Abstract
Thalidomide, bortezomib, and lenalidomide have recently changed the treatment paradigm of myeloma. In young, newly diagnosed patients, the combination of thalidomide and dexamethasone has been widely used as induction treatment before autologous stem cell transplantation (ASCT). In 2 randomized studies, consolidation or maintenance with low-dose thalidomide has extended both progression-free and overall survival in patients who underwent ASCT at diagnosis. In elderly, newly diagnosed patients, 3 independent randomized studies have reported that the oral combination of melphalan and prednisone plus thalidomide (MPT) is better than the standard melphalan and prednisone (MP). These studies have shown better progression-free survival, and 2 have shown improved overall survival for patients assigned to MPT. In refractory-relapsed disease, combinations including thalidomide with dexamethasone, melphalan, doxorubicin, or cyclophosphamide have been extensively investigated. The risks of side effects are greater when thalidomide is used in combination with other drugs. Thromboembolism and peripheral neuropathy are the major concern. The introduction of anticoagulant prophylaxis has reduced the rate of thromboembolism to less than 10%. Immediate thalidomide dose reduction or discontinuation when paresthesia is complicated by pain or motor deficit has decreased the severity of neuropathy. Future studies will define the most effective or the best sequence of combinations which could improve life expectancy.
Introduction
Thalidomide was initially used in Europe as a sedative, tranquilizer, and antiemetic for emesis gravidarum. It was withdrawn from the market in 1961 because of reports of congenital defects when taken during gestation. More recently, thalidomide has been shown to prevent neoangiogenesis in human malignancies and to exert immunomodulatory and anti-inflammatory properties, although the exact mechanism of action is unclear.1-5 Thalidomide was first used 10 years ago to treat patients with multiple myeloma (MM). This review addresses the role of thalidomide in the treatment of MM and the management of its side effects.
Chemistry, clinical pharmacology, and regulatory affairs
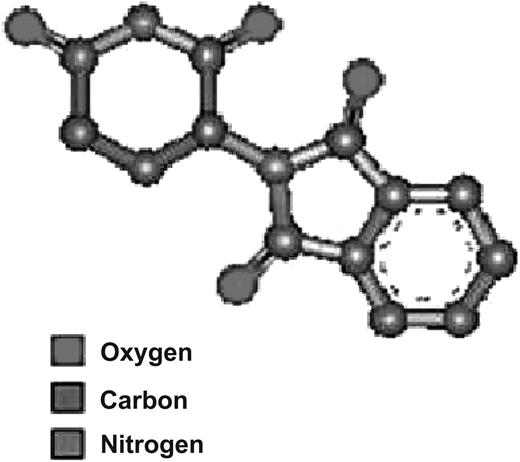
Thalidomide, a synthetic glutamic acid derivative (Figure 1), is poorly soluble in water, and thus no parenteral preparation is available. Thalidomide exhibits linear and dose-dependent pharmacokinetics that are not affected by age, sex, race, food, or smoking status. After a single dose ranging from 50 mg to 400 mg, plasma half-life is roughly 5.5 to 7.3 hours.6
Studies in people have shown little involvement of hepatic enzymes; thalidomide is minimally metabolized by the cytochrome P450 isoenzyme system,7 and interactions with drugs metabolized by this system are unlikely. Products of spontaneous hydrolysis are excreted in the urine. Most of an absorbed oral dose is eliminated through urine and feces.6
Detectable concentrations of thalidomide have been noted in the semen and plasma of 2 of 4 seropositive patients with HIV given thalidomide at 100 mg/day for 8 weeks.8 The presence of thalidomide in semen is important because the risk for birth defects; thalidomide exposure in this context is unknown.
Thalidomide has been licensed for the treatment of relapsed-refractory MM in Australia, New Zealand, Turkey, Israel, and South Korea. The US Food and Drug Administration has approved thalidomide for the acute treatment of the cutaneous manifestation of erythema nodosum leprosum and, in combination with dexamethasone, for treatment of newly diagnosed patients with MM. Thalidomide was designated as an orphan medicinal product by the European Commission in 2001.
Clinical experiences
Single-agent thalidomide
Single-agent thalidomide was used by Singhal and colleagues almost 10 years ago.9 This crucial study showed the future development of the drug, since it first established the in vivo efficacy of thalidomide against myeloma. In relapsed patients, thalidomide monotherapy showed partial response (PR) rates of 25% to 30%. Most responses occurred within 2 months, and the median response duration was 12 months. The 2-year event-free (EFS) and overall survival (OS) were 20% and 48%, respectively.9-11 A systematic review of phase 2 trials identified 42 trials investigating 1674 patients. The PR rate was 29.4%. Minor response rate was 13.8%, and stable disease rate was 11%.12 Patients with extramedullary involvement had a lower response rate than did those without extramedullary disease (0% vs 59%; P < .001).13
In a prospective randomized study,14 400 patients with relapsed-refractory MM received thalidomide at a dose of 100 mg/day or 400 mg/day, and no difference in 1-year OS was recorded (73% vs 69%, respectively). The 2 subgroups who received thalidomide at 100 mg/day or 400 mg/day in combination with dexamethasone also showed identical response rates. The 100 mg/day regimen was better tolerated and somnolence, constipation, and peripheral neuropathy were significantly reduced. The rate of deep-vein thrombosis (DVT) was much the same in both groups.14 These results accord with the use of lower doses of thalidomide in combination with dexamethasone.
Thalidomide and dexamethasone
Advanced multiple myeloma
In various studies,17-19 the thalidomide-dexamethasone (TD) combination (Tables 1,2) induced PR rates ranging from 41% to 65%; the median time to response was lowered to 1 month or less. Hematologic side effects were uncommon; thus, patients with advanced disease and compromised bone marrow function could be treated. Constipation, somnolence, and neuropathy were the most frequent adverse effects. Neurologic side effects were the main reason for thalidomide dose reduction. Thromboembolic events have been reported in 2% to 7% of patients who did not receive any anticoagulant prophylaxis, but dexamethasone was mainly given at low doses (40 mg/day, 4 days/month; Table 3).17-19 In a case-control analysis,17 the efficacy of TD was compared with that of a control group who were given conventional chemotherapy. Results showed that TD was better than chemotherapy; median progression-free survival (PFS) was 17 months in the TD group and 11 months in the control group (P = .002). Three-year OS was 60% after TD and 26% after chemotherapy (P = .001).17 A randomized study20 confirmed the efficacy of the TD regimen compared with high-dose dexamethasone. TD induced higher PR rates (65% vs 28%; P < .001) and longer 1-year PFS (47% vs 31%; P = .009).
Thalidomide in relapsed-refractory patients with MM
| Regimen . | No. patients . | CR, % . | Greater than PR, % . | PFS/EFS/TTP, % . | OS, % . | Reference . |
|---|---|---|---|---|---|---|
| Thalidomide alone | ||||||
| T | 84 | 2 | 25 | 50 at 3 mo | 58 at 12 mo | 9 |
| T | 169 | 14* | 30 | 20 at 24 mo | 48 at 24 mo | 10 |
| T | 400 | ND | ND | ND | 69-73 at 12 mo | 14 |
| T | 1674 | ND | 29 | 50 at 12 mo | 50 at 14 mo | 12 |
| Dexamethasone | ||||||
| TD | 62 | ND | 56 | 50 at 17 mo | 60 at 36 mo | 17 |
| TD | 58 | ND | 46 | 50 at 11 mo | 50 at 19 mo | 17 |
| TD | 77 | ND | 41 | 50 at 12 mo | ND | 18 |
| TD | 44 | ND | 55 | 50 at 4 mo | 50 at 13 mo | 19 |
| TD | 58 | ND | 65 | 47 at 12 mo | ND | 20† |
| Melphalan | ||||||
| TM | 27 | ND | 59 | 61 at 24 mo | 61 at 24 mo | 21 |
| MivPT | 24 | ND | 41 | 50 at 9 mo | 50 at 14 mo | 22 |
| MTD | 21 | 5 | 18‡ | 50 at 9 mo | 50 at 13 mo | 23 |
| Doxorubicin | ||||||
| TAD | 50 | 26 | 76 | 50 at 17 mo | ND | 24 |
| DVd-T | 49 | 20 | 75 | 50 at 16 mo | 50 at 40 mo | 25 |
| Cyclophosphamide | ||||||
| CDT | 52 | 17 | 79 | 34 at 24 mo | 73 at 24 mo | 26 |
| Pulsed CTD | 53 | 5 | 60 | 50 at 8 mo | 50 at 18 mo | 27 |
| ThaCyDex | 71 | 2 | 55 | 57 at 24 mo | 66 at 24 mo | 28 |
| HyperCTD | 60 | 4 | 72 | 50 at 11 mo | 50 at 19 mo | 29 |
| Regimen . | No. patients . | CR, % . | Greater than PR, % . | PFS/EFS/TTP, % . | OS, % . | Reference . |
|---|---|---|---|---|---|---|
| Thalidomide alone | ||||||
| T | 84 | 2 | 25 | 50 at 3 mo | 58 at 12 mo | 9 |
| T | 169 | 14* | 30 | 20 at 24 mo | 48 at 24 mo | 10 |
| T | 400 | ND | ND | ND | 69-73 at 12 mo | 14 |
| T | 1674 | ND | 29 | 50 at 12 mo | 50 at 14 mo | 12 |
| Dexamethasone | ||||||
| TD | 62 | ND | 56 | 50 at 17 mo | 60 at 36 mo | 17 |
| TD | 58 | ND | 46 | 50 at 11 mo | 50 at 19 mo | 17 |
| TD | 77 | ND | 41 | 50 at 12 mo | ND | 18 |
| TD | 44 | ND | 55 | 50 at 4 mo | 50 at 13 mo | 19 |
| TD | 58 | ND | 65 | 47 at 12 mo | ND | 20† |
| Melphalan | ||||||
| TM | 27 | ND | 59 | 61 at 24 mo | 61 at 24 mo | 21 |
| MivPT | 24 | ND | 41 | 50 at 9 mo | 50 at 14 mo | 22 |
| MTD | 21 | 5 | 18‡ | 50 at 9 mo | 50 at 13 mo | 23 |
| Doxorubicin | ||||||
| TAD | 50 | 26 | 76 | 50 at 17 mo | ND | 24 |
| DVd-T | 49 | 20 | 75 | 50 at 16 mo | 50 at 40 mo | 25 |
| Cyclophosphamide | ||||||
| CDT | 52 | 17 | 79 | 34 at 24 mo | 73 at 24 mo | 26 |
| Pulsed CTD | 53 | 5 | 60 | 50 at 8 mo | 50 at 18 mo | 27 |
| ThaCyDex | 71 | 2 | 55 | 57 at 24 mo | 66 at 24 mo | 28 |
| HyperCTD | 60 | 4 | 72 | 50 at 11 mo | 50 at 19 mo | 29 |
TTP indicates time to progression; T, thalidomide; TM, thalidomide-melphalan; MivPT, intravenous melphalan-thalidomide-prednisone; MTD, melphalan-thalidomide-dexamethasone; TAD, thalidomide-pegylated lyposomal doxorubicin-dexamethasone; DVd-T, pegylated lyposomal doxorubicin-vincristine-decreased frequency dexamethasone-thalidomide; CDT, cyclophosphamide-dexamethasone-thalidomide; CTD, cyclophosphamide-thalidomide-dexamethasone; ThaCyDex, thalidomide-cyclophosphamide–dexamethasone; and ND, not determined.
CR plus near CR.
Randomized trial.
Serum monoclonal protein reduction greater than 96%.
Grades 3 to 4 toxic effects of thalidomide in relapsed-refractory patients with MM
| Regimen . | No. patients . | Thrombocytopenia, % . | Neutropenia, % . | Infection, % . | Peripheral neuropathy, % . | Reference . |
|---|---|---|---|---|---|---|
| Thalidomide alone | ||||||
| T | 84 | ND | ND | ND | ND | 9 |
| T | 169 | ND | ND | ND | 9* | 10 |
| TD | 1674 | ND | 11 | ND | 6 | 12 |
| Dexamethasone | ||||||
| TD | 120 | ND | ND | ND | 3 | 17 |
| TD | 44 | ND | ND | ND | ND | 19 |
| Melphalan | ||||||
| TM | 27 | ND | 30† | ND | 40* | 21 |
| MivPT | 24 | 19 | 37 | 8 | 8 | 22 |
| MTD | 21 | 38‡ | 52‡ | ND | 5 | 23 |
| Doxorubicin, TAD | 50 | 2 | 16 | 16 | 2 | 24 |
| Cyclophosphamide | ||||||
| Pulsed CTD | 53 | 2 | 26 | ND | 0 | 27 |
| ThaCyDex | 71 | ND | 10 | 7 | 6* | 28 |
| HyperCTD | 60 | 30 | 86 | 26 | 16 | 29 |
| Regimen . | No. patients . | Thrombocytopenia, % . | Neutropenia, % . | Infection, % . | Peripheral neuropathy, % . | Reference . |
|---|---|---|---|---|---|---|
| Thalidomide alone | ||||||
| T | 84 | ND | ND | ND | ND | 9 |
| T | 169 | ND | ND | ND | 9* | 10 |
| TD | 1674 | ND | 11 | ND | 6 | 12 |
| Dexamethasone | ||||||
| TD | 120 | ND | ND | ND | 3 | 17 |
| TD | 44 | ND | ND | ND | ND | 19 |
| Melphalan | ||||||
| TM | 27 | ND | 30† | ND | 40* | 21 |
| MivPT | 24 | 19 | 37 | 8 | 8 | 22 |
| MTD | 21 | 38‡ | 52‡ | ND | 5 | 23 |
| Doxorubicin, TAD | 50 | 2 | 16 | 16 | 2 | 24 |
| Cyclophosphamide | ||||||
| Pulsed CTD | 53 | 2 | 26 | ND | 0 | 27 |
| ThaCyDex | 71 | ND | 10 | 7 | 6* | 28 |
| HyperCTD | 60 | 30 | 86 | 26 | 16 | 29 |
ND indicates not determined.
Grades 2 to 4.
Leukopenia.
Grade 4 only.
Thromboembolic events during thalidomide treatment
| . | DVT/PE incidence without thromboprophylaxis . | DVT/PE incidence with thromboprophylaxis . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Newly diagnosed patients, % . | Reference . | Relapsed/refractory patients, % . | Reference . | Fixed low-dose warfarin, % . | Full-dose warfarin, % . | Aspirin, % . | LMWH, % . | Reference . | |
| Thalidomide alone | 3 | 30 | 2-3 | 10,12 | ND | ND | ND | ND | — |
| Thalidomide plus dexamethasone | 12-26 | 31-34 | 2-7 | 17,19 | 12-25 | 8 | ND | ND | 15,32,35,36 |
| Thalidomide plus melphalan | 6-20 | 37-39 | 4-11 | 21,22 | ND | ND | ND | 3 | 37 |
| Thalidomide plus doxorubicin | 10-27 | 40-42 | 58* | 43 | 12-14 | ND | 14-18 | 5-9 | 24,43,,–46 |
| Thalidomide plus cyclophosphamide | 3*-11 | 47,48 | 2-8 | 27-29 | 12 | ND | ND | ND | 26 |
| Thalidomide plus multiagent chemotherapy | 16-34 | 49,50 | 15 | 15 | 31 | ND | ND | 15-24 | 49,51 |
| . | DVT/PE incidence without thromboprophylaxis . | DVT/PE incidence with thromboprophylaxis . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Newly diagnosed patients, % . | Reference . | Relapsed/refractory patients, % . | Reference . | Fixed low-dose warfarin, % . | Full-dose warfarin, % . | Aspirin, % . | LMWH, % . | Reference . | |
| Thalidomide alone | 3 | 30 | 2-3 | 10,12 | ND | ND | ND | ND | — |
| Thalidomide plus dexamethasone | 12-26 | 31-34 | 2-7 | 17,19 | 12-25 | 8 | ND | ND | 15,32,35,36 |
| Thalidomide plus melphalan | 6-20 | 37-39 | 4-11 | 21,22 | ND | ND | ND | 3 | 37 |
| Thalidomide plus doxorubicin | 10-27 | 40-42 | 58* | 43 | 12-14 | ND | 14-18 | 5-9 | 24,43,,–46 |
| Thalidomide plus cyclophosphamide | 3*-11 | 47,48 | 2-8 | 27-29 | 12 | ND | ND | ND | 26 |
| Thalidomide plus multiagent chemotherapy | 16-34 | 49,50 | 15 | 15 | 31 | ND | ND | 15-24 | 49,51 |
PE indicates pulmonary embolism; ND, not determined; and —, not applicable.
Includes newly diagnosed and relapsed patients.
Newly diagnosed MM
Two randomized studies have shown that the TD regimen is better than high-dose dexamethasone (Tables 4,5).31,52 Patients received thalidomide at a dose of 200 mg/day plus dexamethasone at a dose of 40 mg/day on days 1 to 4, 9 to 12, and 17 to 20 or dexamethasone alone at the same schedule. In the first study,31 the response rate was significantly higher with TD than with dexamethasone alone (63% vs 41%; P = .001). The rates of any grades 3 to 4 toxic effects were significantly higher with TD (45% vs 21%; P = .001). Both DVT (17% vs 3%) and grades 3 to 4 peripheral neuropathy (7% vs 4%) were more frequent in the TD group than in the dexamethasone group. The investigators concluded that lower doses of dexamethasone could greatly reduce the incidence of adverse events without a negative effect on efficacy, and that anticoagulant prophylaxis should be used in all newly diagnosed patients. No substantial differences in survival were reported.31 The second study showed that time to progression was also improved in the TD group (P < .001).52
Thalidomide in patients with newly diagnosed MM
| Regimen . | No. patients . | CR, % . | Greater than PR, % . | PFS/EFS/TTP, % . | OS, % . | Reference . |
|---|---|---|---|---|---|---|
| Dexamethasone | ||||||
| TD | 235 | 5 | 49 | 50 at 17 mo | ND | 52*† |
| TD | 103 | 4 | 63 | 50 at 22 mo | 72 at 24 mo | 31* |
| TD | 100 | 10 | 76 | ND | ND | 32 |
| TD | 50 | ND | 64 | ND | ND | 34 |
| TD | 100 | ND | 25‡ | ND | ND | 33* |
| TD | 136 | 14 | 68 | 50 at 25 mo | 50 at 45 mo | 35*† |
| Melphalan | ||||||
| MPT | 129 | 16 | 76 | 54 at 24 mo | 80 at 36 mo | 37* |
| MPT | 125 | 13 | 76 | 50 at 28 mo | 50 at 52 mo | 38* |
| MPT | 113 | 7 | 62 | 50 at 24 mo | 50 at 45 mo | 39* |
| Doxorubicin | ||||||
| ThaDD | 50 | 34 | 88 | 57 at 36 mo | 74 at 36 mo | 44 |
| TAD | 193 | ND | 79-81 | ND | ND | 53* |
| DVd-T | 55 | 36 | 83 | 50 at 28 mo | ND | 25 |
| T-VAD doxil | 117 | 15 | 81 | 59 at 24 mo | 77 at 24 mo | 45* |
| T-VAD doxil | 39 | 10 | 74 | 55 at 22 mo | 74 at 22 mo | 40 |
| Cyclophosphamide, CTD | 27 | 15 | 89 | ND | ND | 47 |
| Regimen . | No. patients . | CR, % . | Greater than PR, % . | PFS/EFS/TTP, % . | OS, % . | Reference . |
|---|---|---|---|---|---|---|
| Dexamethasone | ||||||
| TD | 235 | 5 | 49 | 50 at 17 mo | ND | 52*† |
| TD | 103 | 4 | 63 | 50 at 22 mo | 72 at 24 mo | 31* |
| TD | 100 | 10 | 76 | ND | ND | 32 |
| TD | 50 | ND | 64 | ND | ND | 34 |
| TD | 100 | ND | 25‡ | ND | ND | 33* |
| TD | 136 | 14 | 68 | 50 at 25 mo | 50 at 45 mo | 35*† |
| Melphalan | ||||||
| MPT | 129 | 16 | 76 | 54 at 24 mo | 80 at 36 mo | 37* |
| MPT | 125 | 13 | 76 | 50 at 28 mo | 50 at 52 mo | 38* |
| MPT | 113 | 7 | 62 | 50 at 24 mo | 50 at 45 mo | 39* |
| Doxorubicin | ||||||
| ThaDD | 50 | 34 | 88 | 57 at 36 mo | 74 at 36 mo | 44 |
| TAD | 193 | ND | 79-81 | ND | ND | 53* |
| DVd-T | 55 | 36 | 83 | 50 at 28 mo | ND | 25 |
| T-VAD doxil | 117 | 15 | 81 | 59 at 24 mo | 77 at 24 mo | 45* |
| T-VAD doxil | 39 | 10 | 74 | 55 at 22 mo | 74 at 22 mo | 40 |
| Cyclophosphamide, CTD | 27 | 15 | 89 | ND | ND | 47 |
ThaDD indicates thalidomide-pegylated liposomal doxorubicin-dexamethasone; T-VAD doxil, thalidomide-vincristine-pegylated lyposomal doxorubicin; and ND, not determined.
Randomized trial.
Updated information was presented at the American Society of Clinical Oncology and European Hematology Association Congress.
Very good partial response rate.
Grades 3 to 4 toxic effects of thalidomide in patients with newly diagnosed MM
| Regimen . | No. patients . | Thrombocytopenia, % . | Neutropenia, % . | Infection, % . | Peripheral neuropathy, % . | Reference . |
|---|---|---|---|---|---|---|
| Dexamethasone | ||||||
| TD | 235 | 1 | 3 | ND | 2 | 52*† |
| TD | 103 | ND | ND | 7 | 7 | 31* |
| TD | 100 | ND | 0 | 4 | 4 | 32 |
| TD | 50 | ND | ND | ND | 2 | 30 |
| TD | 100 | ND | ND | ND | ND | 33* |
| Melphalan | ||||||
| MPT | 129 | 3 | 16 | 10 | 8 | 37* |
| MPT | 125 | 14 | 48 | 13 | 6 | 38* |
| MPT | 113 | ND | 23‡ | ND | 20‡ | 39* |
| Doxorubicin | ||||||
| ThaDD | 50 | 0 | 12 | 22 | 0 | 44 |
| T-VAD doxil | 117 | 8‡ | 10‡ | 7 | 6‡ | 45* |
| T-VAD doxil | 39 | 15 | 15 | 21 | 5 | 40 |
| Cyclophosphamide, CTD | 27 | ND | 4 | 7 | 4 | 47 |
| Regimen . | No. patients . | Thrombocytopenia, % . | Neutropenia, % . | Infection, % . | Peripheral neuropathy, % . | Reference . |
|---|---|---|---|---|---|---|
| Dexamethasone | ||||||
| TD | 235 | 1 | 3 | ND | 2 | 52*† |
| TD | 103 | ND | ND | 7 | 7 | 31* |
| TD | 100 | ND | 0 | 4 | 4 | 32 |
| TD | 50 | ND | ND | ND | 2 | 30 |
| TD | 100 | ND | ND | ND | ND | 33* |
| Melphalan | ||||||
| MPT | 129 | 3 | 16 | 10 | 8 | 37* |
| MPT | 125 | 14 | 48 | 13 | 6 | 38* |
| MPT | 113 | ND | 23‡ | ND | 20‡ | 39* |
| Doxorubicin | ||||||
| ThaDD | 50 | 0 | 12 | 22 | 0 | 44 |
| T-VAD doxil | 117 | 8‡ | 10‡ | 7 | 6‡ | 45* |
| T-VAD doxil | 39 | 15 | 15 | 21 | 5 | 40 |
| Cyclophosphamide, CTD | 27 | ND | 4 | 7 | 4 | 47 |
ND indicates not determined.
Randomized trial.
Updated information was presented at the American Society of Clinical Oncology and European Haematology Association Congress.
Grades 2 to 4.
In a continuing study,35 274 elderly patients were randomly assigned to TD or oral melphalan and prednisone (MP). Thalidomide was given at 200 to 400 mg/day, and dexamethasone was given at 40 mg/day on days 1 to 4 and 15 to 18 on odd cycles, and on days 1 to 4 on even cycles. TD induced higher PR rates than MP (68% vs 51%; P = .004). In patients older than 72 years, TD induced a higher mortality rate and a worse OS than did MP (median, 45 months vs 58 months; P = .029). Overall survival was much the same in patients younger than 72 years (58 months vs 50 months; P = .186). DVT, neuropathy, and constipation were more frequent in the TD group, whereas hematologic toxic effects were most prominent in the MP group.35
A case-control analysis showed that the TD regimen was better than the vincristine-doxorubicin-dexamethasone (VAD) schema.32 Adverse events were more frequent in the TD group; the DVT rate was 26% in the first 19 patients who did not receive any anticoagulant prophylaxis and 12% in the subsequent 81 patients who received low-dose warfarin as prophylaxis. A prospective randomized study33 compared TD with VAD. The frequency of at least very good partial response (VGPR) was 25% after TD induction compared with 7% after VAD (P = .003). However, the benefit of TD was not confirmed at 6 months after autologous stem- cell transplantation (ASCT), since VGPR rates (44% vs 42%; P = .87) were almost identical. DVT was higher in the TD group (23% vs 8%; P = .004).
Thalidomide and melphalan
Advanced multiple myeloma
Offidani and coworkers21 showed that oral melphalan and thalidomide was better than thalidomide alone (Tables 1,2). Thalidomide has also been associated with low-dose intravenous melphalan (20 mg/m2 every 4 months) in one study,22 and with intermediate-dose intravenous melphalan in another.23 Hematologic toxic effects were manageable in the first study,21 but were quite severe in the other 2 studies.22,23
Newly diagnosed MM
A total of 3 randomized studies assessed the combination of MP and thalidomide (Tables 4,5). In Palumbo et al's study,37 the oral standard MP was compared with MP plus thalidomide (MPT) in patients aged 60 to 85 years. The PR rates were 76.0% in the MPT group and 47.6% in the MP group. The 2-year EFS was 54% after MPT and 27% after MP (P < .001), and 3-year OS was 80% for MPT and 64% for MP (P = .19). The incidence of grades 3 to 4 adverse events was significantly higher in the MPT group than in the MP group (48% vs 25%; P < .001); most frequent were hematological toxic effects (22%), thromboembolism (12%), infections (10%), and peripheral neuropathy (8%). After the introduction of prophylactic enoxaparin, the frequency of DVT was lowered from 20% to 3% (P = .005).
Facon and colleagues38 compared MP with both MPT and ASCT (melphalan, 100 mg/m2) in patients aged 65 to 75 years. PR rates were 35%, 76%, and 65% (P < .001), and median PFSs were 17.8, 27.5, and 19.4 months, respectively; PFS was significantly longer in the MPT group than in the MP (P < .001) and ASCT (P < .001) groups, but no difference was noted between the MP and ASCT groups (P = .25). Median OS was 33.2, 51.6, and 38.3 months in the MP, MPT, and ASCT groups, respectively. Similarly, OS was significantly extended after MPT (P < .001) and ASCT (P = .027), although investigators noted no difference between the MP and ASCT groups (P = .86). The most frequent side effects were neutropenia, thrombocytopenia, infections, constipation, and peripheral neuropathy; thromboembolic events were reported in 12% of patients, but no prophylaxis was adopted.
In Hulin et al's trial,39 patients aged 75 years and older were randomly assigned to MP or MPT. The PR rate was higher in the MPT group than in the MP group (62% vs 31%), and both median PFS (24 months vs 19 months; P = .001) and OS (45 months vs 28 months; P = .03) were significantly longer. Toxic effects were higher in patients assigned to MPT and were mainly because of peripheral neuropathy (20%), neutropenia (23%), and depression (7%); no significant differences in DVT rate between the 2 groups were noted (6% vs 4%).
These 3 independent randomized studies showed virtually identical results, and they represent the experimental evidence that supports MPT as the standard of care for elderly patients not eligible for ASCT.
Thalidomide and doxorubicin
Advanced MM
Several trials have investigated the combination of thalidomide, pegylated lyposomal doxorubicin (PLD), and dexamethasone (Tables 1,2). Offidani and coworkers24 used a VAD-like regimen that substituted vincristine with low-dose thalidomide. Hussein and colleagues25 added thalidomide to a regimen consisting of PLD, vincristine, and decreased-frequency dexamethasone. Complete response (CR) and PR rates were similar between regimens; the toxic effects of these regimens were manageable, although vincristine increased the incidence of severe neuropathy.
Newly diagnosed MM
The association of thalidomide, doxorubicin, and dexamethasone (TAD) has been studied in newly diagnosed patients (Tables 4,5). In a phase 2 study,44 the PR rate was 88%, including 34% CR. Patients with impaired renal function achieved PR, and renal function was improved or restored. The rate of DVT was 14% despite the use of low-dose warfarin. Severe infections were reported in 22% patients, although the introduction of antibiotic prophylaxis decreased the frequency of such adverse events. In 2 parallel phase 3 multicenter German-Dutch studies,53 newly diagnosed patients with MM were randomly assigned to received TAD or the standard VAD regimen as induction treatment before ASCT. An interim analysis showed a better PR rate with TAD than with VAD both in the German study (79% vs 58%) and in the Dutch trial (81% vs 61%). Patients treated with TAD had fewer peripheral blood stem cells; nevertheless, the stem cells obtained were sufficient for double ASCT in 82% of patients in the TAD group.
A randomized trial compared VAD-doxil plus thalidomide (TVAD-doxil) with VAD-doxil.45 Partial response rates were more frequent in the TVAD-doxil group than in the VAD-doxil group (81% vs 63%; P = .003); extended 2-year PFS (59% vs 45%; P = .013) and increased OS (77% vs 64%; P = .037) were reported for patients assigned to TVAD-doxil.
Other studies used vincristine and PLD in association with thalidomide (400 mg) and decreased-frequency dexamethasone,25 or with thalidomide (200 mg) and high-dose dexamethasone.40 The PR rate ranged from 74% to 83%, and major toxic effects included DVT, peripheral neuropathy, palmar-plantar erythrodisestesia, and infections.
Thalidomide and cyclophosphamide
Advanced MM
Cyclophosphamide has been associated with thalidomide and corticosteroids for the treatment of advanced MM (Tables 1,2). Oral weekly cyclophosphamide (300 mg/m2 per week), monthly pulsed low-dose dexamethasone, and thalidomide (300 mg/day; CDT) has been studied. The PR rate was 62%, including 17% CR. A total of 27 percent of patients developed infections, most of which were respiratory, but there were no infection-related deaths.26
In another phase 2 study,27 patients received pulsed cyclophosphamide (150 mg/m2 twice daily, days 1-5), thalidomide (400 mg, days 1-5 and 14-18) and dexamethasone (20 mg/m2, days 1-5 and 14-18). Toxic effects were mild or moderate, and the cumulative frequencies of DVT and peripheral neuropathy were 2% and 4%. Other phase 2 studies showed similar results.28,29
Newly diagnosed MM
The combinations of oral cyclophosphamide (500 mg on days 1, 8, and 15), thalidomide (400 mg), and high-dose dexamethasone (CTD) have been studied in 27 patients and compared with the combination of cyclophosphamide-vincristine-doxorubicin-methylprednisolone (CVAMP) in a case-matched analysis (Tables 4,5). The PR rate was significantly higher with CTD than with CVAMP (89% vs 56%; P = .016). The frequency of DVT was higher in the CTD group (11% vs 4%), but grades 3 to 4 neutropenia (4% vs 60%) and infections (7% vs 26%) were greater in the CVAMP group.47
Thalidomide and ASCT
To assess whether the incorporation of thalidomide into high-dose therapy could improve survival, 668 newly diagnosed patients, who received tandem ASCT, were randomly assigned to receive thalidomide or not.49 Patients given thalidomide had a higher CR rate (62% vs 43%; P < .001) and a higher 5-year EFS rate (56% vs 44%; P = .01). There was no difference in OS because of a significantly shorter survival after relapse in the thalidomide group (median, 1.1 years vs 2.7 years; P = .001). Of note, 83% patients in the control group received thalidomide at relapse. The delivery of thalidomide at diagnosis significantly extended EFS, but the administration of thalidomide at relapse in the control group probably abrogated the OS benefit. Thromboembolic events were more common in the thalidomide group (34% vs 18%; P < .001). The high rate of DVT was not prevented by the introduction later in the study of prophylactic low-molecular-weight heparin (LMWH; 24% in the thalidomide group vs 15% in the control group; P = .064). Peripheral neuropathy (grade 2 or higher) was more common in the thalidomide group (27% vs 17%; P < .001). Syncopal episodes related to sinus bradycardia arose in 12% of patients in the thalidomide group (4% in the control group). Cardiac pacemakers were implanted in nearly a third of patients with symptomatic sinus bradycardia. The treatment-related mortality rate was similar in both groups.49
Post-ASCT thalidomide consolidation-maintenance
High-dose chemotherapy has increased the response rate in patients with MM, but this treatment is not curative, and effective consolidation-maintenance strategies could extend the duration of response.
In a large study,54 2 months after ASCT, patients were randomly assigned to receive no maintenance (group A), pamidronate (group B), or pamidronate plus thalidomide (group C; 400 mg/day, dose reduction to a minimum dose of 50 mg was allowed for treatment-related toxicity). At least VGPR was achieved in 55% of patients (group A), 57% of patients (group B), and 67% of patients (group C; P = .03). The 3-year EFS was 36% (group A), 37% (group B), and 52% (group C; P < .009). The 4-year OS was 77% (group A), 74% (group B), and 87% (group C; P < .04). Patients received thalidomide for a median of 15 months (range, 0.1-50 months). Drug-related adverse events led to discontinuation of thalidomide in 39% of patients, mainly because of peripheral neuropathy. The mean dose of thalidomide was 200 mg/day. Most frequent toxic effects included neuropathy (68%), fatigue (34%), constipation (20%), neutropenia (7%), and cardiac events (4%). The DVT incidence did not differ significantly in the 3 groups.
In an Australian randomized study,55 thalidomide (200 mg/day for a maximum of 12 months) combined with prednisolone (50 mg on alternate days) was compared with prednisolone alone as maintenance after ASCT. At 12 months after randomization, the thalidomide group had higher PR rate (83% vs 52%; P < .01), better 2-year PFS (63% vs 36%; P < .001), and better 3-year OS (86% vs 75%; P = .02).
In a recent study, patients with newly diagnosed MM were randomly assigned to receive either a single ASCT followed by thalidomide maintenance or tandem ASCT. Single ASCT followed by maintenance with thalidomide proved better than tandem ASCT for both 3-year PFS (85% vs 57%; P = .02) and 3-year OS (85% vs 65%; P = .04).56 Whether the most effective strategy is maintenance treatment with thalidomide or therapy with thalidomide at relapse is still debated.
Factors affecting outcome
Factors affecting outcome of patients with MM treated with thalidomide have been assessed. These studies might select patients who most benefit from thalidomide and might allow a risk-based treatment algorithm to be designed.
In the first study into the use of thalidomide monotherapy in advanced MM,9 3 factors were associated with a short EFS: increased LDH, C-reactive protein, and plasma cell labeling index. Short OS was associated with low albumin, chromosome 13 deletion (del13), and high bone marrow plasma cell infiltration. Barlogie and colleagues49 treated patients with ASCT with or without thalidomide. In the thalidomide group, EFS was independent of cytogenetics abnormalities, high LDH, or low serum albumin. Thalidomide was unable to overcome these adverse prognostic factors in terms of OS. The Bologna group57 reported a significantly reduced probability of response to TD in patients with coexisting del13 or t(4;14) cytogenetics abnormalities, but not in those with a single abnormality. These negative results were completely offset by subsequent tandem ASCT.57 In another study of relapsed patients given TD,58 time to first progression of less than 12 months was significantly associated with shorter OS (P = .002), but serum elevated β2-microglobulin and C-reactive protein did not predict poor outcome. Attal and coworkers54 showed that patients without del13 had a significant benefit from thalidomide, whereas patients with del13 did not.
Safety issues
The frequency and severity of thalidomide side effects are dose related and time dependent (Tables 2,3,5). Physicians should grade side effects according to the National Cancer Institute Common Toxicity Criteria for Adverse Events. Generally, once a relation between thalidomide and toxicity is established, no action should be taken if there are nonhematologic grade 1 side effects. If there are grade 2 toxic effects, the thalidomide dose should be reduced to 50%; for grades 3 to 4, it should be discontinued.
Teratogenicity
Birth defects were observed in 10 000 to 12 000 infants before thalidomide was withdrawn.59 Thalidomide does not seem to be carcinogenic, genotoxic, or mutagenic, and it has not been correlated to second-generation birth defects.60,61 Thalidomide administration in pregnant women is absolutely contraindicated. Women of childbearing potential should have a negative pregnancy test before starting thalidomide, use 2 effective forms of birth control, and have a pregnancy test every 4 weeks if their period is regular or every 2 weeks in case of irregularity. Men receiving thalidomide must abstain from sexual intercourse or use a latex condom even if they have undergone vasectomy. The Risk Management Programs are designed to avoid prescription of thalidomide to pregnant women or men with childbearing potential.
Thromboembolism
Thromboembolic events are one of the most important side effects (Table 3). The pathogenic mechanisms of DVT associated with thalidomide have not been clearly established. Acquired activated protein C resistance and a reduction in thrombomodulin level have been associated with an increased risk of DVT.62,63 No specific coagulation test can predict the high risk of DVT. Endothelial injury produced by the combination of thalidomide with chemotherapy and subsequent restoration of endothelial cell PAR-1 expression are probably factors that promote thrombosis.64 Since thalidomide does not cause endothelial damage,65 the frequency of DVT is low when thalidomide is used as monotherapy, whereas it substantially increases when chemotherapy is added.
The goal of thromboprophylaxis should be to reduce the frequency of DVT to less than 10%. Thromboprophylaxis is not recommended for patients who receive single-agent thalidomide or thalidomide maintenance (if no other risk factors for DVT are simultaneously present), but it is strongly recommended in those who receive thalidomide in combination with high-dose dexamethasone or doxorubicin. Thromboprophylaxis should be tailored according to the presence of other risk factors that could further increase the risk of DVT: individual risk factors (age, obesity, comorbidities, surgical procedures), MM-related risk factors (diagnosis, high tumor mass), and treatment-related risk factors (high-dose dexamethasone, doxorubicin, multiagent chemotherapies). There are no data to suggest that one anticoagulant is better than another. Although aspirin is more appealing because of convenience and of ease of administration, the rate of thrombosis is relatively high in particular conditions. Until further evidence becomes available, aspirin should only be recommended for patients with low risk of DVT. In high-risk patients, evidence nowadays supports the use of LMWH or full-dose warfarin. The use of warfarin is limited by its long half-life in patients at risk of concomitant thrombocytopenia. LMWH is a more suitable option because of its short half-life and the decreased risk of secondary bleeding. Ongoing studies will determine which is the most appropriate anticoagulant prophylaxis.
Cardiovascular side effects
Sinus bradycardia occurs in patients given thalidomide because of autonomic neuropathy. Bradycardia can be severe enough to cause syncope.66 Patients should be assessed at the start of treatment to identify previous episodes such as syncope that can be associated with heart disease, especially when thalidomide is used in combination with chemotherapy such as doxorubicin or cyclophosphamide. Symptoms related to bradycardia can resolve or decrease in severity after either discontinuation or dose reduction, but sometimes a pacemaker needs to be implanted.49,67
Hypotension is a possible although infrequent side effect. The concurrent administration of antidepressants could induce or worsen hypotension. The risks of hypotension and bradycardia increase in elderly patients receiving concomitant antihypertensive drugs such as β-blockers. Peripheral edema is more frequent with coadministration of corticosteroids; it is generally mild, reversible, and usually responsive to temporary discontinuation of the drug.66
Neurologic side effects
Many patients with MM present with subclinical or even clinical peripheral neuropathy. These patients can be at increased risk of peripheral neuropathy because of thalidomide treatment. These effects usually arise after prolonged administration of thalidomide (70% of patients treated for 12 months, although effects are mainly grades 1-2).68 Clinical manifestations include sensory symptoms (distal paresthesia and hyperesthesia), motor symptoms, and autonomic dysfunction. Patients suffer from pinprick sensations, numbness, and tingling that initially affect fingers and toes but later extend proximally. Subsequently, vibration perception, deep sensitivity, and position sense are affected, leading to ataxia, progressive gait disturbance, and postural tremor. Neuropathy is closely related to duration of treatment68,69 and cumulative dose,70,71 and it is more frequent in elderly patients.
A randomized study17 reported a greater tolerability of thalidomide in patients who received 100 mg than in those who received 400 mg. Doses of 200 mg/day or less should be considered to keep side effects to a minimum; lower doses (100 mg/day) are better tolerated in elderly patients. Thalidomide discontinuation increases the probability of recovery, which usually occurs within 3 weeks. If treatment is not stopped, the neuropathy progresses and could become irreversible. Patients should be taught to recognize peripheral neuropathy and to immediately decrease the dose or discontinue thalidomide when sensory paresthesia is complicated by pain, motor deficit, or interference with daily function. A practical rule is to maintain the assigned dose if neuropathy is grade 1, to reduce the dose by 50% if neuropathy is grade 2, to discontinue the drug if neuropathy is grade 3, and to eventually resume thalidomide at a decreased dose if neuropathy reaches grade 1. Symptomatic therapy for paresthesia includes gabapentin, pregabalin, L-carnitine, and tricyclic antidepressants.
Tremors arise quite frequently but rarely interfere with daily activity. Depression has been reported, especially in patients with a previous history of depression. Headache is possible, it is not usually dose-dependent and can be relieved with nonsteroidal anti-inflammatory drugs.66
Constipation
Constipation is the most common gastrointestinal side effect (more than 50% of patients), and it seems to be dose dependent. This adverse event appears early after treatment is started (2-4 days), and is most severe in elderly patients and in those receiving opioid analgesics.66 Patients should maintain a high fluid intake, a high fiber diet, and sufficient exercise. Drugs that reduce bowel motility should be avoided if possible. Stool softeners and osmotic laxative are usually sufficient for moderate constipation. For severe constipation, a 50% dose reduction is needed.
Sedation
Some amount of sedation is universal. It generally appears within 15 days after thalidomide treatment is started and is usually mild and dose dependent. Benzodiazepines, opiates, phenytoin, or sedative neuroleptics can enhance sedation, and thalidomide itself can increase the sedative activity of barbiturates and alcohol, and the catatonic activity of chlorpromazine and reserpine.66 Thalidomide does not produce central nervous system (CNS) or respiratory depression.59
Patients should be instructed to take thalidomide in the evening and to expect assumption in late afternoon if somnolence persists the following morning. Cautions should be given in the first 2 weeks for activities requiring great attention (eg, driving), and concomitant drugs with sedative activity should be avoided.
Dermatologic side effects
Adverse dermatologic effects include rash, atrophic lesions, dry skin and mouth, and, rarely, toxic epidermic necrolysis and the Stevens-Johnson syndrome. The most common dermatologic side effect is a pruritic maculopapular rash, starting on the trunk and extending to the back and proximal limbs; it does not seem to be dose related and has been reported within 10 to 14 days after the start of treatment. Vesicular eruptions, usually pruritic, occur in 25% of patients given doses greater than 400 mg/day after 30 to 60 days of therapy. Temporary discontinuation leads to resolution of the rash and allows readministration at a reduced dose.
The most serious hypersensitivity reaction is toxic epidermic necrolysis, associated with mortality rates greater than 30%. The appearance of a maculopapular rash that covers the entire body is a potential early sign.72 Because of these possible dermatologic effects, coadministration of thalidomide with sulphonamides, allopurinol, or cotrimoxazole should be undertaken with caution.73
Thyroid dysfunction
Thalidomide can rarely be thyrotoxic or provoke an immune reaction against the thyroid gland.66 Evidence of reduction in thyroid function, most of which is subclinical, has been reported from 2% to 35%.29,74 Subclinical hypothyroidism might contribute to the adverse effect profile of thalidomide, especially constipation, fatigue, neuropathy, skin rush, and bradycardia. Measurements of thyroid-stimulating hormone levels at baseline and at regular intervals (every 3 months) could detect subclinical hypothyroidism. Low-dose thyroxin supplementation should be given for cases of clinical and subclinical hypothyroidism.
Hematologic side effects and infections
Thalidomide can induce neutropenia, usually mild, in 3% to 15% of patients.9,75 The frequency increases when thalidomide is combined with myelotoxic chemotherapy. Thrombocytopenia is rare and generally associated with neutropenia. Impairment of marrow function is not a contraindication but rather an argument for the use of thalidomide and corticosteroids, since this combination does not induce substantial hematologic toxic effects.
Infections are an important complication: immunosuppression, exacerbated by the concomitant use of corticosteroids, is probably the main cause. Grades 3 to 4 infections were reported in 16% to 22% of patients given thalidomide, dexamethasone, and doxorubicin. Most infectious complications occurred during the first 3 courses of treatment; prophylaxis with ciprofloxacin reduced the infection rate to less than 10%.24,44
Liver and renal function
Thalidomide-associated hepatitis, resolved after drug withdrawal, has been reported, although it is rare.76 Since thalidomide is spontaneously and nonspecifically hydrolyzed, plasma concentrations should not change in patients with compromised liver function. No data suggest that patients with damaged liver function need a dose reduction. Regular monitoring of liver enzymes (ie, during the first months of therapy and every 3 months thereafter) might be reasonable.
Renal adverse effects are rare. In patients with renal impairment, pharmacokinetics of thalidomide was similar to that reported in those with normal renal function.77 These data suggest that a thalidomide dose adjustment is not needed in patients with renal impairment. In patients with renal impairment (defined as serum creatinine greater than 130 mM/L), the frequency of side effects was comparable to that observed in patients with normal renal function.78
Conclusions
After 40 years, thalidomide is the first new drug that yields high response rates and improves remission duration and survival for patients with MM.
TD is a new induction therapy for patients eligible for ASCT for whom stem-cell harvest is efficient. The response rate is better than it is with VAD before high-dose melphalan, but almost identical after ASCT. EFS data are needed before the benefit of this approach can be clearly established. Preliminary data suggest that thalidomide combinations including doxorubicin or cyclophosphamide might be more effective than TD. Maintenance treatment with low-dose thalidomide should be at least offered to patients not achieving VGPR after tandem ASCT. In elderly patients, MPT is now regarded as the standard of care after 3 independent randomized studies37-39 showed that MPT was better than the standard MP regimen. Combinations including thalidomide with dexamethasone, melphalan, doxorubicin, or cyclophosphamide are effective options for the management of relapsed-refractory disease.
An improved ratio of efficacy to side effects can be obtained when low-dose thalidomide is combined with steroids or chemotherapy. Somnolence and constipation are clearly dose dependent; DVT is a major side effect when thalidomide is used in combination with other drugs. Antithrombotic prophylaxis is mandatory in newly diagnosed patients for at least the first 6 months of therapy. Peripheral neuropathy is dose and time dependent; although mild symptoms might be acceptable, more severe symptoms (greater than grade 1) need a thalidomide dose reduction and discontinuation. Thalidomide can be safely given to patients with renal impairment.
In the future, the main issues will be to define the subgroups of patients who might benefit most from thalidomide-based regimens and to explore new combinations including bortezomib or lenalidomide. Both agents can be given to patients with established resistance to thalidomide. Lenalidomide showed little degree of cross-resistance with thalidomide, and bortezomib can have some restrictions in patients with preexisting thalidomide-related neuropathy. Whether these new combinations will replace ASCT or whether they should be incorporated to further improve the efficacy of standard ASCT is unknown. Finally, further studies should clarify whether tumor eradication rather than maintaining of an asymptomatic disease is the most effective strategy for MM.
Acknowledgment
We thank Tiziana Marangon for her technical assistance in the preparation of the manuscript.
Authorship
Contribution: A.P., T.F., P.S., J.B., P.M., A.W., A.S., H.L., M.B., and J.-L.H. revised material and wrote the paper; and M.O. and F.G. collected data, revised material, and wrote the paper.
Conflict-of-interest disclosure: A.P., T.F., and M.B. have received scientific advisor board and lecture fees from Pharmion, Celgene, and Janssen-Cilag. P.S. has received scientific advisor board and lecture fees from Celgene. P.M. received honoraria from Pharmion in 2007. A.W. is a member of the Advisory Board, Nordic branch of Pharmion Speakers Bureau, Janssen-Cilag. J.-L.H. is a member of the Speakers Bureau and Advisory Board of Orthobiotech, Pharmion, and Celgene. All other authors declare no competing financial interests.
Correspondence: Antonio Palumbo, Divisione di Ematologia dell'Università di Torino, Azienda Ospedaliera S Giovanni Battista, Via Genova 3, 10126 Torino, Italy; e-mail: appalumbo@yahoo.com.