Abstract
Although detection of autoantibodies in the peripheral blood from patients with immune-mediated endothelial dysfunctions has so far failed to provide tools of diagnostic or pathogenetic value, putative bioindicators include anti-endothelial cell antibodies, a heterogeneous family of antibodies that react with autoantigens expressed by endothelial cells. In this study, to identify endothelial autoantigens involved in the autoimmune processes causing endothelial damage, we screened a human microvascular endothelial cell cDNA library with sera from patients with Behçet's disease. We identified antibodies to the C-terminus of Ral binding protein1 (RLIP76), a protein that catalyzes the ATP-dependent transport of glutathione (GSH) conjugates including GSH-4-hydroxy-t-2,3-nonenal, in the serum of a significant percentage of patients with various diseases characterized by immune-mediated endothelial dysfunction, including Behçet disease, systemic sclerosis, systemic lupus erythematosus and carotid atherosclerosis. These autoantibodies increased intracellular levels of 4-hydroxy-t-2,3-nonenal, decreased levels of GSH and activated C-Jun NH2 Kinase signaling (JNK), thus inducing oxidative stress-mediated endothelial cell apoptosis. The dietary antioxidant alpha-tocopherol counteracted endothelial cell demise. These findings suggest that autoantibodies to RLIP76 play a pathogenetic role in immune-mediated vascular diseases and represent a valuable peripheral blood bioindicator of atherosclerosis and immune-mediated vascular diseases.
Introduction
Autoantibodies directed against normal host antigens are a common feature of many autoimmune diseases. Some of the markers are pathogenic, whereas others they may be merely an epiphenomenon due to tissue damage and serve as markers for organ involvement or outcomes.1 Pathogenic autoantibodies may act directly on target organs by immune-complex deposition, by complement activation, or by binding distinct soluble or membrane proteins blocking or activating their biologic activity.2 Anti-endothelial cell antibodies (AECA) are a heterogeneous group of antibodies detected in autoimmune vasculitis, vasculopathies, atherosclerosis, and other diseases caused by vessel-wall damage.3 AECA induce an endothelial perturbation in vitro, increasing adhesion molecule expression and secretion of proinflammatory cytokines and chemokines. Some evidence suggests that AECA favor ischemic events by inducing apoptosis.4-7 The pathogenetic role of AECA in ischemia receives support from their frequent association with disease activity in several autoimmune vasculitis and vasculopathies including Behçet disease (BD), systemic lupus erythematosus (SLE), and systemic sclerosis (SS).3,8,9 Until recently, few published data were available on the endothelial cell autoantigens recognized by AECA.10 Identifying endothelial autoantigens involved in the immune-mediated processes during endothelial dysfunctions could help to explain how chronic inflammation of the vascular wall initiates and progresses. Screening a cDNA expression library is a powerful technique that identifies previously uncharacterized antigens from patients' sera containing antibodies
In this study, designed to identify new antigenic targets of AECA, we screened a human microvascular endothelial cell (HMVEC) cDNA expression library with sera from patients with BD, a systemic form of primary vasculitis, and identified a strongly reactive clone encoding Ral binding protein1 (RLIP76/RALBP1). RLIP76 is a Ral effector, GTPase-activating protein11 expressed in several malignant cell lines and, in smaller amounts, in nonmalignant human cell lineages of endothelial, epithelial, and aortic smooth muscle origin, as well as in erythrocytes.12,13 Like ABC protein, it catalyzes ATP-dependent transport and extrusion from the cell of anionic (eg, glutathione [GSH]) conjugates, such as GSH-4-hydroxy-t-2,3-nonenal (GS-HNE), leukotrienes, and weakly cationic compounds, that is, anthracyclins. 4-hydroxy-t-2,3-nonenal (4-HNE) is an end product of lipid peroxidation that induces oxidative stress, causes apoptosis, activates several signaling pathways and is conjugated with GSH.14-21 By lowering GS-HNE, RLIP76 helps to maintain cell homeostasis.22 Cells subjected to mild, transient oxidative stress redistribute RLIP76 on the membrane surface thus expelling 4-HNE at a higher rate.23 In experiments to identify the immunoreactive region of RLIP76, we cloned and expressed the N- and C-terminal regions and by enzyme-linked immunosorbent assay (ELISA) we measured IgG specific to RLIP76 in patients with immune-mediated endothelial dysfunction, BD, SLE, SS, and carotid atherosclerosis. Second, we analyzed the endothelial RLIP76 expression, localized the protein on endothelial cells in physiologic conditions and after mild oxidative stress, and investigated the pathogenic effects of the specific autoantibodies. In particular, we studied the anti-RLIP76 antibody–induced intracellular levels of 4-HNE and GSH and C-Jun NH2 kinase signaling activation (JNK) and apoptosis. The data we present here provide evidence that sera from patients with various diseases characterized by immune-mediated endothelial dysfunctions contain autoantibodies specific to the C-terminal region of RLIP76. These autoantibodies may have a pathogenetic role inducing oxidative stress-mediated apoptosis in endothelial cells.
Methods
Patients
We studied 37 unselected outpatients with BD (10 women, 27 men; mean age, 42.2 years, range, 27-58 years; mean disease duration, 7.9 years, range, 0-24 years), 40 consecutive patients with SLE (35 women, 5 men; mean age, 40.1 years, range, 19-71 years; mean disease duration, 8.2 years, range, 0.4-24 years), 65 consecutive patients with SS (60 women, 5 men; mean age, 57 years; range, 20-77 years; mean disease duration, 6.2 years; range, 0.9-30 years) attending the Rheumatology Division of Sapienza University of Rome. All patients with BD fulfilled the diagnostic criteria of the International Study Group for BD.24 Glucocorticoids were used in 46.1% of patients with BD, immunosuppressive drugs (cyclosporine A, methotrexate, azathioprine, chlorambucil) in 56.4%, infliximab in 5.1%, interferon α in 5.1%, and 10.2% of the patients with BD were not treated. Patients who had 2 of the 7 findings (oral and genital ulcerations, skin lesions, eye involvement, positive pathergy test, thrombophlebitis, and arthritis), or multiple erythema nodosum with severe inflammation and with an elevated erythrocyte sedimentation rate and positive C-reactive protein were assumed to have active disease. According to these criteria, 44.4% of patients had active disease. The frequency of the HLAB51 allele was 68.7%. SLE was diagnosed in accordance with the American College of Rheumatology revised criteria.25 Glucocorticoids were used in 74% of patients with SLE, hydroxychloroquine in 48.1%, and immunosuppressive drugs (azathioprine, cyclophosphamide, cyclosporine A, methotrexate, mycophenolate mofetil) in 37%; 11.1% were not treated. SS was diagnosed in accordance with the criteria of the American Rheumatism Association.26 Of the 65 patients with SS, 15 were receiving low doses of glucocorticoids (< 10 mg prednisone daily) and 9 patients were also undergoing immunosuppressive therapy (cyclosporine A or cyclophosphamide). As controls, we also enrolled 43 patients with infectious mononucleosis and 46 healthy subjects (27 women, 19 men; mean age, 45 years; range, 34-53 years).
We also enrolled 66 consecutive patients with carotid atherosclerosis undergoing carotid endarterectomy at the Department of Surgical Sciences of Sapienza University of Rome. The indications for surgery, based on the recommendations published by the Asymptomatic Carotid Atherosclerosis Study and the North American Symptomatic Carotid Endarterectomy Trial, were clinically asymptomatic, severe or preocclusive carotid-artery stenosis of 70% or more, clinically asymptomatic stenosis with ipsilateral signs of cerebral ischemia on computed tomographic scan, and clinically symptomatic stenosis.27,28 To correct cardiovascular risk factors, all patients received 150 mg of aspirin for 4 weeks before endarterectomy. Exclusion criteria were recent infection (< 1 month), autoimmune disease, malignancy, and inflammatory diseases. We also excluded patients receiving statins. Venous peripheral blood was drawn from patients before endarterectomy and from 25 sex- and age-matched healthy subjects with no ultrasonographically evident carotid atherosclerotic disease recruited as controls. Informed consent was obtained from each patient in accordance with the Declaration of Helsinki, and the local ethics committee approved the study.
Immunoscreening of the cDNA expression library
A commercially available HMVEC cDNA library (Stratagene, Cambridge, United Kingdom) was screened with the serum from 2 of the 37 patients with BD, essentially as previously described.29 The 2 patients' sera were selected on the basis of their AECA positive immune reaction and disease activity. Positive plaques were rescreened with the same pool of sera to obtain the clonality and phages were recovered as pBluescript by single-stranded rescue using the helper phage (Stratagene) according to the manufacturer's instructions and used to transform SolR XL1 cells.
Identification, amplification, and cloning in expression vector of the cDNA subunits
The nucleotide sequence of the cloned cDNA insertions was sequenced with automated sequencer ABI Prism 310 collection (Applied Biosystems, Foster City, CA) and the sequence compared with the GenBank sequence database using the Blast program revealed 100% identity with RLIP76 (NM 006788). The cDNA insertion was amplified by PCR to obtain the N- and C-terminal regions using as primers the oligonucleotides with restriction sites. For the N-terminal region we used: forward (BamH I restriction site) 5′-GCATGGATCCATGACTGAGTGCTTCCTG-3′, reverse (Hind III restriction site) 5′-GCATAAGCTTAGTTCCTTTGCAATGACATG-3′; for the C-terminal subunit we used: forward (BamH I restriction site) 5′-GCATGGATCCCCAGAATGTAACTATCTTCTG-3′, reverse (Hind III restriction site) 5′-GCATAAGCTTTCAGATGGACGTCTCCTT-3′. The amplified fragments were run in 2% agarose gel, purified by Qiaex kit following the manufacturer's instruction (Qiagen, Hilden, Germany) and after digestion with the restriction enzymes (Promega, Madison, WI) cloned in pQE (Qiagen) expression vector.
Expression and purification of the recombinant antigens
The fusion proteins were expressed in Escherichia coli SG130009 cells, purified by affinity of NI-NTA resin for the 6-histidine tail, and eluted under denaturing conditions according to the manufacturer's instruction (Qiagen) using a protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO).
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting
After 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, immunoblotting was performed as previously described.30 In brief, the antigen was loaded at concentrations of 3 μg/lane and was revealed by human sera diluted 1:100 and by a monoclonal antibody to 6-histidine tail (Qiagen). Peroxidase-conjugated goat anti–human IgG and anti–mouse IgG sera (Bio-Rad, Richmond, CA) were used as second antibodies. Strips were developed with 3-3′-diaminobenzidine (Sigma-Aldrich).
ELISA
ELISA was developed essentially as previously described.31 In brief, polystyrene plates (Maxisorp; Nunc, Rochester, NY) were coated with the antigen (0.1 μg/well) in 0.05 M NaHCO3 buffer, pH 9.5, and incubated overnight at 4°C. Plates were blocked with 100 μL/well of phosphate-buffered saline (PBS) with 0.05% Tween20 (PBS-Tween) containing 3% milk, for 1 hour at 37°C. Optimal serum dilution was established in preliminary experiments (1:10-1:500). For the RLIP76 C-terminal subunit serum reactivity peaked at a dilution of 1:100 and remained unchanged at higher concentrations whereas for the RLIP76 N-terminal subunit it remained below detectable values at each serum dilution tested (data not shown). After blocking with 3% milk, plates were therefore incubated with human sera diluted 1:100 in PBS-Tween and 1% milk. Peroxidase conjugated goat anti–human IgG (Bio-Rad) diluted 1:3000 in PBS-Tween containing 1% milk was incubated 1 hour at room temperature. O-phenylenediamine dihydrochloride (Sigma-Aldrich) was used as substrate and the optical density (OD) was measured at 490 nm. Means plus 2 standard deviations of the OD reading of the healthy controls were considered as the cutoff level for positive reactions. All assays were performed in quadruplicate. Data were presented as the mean OD corrected for background (wells without coated antigen). The results of unknown samples on the plate were accepted if internal controls (2 serum samples, one positive and one negative) had an absorbance reading within mean plus or minus 10% of previous readings. To inhibit specific IgG, the sera from 2 patients with BD were incubated overnight at room temperature with 10 μg/mL of the same antigen used to coat ELISA plates according to the method reported by Huang and colleagues.32 As negative controls for the inhibition analysis, the sera were preincubated with 10 μg/mL of an unrelated recombinant antigen or 40 μg/mL of bovine serum albumin (BSA).
Cultures of human umbilical-vein endothelial cells (HUVECs) at the third to fourth passage were used to detect AECA (IgG), using a cell-surface ELISA on living cells, as previously reported.33
Antibodies specific to RLIP76
Antibodies from patients' sera were purified as previously described.29 In brief, antigen (50 μg) was spotted onto a nitrocellulose filter and incubated with the sera from patients with BD used for the immunoscreening. The bound antibodies were eluted with glycine 100 mM, pH 2.5, mixed for 10 minutes and neutralized with Tris-HCl 1 M, pH 8. Antibodies from a preparation of intravenous immunoglobulin (IVIG) precipitated by saturated ammonium sulfate solution (SAS) were used as control. Endotoxin contamination of antibodies, as determined by the quantitative chromogenic Limulus amebocyte lysate assay (QCL-1000; BioWhittaker, Walkersville, MD) was less than 0.03 EU/μg of protein.
Mouse polyclonal antibodies to RLIP76 C-ter obtained by a standard immunization protocol and mouse monoclonal antibody to 6-histidine (Qiagen) were used as positive controls.
Culture conditions of endothelial cells
The primary cultures HMVEC-L (Provitro, Berlin, Germany) or the immortalized hybridoma cell line EAhy926 or HUVEC isolated by collagenase perfusion from normal-term umbilical cord veins were used as endothelial cells. Cells were grown to 60% to 70% confluence and seeded at 5 × 106 well on glass coverslips. To induce mild oxidative stress, cells were treated with 30 μM H2O2 for 30 minutes. After this time cells were incubated for an additional 30 minutes, 6 hours, and 24 hours in H2O2-free medium or with human anti-RLIP76 C-ter antibodies at a concentration of 40 μg/mL, reported by Singhal et al as the optimal concentration for inducing apoptosis.12 As a control we used the same concentration of human IgG in the medium. To rule out endotoxin contamination, the same experiments were run in the presence of polymyxin B (10 μg/mL; Sigma-Aldrich). In some experiments, cells were also preincubated for 2 hours with 30 μM pan-caspase inhibitor zVAD (Alexis, San Diego, CA) or for 24 hours with 30 μM alpha-tocopherol (α-TCPH; Sigma-Aldrich).
Cellular localization of RLIP76
An indirect immunofluorescence assay was developed on endothelial cells, as previously described.34 Cells were permeabilized with acetone/methanol 1/1 (vol/vol) for 10 minutes at 4°C, soaked in balanced salt solution (Sigma-Aldrich) for 30 minutes at 25°C, and then incubated for 30 minutes at 25°C in the blocking buffer (2% BSA in PBS, containing 5% glycerol and 0.2% Tween-20). After washing 3 times with PBS, cells were incubated for 1 hour at 4°C with human anti-RLIP76 antibodies and with control human IgG (0.1 μg/μL) in PBS containing 1% BSA. Fluorescein isothiocyanate-conjugated anti–human IgG (γ-chain specific; Sigma-Aldrich) was then added and incubated at 4°C for 30 minutes. After washing with PBS, fluorescence was analyzed with an Olympus U RFL microscope (Olympus, Hamburg, Germany) or by a flow cytometer.
RLIP76 immunoprecipitation
Cell-free lysates from EAhy926 were immunoprecipitated with mouse polyclonal anti-RLIP76 C-ter antibodies. In brief, cells were lysed in lysis buffer (20 mM HEPES, pH 7.2, 1% Nonidet P-40, 10% glycerol, 50 mM NaF, including protease inhibitors). To preclear nonspecific binding, cell-free lysates were mixed with protein A-acrylic beads (Bio-Rad) and stirred in a rotary shaker for 1 hour at 4°C. After centrifugation (500g for 1 minute), the supernatant was immunoprecipitated with mouse polyclonal anti-RLIP76 C-ter antibodies (3 μg) plus protein A-acrylic beads. The immunoprecipitates were subjected to 7.5% SDS-PAGE and immunoblotting with human anti-RLIP76 antibodies. Immunoreactivity was assessed by the chemiluminescence reaction using the enhanced chemoluminescence (ECL) Western blotting system (GE Healthcare, Little Chalfont, United Kingdom).
Immunohistochemistry
The superior thyroid artery, obtained from a patient after thyroidectomy, was immediately frozen. Cryostat sections of the artery were incubated with mouse anti-RLIP76 serum and with a biotinylated antimouse antibody and peroxidase-labeled streptavidin. Specimens were reincubated with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich) and nuclei were counterstained with Mayer haematoxylin. Controls included isotype-matched IgG and elimination of the primary antibody step.
4-Hydroxynonenal quantification
To evaluate the formation of 4-HNE adducts with histidine, cells fixed with 4% paraformaldehyde and permeabilized with 0.5% Triton X-100 (Sigma-Aldrich) were stained with specific monoclonal antibody against 4-HNE (10 μg/mL, R&D Systems, Minneapolis, MN) for 1 hour at 4°C. After washing, cells were incubated with an antimouse antiserum conjugated with Alexa-488 (Molecular Probes, Eugene, OR). After 30 minutes at 37°C, cells were washed twice and then analyzed on a cytometer. For fluorescence microscopic observations, cells were also counterstained with Hoechst and mounted with glycerol:PBS (2:1) before analyses by a Nikon Microphot equipped with intensified video microscopy (IVM) by a CCD camera (Carl Zeiss, Oberkochen, Germany). Micrographs were captured using IAS2000 software (Delta Sistemi, Rome, Italy).
Staining for intracellular GSH
Intracellular GSH was detected by monochlorobimane (Molecular Probes) staining as previously described.35 Samples were analyzed with an LRS II cytometer (Becton Dickinson, San Jose, CA) equipped with a UVB laser. Data obtained were analyzed by DIVA software (Becton Dickinson).
Annexin-V assay
Apoptosis was quantitatively evaluated by flow cytometry with the annexin-V–fluorescein isothiocyanate apoptosis detection kit (Eppendorf, Milan, Italy), which distinguishes early apoptotic (single annexin-V positive), late apoptotic (double annexin-V/propidium iodide positive) and necrotic cells (single propidium iodide positive).
Activation of caspase-3
The activation state of caspase-3 was evaluated with the CaspGLOW fluorescein active caspase staining Kit (MBL, Woburn, MA). Control and treated cells were incubated with FITC-conjugated caspase-3 inhibitor (DEVD-FMK) for 1 hour at 37°C, following the manufacturer's instructions. Samples were thereafter washed 3 times and immediately analyzed on a cytometer equipped with an FL-1 channel.
Activation of JNK
To evaluate the activation state of JNK by flow cytometry, we used a rabbit anti-JNK polyclonal antibody (BD/Pharmingen, Oxford, United Kingdom) able to recognize human JNK1 phosphorylated at T183 and Y185. Cells were fixed with paraformaldehyde (4% in PBS), permeabilized with Triton X-100 (0.05% in PBS), and then stained with anti-JNK (pT183/pY185) followed by addition of FITC-conjugated antirabbit for 45 minutes at 4°C. After washings, cells were resuspended in PBS and analyzed on a cytometer.
Statistical analysis
For the analysis of the associations between the clinical characteristics of patients and anti-RLIP76 antibodies, chi-square test was used to evaluate differences between percentages and the Mann-Whitney unpaired test was used to compare quantitative variables. Linear regression analysis (r correlation coefficient) was used to identify significant correlations. For the flow cytometric studies, at least 20 000 events were acquired. Data were recorded and statistically analyzed with a Macintosh computer using CellQuest Software (Becton Dickinson). Student t test was used for statistical analysis of mean values of the biologic variants analyzed in endothelial cells under the different treatments. Statistical significance of flow cytometric studies was calculated with the nonparametric Kolmogorov-Smirnov (K/S) test. Unless otherwise indicated, P values of less than .01 were considered significant.
Results
Identification of RLIP76 by immunoscreening of the HMVEC expression library and its characterization
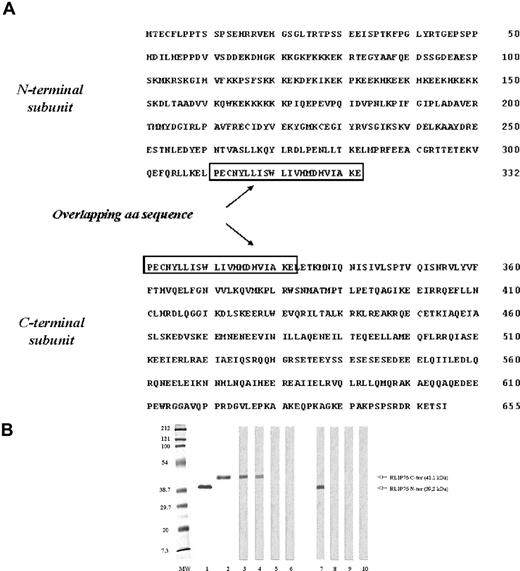
To identify genes encoding putative endothelial antigens, we immunoscreened a HMVEC expression library with IgG from the serum of 2 patients with BD. Besides clones with cDNA insertion of Sip1, a known BD autoantigen,29 we identified other strongly reactive clones with a 1968 base-pair open reading frame and a predicted amino acid sequence 655 residues long that had 100% identity with the glutathione conjugate transporter RLIP76. To identify the immunoreactive region of RLIP76, we cloned and expressed 2 distinct overlapping subunits corresponding to the N- and the C-terminal fragments (Figure 1A). These fragments showed the expected molecular sizes of 41.1 kDa for the C-terminal region and 39.2 kDa for the N-terminal region by 10% SDS-PAGE. In immunoblotting analysis, the patients' serum IgG used for immunoscreening the library recognized only the C-terminal fragment of RLIP76 (RLIP76 C-ter; Figure 1B).
The amino acid sequence and the immunochemical characterization of the N- and C-terminal regions of RLIP76. (A) The nucleotide sequence of the cloned cDNA (GenBank accession number NM 006788) was divided in 2 subunits by PCR with specific primers. The cDNA subunits were cloned in an expression vector and the N- and C-terminal regions of the protein were expressed and purified. The overlapped amino acids were squared. (B) The molecular size and the purity of the expressed proteins were confirmed by 10% SDS-PAGE stained by Coomassie blue (lane 1, N-terminal region; lane 2, C-terminal region) and serum immunoreactivity was analyzed by immunoblotting (lanes 3-6, C-terminal region; lanes 7-10, N-terminal region). Lanes 3,7: monoclonal antibody specific to 6-histidine tail; lanes 4,8: serum pool from the 2 patients with Behçet disease (BD) used in screening the library; lane 5,9: representative serum from a healthy subject; lanes 6,10: control without serum.
The amino acid sequence and the immunochemical characterization of the N- and C-terminal regions of RLIP76. (A) The nucleotide sequence of the cloned cDNA (GenBank accession number NM 006788) was divided in 2 subunits by PCR with specific primers. The cDNA subunits were cloned in an expression vector and the N- and C-terminal regions of the protein were expressed and purified. The overlapped amino acids were squared. (B) The molecular size and the purity of the expressed proteins were confirmed by 10% SDS-PAGE stained by Coomassie blue (lane 1, N-terminal region; lane 2, C-terminal region) and serum immunoreactivity was analyzed by immunoblotting (lanes 3-6, C-terminal region; lanes 7-10, N-terminal region). Lanes 3,7: monoclonal antibody specific to 6-histidine tail; lanes 4,8: serum pool from the 2 patients with Behçet disease (BD) used in screening the library; lane 5,9: representative serum from a healthy subject; lanes 6,10: control without serum.
Serum IgG immunoreactivity to RLIP76
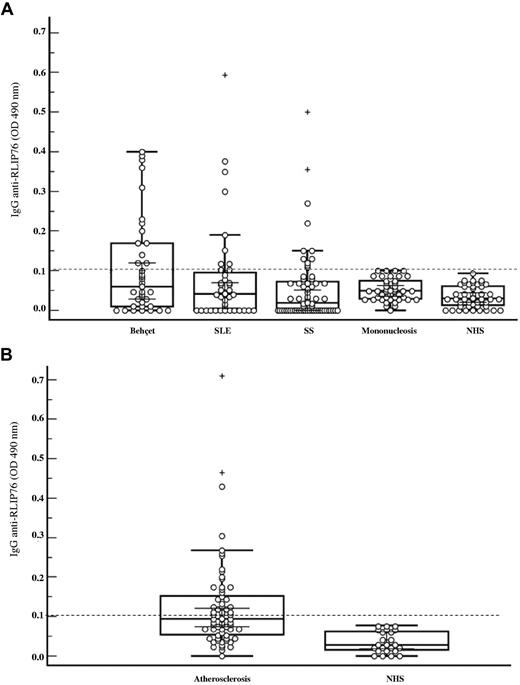
We analyzed serum IgG immunoreactivity to the N- and C-terminal regions of RLIP76. When we investigated the prevalence of serum anti-RLIP76 C-ter antibodies in patients with diseases characterized by endothelial dysfunction and controls, ELISA detected IgG specific to RLIP76 C-ter in sera from all the groups of patients studied (11/37 [30%] patients with BD, 11/65 [17%] patients with SS, and 10/40 [25%] patients with SLE) but in no sera from controls (patients with mononucleosis or age- and sex-matched healthy subjects; Figure 2A). ELISA also detected serum anti-RLIP76 C-ter antibodies in 27 of the 66 patients with carotid atherosclerosis (41%) but in no age- and sex-matched healthy subjects (Figure 2B). Preabsorption with RLIP76 C-ter itself of the sera from 2 patients with BD completely inhibited the antibody immunoreactivity thus confirming the specificity of ELISA (data not shown). No tested patients' or controls' sera reacted with RLIP76 N-ter (OD490 < .05). To assess the association of the clinical features in each disease with anti-RLIP76 C-ter antibody reactivity, we then subgrouped the patients according to the presence of serum anti-RLIP76 C-ter antibodies. For BD we considered ocular, genital, skin, or vascular involvement (Table 1); for SLE, SLE Disease Activity Index (SLEDAI), skin or kidney involvement, neuropsychiatric manifestations and serologic markers (triglycerides, cholesterol, HDL, LDL) (Table 2); for SS, lung fibrosis and skin score (Table 3); and for carotid atherosclerosis, diabetes, hypertension, cardiovascular diseases in relatives and hypercholesterolemia (Table 4). Although no significant difference was found between the presence of serum anti-RLIP76 C-ter antibodies and clinical variables, in the SS group, sera from patients with lung fibrosis more frequently contained RLIP76 C-ter antibodies than sera from patients without (6/11 [54%] vs 18/54 [33%]). Considering as indicators of disease activity SLEDAI for patients with SLE, skin score and lung fibrosis for patients with SS, and the criteria defined in “Patients” for patients with BD, we found no significant association between the presence of serum anti-RLIP76 antibodies and disease activity. No association was found between the presence of serum anti-RLIP76 antibodies and serum AECA or therapeutic regimen in the various subgroups (data not shown). Overall these data suggest that anti-RLIP76 antibodies are a new immunologic marker shared by patients with various immune-mediated endothelial diseases.
Anti-RLIP76 C-ter antibodies in patients and healthy controls. (A) Box-whisker plot of anti-RLIP76 C-ter IgG in patients with BD, SLE, SS, infectious mononucleosis, and from sex- and age-matched healthy donors (NHS). (B) Box-whisker plot of anti-RLIP76 C-ter IgG in patients with carotid atherosclerosis and from sex- and age-matched healthy donors (NHS). Median, quartiles, range, and possibly extreme values are indicated. The broken line represents the cutoff (mean + 2 SD for the healthy controls). Outliers are represented as +.
Anti-RLIP76 C-ter antibodies in patients and healthy controls. (A) Box-whisker plot of anti-RLIP76 C-ter IgG in patients with BD, SLE, SS, infectious mononucleosis, and from sex- and age-matched healthy donors (NHS). (B) Box-whisker plot of anti-RLIP76 C-ter IgG in patients with carotid atherosclerosis and from sex- and age-matched healthy donors (NHS). Median, quartiles, range, and possibly extreme values are indicated. The broken line represents the cutoff (mean + 2 SD for the healthy controls). Outliers are represented as +.
Clinical characteristics of patients with BD divided according to the presence of serum anti-RLIP76 IgG immunoreactivity
| Characteristics . | Patients with serum anti-RLIP76 IgG, n = 11 . | Patients without serum anti-RLIP76 IgG, n = 26 . |
|---|---|---|
| Median age, y (range) | 33 (28-54) | 43.5 (27-58) |
| Sex, M/F | 8/3 | 19/7 |
| Median disease duration, y (range) | 4.8 (0-14) | 6 (0-24) |
| Median C-reactive protein, mg/L | 3.2 (3.2-15.2) | 4.4 (0-129) |
| (range) | ||
| Genital ulcerations, % | 45.4 | 38.5 |
| Skin lesions, % | 54.5 | 61.5 |
| Eye involvement, % | 54.5 | 69.2 |
| Vascular manifestations, % | 36.4 | 20.8 |
| Arthrites, % | 9 | 23.1 |
| HLA B51, % | 75 | 66.6 |
| Active disease, % | 45.4 | 42.3 |
| Characteristics . | Patients with serum anti-RLIP76 IgG, n = 11 . | Patients without serum anti-RLIP76 IgG, n = 26 . |
|---|---|---|
| Median age, y (range) | 33 (28-54) | 43.5 (27-58) |
| Sex, M/F | 8/3 | 19/7 |
| Median disease duration, y (range) | 4.8 (0-14) | 6 (0-24) |
| Median C-reactive protein, mg/L | 3.2 (3.2-15.2) | 4.4 (0-129) |
| (range) | ||
| Genital ulcerations, % | 45.4 | 38.5 |
| Skin lesions, % | 54.5 | 61.5 |
| Eye involvement, % | 54.5 | 69.2 |
| Vascular manifestations, % | 36.4 | 20.8 |
| Arthrites, % | 9 | 23.1 |
| HLA B51, % | 75 | 66.6 |
| Active disease, % | 45.4 | 42.3 |
P > .05 for all characteristics.
Clinical characteristics of patients with SLE divided according to the presence of serum anti-RLIP76 IgG immunoreactivity
| Characteristics . | Patients withanti-RLIP76 IgG, n = 10 . | Patients without anti-RLIP76 IgG,n = 30 . | P . |
|---|---|---|---|
| Median age, y (range) | 36 (19-44) | 41 (26-71) | .03 |
| Sex, M/F | 0/10 | 5/25 | > .05 |
| Median disease duration, y (range) | 4 (0.5-24) | 7 (0.4-22) | > .05 |
| Median SLEDAI (range) | 0 (0-18) | 2 (0-25) | > .05 |
| Skin lesions, % | 60 | 46.6 | > .05 |
| Arthrites, % | 80 | 66.6 | > .05 |
| Neuropsychiatric manifestations, % | 30 | 26.6 | > .05 |
| Kidney involvement, % | 20 | 30.0 | > .05 |
| Cytopenia, % | 70 | 73.3 | > .05 |
| Serositis, % | 20 | 36.7 | > .05 |
| Antiphospholipid syndrome, % | 20 | 36.7 | > .05 |
| Characteristics . | Patients withanti-RLIP76 IgG, n = 10 . | Patients without anti-RLIP76 IgG,n = 30 . | P . |
|---|---|---|---|
| Median age, y (range) | 36 (19-44) | 41 (26-71) | .03 |
| Sex, M/F | 0/10 | 5/25 | > .05 |
| Median disease duration, y (range) | 4 (0.5-24) | 7 (0.4-22) | > .05 |
| Median SLEDAI (range) | 0 (0-18) | 2 (0-25) | > .05 |
| Skin lesions, % | 60 | 46.6 | > .05 |
| Arthrites, % | 80 | 66.6 | > .05 |
| Neuropsychiatric manifestations, % | 30 | 26.6 | > .05 |
| Kidney involvement, % | 20 | 30.0 | > .05 |
| Cytopenia, % | 70 | 73.3 | > .05 |
| Serositis, % | 20 | 36.7 | > .05 |
| Antiphospholipid syndrome, % | 20 | 36.7 | > .05 |
Clinical characteristics of patients with SS divided according to the presence of serum anti-RLIP76 IgG immunoreactivity
| Characteristics . | Patients with anti-RLIP76 IgG, n = 11 . | Patients without anti-RLIP76 IgG, n = 54 . |
|---|---|---|
| Median age, y (range) | 57 (47-65) | 52 (20-77) |
| Sex, M/F | 1/10 | 4/50 |
| Median disease duration, y (range) | 6.5 (1-19) | 6 (0.9-30) |
| Median erythrocyte sedimentation rate, | 16 (10-30) | 16 (2-64) |
| mm/hr (range) | ||
| Median C-reactive protein, mg/L (range) | 3 (0-72) | 3 (0-48) |
| Median C3 (range) | 144 (72-179) | 113 (69-286) |
| Median C4 (range) | 22 (11-51) | 22 (14-46) |
| Lung fibrosis, % | 54.5 | 33.3 |
| Median skin score (range)* | 13 (4-30) | 8 (4-27) |
| Characteristics . | Patients with anti-RLIP76 IgG, n = 11 . | Patients without anti-RLIP76 IgG, n = 54 . |
|---|---|---|
| Median age, y (range) | 57 (47-65) | 52 (20-77) |
| Sex, M/F | 1/10 | 4/50 |
| Median disease duration, y (range) | 6.5 (1-19) | 6 (0.9-30) |
| Median erythrocyte sedimentation rate, | 16 (10-30) | 16 (2-64) |
| mm/hr (range) | ||
| Median C-reactive protein, mg/L (range) | 3 (0-72) | 3 (0-48) |
| Median C3 (range) | 144 (72-179) | 113 (69-286) |
| Median C4 (range) | 22 (11-51) | 22 (14-46) |
| Lung fibrosis, % | 54.5 | 33.3 |
| Median skin score (range)* | 13 (4-30) | 8 (4-27) |
P > .05 for all characteristics.
Skin score is the assessment of skin thickening.
Clinical characteristics of patients with carotid atherosclerosis divided according to the presence of serum anti-RLIP76 IgG immunoreactivity
| Characteristics . | Patients with anti-RLIP76 IgG, n = 27 . | Patients without anti-RLIP76 IgG, n = 39 . |
|---|---|---|
| Median age, y (range) | 76 (68-82) | 71.5 (65-80) |
| Sex, M/F | 19/8 | 28/11 |
| Diabetes,* % | 23 | 28 |
| Smoking,† % | 70 | 62.5 |
| Hypertension,‡ % | 67 | 87 |
| Cardiovascular diseases in | 57 | 40 |
| relatives, % | ||
| Hypercholesterolemia,§ % | 14 | 14 |
| Characteristics . | Patients with anti-RLIP76 IgG, n = 27 . | Patients without anti-RLIP76 IgG, n = 39 . |
|---|---|---|
| Median age, y (range) | 76 (68-82) | 71.5 (65-80) |
| Sex, M/F | 19/8 | 28/11 |
| Diabetes,* % | 23 | 28 |
| Smoking,† % | 70 | 62.5 |
| Hypertension,‡ % | 67 | 87 |
| Cardiovascular diseases in | 57 | 40 |
| relatives, % | ||
| Hypercholesterolemia,§ % | 14 | 14 |
P > .05 for all characteristics.
Diabetes is defined as fasting glucose levels ≥140 mg/dL or need for antidiabetic medications.
Smoking is defined as current smokers.
Hypertension is defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or need for hypertensive medication.
Hypercholesterolemia is defined as total cholesterol >200 mg/dL or need for lipid-lowering therapy.
Localization of RLIP76 in EAhy926 cells under physiologic conditions and after oxidative stress
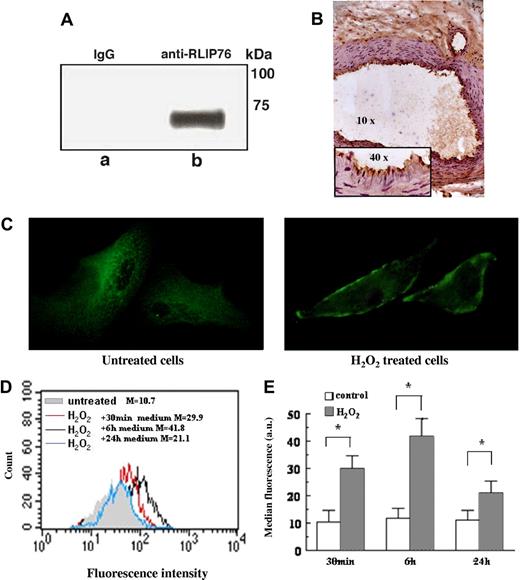
We analyzed RLIP76 expression in EAhy926 endothelial cells and in vascular tissue. Immunoprecipitation analysis showed that RLIP76 antibodies can immunoprecipitate the protein from EAhy926 endothelial cells in vitro (Figure 3A). Immunohistochemistry provided evidence that this protein is expressed on vascular endothelium in vivo (Figure 3B). To find out whether oxidative stress induces a redistribution of RLIP76 from the intracellular compartment into the membrane surface of endothelial cells, and to identify the extracellular region, we analyzed qualitatively (by fluorescence microscopy) and quantitatively (by flow cytometry) RLIP76 C-ter in EAhy926 cells under physiologic conditions and under mild oxidative stress.23,36 Immunofluorescence analysis with human purified antibodies specific to RLIP76 C-ter disclosed a redistribution of RLIP76 C-ter on the surface membrane of oxidative-stressed cells (Figure 3C). Accordingly, semiquantitative analysis of surface expression clearly showed that under physiologic conditions, RLIP76 C-ter was weakly expressed in the membrane. Under mild oxidative stress, membrane expression rapidly increased at 30 minutes, peaked at 6 hours, and diminished at 24 hours (Figure 3D,E).
RLIP76 expression and localization.(A) EAhy926 were immunoprecipitated with mouse polyclonal anti-RLIP76 C-ter antibodies. The immunoprecipitates were analyzed by Western blotting, using anti–human RLIP76 C-ter antibodies. Bound antibodies were visualized with HRP-conjugated anti–human IgG and immunoreactivity was assessed by ECL. Virtually no reactivity was found with immunoprecipitates obtained using non RLIP76-specific IgG (irrelevant). (B) RLIP76 expression in vascular tissue was detected by immunohistochemistry on tissue arrays with histologic sections from normal vascular human tissue incubated with mouse anti-RLIP76 C-ter antibodies. Intense immunoreactivity was observed in vascular endothelium. (C) Immunofluorescence analysis of RLIP76 distribution in EAhy926 untreated cells (left panel) or treated with 30 μM H2O2 30 minutes (right panel). Original magnification 500×, objective 100×, numeric aperture 1.4. (D,E) Flow cytometric analysis after surface staining of EAhy926 cells with antibodies to the RLIP76 C-terminus. (D) Results obtained in a representative experiment. Full light-gray histogram: untreated control cells; red histogram: H2O2-treated cells incubated for 30 minutes with fresh medium; black histogram: H2O2-treated cells incubated for 6 hours with fresh medium; blue histogram: H2O2-treated cells incubated for 24 hours with fresh medium. (E) Time-course evaluation of RLIP76 expression in untreated cells and in cells treated with H2O2 and then incubated for 30 minutes, 6 hours, and 24 hours in fresh medium (mean ± SD of the results obtained from 3 different experiments). *P < .01 by Student t test.
RLIP76 expression and localization.(A) EAhy926 were immunoprecipitated with mouse polyclonal anti-RLIP76 C-ter antibodies. The immunoprecipitates were analyzed by Western blotting, using anti–human RLIP76 C-ter antibodies. Bound antibodies were visualized with HRP-conjugated anti–human IgG and immunoreactivity was assessed by ECL. Virtually no reactivity was found with immunoprecipitates obtained using non RLIP76-specific IgG (irrelevant). (B) RLIP76 expression in vascular tissue was detected by immunohistochemistry on tissue arrays with histologic sections from normal vascular human tissue incubated with mouse anti-RLIP76 C-ter antibodies. Intense immunoreactivity was observed in vascular endothelium. (C) Immunofluorescence analysis of RLIP76 distribution in EAhy926 untreated cells (left panel) or treated with 30 μM H2O2 30 minutes (right panel). Original magnification 500×, objective 100×, numeric aperture 1.4. (D,E) Flow cytometric analysis after surface staining of EAhy926 cells with antibodies to the RLIP76 C-terminus. (D) Results obtained in a representative experiment. Full light-gray histogram: untreated control cells; red histogram: H2O2-treated cells incubated for 30 minutes with fresh medium; black histogram: H2O2-treated cells incubated for 6 hours with fresh medium; blue histogram: H2O2-treated cells incubated for 24 hours with fresh medium. (E) Time-course evaluation of RLIP76 expression in untreated cells and in cells treated with H2O2 and then incubated for 30 minutes, 6 hours, and 24 hours in fresh medium (mean ± SD of the results obtained from 3 different experiments). *P < .01 by Student t test.
Effects of anti-RLIP76 C-ter antibodies on intracellular 4-HNE and GSH levels in EAhy926 endothelial cells
To find out more about the role of RLIP76 in the cell response to oxidative stress, we studied the effects of anti-RLIP76 C-ter antibodies on EAhy926 endothelial cells under physiologic conditions and after mild oxidative stress.
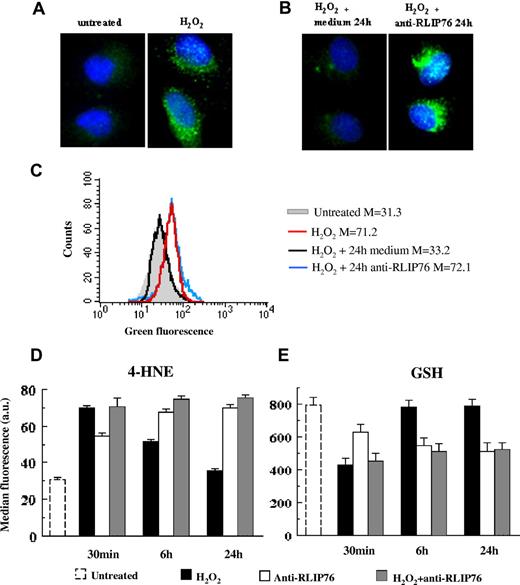
First, we analyzed the formation of 4-HNE adducts with histidine (Figure 4A-C). As expected, when endothelial cells were exposed to H2O2, 4-HNE formation increased (Figure 4A,C). When cells were allowed to recover for 24 hours in fresh culture medium, 4-HNE levels returned to baseline. Adding anti-RLIP76 C-ter antibodies to the medium completely prevented endothelial cell recovery (Figure 4B,C). A time-course analysis in H2O2-treated cells clearly confirmed that the time-dependent recovery of 4-HNE to baseline levels was abolished by adding anti-RLIP76 C-ter antibodies to the medium (Figure 4D). Levels of 4-HNE were significantly higher in cells incubated for 6 hours and 24 hours with anti-RLIP76 C-ter antibodies than in cells cultivated without. In untreated endothelial cells, anti-RLIP76 C-ter antibodies also induced per se a time-dependent increase in 4-HNE (Figure 4D).
Intracellular 4-HNE and GSH levels in EAhy926 endothelial cells. (A,B) Immunofluorescence analysis of the formation of 4-HNE adducts with histidine in EAhy926 cells stained with a 4-HNE specific antibody and counterstained with Hoechst dye to reveal nuclei. Panel A left, untreated cells; panel A right, H2O2-treated cells; panel B left, H2O2-treated cells incubated for 24 hours with fresh medium; panel B right, H2O2-treated cells incubated for 24 hours with medium containing anti-RLIP76 C-ter antibodies. Magnification 1500×, objective 100×, numeric aperture 1.4. (C) Quantitative analysis of 4-HNE adducts by flow cytometry in a representative experiment. Gray histogram, untreated control cells; red histogram, H2O2-treated cells; black histogram, H2O2-treated cells incubated for 24 hours with fresh medium; blue histogram, H2O2-treated cells incubated for 24 hours with medium containing anti-RLIP76 C-ter antibodies. Numbers represent median values of fluorescence intensity. (D) Quantitative time-course evaluation of 4-HNE intracellular content in untreated control cells; in cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies; in cells treated with H2O2; and in cells treated with H2O2 and then incubated for an additional 30 minutes, 6 hours, and 24 hours in fresh medium or in medium containing anti-RLIP76 C-ter antibodies. Statistical analysis performed by Student t test indicated P < .01 for: control untreated cells versus cells incubated with anti-RLIP76 C-ter antibodies for 30 minutes, 6 hours, and 24 hours; untreated cells versus H2O2-treated cells incubated for 30 minutes and 6 hours in fresh medium; H2O2-treated cells incubated in fresh medium versus H2O2-treated cells incubated for 6 hours and 24 hours with medium containing anti-RLIP76 C-ter antibodies. (E) Quantitative time-course evaluation of GSH intracellular content. Statistical analysis performed by Student t test indicated P < .01 for: untreated cells versus H2O2-treated cells incubated for 30 minutes in fresh medium; untreated cells versus cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies; H2O2-treated cells incubated in fresh medium versus H2O2-treated cells incubated for 6 hours and 24 hours with medium containing anti-RLIP76 C-ter antibodies. Data reported in panels D and E are the mean plus or minus SD of the results obtained from 3 different experiments.
Intracellular 4-HNE and GSH levels in EAhy926 endothelial cells. (A,B) Immunofluorescence analysis of the formation of 4-HNE adducts with histidine in EAhy926 cells stained with a 4-HNE specific antibody and counterstained with Hoechst dye to reveal nuclei. Panel A left, untreated cells; panel A right, H2O2-treated cells; panel B left, H2O2-treated cells incubated for 24 hours with fresh medium; panel B right, H2O2-treated cells incubated for 24 hours with medium containing anti-RLIP76 C-ter antibodies. Magnification 1500×, objective 100×, numeric aperture 1.4. (C) Quantitative analysis of 4-HNE adducts by flow cytometry in a representative experiment. Gray histogram, untreated control cells; red histogram, H2O2-treated cells; black histogram, H2O2-treated cells incubated for 24 hours with fresh medium; blue histogram, H2O2-treated cells incubated for 24 hours with medium containing anti-RLIP76 C-ter antibodies. Numbers represent median values of fluorescence intensity. (D) Quantitative time-course evaluation of 4-HNE intracellular content in untreated control cells; in cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies; in cells treated with H2O2; and in cells treated with H2O2 and then incubated for an additional 30 minutes, 6 hours, and 24 hours in fresh medium or in medium containing anti-RLIP76 C-ter antibodies. Statistical analysis performed by Student t test indicated P < .01 for: control untreated cells versus cells incubated with anti-RLIP76 C-ter antibodies for 30 minutes, 6 hours, and 24 hours; untreated cells versus H2O2-treated cells incubated for 30 minutes and 6 hours in fresh medium; H2O2-treated cells incubated in fresh medium versus H2O2-treated cells incubated for 6 hours and 24 hours with medium containing anti-RLIP76 C-ter antibodies. (E) Quantitative time-course evaluation of GSH intracellular content. Statistical analysis performed by Student t test indicated P < .01 for: untreated cells versus H2O2-treated cells incubated for 30 minutes in fresh medium; untreated cells versus cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies; H2O2-treated cells incubated in fresh medium versus H2O2-treated cells incubated for 6 hours and 24 hours with medium containing anti-RLIP76 C-ter antibodies. Data reported in panels D and E are the mean plus or minus SD of the results obtained from 3 different experiments.
Because intracellular 4-HNE detoxification involves the most abundant cellular thiol-containing peptide, GSH, we analyzed the time courses of endothelial intracellular GSH content.37 At 30 minutes after cells had been exposed to H2O2, intracellular levels of GSH decreased significantly and at 6 hours and 24 hours recovered to baseline levels. The time-dependent recovery of GSH levels was abolished by adding anti-RLIP76 C-ter antibodies to the medium. In untreated cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies, GSH levels significantly decreased (Figure 4E). Linear regression analysis showed a strong negative correlation between intracellular 4-HNE and GSH (r = −0.94, P < 10−4). Control purified total IgG from healthy subjects left 4-HNE and GSH levels appreciably unchanged (data not shown). Collectively, these data suggest that anti-RLIP76 C-ter antibodies not only lower physiologic cellular defenses against oxidative stress but also directly per se increase the formation of oxidative by-products.
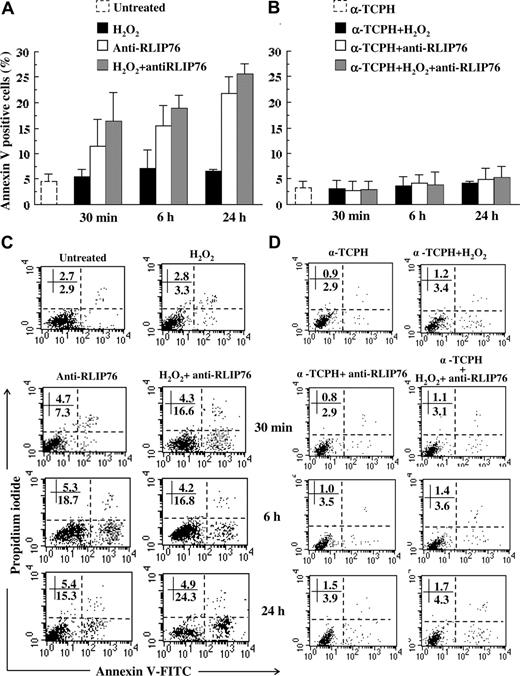
Effects of anti-RLIP76 C-ter on induction of cellular apoptosis
The well-known relationship among apoptosis, 4-HNE and GSH prompted us next to analyze the possible role of anti-RLIP76 C-ter antibodies as an apoptotic inducer in endothelial cells.38 Anti-RLIP76 C-ter antibodies induced apoptosis in a significant percentage of H2O2-treated and untreated cells (P < .01 after 30 minutes, 6 hours, and 24 hours of antibody incubation; Figure 5A,C). Under all experimental conditions, apoptosis correlated positively with 4-HNE levels (r = 0.8, P < 10−4) and negatively with GSH (r = −0.75, P < 10−4). In experiments incubating cells with purified total IgG from healthy subjects, endothelial cell apoptosis remained unchanged (data not shown).
Induction of apoptosis by anti-RLIP76 C-ter antibodies in EAhy926 endothelial cells.(A,C) Flow cytometric analysis after double staining with annexin V/propidium iodide of untreated control cells; cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies; and in cells treated with H2O2 and then incubated additional 30 minutes, 6 hours, and 24 hours in fresh medium or with medium containing anti-RLIP76 C-ter antibodies. (B,D) Cells were also pretreated with α-TCPH. (A,B) Results obtained from 3 independent experiments are reported as mean plus or minus SD. (C,D) Dot plots from a representative experiment. Numbers represent the percentage of annexin V single positive (early apoptosis, bottom right quadrant) or annexin V/PI double positive cells (late apoptosis, bottom right quadrant). Statistical analysis performed by Student t test indicated P < .01 for: control untreated cells versus H2O2-treated cells; control untreated cells versus cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies; and cells treated with H2O2 and then incubated in fresh medium versus cells treated with H2O2 and then incubated for 6 hours and 24 hours with medium containing anti-RLIP76 C-ter antibodies. P < .01 for any treatment (panel A) versus the same treatment performed after α-TCPH preincubation (panel B).
Induction of apoptosis by anti-RLIP76 C-ter antibodies in EAhy926 endothelial cells.(A,C) Flow cytometric analysis after double staining with annexin V/propidium iodide of untreated control cells; cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies; and in cells treated with H2O2 and then incubated additional 30 minutes, 6 hours, and 24 hours in fresh medium or with medium containing anti-RLIP76 C-ter antibodies. (B,D) Cells were also pretreated with α-TCPH. (A,B) Results obtained from 3 independent experiments are reported as mean plus or minus SD. (C,D) Dot plots from a representative experiment. Numbers represent the percentage of annexin V single positive (early apoptosis, bottom right quadrant) or annexin V/PI double positive cells (late apoptosis, bottom right quadrant). Statistical analysis performed by Student t test indicated P < .01 for: control untreated cells versus H2O2-treated cells; control untreated cells versus cells incubated for 30 minutes, 6 hours, and 24 hours with anti-RLIP76 C-ter antibodies; and cells treated with H2O2 and then incubated in fresh medium versus cells treated with H2O2 and then incubated for 6 hours and 24 hours with medium containing anti-RLIP76 C-ter antibodies. P < .01 for any treatment (panel A) versus the same treatment performed after α-TCPH preincubation (panel B).
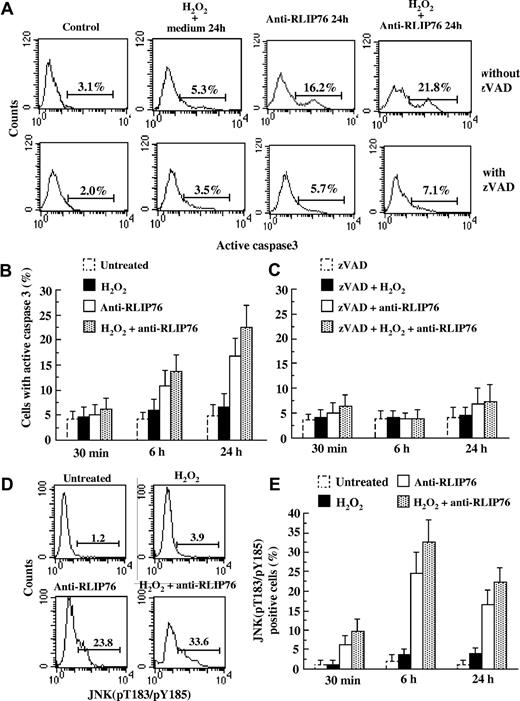
Because the foregoing results suggested that apoptosis induced by anti-RLIP76 C-ter antibodies could be mediated at least in part by 4-HNE (and possibly by other oxidized lipids), we analyzed the same time course in cells pretreated with α-TCPH, the most active form of vitamin E in humans, known to prevent lipid oxidation. As expected, under all the experimental conditions, cell pretreatment with α-TCPH completely prevented apoptosis induced by anti-RLIP76 C-ter antibodies (Figure 5B,D). We also found that apoptosis induced by anti-RLIP76 C-ter antibodies was caspase-dependent as evidenced by detection of caspase-3 enzymatic activity (Figure 6A,B). The pan-caspase inhibitor zVAD significantly prevented anti-RLIP76 C-ter antibody–induced caspase-3 activation (Figure 6C) and apoptosis (data not shown).
Apoptotic pathway induced by anti-RLIP76 C-ter in EAhy926 cell line. (A) Flow cytometric data were obtained in the absence (first row) or in the presence (second row) of 30 μM pan-caspase inhibitor zVAD. The numbers in each panel refer to the percentage of cells containing caspase 3 in its active form. Results obtained in a representative experiment are reported. (B,C) Graphs showing the mean plus or minus SD of the percentages of cells with the active form of caspase 3 obtained from 3 different experiments done without (panel B) or with (panel C) zVAD. Statistical analyses indicate a significant (P < .01) decrease in caspase 3 activity in cells pretreated with zVAD before anti-RLIP76 C-ter antibody exposure. (D) Quantitative flow cytometric analysis investigating the JNK activation state 6 hours after the various treatments with a polyclonal antibody able to identify JNK (pT183/pY185). The numbers in each panel refer to the percentage of cells containing JNK in its active form. Results obtained in a representative experiment are reported. (E) Time-course analysis of the activation state of JNK. Statistical analyses of the results obtained from 3 independent experiments (reported as mean ± SD) indicate a significant difference (P < .01) between cells treated with RLIP76 C-ter antibodies, alone or in combination with H2O2, and both untreated and H2O2-treated cells at any time point considered.
Apoptotic pathway induced by anti-RLIP76 C-ter in EAhy926 cell line. (A) Flow cytometric data were obtained in the absence (first row) or in the presence (second row) of 30 μM pan-caspase inhibitor zVAD. The numbers in each panel refer to the percentage of cells containing caspase 3 in its active form. Results obtained in a representative experiment are reported. (B,C) Graphs showing the mean plus or minus SD of the percentages of cells with the active form of caspase 3 obtained from 3 different experiments done without (panel B) or with (panel C) zVAD. Statistical analyses indicate a significant (P < .01) decrease in caspase 3 activity in cells pretreated with zVAD before anti-RLIP76 C-ter antibody exposure. (D) Quantitative flow cytometric analysis investigating the JNK activation state 6 hours after the various treatments with a polyclonal antibody able to identify JNK (pT183/pY185). The numbers in each panel refer to the percentage of cells containing JNK in its active form. Results obtained in a representative experiment are reported. (E) Time-course analysis of the activation state of JNK. Statistical analyses of the results obtained from 3 independent experiments (reported as mean ± SD) indicate a significant difference (P < .01) between cells treated with RLIP76 C-ter antibodies, alone or in combination with H2O2, and both untreated and H2O2-treated cells at any time point considered.
To find out whether anti-RLIP76 C-ter antibodies activate the typical oxidative signaling pathway, we investigated JNK phosphorylation. Treatment with anti-RLIP76 C-ter antibodies, either alone or in combination with H2O2, led to the phosphorylation of JNK in a large percentage of cells. JNK activation started after 30 minutes, peaked after 6 hours, and decreased 24 hours after exposure to anti-RLIP76 C-ter antibodies (Figure 6D,E).
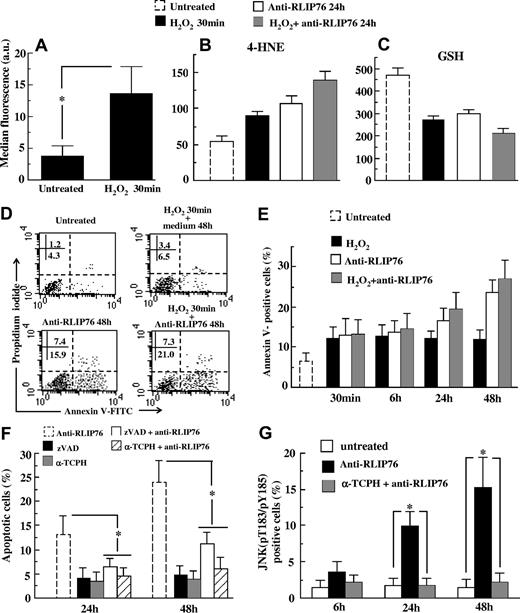
Effects of anti RLIP76 C-ter antibodies on microvascular cells
Besides using the EAhy926 cell line, a model of macrovascular endothelium, we analyzed the biological effects of anti-RLIP76 C-ter antibodies on a primary microvascular cell line (HMVEC-L). When these cells were subjected to mild H2O2-induced oxidative stress, RLIP76 expression at the cell surface increased (Figure 7A). In H2O2-treated and in untreated HMVEC-L, anti-RLIP76 C-ter antibodies exposure for 24 hours induced 4-HNE adduct formation (Figure 7B), GSH depletion (Figure 7C), and apoptosis (Figure 7D,E). Both α-TCPH and the caspase inhibitor zVAD completely prevented RLIP76 C-ter antibody–induced apoptosis in HMVEC-L cells (Figure 7F). JNK-mediated signaling started 24 hours after cells were exposed to anti-RLIP76 C-ter antibodies (Figure 7G).
Effects of anti-RLIP76 C-ter antibodies on human microvascular primary cells. (A) Flow cytometric analysis of HMVEC-L cells, either untreated or H2O2-treated cells, after surface staining with antibodies to RLIP76. Mean plus or minus SD of the results obtained from 3 different experiments. *P < .01 by Student t test. (B,C) Quantitative flow cytometric analysis of 4-HNE adducts and GSH intracellular content in: untreated control cells, cells treated with H2O2 for 30 minutes, cells incubated with anti-RLIP76 C-ter antibodies for 24 hours, and cells treated with H2O2 for 30 minutes and then incubated for an additional 24 hours in medium containing anti-RLIP76 C-ter antibodies. Data reported in panels B and C are the mean plus or minus SD of the results obtained from 3 different experiments. Student t test indicated P < .01 for: control untreated cells versus H2O2-treated cells; untreated cells versus cells incubated with anti-RLIP76 C-ter antibodies for 24 hours; and untreated cells versus H2O2-treated cells incubated for 24 hours with medium containing anti-RLIP76 C-ter antibodies. (D) Dot plots from a representative experiment performed 48 hours after different treatments. Numbers represent the percentage of annexin V single positive (early apoptosis, bottom right quadrant) or annexin V/PI double positive cells (late apoptosis, bottom right quadrant). (E) Flow cytometric analysis of apoptosis after double staining with annexin V/propidium iodide of untreated control cells; cells treated with H2O2 and then incubated at different time points with fresh medium or with medium containing anti-RLIP76 C-ter antibodies. Cells were also treated for the same times with anti-RLIP76 C-ter antibodies given alone. Results obtained from 3 independent experiments are reported as mean plus or minus SD. Student t test indicated P < .01 for: control untreated cells versus cells incubated for 24 and 48 hours with anti-RLIP76 C-ter antibodies, and cells treated with H2O2 and then incubated in fresh medium versus cells treated with H2O2 and then incubated for 24 and 48 hours with medium containing anti-RLIP76 C-ter antibodies. (F) Quantitative flow cytometric analysis of apoptosis in cells treated with anti-RLIP76 C-ter antibodies for 24 and 48 hours pretreated or not with zVAD or α-TCPH as indicated in “Methods.” As control, cells were also treated at the same time points with zVAD or α-TCPH given alone. Results obtained from 3 independent experiments are reported as mean plus or minus SD. (G) Quantitative flow cytometric analysis of the JNK activation state obtained with a polyclonal antibody specific for active form of JNK (pT183/pY185) in cells treated with anti-RLIP76 C-ter antibodies in the presence or absence of α-TCPH. * represents P < .01 by Student t test.
Effects of anti-RLIP76 C-ter antibodies on human microvascular primary cells. (A) Flow cytometric analysis of HMVEC-L cells, either untreated or H2O2-treated cells, after surface staining with antibodies to RLIP76. Mean plus or minus SD of the results obtained from 3 different experiments. *P < .01 by Student t test. (B,C) Quantitative flow cytometric analysis of 4-HNE adducts and GSH intracellular content in: untreated control cells, cells treated with H2O2 for 30 minutes, cells incubated with anti-RLIP76 C-ter antibodies for 24 hours, and cells treated with H2O2 for 30 minutes and then incubated for an additional 24 hours in medium containing anti-RLIP76 C-ter antibodies. Data reported in panels B and C are the mean plus or minus SD of the results obtained from 3 different experiments. Student t test indicated P < .01 for: control untreated cells versus H2O2-treated cells; untreated cells versus cells incubated with anti-RLIP76 C-ter antibodies for 24 hours; and untreated cells versus H2O2-treated cells incubated for 24 hours with medium containing anti-RLIP76 C-ter antibodies. (D) Dot plots from a representative experiment performed 48 hours after different treatments. Numbers represent the percentage of annexin V single positive (early apoptosis, bottom right quadrant) or annexin V/PI double positive cells (late apoptosis, bottom right quadrant). (E) Flow cytometric analysis of apoptosis after double staining with annexin V/propidium iodide of untreated control cells; cells treated with H2O2 and then incubated at different time points with fresh medium or with medium containing anti-RLIP76 C-ter antibodies. Cells were also treated for the same times with anti-RLIP76 C-ter antibodies given alone. Results obtained from 3 independent experiments are reported as mean plus or minus SD. Student t test indicated P < .01 for: control untreated cells versus cells incubated for 24 and 48 hours with anti-RLIP76 C-ter antibodies, and cells treated with H2O2 and then incubated in fresh medium versus cells treated with H2O2 and then incubated for 24 and 48 hours with medium containing anti-RLIP76 C-ter antibodies. (F) Quantitative flow cytometric analysis of apoptosis in cells treated with anti-RLIP76 C-ter antibodies for 24 and 48 hours pretreated or not with zVAD or α-TCPH as indicated in “Methods.” As control, cells were also treated at the same time points with zVAD or α-TCPH given alone. Results obtained from 3 independent experiments are reported as mean plus or minus SD. (G) Quantitative flow cytometric analysis of the JNK activation state obtained with a polyclonal antibody specific for active form of JNK (pT183/pY185) in cells treated with anti-RLIP76 C-ter antibodies in the presence or absence of α-TCPH. * represents P < .01 by Student t test.
Discussion
In this study, using a molecular cloning strategy, we identified a new antigenic target of AECA involved in the autoimmune processes causing endothelial damage. The main finding in this study is that the C-terminal subunit of RLIP76 we cloned is a novel autoantigen in various immune-mediated diseases characterized by endothelial dysfunction, BD, SLE, SS, and carotid atherosclerosis. Our findings strongly suggest that autoantibodies specific to the RLIP76 C-ter exert pathogenetic effects on the endothelium by inducing oxidative stress-mediated apoptosis.
Our results extend current knowledge about the glutathione conjugate transporter RLIP76. We provide evidence that the C-terminal immunoreactive region of RLIP76 may be accessible to serum antibodies on the surface of both macrovascular and microvascular endothelium. Confirming published data,23 we also found that after mild, transient oxidative stress, RLIP76 redistributes from the intracellular compartment to the plasma cell membrane. More important, we provide new evidence showing anti-RLIP76 C-ter antibodies in sera from patients with immune-mediated vascular diseases. In our in vitro experiments, by blocking the physiologic function of RLIP76 to throw out GS-HNE, these autoantibodies depleted cellular antioxidant defenses, thereby causing oxidative imbalance increasing 4-HNE intracellular levels, decreasing GSH levels, and ultimately inducing apoptosis in endothelial cells under physiologic conditions and after mild oxidative stress (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These findings are consistent with previous studies suggesting that inhibition of RLIP76 with rabbit specific IgG induced apoptosis.39,40 In our experiments, the antioxidant α-TCPH, the main component of vitamin E, counteracted the effects of anti-RLIP76 C-ter antibodies on cell apoptosis confirming that these autoantibodies induce oxidative stress-mediated apoptosis.
Oxidative stress, expressed by increased 4-HNE level, was found in several degenerative and immune-mediated diseases, confirming the idea that it could have a pathogenetic role in such diseases.41-46 Our findings suggest that 4-HNE could induce apoptosis also through immune-mediated mechanisms. Although the precise mechanism of 4-HNE–induced apoptosis is unclear, several studies show that 4-HNE reduces cellular GSH.38 GSH is an endogenous thiol that plays an important role as antioxidant in regulating cellular redox status.47 In particular, GSH has a pivotal role in protecting macromolecules, such as DNA, from cyclic adduction by 4-HNE, suppressing the oxidation of lipids and, consequently, reducing the formation of 4-HNE. Hence, depletion of GSH in tissues finally leads to an increased formation of oxidized products.48 Decreased GSH levels have been found in numerous diseases such as cancer, viral infections, and immune dysfunctions.49
As expected, cytofluorimetric analysis showed that anti-RLIP76 C-ter antibodies activate JNK, thus inducing the signaling pathway typical of oxidative damage.23,39 Oxidative stress–mediated JNK activation is a critical component that decides cell fate in response to various stress stimuli.50
Our study provides new evidence suggesting that the various diseases related to endothelial dysfunction may arise through a common pathogenetic mechanism, possibly involving anti-RLIP76 C-ter antibodies. We found anti-RLIP76 C-ter antibodies not only in the sera from patients with autoimmune vasculitis and vasculopathies but also in the sera of a high percentage (41%) of patients with carotid atherosclerosis. The presence of pathogenetic serum autoantibodies specific to the RLIP76 C-terminus in patients with atherosclerosis supports the recently emerging role of the immune system in the development and progression of atherosclerosis. Atherosclerosis arises as a vascular wall response to endothelial injury. Endothelial cell apoptosis may be an important mechanism of vascular injury leading to the disruption of the endothelial barrier with vascular leak, extravasation of plasma proteins, and exposure of the prothrombotic subendothelial matrix.51,52 Accumulating evidence suggests that endothelial cell apoptosis could play a critical role as an initial pathogenic event in atherosclerosis, SS, and SLE.53-56
In this study we found that anti-RLIP76 C-ter antibodies bound RLIP76 on endothelial cells. We cannot exclude the possibility that anti-RLIP76 C-ter antibodies may induce oxidative damage or complement-mediated cytoxicity, or both, in other cellular types besides endothelial cells. In fact, RLIP76 protein is expressed by various types of cells including endothelial cells even though contrasting data have been reported on its expression on endothelium in brain.57,58
The potent activity of anti-RLIP76 C-ter antibodies we observed in vitro clearly disagrees with their lack of correlation with clinical disease activity. One explanation might be that anti-RLIP76 C-ter antibodies in vivo failed to induce oxidant-mediated damage because this protein is poorly expressed on the cell surface. Our experiments showed RLIP76 expression and anti-RLIP76 C-ter antibodies induced cellular damage in unperturbed endothelial cells in culture. Cells in culture can nevertheless spontaneously undergo oxidative stress, thus increasing surface expression of RLIP76. Hence, we consider it unlikely that in vivo anti-RLIP76 C-ter antibodies stimulate cellular damage without a preexisting oxidant-mediated damage caused, for instance, by inflammation or infections, and further studies will be necessary to clarify whether the presence of anti-RLIP76 C-ter antibodies in the serum could be a risk factor for the onset of vascular diseases. A combination of several antibodies rather than a single autoantibody alone could be used as biologic markers to monitor disease manifestations and progression. In single patients, an antibody panel that mirrors the various stages of disease might be needed to optimize therapy and prevent disability. Studies are in progress to clarify the effective role of anti-RLIP76 C-ter antibodies in vivo and to provide new insights into their potential pathogenicity. An important question our study leaves unanswered is whether a diet rich in antioxidant elements, in particular vitamin E, counteract the pathogenic effect of anti-RLIP76 C-ter antibodies.
How the RLIP76 C-ter becomes autoantigenic remains unclear. Our study leaves open the possibility that RLIP76 becomes antigenic through mechanisms of molecular mimicry. Further investigations will clarify the possible cross-reaction with molecules from microorganisms associated with immune-mediated vascular diseases and RLIP76.
Overall, our study suggests that future research should be aimed at evaluating the potential therapeutic effectiveness of antioxidant agents in patients with endothelial dysfunction who have serum anti-RLIP76 C-ter antibodies. A valuable therapeutic approach in these patients could be to immunomodulate or block the anti-RLIP76 immune response. Besides, natural products having an antioxidant activity, such as vitamin E, could represent an optional complementary therapeutic tool. Finally, autoantibodies specific to the RLIP76 C-ter might in the long run become a marker of prognostic value in these human diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Angela Tagliani for help in immunohistochemical analysis.
This work was supported by a research grant from the Italian Ministry of Health (project no. 6ACF/1) to E.O., grant ISS/National Institutes of Health to W.M., and Fondazione Umberto Di Mario, Organizzazione Non Lucrativa di Utilitá Sociale to G.V.
National Institutes of Health
Authorship
Contribution: P. Margutti and E.O. conceived the idea; P. Matarrese conducted the cytofluorimetric experiments on endothelial cell lines and participated in the design of the study and analysis of data; P. Margutti and T.C. screened the library, conducted the ELISA experiments, and participated in the design of the study and analysis of the data; F.D. cloned and sequenced cDNA, purified the recombinant protein, and helped to interpret the data; F.C. and C.A. participated in the design of the study and analysis of data, helped to draft the manuscript, and were responsible for the autoimmune patient selection; R.R. and E.P. participated in the design and revision of the study; A.S. participated in the analysis and interpretation of data and helped to draft the manuscript; B.S. was responsible for the selection of patients with atherosclerosis; G.V. participated in the design of the study and in the revision of the manuscript; A.C. and T.G. conducted the immunofluorescence analysis on endothelial cell lines and participated in the design of the study; P. Margutti, E.O., M.S., and W.M. coordinated the study and drafted the manuscript; all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elena Ortona, Department of Infectious, Para-sitic and Immune-Mediated Diseases; Section of Immune-Mediated Diseases; Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Rome, Italy; e-mail: elena.ortona@iss.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal