATCL can be CD56+ or CD56− and may express CD30. There is a recently recognized association of EATCL with gains in chromosome 9q33-34, and it has been suggested that FISH for these abnormalities could assist in confirming a diagnosis. Intramucosal lymphocytes in celiac disease can demonstrate a similar unusual phenotype to that of EATCL, and may demonstrate clonal TCR gene rearrangement. Therefore, a diagnosis of EATCL usually requires the presence of a destructive infiltrate.
HSTCL is usually negative for CD4, CD8, and CD5, positive for CD56, demonstrates variable expression of CD16, is mostly CD57−, and demonstrates a nonactivated cytotoxic phenotype with expression of TIA-1 but absence of granzyme-B and perforin.84 Although many patients with HSTCL are TCR γ/δ+, TCR α/β+ does occur and is considered part of the same entity. HSTCL is frequently associated with the cytogenetic abnormality isochromosome 7q.
Nonhepatosplenic γ/δ T-cell lymphoma demonstrates a similar phenotype to HSTCL, but usually demonstrates an activated cytotoxic phenotype: CD4−, CD8−, CD2+, CD3+, CD5−, CD56+, mostly CD57−, TIA-1+, granzyme-B+, and perforin+.71,84 This group of diseases includes mucosal and cutaneous lymphomas and typically has an aggressive clinical course. A significant number of mucosal γ/δ T-cell lymphomas are EBV associated, whereas the cutaneous γ/δ T-cell lymphomas are usually EBV−.
Extranodal T/NK-cell lymphoma–nasal type most frequently falls under the mature NK-cell neoplasms rather than the mature T-cell lymphoid neoplasms because of lack of surface CD3 expression and expression of the NK-associated antigen CD56 (see “Mature NK-cell neoplasms”).95 However, patients with a mature T-cell phenotype expressing CD56 and cytotoxic granule–associate proteins should probably be included in the extranodal T/NK-cell lymphoma–nasal type category.
Mature NK-cell neoplasms
NK-cell neoplasms include extranodal T/NK-cell lymphoma–nasal type, aggressive NK-cell leukemia, and a subset of LGL. Although these subtypes have overlapping features, it is probably most important to distinguish the more aggressive neoplasms from the subset of NK-cell LGL that follows a more indolent course. In contrast to the other NK-cell malignancies, indolent NK-cell LGL often expresses CD57 in addition to CD56, is EBV−, and more often follows a course similar to T-LGL.
Extranodal T/NK-cell lymphoma–nasal type is one of the best characterized NK-cell malignancies and usually requires assessment for CD56 and EBV.95 Typical extranodal T/NK-cell lymphoma–nasal type is CD56+ and EBV+; expresses cytotoxic granules TIA-1, granzyme-B, and perforin; presents in the nasal cavity and surrounding structures; and demonstrates angiocentric and angiodestructive growth (CD4−, CD8−, CD3s−, CD5−, CD7+, CD56+, TIA-1+, granzyme-B, or perforin+, EBV+). Although the boundaries of this disease entity are not well defined, it has been recommended that atypical disease that presents in the nasal location and are EBV+ but lack CD56 or demonstrate evidence of T-cell lineage should still be included in this grouping. Disease that present at other sites, including lymph node, but are in all other respects typical, can also be included with the extranodal T/NK-cell lymphoma–nasal type. However, it is uncertain how best to classify diseases that are more atypical, such as nonnasal disease that lacks evidence of EBV.
Aggressive NK-cell leukemia and extranodal T/NK-cell lymphoma–nasal type share many features, including an NK-cell phenotype, expression of CD56, and the frequent presence of EBV.95 The distinction is usually made on clinical grounds. Whereas extranodal T/NK-cell lymphoma–nasal type is tissue based, aggressive NK-cell leukemia presents with bone marrow involvement, cytopenias, a few circulating neoplastic cells, constitutional symptoms, and elevated liver function tests.
Plasma cell disorders
Plasma cell disorders are often identified through increased serum or urine gamma globulins and can be divided into polyclonal reactive proliferations and those producing monoclonal gammopathy. The monoclonal gammopathies can be further divided into monoclonal gammopathy of undetermined significance (MGUS) and overt PCN, including plasmacytoma, plasma cell myeloma and variants, plasma cell leukemia, amyloidosis, and immunoglobulin light and heavy chain diseases. The diagnosis of a PCN usually requires identification of increased plasma cells (greater than 10% of marrow cells), demonstration of an abnormal phenotype and/or clonality, and further classification using a combination of morphologic, laboratory, radiologic, and other clinical findings. Flow cytometric immunophenotyping is a useful tool for the identification of abnormal plasma cells and for the distinction between lymphoid and plasma cell neoplasms. In addition, flow cytometric testing may provide additional prognostic information.96,97 Table 5 outlines the normal pattern of staining and clinical utility of reagents recommended by the 2006 Bethesda consensus group for the evaluation of plasma cells.
Reagents of clinical utility in the evaluation of plasma cell disorders
| Reagent . | Normal distribution . | Clinical utility in plasma cell neoplasms . | Comments . |
|---|---|---|---|
| CD19 | All B cells including lymphoblasts, mature B-lymphoid cells, and most plasma cells. | PCN often CD19−, mature B-cell lymphoid neoplasms with plasmacytic differentiation+. | Aberrant expression on myeloid cells. |
| CD38 | Precursor B cells (hematogones), normal follicle center B cells, immature and activated T cells, plasma cells (bright intensity), myeloid and monocytic cells, and erythroid precursors. | Identification of plasma cells with bright intensity staining, often in combination with dim to negative CD45. | Not specific for plasma cells. |
| CD45 | All B cells (weaker intensity on precursors and some plasma cells), all T cells (weaker intensity on precursors). | Identification of plasma cells often in combination with CD38. | — |
| CD56* | NK cells, NK-like T cells. | May be aberrantly expressed in PCN. | CD56− PCN more often leukemic. |
| CD10* | Immature T cells and B cells, subset of mature T cells and B cells, and neutrophils. | May be aberrantly expressed in PCN. | |
| CD117* | Immature myeloid, mast cells. | May be aberrantly expressed in PCN. | Also described in MGUS. |
| CD138* | Plasma cells. | Identification of plasma cells. | More specific than CD38, but not as sensitive. |
| Kappa and* lambda, cytoplasmic | Plasma cells. | Light chain restriction in cells with plasmacytic differentiation. | Most flow cytometric assays detect surface and cytoplasmic Ig. |
| Reagent . | Normal distribution . | Clinical utility in plasma cell neoplasms . | Comments . |
|---|---|---|---|
| CD19 | All B cells including lymphoblasts, mature B-lymphoid cells, and most plasma cells. | PCN often CD19−, mature B-cell lymphoid neoplasms with plasmacytic differentiation+. | Aberrant expression on myeloid cells. |
| CD38 | Precursor B cells (hematogones), normal follicle center B cells, immature and activated T cells, plasma cells (bright intensity), myeloid and monocytic cells, and erythroid precursors. | Identification of plasma cells with bright intensity staining, often in combination with dim to negative CD45. | Not specific for plasma cells. |
| CD45 | All B cells (weaker intensity on precursors and some plasma cells), all T cells (weaker intensity on precursors). | Identification of plasma cells often in combination with CD38. | — |
| CD56* | NK cells, NK-like T cells. | May be aberrantly expressed in PCN. | CD56− PCN more often leukemic. |
| CD10* | Immature T cells and B cells, subset of mature T cells and B cells, and neutrophils. | May be aberrantly expressed in PCN. | |
| CD117* | Immature myeloid, mast cells. | May be aberrantly expressed in PCN. | Also described in MGUS. |
| CD138* | Plasma cells. | Identification of plasma cells. | More specific than CD38, but not as sensitive. |
| Kappa and* lambda, cytoplasmic | Plasma cells. | Light chain restriction in cells with plasmacytic differentiation. | Most flow cytometric assays detect surface and cytoplasmic Ig. |
Reagents included in this table were recommended in the consensus guidelines.
— indicates not applicable.
These reagents may be considered for secondary evaluation, after other reagents listed have been used in the initial evaluation.
Identification of abnormal plasma cells
Two antigens are commonly used to identify plasma cells: CD38 (bright intensity) and CD138. In addition to plasma cells, CD38 is expressed by a wide variety of cell types: hematogones, some mature B cells, activated T cells, and myeloid cells. However, in contrast to the bright CD38 staining characteristic of plasma cells, these other cell types typically express CD38 at a lower intensity. CD138 expression is restricted to plasma cells and some carcinoma cells, but is less sensitive for the detection of plasma cells than CD38. Therefore, evaluation for both CD38 and CD138 provides the most sensitive and specific means of detecting plasma cells. These markers are often used in combination with CD45. Normal tonsillar and peripheral blood plasma cells are CD45+.98 In contrast, normal bone marrow usually contains 2 subsets of plasma cells: a CD45+ subset that appears to be proliferative, and a small subset that is negative or only expresses CD45 at a low intensity.98
The phenotype of neoplastic plasma cells usually differs from that of reactive plasma cells. Neoplastic plasma cells often demonstrate lower levels of expression of CD38 and CD138, and include a larger CD45 negative to low-intensity population with only a small CD45+ population.99,100 In addition, PCNs usually demonstrate an abnormal CD19−, CD20− phenotype that differs from most normal CD19+, CD20− bone marrow plasma cells and CD19+, CD20+ B-cell lymphoid neoplasms. Although approximately 10% of PCNs express CD20, and a smaller subset are CD19+, expression of both CD19 and CD20 is rare and should raise the possibility of a mature lymphoid B-cell neoplasm. CD20 expression in myeloma has been associated with a more “lymphoid” appearance, presence of the translocation t(11;14), and cyclin-D1 protein staining by paraffin section IHC.96
Aberrant CD56 expression is identified in most patients with myeloma.96 CD56− myeloma appears to more frequently involve the peripheral blood and may meet the criteria for plasma cell leukemia.101 Abnormal gain in expression of CD28 has been demonstrated on approximately one-half of patients with myeloma at diagnosis. Loss of expression of CD27 occurs on a similar proportion of all patients with myeloma, but is often associated with disease progression.102 Approximately 20% of PCNs demonstrate staining for CD117. CD117 is more typically thought of as a marker of immature myeloid cells and mast cells. Therefore, if plasma cell markers are not included in the analysis, the presence of a population staining for CD117 could be misinterpreted.96
Role of flow cytometric immunophenotyping in the diagnosis and classification of plasma cell disorders
Although flow cytometric immunophenotyping is a useful tool for the identification and characterization of plasma cells, and distinction between PCNs and mature B-lymphoid malignancies with plasmacytic differentiation, its diagnostic utility is limited by difficulties encountered in enumerating plasma cells.96,97 Even using sensitive techniques, flow cytometric immunophenotyping usually identifies fewer plasma cells than paraffin section IHC of biopsy sections.103 This discrepancy appears to reflect a combination of sampling differences, loss of plasma cells during processing for flow cytometric studies, and difficulty encountered in including all surviving plasma cell populations in the flow cytometric analysis. Because of the relative paucity of plasma cells identified using flow cytometric techniques, many of the studies characterizing PCN and MGUS have used sensitive techniques that permit accurate detection of less than 1% plasma cells, and often down to 0.01%.
The phenotype of MGUS overlaps that of overt PCN. Sensitive flow cytometric studies can usually identify a population of abnormal immunoglobulin light chain–restricted plasma cells in MGUS, and there are even reports of CD56 and CD117 expression.104 In addition, because of loss of plasma cells during processing, the identification of less than 10% bone marrow plasma cells using flow cytometric immunophenotyping cannot be used to distinguish MGUS from an overt PCN. The most useful distinguishing feature between MGUS and PCN is the identification of a significant proportion of phenotypically normal plasma cells admixed with the abnormal cells.105 Although nonneoplastic plasma cells can be seen in some instances of overt PCN, they represent a smaller proportion of the total cells; in one study, they represented less than 3% of all plasma cells in the majority of patients.105
Immunophenotypic information of additional prognostic value in plasma cell disorders
In addition to establishing the diagnosis of a PCN, several studies have reported an association between the phenotype of neoplastic plasma cells and prognosis. Lack of CD56 expression in myeloma has been associated with a worse prognosis, expression of CD28 is associated with reduced event-free survival but not overall prognosis, and lack of CD27 and CD45 have been associated with an adverse prognosis.102,106 In addition, although not widely performed, S-phase fraction of neoplastic plasma cells remains one of the strongest independent predictors of survival in myeloma.107
Flow cytometric evaluation of peripheral blood has been proposed as a prognostic marker in myeloma. The number of circulating abnormal plasma cells identified at the time of diagnosis has been shown to have an inverse relationship with overall survival.108 In addition, detection of abnormal plasma cells in the peripheral blood prior to autologous stem cell transplantation has been associated with decreased overall survival and time to disease progression.109 However, the detection of circulating plasma cells requires sensitive techniques similar to those used for the detection of MRD, and the number of circulating cells that is clinically significant probably depends on the procedure used.
Sensitive flow cytometric techniques provide a quick, inexpensive tool for the detection of residual involvement by a PCN following therapy.110 Although not as sensitive as allele-specific oligonucleotide–polymerase chain reaction, flow cytometry techniques have been developed that can reliably detect 0.01% abnormal cells. However, because plasma cell neoplasms usually involve the bone marrow in a relatively patchy fashion, caution should be used in interpreting changes in the number of detectable bone marrow plasma cells following treatment.
Blastic neoplasms
Acute leukemia and lymphoblastic lymphoma can present with blasts or other abnormal cells in the peripheral blood or body fluids, cytopenias related to bone marrow replacement, and infiltration at extramedullary sites. Flow cytometric immunophenotyping can assist in the identification of immature or abnormal cells, their distinction from immature cells normally present in the bone marrow and thymus, and determination of lineage in order to differentiate between ALL and AML, and occasionally can aid in further classification.
Identification and enumeration of blasts
Blasts often differ from more mature cells by expressing markers of immaturity and lacking antigens expressed by more mature cells. For example, myeloblasts can be distinguished from maturing myeloid cells if they display low orthogonal (side) light scatter, markers of immaturity such as CD34 and CD117, and lack markers of maturation such as CD11b, CD15, and CD16. Immature B-lymphoid cells can be distinguished from mature B-lymphoid cells if they express CD34 and TdT, and lack surface immunoglobulin and CD20. Immature T-lymphoid cells can be distinguished from mature T-lymphoid cells if they express CD34, TdT, or CD1a, or lack surface expression of CD3. A plot of CD45 versus orthogonal (side) light scatter is very useful in identifying blasts by their low side light scatter and weak intensity expression of CD45. This representation can help to distinguish blasts from lymphocytes (bright CD45), erythroid precursors (essentially negative CD45), neutrophilic precursors and eosinophils (higher side light scatter), and monocytes (higher side light scatter and brighter CD45). However, basophils usually fall within the blast region in this plot due to loss of granules during processing.
Through the analysis of many thousands of cells, flow cytometric studies can determine the precise percentage of the total cells analyzed that demonstrate phenotypic features of immature cells. However, the percentage of immature cells determined by flow cytometry often differs from the blast count determined by manual differential counting performed on aspirate smears. There are several possible explanations for discrepant manual and flow cytometric counts.
Flow cytometric count lower than manual count.
The portion of the bone marrow aspirate specimen submitted for flow cytometric studies often contains fewer bone marrow spicules and greater dilution with peripheral blood than the portion used to prepare smears. Some blasts lack expression of CD34 and CD117 and can be difficult to distinguish from more mature cells. For example, it may be difficult to distinguish CD34− monoblasts from more mature monocytes. For some hematologic neoplasms, the current classification schemes require inclusion of cells other than blasts in the morphologic blast count, such as promonocytes for AML with monocytic differentiation. The manual count may include at least some normal immature precursors (hematogones) in addition to leukemic blasts.
Flow cytometric count higher than manual count.
The denominator used to determine the blast percentage for manual counts is different from that used for flow cytometric counts. For manual counts, blasts are determined as a percentage of all nucleated cells. For flow cytometric studies, the lysis step that is usually performed to remove anucleate red blood cells also removes a varying number of more mature nucleated erythroid precursors. Therefore, flow cytometric counts are determined as a percentage of all cells analyzed or all nonerythroid cells. This difference also affects the use of flow cytometric studies to assess for erythroleukemia, erythroid/myeloid type, where the criteria require greater than 50% erythroid precursors in the entire nucleated cell population and greater than 20% myeloblasts in the nonerythroid cell population. The use of other cell-separation procedures, such as Ficoll Hypaque separation, leads to further alteration in the proportion of cells, and is strongly discouraged. Also: blasts may be difficult to recognize by manual counting or may be disrupted during smear preparation; CD117 can be expressed on some maturing myeloid cells and mast cells, in addition to blasts; and hypogranular mature myeloid cells may have decreased orthogonal (side) light scatter and fall in the “blast region” in the CD45 versus side scatter plot.
Despite inaccuracies inherent in manual differential counting, this method is still used in the current classification schemes and therefore remains the gold standard for determining blast percentage. However, flow cytometric analysis can certainly assist in identifying immature or neoplastic cells that might otherwise be overlooked because of atypical morphologic features or less than optimal smears. Therefore, it is prudent to perform both flow cytometric and morphologic blast enumeration and investigate significant discrepancies.
Identification of abnormal blasts
Neoplastic blasts often have an abnormal phenotype that permits their distinction from normal immature cells. Phenotypic abnormalities include expression of markers not normally present on cells of that lineage, such as myeloid markers on lymphoblasts or lymphoid markers on myeloblasts, and deviations from the well-coordinated gain and loss of antigens seen with normal maturation. Normal immature B-lineage cells in the bone marrow (hematogones) demonstrate organized maturation from B-lineage–committed progenitor cells through to cells with a mature surface immunoglobulin–positive phenotype.111 Although ALL may closely resemble one stage of B-cell maturation, such as precursor B cells, the phenotype usually contains some unexpected features for that stage of maturation, such as surface CD20 expression.111 Similar maturation abnormalities have been identified in T-cell ALL and can be used to distinguish neoplastic blasts from normal thymocytes either derived from nonneoplastic thymic tissue or thymoma.112 In addition, phenotypic aberrancies in AML can be used in the distinction of neoplastic blasts from normal myeloid and monocytic blasts.113 However, a subset of nonneoplastic immature myeloid and monocytic cells may demonstrate an unusual phenotype following growth factor stimulation or regeneration, such as low level expression of CD56 and possible CD5.113
Assignment of blast lineage
Reliable distinction between AML and ALL is important for the selection of appropriate therapy. Leukemic blasts often resemble a population of normal cells and therefore can be easily assigned to that lineage. AML usually expresses antigens characteristic of neutrophilic or monocytic differentiation such as CD13, CD15, CD33, CD64, CD117, and myeloperoxidase. In ALL, CD19 has the highest sensitivity and specificity for the detection of B-cell lineage and cytoplasmic CD3 for the detection of T-cell lineage. Cytoplasmic CD22 is also a sensitive and specific B-lineage marker, whereas surface CD22 staining is often weak and, at least with some antibodies, may be seen on other cell types.114 Although CD79a was proposed as a specific marker of B-cell lineage, subsequent studies have demonstrated staining of some cases of precursor T-cell ALL and AML.115
As described, leukemic blasts may aberrantly express antigens from another lineage. Lymphoid antigens that are frequently expressed in AML include CD7, CD56, CD2, and CD19. ALL frequently demonstrates expression of one or more myeloid antigen. Although the detection of 1 or 2 myeloid antigens on lymphoblasts can assist in the identification of the abnormal cells, it does not appear to be of independent prognostic significance in ALL, and should not be used to diagnose biphenotypic leukemia.
Determination of lineage can be difficult if the leukemic blasts express antigens from more than one cell line or demonstrate very few lineage-associated antigens. With the advent of detailed flow cytometric immunophenotyping, approximately 5% of acute leukemias were found to demonstrate lineage heterogeneity, either biphenotypic leukemia (expression of antigens from more than one lineage by a single population of blasts) or bilineal leukemia (2 populations of blasts from different lineages). To assist in standardization, a scoring system was proposed that gave weight to phenotypic findings in the assignment of lineage.116 For example, cytoplasmic expression of CD3 is a strong indicator of T-cell lineage, whereas CD7 is frequently expressed in AML and therefore carries less weight for the assignment of a T-cell lineage. However, this scoring scheme does not include all the markers used in current clinical practice and does not address the weighting of partial expression or intensity of expression of antigens. Therefore, when faced with a blastic malignancy expressing antigens from more than one lineage, the leukemic cells should be thoroughly characterized using a multiparametric approach including morphology, cytochemical staining, flow cytometric immunophenotyping, and cytogenetic analysis. Neoplasms with recurrent genetic abnormalities recognized in the WHO classification are more easily categorized. For the remaining neoplasms, the information should be weighed-up and, where possible, a diagnosis of AML or ALL favored. A similar multiparameter approach can be taken to the characterization of patients expressing very few antigens, in an attempt to minimize the number of patients falling in the acute undifferentiated leukemia category.
There is an ongoing debate about the existence and identification of acute leukemia of NK-cell lineage. Several somewhat overlapping disorders have been described in the literature, including thymic lymphoblastic lymphoma of NK origin, myeloid/NK-cell acute leukemia, blastic NK-cell lymphoma, and CD4+ CD56+ HDN. Some of the difficulties encountered in diagnosis relate to the criteria used to define NK-cell differentiation. Although early studies used CD56 expression as an indicator of NK-cell lineage, CD56 is also found on T cells, some dendritic cells, and some normal myeloid progenitors such as during active marrow regeneration, and approximately 20% to 30% of AMLs with a predilection for those with monocytic differentiation.6,117 In addition, it is becoming recognized that there is a complex dynamic relationship between cells of myeloid, monocytic, and dendritic lineage and their progenitors. Despite these difficulties, it appears that there might be a group of diseases referred to as NK/myeloid leukemia that may have extramedullary involvement and express the following phenotype: CD56+, CD7+, possible CD2+, CD13+, CD33+, myeloperoxidase-negative, and CD3− (surface and cytoplasmic).118
CD4+CD56+ hematodermic tumor (HDT) is a recently recognized malignancy that usually has a blastic appearance and is thought to be derived from plasmacytoid dendritic cells.86,119 It frequently presents in the skin and often has low-level bone marrow and peripheral blood involvement, although a primarily leukemic form has been recognized. HDT usually has the following phenotype: CD4+, CD56+, absence of B- or T-lineage markers, TCR−, and negative to weak cytoplasmic CD3, variable TdT, myeloperoxidase-negative, lysozyme-negative, variable CD33 and CD68, CD123+ (uniform bright intensity), CD43+, HLA-DR+, CD45RA+, TCL-1+, and BDCA-2+. Although HDT has been proposed to represent a distinct entity, the distinction from AML can be difficult, and there may be preceding, concurrent, subsequent, or apparent transformation to myelomonocytic leukemia.
Role of flow cytometric immunophenotyping in the diagnosis and classification of AML
The identification of recurrent genetic abnormalities has assumed priority in the classification of AML. However, flow cytometric immunophenotypic studies remain of value in its distinction from ALL. In addition, flow cytometric studies are also of value in the identification of megakaryocytic differentiation with expression of CD41, CD61, and pure erythroid leukemia with expression of CD235a (glycophorin A) or CD36 in the absence of CD64, myeloperoxidase, and other myeloid-associated antigens. Although flow cytometric studies can also evaluate for monocytic differentiation, cytochemical stains remain part of the current WHO classification scheme.120 Flow cytometric evaluation for CD14 lacks sensitivity for the detection of monocytic differentiation.120 However, it has been suggested that the sensitivity of the flow cytometric assay can be improved by evaluation of other monocyte-associated antigens such as coexpression of CD36 and CD64 bright, intermediate CD15 plus bright CD33, and different antibodies directed against different CD14 epitopes (Figure 3).121
Acute myeloid leukemia with MLL rearrangement and monocytic differentiation. Although there is minimal staining with CD14, flow cytometric studies demonstrate other features associated with monocytic differentiation and a butyrate esterase cytochemical stain is positive. (A) Bone marrow aspirate smear demonstrating abnormal cells with moderately abundant cytoplasm, a few cytoplasmic granules, and some irregularity in nuclear outlines. Wright Giemsa stain, magnification ×100. (B) Representative flow cytometric dot plots: CD45 versus side scatter demonstrates a small population of lymphoid cells indicated in red, and a population of interest highlighted in green with weak intensity CD45 and variable side (orthogonal) light scatter; CD14 versus CD13+33 demonstrates staining for the myeloid antigens CD13 and/or CD33 and minimal staining for CD14; CD13+33 versus CD34 demonstrates absence of staining for CD34; CD15 versus CD33 demonstrates relatively bright staining for CD33 and variable intensity staining for CD15; CD36 versus CD64 demonstrates staining for both CD36 and CD64; and CD13+33 versus CD56 demonstrates partial aberrant expression of CD56. (C) Butyrate esterase cytochemical stain demonstrating many positive cells; magnification ×100. Staining was inhibited with fluoride incubation (not shown). (D) Classical cytogenetic studies demonstrating 47,XX,+8,t(11,19)(q23;p13.3). Courtesy of the Pittsburgh Cytogenetics Laboratory, Magee-Womens Hospital, Pittsburgh, PA. (E) FISH studies demonstrating an MLL gene rearrangement. Hybridization with the LSI MLL dual color DNA probe demonstrates one cell (lower left) with one fusion signal (corresponding to the unrearranged chromosome 11 at band 11q23) and separate green and red signals corresponding to the split MLL gene, and one normal cell (top right) with 2 fusion signals. Courtesy of the Pittsburgh Cytogenetics Laboratory, Magee-Womens Hospital, Pittsburgh, PA. Images were acquired as in Figure 1.
Acute myeloid leukemia with MLL rearrangement and monocytic differentiation. Although there is minimal staining with CD14, flow cytometric studies demonstrate other features associated with monocytic differentiation and a butyrate esterase cytochemical stain is positive. (A) Bone marrow aspirate smear demonstrating abnormal cells with moderately abundant cytoplasm, a few cytoplasmic granules, and some irregularity in nuclear outlines. Wright Giemsa stain, magnification ×100. (B) Representative flow cytometric dot plots: CD45 versus side scatter demonstrates a small population of lymphoid cells indicated in red, and a population of interest highlighted in green with weak intensity CD45 and variable side (orthogonal) light scatter; CD14 versus CD13+33 demonstrates staining for the myeloid antigens CD13 and/or CD33 and minimal staining for CD14; CD13+33 versus CD34 demonstrates absence of staining for CD34; CD15 versus CD33 demonstrates relatively bright staining for CD33 and variable intensity staining for CD15; CD36 versus CD64 demonstrates staining for both CD36 and CD64; and CD13+33 versus CD56 demonstrates partial aberrant expression of CD56. (C) Butyrate esterase cytochemical stain demonstrating many positive cells; magnification ×100. Staining was inhibited with fluoride incubation (not shown). (D) Classical cytogenetic studies demonstrating 47,XX,+8,t(11,19)(q23;p13.3). Courtesy of the Pittsburgh Cytogenetics Laboratory, Magee-Womens Hospital, Pittsburgh, PA. (E) FISH studies demonstrating an MLL gene rearrangement. Hybridization with the LSI MLL dual color DNA probe demonstrates one cell (lower left) with one fusion signal (corresponding to the unrearranged chromosome 11 at band 11q23) and separate green and red signals corresponding to the split MLL gene, and one normal cell (top right) with 2 fusion signals. Courtesy of the Pittsburgh Cytogenetics Laboratory, Magee-Womens Hospital, Pittsburgh, PA. Images were acquired as in Figure 1.
Some phenotypes in AML are associated with the presence of recurrent genotypic abnormalities. For example, AML with t(8;21)(q22;q22) is associated with aberrant expression of CD19, CD56, and sometimes TdT. Acute promyelocytic leukemia with t(15;17)(q22;q12) often has the following phenotype: CD34− or only partially positive, HLA-DR− or only partially positive, CD11b−, CD13 heterogeneous, CD117+, CD33+ (homogeneous bright staining), and CD15− or weak intensity staining. Recently, a similar CD34−, HLA-DR− phenotype has been described in a subset of AML with cup-shaped nuclear invaginations, normal cytogenetics, and an apparent association with FLT-3 gene internal tandem duplication.122 Additional phenotypic characteristics of these patients include frequent aberrant expression of CD56 and uniform expression of CD123 without CD133.122 Therefore, although flow cytometric immunophenotypic studies may be used as a screening tool, they lack specificity and sensitivity for the detection of genotypic abnormalities.123
Flow cytometric immunophenotyping of AML is also of value in patients being considered for gemtuzumab ozogamicin therapy by demonstrating expression of the target antigen CD33.8
Role of flow cytometric immunophenotyping in the diagnosis and classification of ALL
Flow cytometric immunophenotyping is important for the distinction between ALL and AML, identification of B-cell or T-cell lineage, and assessing response to treatment, including the identification of early responders and the detection of MRD.114 Some phenotypes in ALL are associated with the presence of prognostically significant cytogenetic and molecular abnormalities. For example, B-cell ALL with a CD9+, CD10+, CD19+, CD20− or only partial, CD34− phenotype is a sensitive marker for t(1;19)(q23;p13), but lacks specificity.124 B-cell ALL with a CD10−, CD15+, CD24− or partial phenotype is associated with t(4;11)(q21;q23).114 However, flow cytometric immunophenotyping does not provide a suitable surrogate tool for detection of these subtypes of ALL.
Maturing myeloid and monocytic neoplasms
The myelodysplastic syndromes (MDSs) and chronic myeloproliferative disorders (CMPDs) are a diverse group of neoplasms composed of maturing hematopoietic precursors. MDSs often present with symptoms and signs related to anemia, thrombocytopenia, and neutropenia. Patients with CMPD usually have elevation of peripheral blood counts for at least one cell line and may present with splenomegaly and signs of defective cell function such as thrombosis or bleeding. The diagnosis of maturing myeloid neoplasms involves enumeration of blasts for exclusion of AML, to provide additional prognostic information, and for further classification. As described previously for blastic malignancies, flow cytometric immunophenotyping can assist in the enumeration of blasts, but for a number of reasons has not replaced morphologic blast counts. However, flow cytometric immunophenotyping is becoming increasingly recognized as a tool for the distinction between maturing myeloid neoplasms and reactive disorders through the identification of phenotypic abnormalities.
The study of Stetler-Stevenson and colleagues published in 2001 was the first to demonstrate that the identification of phenotypic abnormalities by flow cytometry is useful in establishing a diagnosis of a MDS when the results of morphologic evaluation and cytogenetic studies are indeterminate.125 This study evaluated granulocytic, erythroid, and megakaryocytic cells for immunophenotypic evidence of dysplasia. Although flow cytometry was documented to be more sensitive than morphology for the detection of abnormalities of granulocytic cells, morphology appeared to be more sensitive at picking up dysplasia in erythroid and megakaryocytic lines.125 These observations may reflect the presence of only a few antigens that can be evaluated for erythroid and megakaryocytic cells and the relative paucity of megakaryocytes. Subsequent studies, as outlined in the section below (“Identification of abnormal maturing myeloid and monocytic cells”), have focused on evaluation of neutrophilic and monocytic lineages. Table 6 outlines the normal pattern of staining and clinical utility of reagents recommended by the 2006 Bethesda consensus group for the evaluation of myeloid and monocytic cells.
Reagents of clinical utility in the evaluation of maturing myeloid and monocytic neoplasms
| Reagent . | Normal distribution . | Clinical utility in myeloid and monocytic neoplasms . | Comments . |
|---|---|---|---|
| CD11b | Maturing neutrophilic and monocytic cells, some lymphoid cells. | May be aberrantly expressed in AML, MDS, and MPD. | — |
| CD13 | Neutrophilic and monocytic cells. | Indicator of neutrophilic and monocytic lineage in acute leukemia. May be aberrantly expressed in AML, MDS, and MPD. | |
| CD14 | Monocytes. | Indicator of monocytic differentiation. | Not a sensitive marker of immature monocytes. |
| CD15 | Maturing neutrophilic cells and monocytes. | May be aberrantly expressed in AML, MDS, and MPD. | — |
| CD16 | Maturing neutrophilic cells, monocytes and NK cells. | May be aberrantly expressed in AML, MDS, and MPD. | — |
| CD33 | Neutrophilic and monocytic cells. | May be aberrantly expressed in AML, MDS, and MPD. | Some normal variability in intensity of expression. |
| CD34 | B-cell and T-cell precursors and myeloblasts. | Identification and enumeration of blasts. | Not all blasts are CD34+. |
| CD45 | All B cells (weaker intensity on precursors and plasma cells), all T cells (weaker intensity on precursors). | Identification of blasts (CD45 gating often with low orthogonal (side) light scatter). | — |
| CD56 | NK cells and NK-like T cells. | May be aberrantly expressed in AML, MDS, and MPD. | Low level of expression on regenerating normal neutrophilic and monocytic cells and with growth factor stimulation. |
| CD117 | Immature neutrophilic cells and mast cells. | Identification myeloblasts and mast cells. | May be present in myeloma and some T-cell neoplasms. |
| HLA-DR | Myeloblasts, monocytes, all B cells, activated T cells. | Identification of promyelocytes, such as in APL. May be aberrantly expressed in AML, MDS, and MPD. | Non-APL AML may also be negative. |
| CD2* | T cells, NK cells. | May be aberrantly expressed in AML; some association with inv16. May be aberrantly expressed in systemic mastocytosis. | — |
| CD4* | T-cell subset, monocytic. | Often positive in AML, particularly with monocytic differentiation. | Also mature T-cell neoplasms and HDN. |
| CD7* | T cells and NK cells. | May be aberrantly expressed in AML, MDS, and MPD. | |
| CD25* | Activated B cells and T cells. | May be aberrantly expressed in systemic mastocytosis. | Reported association with BCR/ABL+ ALL. |
| CD36* | Monocytes, erythroid cells, megakaryocytes and platelets. | When used in combination with CD64 is a more sensitive marker of monocytic differentiation than CD14. | — |
| CD38* | Precursor B cells (hematogones), normal follicle center B cells, immature and activated T cells, plasma cells (bright intensity), myeloid and monocytic cells, and erythroid precursors. | Identification of early bone marrow progenitor cell populations for further evaluation of phenotypic abnormalities. | — |
| CD41* | Megakaryocytes and platelets. | Megakaryocytic differentiation. | May detect nonspecific binding of platelet proteins to other cells such as monocytes. |
| CD61* | Megakaryocytes and platelets. | Megakaryocytic differentiation. | May detect nonspecific adherence of platelet proteins to other cells such as monocytes. Sometimes combined with CD42b to distinguish platelets from blasts. |
| CD61, cytoplasmic* | Megakaryocytes and platelets. | Megakaryocytic differentiation. | May demonstrate fewer problems with adherence of platelet proteins. |
| CD64* | Monocytes and intermediate neutrophilic precursors. | Identification of monocytic differentiation. May be aberrantly expressed in AML, MDS, and MPD. | Gained on mature neutrophils with sepsis. |
| CD71* | Erythroid precursors (bright), myeloid, activated lymphoid, proliferating cells. | Identification of immature erythroid cells. Possibly expressed in MDS. | — |
| cMPO* | Neutrophilic and monocytic cells. | Indicator of myeloid differentiation. | In contrast to cytochemical stain, measures the presence of antigen, not enzyme activity. |
| CD117* | Immature neutrophilic cells and mast cells. | Identification myeloblasts. | May be expressed by cells more mature cells than blasts. |
| CD123* | Monocytes, neutrophils, basophils, megakaryocytes, and plasma cytoid dendritic cells (bright). | Identification HDN. Positive some AML, especially with monocytic differentiation. | Plasmacytoid dendritic cells may be increased in some reactive conditions such as Castleman disease and Kikuchi lymphadenitis and in association with MPD. |
| CD163* | Monocyte, macrophage. | Indicator of monocytic differentiation. | — |
| CD235a* | Erythroid precursors. | Indicator of erythroid maturation. | Not present on some immature erythroid precursors. |
| Reagent . | Normal distribution . | Clinical utility in myeloid and monocytic neoplasms . | Comments . |
|---|---|---|---|
| CD11b | Maturing neutrophilic and monocytic cells, some lymphoid cells. | May be aberrantly expressed in AML, MDS, and MPD. | — |
| CD13 | Neutrophilic and monocytic cells. | Indicator of neutrophilic and monocytic lineage in acute leukemia. May be aberrantly expressed in AML, MDS, and MPD. | |
| CD14 | Monocytes. | Indicator of monocytic differentiation. | Not a sensitive marker of immature monocytes. |
| CD15 | Maturing neutrophilic cells and monocytes. | May be aberrantly expressed in AML, MDS, and MPD. | — |
| CD16 | Maturing neutrophilic cells, monocytes and NK cells. | May be aberrantly expressed in AML, MDS, and MPD. | — |
| CD33 | Neutrophilic and monocytic cells. | May be aberrantly expressed in AML, MDS, and MPD. | Some normal variability in intensity of expression. |
| CD34 | B-cell and T-cell precursors and myeloblasts. | Identification and enumeration of blasts. | Not all blasts are CD34+. |
| CD45 | All B cells (weaker intensity on precursors and plasma cells), all T cells (weaker intensity on precursors). | Identification of blasts (CD45 gating often with low orthogonal (side) light scatter). | — |
| CD56 | NK cells and NK-like T cells. | May be aberrantly expressed in AML, MDS, and MPD. | Low level of expression on regenerating normal neutrophilic and monocytic cells and with growth factor stimulation. |
| CD117 | Immature neutrophilic cells and mast cells. | Identification myeloblasts and mast cells. | May be present in myeloma and some T-cell neoplasms. |
| HLA-DR | Myeloblasts, monocytes, all B cells, activated T cells. | Identification of promyelocytes, such as in APL. May be aberrantly expressed in AML, MDS, and MPD. | Non-APL AML may also be negative. |
| CD2* | T cells, NK cells. | May be aberrantly expressed in AML; some association with inv16. May be aberrantly expressed in systemic mastocytosis. | — |
| CD4* | T-cell subset, monocytic. | Often positive in AML, particularly with monocytic differentiation. | Also mature T-cell neoplasms and HDN. |
| CD7* | T cells and NK cells. | May be aberrantly expressed in AML, MDS, and MPD. | |
| CD25* | Activated B cells and T cells. | May be aberrantly expressed in systemic mastocytosis. | Reported association with BCR/ABL+ ALL. |
| CD36* | Monocytes, erythroid cells, megakaryocytes and platelets. | When used in combination with CD64 is a more sensitive marker of monocytic differentiation than CD14. | — |
| CD38* | Precursor B cells (hematogones), normal follicle center B cells, immature and activated T cells, plasma cells (bright intensity), myeloid and monocytic cells, and erythroid precursors. | Identification of early bone marrow progenitor cell populations for further evaluation of phenotypic abnormalities. | — |
| CD41* | Megakaryocytes and platelets. | Megakaryocytic differentiation. | May detect nonspecific binding of platelet proteins to other cells such as monocytes. |
| CD61* | Megakaryocytes and platelets. | Megakaryocytic differentiation. | May detect nonspecific adherence of platelet proteins to other cells such as monocytes. Sometimes combined with CD42b to distinguish platelets from blasts. |
| CD61, cytoplasmic* | Megakaryocytes and platelets. | Megakaryocytic differentiation. | May demonstrate fewer problems with adherence of platelet proteins. |
| CD64* | Monocytes and intermediate neutrophilic precursors. | Identification of monocytic differentiation. May be aberrantly expressed in AML, MDS, and MPD. | Gained on mature neutrophils with sepsis. |
| CD71* | Erythroid precursors (bright), myeloid, activated lymphoid, proliferating cells. | Identification of immature erythroid cells. Possibly expressed in MDS. | — |
| cMPO* | Neutrophilic and monocytic cells. | Indicator of myeloid differentiation. | In contrast to cytochemical stain, measures the presence of antigen, not enzyme activity. |
| CD117* | Immature neutrophilic cells and mast cells. | Identification myeloblasts. | May be expressed by cells more mature cells than blasts. |
| CD123* | Monocytes, neutrophils, basophils, megakaryocytes, and plasma cytoid dendritic cells (bright). | Identification HDN. Positive some AML, especially with monocytic differentiation. | Plasmacytoid dendritic cells may be increased in some reactive conditions such as Castleman disease and Kikuchi lymphadenitis and in association with MPD. |
| CD163* | Monocyte, macrophage. | Indicator of monocytic differentiation. | — |
| CD235a* | Erythroid precursors. | Indicator of erythroid maturation. | Not present on some immature erythroid precursors. |
Reagents included in this table were recommended in the consensus guidelines.
— indicates no entry.
These reagents may be considered for secondary evaluation after other reagents listed have been used in the initial evaluation.
Identification of abnormal maturing myeloid and monocytic cells
Abnormal maturing myeloid and monocytic cells can be recognized by aberrant expression of antigens.113,117,125,126 Reported aberrancies include the presence of antigens that are not normally present, such as lymphoid antigens, and altered expression of myeloid or monocytic antigens, either in a single population of cells, or within a generation of maturing cells. In addition, decreased orthogonal (side) light scatter has been used as an indicator of abnormal hypogranularity of maturing neutrophilic cells.
Aberrant expression of lymphoid antigen can be seen on neoplastic myeloid or monocytic cells at any or all stages of maturation. As for AML, CD7 is one of the most frequently expressed lymphoid antigen in MDS.113 Although CD56 expression has been described on neoplastic monocytes and myeloid cells, it can also be expressed at a low level during marrow regeneration and following growth factor stimulation.113 Other lymphoid antigens that are expressed less frequently on neoplastic myeloid and monocytic cells include CD2, CD5, and CD19.113
Neoplastic myeloid and monocytic cells may also demonstrate abnormal levels of expression of antigens normally expected on those lineages. Reported abnormalities include increased, decreased, and more homogeneous intensity of staining of antigens, and asynchronous gain and loss of antigens during maturation.113,117 Frequently reported abnormalities of neutrophilic cells in MDS include alterations in intensity of expression of CD64, CD33, CD16, CD14, CD13, CD11b, and CD10.113 Although most of the studies have evaluated bone marrow, the study of Cherian and colleagues demonstrated that flow cytometric evaluation of peripheral blood neutrophils could also assist in the diagnosis of MDS.126 Abnormalities of neoplastic monocytes include altered intensity of HLA-DR, CD36, CD33, CD15, CD14, CD13, and CD11b.113 However, it is important to recognize that the level of expression of many of these myeloid and monocytic antigens varies with the stage of maturation of the cells. For example, although low-intensity expression of CD13 is abnormal for myeloblasts, it is characteristic of myelocytes and metamyelocytes. Therefore, for the evaluation of both myeloid and monocytic cells, it is important to consider the stages of maturation that are represented in the specimen and assess for the expected maturation pattern with synchronous gain and loss of antigens.
For neutrophilic cells, an altered maturation pattern is best demonstrated by plotting CD13 versus CD16.113,125 During maturation, neutrophilic cells normally acquire increasing levels of CD16 that are initially accompanied by a decrease in CD13 expression, as cells mature from blasts through the myelocyte and metamyelocyte stages of maturation, followed by intermediate levels of CD13 in band forms and high levels in segmented neutrophils. Many of the maturation abnormalities identified by flow cytometry in MDS relate to altered expression of CD16.113 Although a similar evaluation can also be performed for CD11b versus CD16, abnormalities in CD11b expression appear to be less frequent in MDS.
The identified phenotypic aberrancies appear to carry differing weight toward the distinction between maturing myeloid and monocytic malignancy and nonneoplastic disorders. Therefore, some studies have used a scoring scheme to quantitate the number and severity of the abnormalities identified.117,126-128 In general, expression of nonmyeloid antigens by a significant proportion of myeloid or monocytic cells carries more weight than altered expression of myeloid or monocytic antigens, and the presence of multiple abnormalities in myeloid or monocytic antigen expression has a higher predictive value for MDS than single abnormalities. Using this type of approach to evaluate bone marrows from patients with peripheral blood cytopenias, flow cytometric immunophenotyping has been reported to have a sensitivity of 89% and specificity of 88% for the diagnosis of MDS.117 In the evaluation for chronic myelomonocytic leukemia (CMML) it has been reported that the identification of CD56 expression on monocytes plus underexpression of 2 myeloid-associated antigens shows high specificity for the diagnosis of CMML although it lacks sensitivity.129
Although identification of these abnormalities can assist in the distinction between normal and neoplastic cells, detection and interpretation of altered expression of myeloid antigens is often made difficult by the following confounding factors: (1) lack of adequate daily standardization of flow cytometric instruments and procedures, making it difficult to compare results with historic controls; (2) contamination of the population being evaluated with cells from another lineage (eg, eosinophils or monocytes in a region being used to evaluate neutrophilic antigen expression, or basophils contaminating a blast region); (3) difficulty in defining the permitted normal range of antigen expression for each cell type or generation of cells, and determining what constitutes a clinically significant abnormality; (4) difficulty in distinguishing deviations in phenotype from variations related to the relative proportions of bone marrow hematopoietic precursors, such as seen with a “left shift,” maturation arrest, or dilution of bone marrow specimens with peripheral blood (eg, an incomplete granulocyte maturation pattern might represent either a relative lack of segmented neutrophils or the presence of neutrophils with an abnormal phenotype); (5) sensitivity of some myeloid antigens to anticoagulation, temperature changes, and delays in specimen processing130 ; (6) normal variation between individuals in the intensity of expression of some antigens such as CD33; and (7) alterations in antigen expression in nonneoplastic disorders such as following growth factor stimulation or with marrow regeneration (eg, CD56 expression is seen on a subset of granulocytes and monocytes during active marrow regeneration and increased neutrophil HLA-DR and CD64 expression can be seen with cell activation).
These difficulties have delayed the widespread adoption of flow cytometric immunophenotyping for the identification of maturing myeloid neoplasms. The following strategies have been proposed to address some of these issues: (1) adoption of rigorous quality control and assurance programs to improve standardization; (2) acquisition of at least 100 000 cells to facilitate recognition of small populations of cells; (3) simultaneous evaluation of multiple antibodies to assist in identification and isolation of cell populations, and to enhance the evaluation of antigen acquisition and loss during maturation; (4) use of population analysis strategies to identify, isolate, and critically evaluate discrete populations of cells; (5) comparison of antigen intensity between cell populations to overcome individual variation, such as use of CD33 expression on monocytes as an internal control for comparison with staining of blasts and neutrophils113 ; (6) familiarity with the changes that can be seen during bone marrow regeneration, growth factor stimulation, and with delay in specimen delivery; and (7) establishment of a scoring system for different phenotypic abnormalities to indicate the relative weight that should be applied toward establishing a diagnosis of a maturing myeloid and monocytic neoplasm.
Ongoing advances in flow cytometric instrumentation such as faster acquisition, decreased carry-over, increased sensitivity, simultaneous detection of more fluorochromes, and software enhancements allowing improved population analysis will undoubtedly permit the more widespread adoption of flow cytometry as a tool for the diagnosis of maturing myeloid malignancies.
Role of flow cytometric immunophenotyping in the diagnosis and classification of MDSs
Assessment of bone marrow smears for dyspoietic changes remains the gold standard for the diagnosis of MDS. However, similar morphologic features can be seen with nutritional deficiency (vitamin B12 or folate), infection, toxic exposure, medication, and some immune mediated phenomenon. Although identification of clonal abnormalities using conventional cytogenetic or fluorescence in situ hybridization studies can assist in reaching a definitive diagnosis, many patients lack an identifiable clonal abnormality. Flow cytometric immunophenotyping may assist in identification of MDS through the identification of abnormal maturing myeloid cells. In the recent report from the 2006 Working Conference on MDS, an abnormal phenotype determined by flow cytometry has been included in the minimal diagnostic criteria in MDS that can be used to establish a definitive diagnosis in the absence of significance morphologic dysplasia or increased blasts.131
Following a definitive diagnosis, MDS is divided into subtypes that relate to survival and incidence of evolution to acute leukemia. The WHO morphologic classification is based on the percentage of blasts (less than 5%, 5%-9%, and 10%-19%), presence of multilineage dysplasia (affecting greater than 10% of cells in 2 or more myeloid lineages), and ringed sideroblasts affecting greater than 15% of erythroid precursors. Although flow cytometric immunophenotyping is often used to confirm the morphologic blast count, it has not taken on a primary role in the classification of MDS. However, some phenotypic abnormalities have been reported to correlate with the International Prognostic Scoring System (IPSS) score,128 and an association between the number of phenotypic abnormalities expressed as a score and prognosis following bone marrow transplantation for MDS has been reported.127
Role of flow cytometric immunophenotyping in the diagnosis and classification of CMPDs
Flow cytometric immunophenotyping has an even less well-defined role in the diagnosis of CMPD. Abnormalities in myeloid antigen expression have been described in CMPD, particularly chronic idiopathic myelofibrosis, and may assist in the distinction from reactive hematopoietic proliferations. In addition, the enumeration of blasts remains important in the distinction from acute leukemia and an accelerated phase of CMPD. However, genotypic studies have received greater attention in the identification and classification of CMPD, including the Philadelphia chromosome and BCR/ABL translocation for the diagnosis of chronic myeloid leukemia (CML), the JAK2 V617F mutation for the identification of a significant proportion of non-CML CMPD, and PDGFR-α and -β in the identification of subsets of myeloid neoplasms associated with eosinophilia.
Mast cell neoplasms
Mast cell neoplasms are uncommon disorders that may be limited to the skin (cutaneous mastocytosis) or involve one or more extracutaneous organs (systemic mastocytosis). Symptoms relate to tissue infiltration, with or without the release of biochemical mediators such as histamine, and bone marrow infiltration with secondary cytopenias. In the last 10 years there have been significant advances in the characterization of normal mast cells and mast cell neoplasms, including the recognition of frequent phenotypic aberrancies. This understanding has led to changes in the criteria used for diagnosis and classification, as outlined in the WHO classification scheme.
Identification of abnormal mast cells
Mast cells are recognized by their unique phenotype: CD117+ (strong intensity), CD34−, FcEr1+, CD45+, CD38−, CD138−, CD33+, CD13+/−, CD15−, CD16−, CD11c+, CD11b+/−, CD71+, CD25−, and CD2−.132 Normally, mast cells account for less than 0.05% of bone marrow cells, and even in systemic mastocytosis usually account for less than 2% of aspirated cells. Therefore, flow cytometric analysis for mast cells usually requires strategies similar to those used for the detection of MRD, including acquisition of many thousands of events. Neoplastic mast cells usually demonstrate an abnormal phenotype with aberrant expression of markers not normally expressed by mast cells, such as CD25 and CD2, and altered intensity of expression of antigens normally expressed on mast cells, such as increased intensity for CD33, CD11c, CD35, CD59, and CD69 and decreased intensity of CD117.132
Role of flow cytometric immunophenotyping in the diagnosis and classification of mast cell neoplasms
Using the current WHO classification scheme, mast cell neoplasms are distinguished from reactive mastocytosis using major and minor criteria that include morphologic features, such as the presence of multifocal dense aggregates of mast cells and abnormal cytologic appearance, an abnormal immunophenotype with aberrant expression of CD25 and/or CD2, presence of the D816V KIT mutation, and serum tryptase level.3 Morphologic findings form an important part of the diagnostic criteria, and are often supplemented by paraffin section immunohistochemical stains that can identify mast cell aggregates such as tryptase or CD117, and detect the presence of aberrant staining for CD2 and CD25. Flow cytometric immunophenotyping provides a more sensitive method for the detection of phenotypic aberrancy, but often requires application of a procedure that is designed for the detection and evaluation of mast cells. Flow cytometric immunophenotyping can also assist in the detection of another coexisting hematologic malignancy. Systemic mastocytosis is associated with other clonal hematologic non–mast cell lineage disease (SM-AHNMD), such as MDS, CMPD, AML, and lymphoma, in approximately one-third of adult patients.133 The recognition of SM-AHNMD is important because the clinical course is usually determined by the non–mast cell hematologic disorder.
Paroxysmal hemoglobinuria
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare clonal stem cell disorder of hematopoietic cells that is associated with intravascular hemolysis, venous thrombosis, and cytopenias related to bone marrow aplasia. PNH results from acquired somatic mutation of the phosphatidylinositol glycan complementation class A (PIG-A) gene, leading to partial or complete absence of the glycosylphosphatidylinositol (GPI) anchor that is responsible for linking a large number of proteins to the cell membrane, including CD59 (membrane inhibitor of reactive lysis) and CD55 (decay accelerating factor). Flow cytometric immunophenotyping has become the gold standard for the diagnosis and monitoring of PNH. In addition, with the advent of sensitive flow cytometric techniques, populations of cells with a PNH-like phenotype have been recognized in MDS, aplastic anemia, and at a low level in healthy individuals. The significance of PNH-clones in clinical settings other then PNH is still under investigation.
Identification of cells with a PNH-like phenotype
Most flow cytometric assays for PNH are based on the detection of cells deficient in GPI-linked antigens.134 However, because of rare inherited deficiencies of single GPI-linked antigens a diagnosis of PNH usually requires demonstration of at least 2 deficiencies involving 2 cell lines. Therefore, analysis of both red blood cells (RBCs) and white blood cells (WBCs) is recommended.
Flow cytometric analysis of RBCs for PNH most frequently involves evaluation of CD55 and CD59 for normal expression (type 1 cells), partial deficiency (type II cells), and complete deficiency (type III cells). Single colored assays can usually detect deficiencies that affect at least 2% to 3% of RBCs, but may miss smaller PNH-clones seen in MDS and aplastic anemia. WBC analysis for PNH by flow cytometry is performed using multicolor evaluation of peripheral blood neutrophils and monocytes for several GPI-linked antigens. In addition, some assays include evaluation of in vitro binding of a fluorescent-labeled modified toxin (FLAER) that is dependent on the presence of GPI anchors. It is recommended that all assays incorporate a method for adequately separating neutrophils and monocytes, such as CD33 versus side scatter or CD15 versus side scatter, because these cells normally have different levels of antigen expression. The monocyte marker CD14 should not be used for this distinction because it is one of the GPI-anchored antigens. The level of antigen expression may also differ during maturation; therefore, peripheral blood is preferred over bone marrow specimens.
Role of flow cytometric immunophenotyping in the diagnosis and monitoring of PNH
The percentage of cells with a PNH-like phenotype identified using flow cytometric assays correlate to some extent with the clinical manifestations of the disease.134 Patients with greater than 20% type III–deficient RBCs almost always have evidence of hemolysis. Patients with greater than 10% deficient granulocytes have an increased risk of thrombosis, and it has been reported that approximately 44% of patients with greater than 50% deficient granulocytes will develop venous thrombosis in the first 10 years after diagnosis.135 Following diagnosis, serial monitoring of the size of the PNH-clone by flow cytometry may be of value in determining the clinical course of disease and when to initiate treatment or anticoagulation. In addition, flow cytometry has been used to monitor response to eculizumab monoclonal antibody therapy directed against the complement component C5.136 Successful therapy with eculizumab has been associated with increase in the RBC clone to a level that approaches the granulocyte clone, presumably due to prolonged RBC survival.137
Role of flow cytometric immunophenotyping in the detection of MRD
Improvements in flow cytometry instrumentation and software have made it feasible for clinical laboratories to offer assays for the detection of MRD following therapy.138 For many years, flow cytometry laboratories have acquired only 10 000 events with the goal of characterizing populations of cells that predominate. This traditional approach permits reliable detection of populations that represent down to approximately 1% of the acquired events. Over recent years, enhancements in instrumentation have made it feasible for clinical laboratories to consider acquiring enough events for MRD detection and use 4 or more colors to reliably identify populations of phenotypically abnormal cells. Clinical flow cytometric assay have been developed to reliably detect populations representing 0.01% of events (1 cell in 104) and therefore can complete with PCR-based methods. Although in many situations there is concordance between the 2 techniques, flow cytometric methods have the advantage over PCR of discriminating viable and dead cells and directly measuring the proportion of positive cells, rather than using an amplification method. However, several factors need to be taken into consideration in developing a successful flow cytometric assay for the detection of MRD. (1) Number of events—although increasing the number of acquired events improves sensitivity, it is limited by the speed of acquisition. At present, most MRD assays aim to detect 1 cell in 104 through the analysis of 500 000 to 1 million cells and the goal of detecting at least 50 to 100 events of interest. (2) Limit of detection—if no disease is identified, the limit of detection should be determined to take into account the number of cells analyzed, presence of contaminating cells, and background noise. (3) Carryover between tubes should be eliminated though the addition of wash steps. (4) The analysis should be designed to identify phenotypic features characteristic of the disease of interest and facilitate distinction from other cell populations in the specimen. Phenotypes often change over time and with treatment, and therefore the MRD assay should not rely on an exact match between the phenotype of the residual disease and the original diagnostic specimen. Therefore the antibody combinations should be chosen to maximize detection of disease, limit the impact of phenotypic variation, and permit detection of disease following antibody directed therapy.139
Flow cytometric immunophenotyping has an established role in the detection of minimal residual ALL, an emerging role CLL/SLL, and potential role in some other hematopoietic and lymphoid malignancies, such as AML and PCN.
Role of flow cytometric immunophenotyping in the detection of minimal residual ALL
Several studies have demonstrated that MRD detected by flow cytometric immunophenotyping is an independent adverse prognostic factor in pediatric ALL.140 The presence of MRD in bone marrow samples with no morphologic evidence of disease is associated with a greater risk of relapse, and this risk increases with the level of disease detected. Although there is less data for adult ALL, the detection of MRD appears to be an independent risk factor for relapse.141 In theory, this information could assist in identifying high-risk patients who might benefit from additional therapy, or those low-risk patients who could be treated with a less intense regimen with lower toxicity. Although there is currently no data establishing a benefit of such tailored therapy, this is being actively evaluated by the Children's Oncology Group.142
Role of flow cytometric immunophenotyping in the detection of minimal residual AML
Although less well-established than for ALL, flow cytometric evaluation for MRD in AML is becoming more widespread.113 As for ALL, MRD detection in AML involves the identification of phenotypically abnormal populations. Because of frequent changes in phenotype over time and with therapy, it is not recommended to restrict evaluation to detection of abnormalities identified at diagnosis. A further complication is recognition that regenerating marrow may contain populations of cells with an unusual phenotype, such as a low level of CD56 expression on myeloid precursors. However, using multicolor flow cytometric immunophenotyping it is possible to detect residual AML at levels of 0.1% to 0.01%.113 In most studies, the documentation of residual AML has been associated with a poor prognosis.143,144 Again, one of the main challenges is identifying effective therapy for these high-risk patients.
Role of flow cytometric immunophenotyping in the detection of minimal residual CLL/SLL
Traditionally, the goals of treatment in CLL/SLL have been palliation, delayed progression, and decreased disease burden. More recently, combination chemoimmunotherapy have been developed that can achieve MRD− remission, and initial studies have shown longer duration of response or longer survival for patients without detectable MRD.145 Minimal residual CLL/SLL can be detected by PCR for clonal immunoglobulin gene rearrangement and flow cytometric immunophenotyping. A standardized multiparameter flow cytometric procedure has been developed for CLL/SLL MRD that can detect disease at a level similar to conventional PCR (ie, 1 × 10−4 or 0.01%).10 Although quantitative allele-specific oligonucleotide PCR is more sensitive, it requires the generation of patient specific primers, is not widely available, and for disease down to a level of 0.01% is closely correlated with the results of flow cytometric immunophenotyping. Therefore, standardized multiparameter flow cytometric immunophenotyping has been proposed to be the preferred method for the detection of CLL/SLL MRD10,145 (Figure 4).
Flow cytometric detection of minimal residual CLL Detection using the protocol published by Rawstron and colleagues.10 Top row of plots demonstrate preliminary gating on CD19+ B cells with low orthogonal (side) light scatter and refinement of the gate using a plot of forward versus low orthogonal (side) light scatter. Bottom 3 rows of plots demonstrate representative dot-plots from 3 separate tubes evaluating CD5, CD20, and CD38, (second row); CD5, CD22, and CD81 (third row); and CD5, CD43, and CD79b (bottom row). Only the CD19+ gated events are displayed. Regions are placed around events with phenotypic features characteristic of CLL: R5 indicates dim CD20 and CD5+; R6, dim CD20 and dim to moderate intensity CD38 staining; R7, CD5+ and CD22+; R8, CD22+ and dim CD81; R9, CD5+; and CD79bdim, R10, CD5+ and CD43+. Residual CLL is identified by more than 50 events meeting the criteria for 2 or more of the following: R5 and R6 (displayed in second row right dot plot), R7 and R8 (displayed in third row right dot plot), R9 and R10 (displayed in bottom row right dot plot). The flow cytometric data were acquired using a BD FACS Calibur flow cytometer (BD Biosciences) and the dot plots were created using BD CellQuest software v3.3 (BD Biosciences).
Flow cytometric detection of minimal residual CLL Detection using the protocol published by Rawstron and colleagues.10 Top row of plots demonstrate preliminary gating on CD19+ B cells with low orthogonal (side) light scatter and refinement of the gate using a plot of forward versus low orthogonal (side) light scatter. Bottom 3 rows of plots demonstrate representative dot-plots from 3 separate tubes evaluating CD5, CD20, and CD38, (second row); CD5, CD22, and CD81 (third row); and CD5, CD43, and CD79b (bottom row). Only the CD19+ gated events are displayed. Regions are placed around events with phenotypic features characteristic of CLL: R5 indicates dim CD20 and CD5+; R6, dim CD20 and dim to moderate intensity CD38 staining; R7, CD5+ and CD22+; R8, CD22+ and dim CD81; R9, CD5+; and CD79bdim, R10, CD5+ and CD43+. Residual CLL is identified by more than 50 events meeting the criteria for 2 or more of the following: R5 and R6 (displayed in second row right dot plot), R7 and R8 (displayed in third row right dot plot), R9 and R10 (displayed in bottom row right dot plot). The flow cytometric data were acquired using a BD FACS Calibur flow cytometer (BD Biosciences) and the dot plots were created using BD CellQuest software v3.3 (BD Biosciences).
Concluding remarks
In the past 10 years, clinical flow cytometry has evolved from a technique primarily used to characterize large populations of abnormal cells to one that can routinely evaluate small populations of cells for subtle aberrancies in antigen expression. As outlined in this review, these advances have expanded and refined the clinical applications of flow cytometric immunophenotyping. Adoption of these more sophisticated techniques has reinforced the need for optimization of flow cytometric procedures and for interpretation by individuals who are familiar with all aspects of the testing that may affect the quality of the data.146 In addition, it is important that interpreters of flow cytometric data have a thorough knowledge of the phenotypes of diverse normal cell populations, can recognize deviations from normal, and are able to discuss the potential clinical significance of the flow cytometric findings.
Acknowledgments
The authors thank Dr Steven Swerdlow for his support and helpful suggestions and Dr Sara Monaghan for her thorough review of the manuscript and constructive comments.
Authorship
Contribution: Both authors contributed equally to the preparation of this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Fiona E. Craig, UPMC-Presbyterian Hospital, C604, 200 Lothrop St, Pittsburgh, PA 15213; e-mail: craigfe@upmc.edu.

![Figure 1. Mantle cell lymphoma. (A) Histologic section from a submandibular gland biopsy specimen demonstrating an abnormal diffuse infiltrate of small to intermediate-size lymphoid cells. Several mitotic figures are present. Hematoxylin & eosin stain, magnification ×40. (B) Representative flow cytometric dot plots with population of interest highlighted in green: CD19 versus CD5 demonstrates CD5+ B-cell population with weak intensity staining for CD19; FMC-7 versus CD5 demonstrates positivity for FMC-7; CD20 versus kappa and CD20 versus lambda demonstrate moderate intensity staining for CD20 and kappa immunoglobulin light chain restriction. In addition, B cells were CD10− and CD23− (data not shown). The flow cytometric data was acquired using a BD FACS Calibur flow cytometer (BD Biosciences, San Jose, CA) and the dot plots were created using BD FACSDiva software v5.0.2 (BD Biosciences). (C) Cyclin-D1 paraffin section immunohistochemical stain, demonstrating many positive cells with characteristic nuclear staining; magnification ×40. (D) FISH studies demonstrating the IGH/CCND1 [t(11,14)(q13;q32)] rearrangement. Hybridization with the LSI IGH/CCND1-XT dual color, dual fusion DNA probe demonstrates one green signal from the unrearranged chromosome 14q32, one red signal from the unrearranged 11q13, and 3 fusion signals: one from the derivative chromosome 11, one from the derivative chromosome 14, and an extra signal suggesting the presence of an additional copy of all or part of one of the derivative chromosomes involved in the IGH/CCND1rearrangement. Courtesy of the Pittsburgh Cytogenetics Laboratory, Magee-Womens Hospital, Pittsburgh, PA. The images were taken through an Olympus BX40 microscope (Olympus, Tokyo, Japan) and acquired with a SPOT Insight 2 megapixel 3-shot color camera and SPOT Advanced imaging software (Diagnostic Instruments, Sterling Heights, MI).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/8/10.1182_blood-2007-11-120535/6/m_zh80060817010001.jpeg?Expires=1771021750&Signature=izTVRGAaRPmCCHh1eE0zpPD4lLK7GXVKVkY9S86uUgDeIJpmAt7qxqfBN2rouN23VocwEsLtLIXXNNJPGtzPGU9v7RTjtKJJFr9XkycGeQ0DGV0oOBQCGqq7N1lEMYhBCBBFTaN3~o2VMnf~hJ5y2JM72apMXTJPrQcKRA5Nexfp3UVxGfEyXGx0ww7unDnLupqNkElKXold23~WhB75anPPsSzEB3xysZQ75WhoiJb3S7XnoHswvENP-pNMGiJHAukbstRGTktcF1asr57LAt65a8AbZalfcw74fb0ltK8BSPwE0QpGzd20wmLBZZMk~ENg5fpXweVaQtZ9jWv6fA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
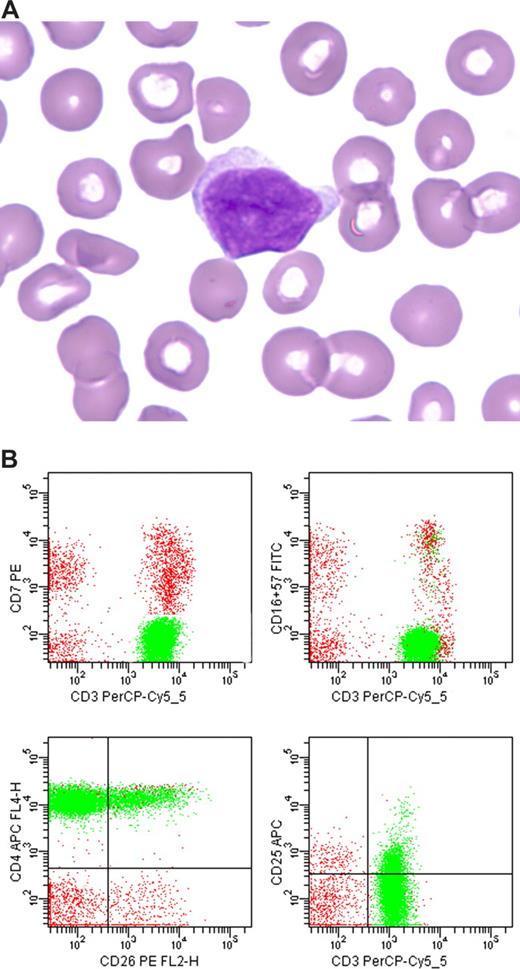
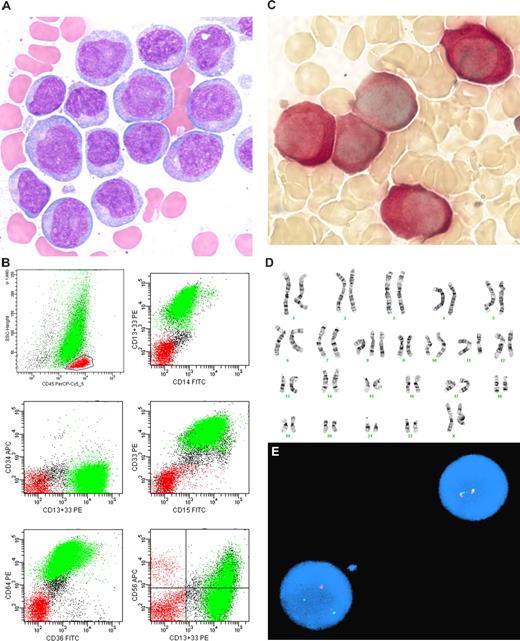
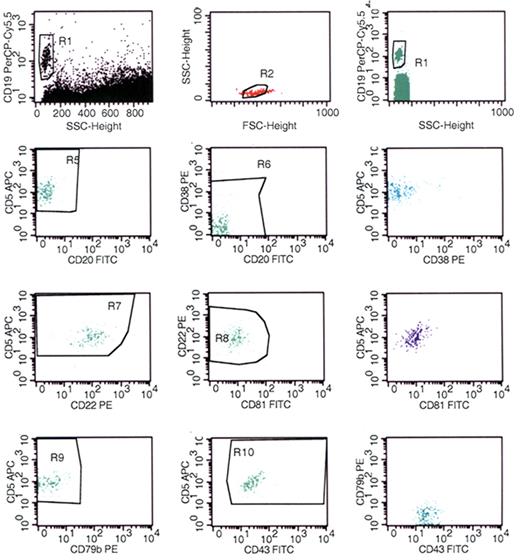
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal