Abstract
Endothelial cells (ECs) presenting minor histocompatibility antigen (mhAg) are major target cells for alloreactive effector CD8+ T cells during chronic transplant rejection and graft-versus-host disease (GVHD). The contribution of ECs to T-cell activation, however, is still a controversial issue. In this study, we have assessed the antigen-presenting capacity of ECs in vivo using a transgenic mouse model with beta-galactosidase (β-gal) expression confined to the vascular endothelium (Tie2-LacZ mice). In a GVHD-like setting with adoptive transfer of β-gal–specific T-cell receptor–transgenic T cells, β-gal expression by ECs was not sufficient to either activate or tolerize CD8+ T cells. Likewise, transplantation of fully vascularized heart or liver grafts from Tie2-LacZ mice into nontransgenic recipients did not suffice to activate β-gal–specific CD8+ T cells, indicating that CD8+ T-cell responses against mhAg cannot be initiated by ECs. Moreover, we could show that spontaneous activation of β-gal–specific CD8+ T cells in Tie2-LacZ mice was exclusively dependent on CD11c+ dendritic cells (DCs), demonstrating that mhAgs presented by ECs remain immunologically ignored unless presentation by DCs is granted.
Introduction
Endothelial cells (ECs) act as the major interface between blood and tissues. Forming the inner lining of blood vessels, they are uniquely positioned between circulating lymphocytes and the periphery and thereby regulate the trafficking of T lymphocytes from the bloodstream to sites of infection and inflammation. Following transplantation of vascularized organs, ECs are the first graft cells encountered by activated host lymphocytes and are therefore primary targets of alloreactive cytotoxic T lymphocytes (CTLs).1,2 Since donor ECs persist in vascularized organ transplants, they may contribute to chronic immune stimulation and thereby fuel the process of chronic rejection. Such late graft failure is a major problem in transplantation medicine that frequently necessitates retransplantation.3 Furthermore, ECs are important target cells for activated alloreactive CTLs during graft-versus-host disease (GVHD),4 which is characterized by large numbers of circulating minor histocompatibility antigen (mhAg)–specific CTLs.5
ECs can act as antigen-presenting cells (APCs) to CD8+ T cells both via the direct pathway (ie, recognition of allo–major histocompatibility complex [MHC]/peptide complexes) or via the indirect pathway involving cross-presentation of exogenous antigens.6-8 In vitro studies have demonstrated that both human9 and murine10 ECs can activate resting allogeneic CD8+ T cells, suggesting that ECs critically contribute to the initial stimulation of alloreactive T lymphocytes.1 Moreover, ECs exhibit important functions of professional APCs, including expression of MHC class II and costimulatory molecules11 and cross-presentation of minor histocompatibility antigens.6,8 The notion that ECs may under particular circumstances act as professional APCs has been supported by the finding that nonhematopoietic cells within vascularized grafts—presumably ECs—are able to initiate CTL responses that mediate allograft rejection.12
There are, however, a number of reports challenging the view that ECs may act as immune activators. Murine lung ECs, for example, have been shown to negatively regulate CD8+ T-cell function.13 Furthermore, liver sinusoidal ECs can induce CD8+ T-cell tolerance to soluble8,14 or tumor-derived antigens.15 A third possible form of EC-CTL interaction is that of immunologic ignorance. Indeed, aly/aly mice lacking secondary lymphoid organs fail to reject vascularized organ transplants, even in an allogeneic setting,16 suggesting that the environment of organized lymphoid tissues is critical for primary activation of T-cell responses. To a large extent, these contradictory findings can be explained by the use of in vitro coculture systems or the lack of an appropriate in vivo model with truly EC-restricted antigen presentation. An experimental in vivo system with expression of well-defined antigens exclusively in vascular ECs may therefore be helpful to solve the question whether antigen presentation by vascular ECs can lead to activation or tolerization of antigen-specific CD8+ T cells.
The use of antigen-transgenic mice combined with the power of T-cell receptor (TCR)–transgenic animals has provided important insight into the basic principles of autoimmunity17,18 and tumor immunity.19,20 Recently, similar systems have been exploited to analyze T-cell responses in different allograft transplantation6,21-23 and GVHD models.24 However, despite significant advances in our understanding of the antigen-presenting function of nonhematopoietic cells during allograft reactions21 or the importance of T-cell frequencies for solid organ graft rejection,23,25 the precise role of mhAg presentation by ECs has remained elusive. We have used here Tie2-LacZ mice to model mhAg presentation by ECs. In these mice, the tie2 promotor drives the expression of the beta-galactosidase (β-gal) antigen in ECs in all tissues.26 In vivo analysis of antigen-specific interaction between ECs and CD8+ T cells has been facilitated by using high-affinity β-gal–specific TCR-transgenic CD8+ T cells (Bg1 cells). Adoptive transfer of Bg1 CD8+ T cells into Tie2-LacZ mice revealed that mhAg presentation by ECs did not suffice to activate or to tolerize CD8+ T cells. Furthermore, β-gal expression by ECs in heterotopically transplanted Tie2-LacZ hearts or orthotopically transplanted Tie2-LacZ livers did not result in CD8+ T-cell activation in naive recipients. Finally, generation of bone marrow (BM) chimeric mice that facilitated selective ablation of CD11c+ dendritic cells (DCs) revealed that EC-associated mhAg has to be cross-presented by DCs in order to elicit CD8+ T-cell activation.
Methods
Mice
Male and female C57BL/6 mice were obtained from Charles River (Sulzfeld, Germany). Tie2-LacZ mice26 had been backcrossed with C57BL/6 mice at least 14 times. B6.C-H2bm1 mice were provided by Christian Kurts (University of Bonn, Germany). Bg1 mice were produced with TCR cassette vectors generously provided by Dr Diane Mathis (Brigham and Women's Hospital, Boston, MA). RNA was isolated from a β-gal96-103–specific CD8+ T-cell clone, generated by limiting dilution, using silica matrix columns (Qiagen, Valencia, CA). Known TCR α and β constant region sequences were used to perform 5′ rapid amplification of cDNA ends (Invitrogen, Carlsbad, CA), and TCR sequences were then cloned into pCR4TOPO TA cloning sequencing vectors (Invitrogen). The TCR α and β transcripts were sequenced using an ABI Prism (Perkin-Elmer, Wellesley, MA), and these sequences were compared with available sequences to develop genomic cloning polymerase chain reaction (PCR) primers. These cloning primers provide amplification of the variable domains consisting of 10 to 20 bp upstream of the start codon through 200 to 300 bp of intronic sequence downstream of the junctional regions, thereby preserving splice donor/acceptor sites. The α and β genomic variable domains were PCR-amplified (Perkin-Elmer) and TA-cloned into a sequencing vector (Invitrogen). The genomic variable domains were sequenced (Vα1/JαTA13/Cα and Vβ7S1/Jβ2S4/Cβ2) and subcloned into the TCR cassette vectors. The α and β cassette vectors were coinjected into fertilized C57BL/6 embryos (SAIC, Frederick, MD), and founders were obtained. The resulting mice, named Bg1, were maintained as heterozygotes, as a high rate of lymphoma in homozygotes reduced their life span. Heterozygotes were bred to B6.SJL mice and transgene expression was monitored by staining of blood cells with anti-Vβ7 by flow cytometry. Bg1 mice were further crossed with C57BL/6 mice expressing the congenic marker Thy1.1. Mice expressing the human high-affinity diphteria toxin receptor (DTR) under the control of the CD11c promoter27 were provided by Steffen Jung (The Weizmann Institute of Science, Rehovot, Israel). The presence of the β-gal and DTR transgenes was determined by PCR from genomic DNA; the presence of the H2-Kbm1 molecule was determined by flow cytometry of blood lymphocytes using the 5F1 antibody.28 All animals were kept under conventional conditions in individually ventilated cages and fed with normal chow diet. Experiments were carried out with age-matched (6-8 weeks) and sex-matched animals. Experiments were performed in accordance with Swiss Kantonal and federal legislations and were approved by the Veterinary Officer of the Kanton of St Gallen.
Viruses and peptides
Recombinant murine cytomegalovirus (MCMV) expressing the β-gal protein under the transcriptional control of the human CMV ie1/ie2 promoter-enhancer (MCMV-LacZ RM42729 ) was kindly provided by Prof E. S. Mocarski (Stanford University, Stanford, CA). MCMV-LacZ was propagated and titrated on NIH 3T3 cells (European Collection of Cell Cultures, Salisbury, United Kingdom) and injected intravenously at a dose of 2 × 106 pfu. β-gal96-103 (DAPIYTNV),30 β-gal497-504 (ICPMYARV),31 and MCMV M45985-993 (HGIRNASFI)32 peptides were purchased from Neosystem (Strasbourg, France).
Generation of BM chimeric mice
Recipient mice were lethally irradiated with 9 Gy (900 rad) from a linear accelerator (Clinic of Radio-Oncology, Kantonal Hospital, St Gallen) and intravenously injected 1 day later with 2 × 107 of the indicated donor BM cells. Chimeric mice were maintained on antibiotic water containing sulfadoxin and trimethoprim (Veterinaria, Zurich, Switzerland) for the following 3 weeks. Recipient mice carrying the Kbm1 mutation received CD4+ and CD8+ T cell–depleted BM and were further depleted of NK 1.1+ cells by intraperitoneal injection of 20 μL anti-asialo GM1 antibody (Wako Pure Chemical Industries, Tokyo, Japan) on the day before and in weekly intervals for 6 weeks following irradiation. Mice were used for experiments 8 to 10 weeks after BM reconstitution.
Antibodies and flow cytometry
Anti-CD8–FITC, anti-CD4–PerCP, anti-Vβ7–FITC, anti-CD90.1–PE, anti-CD44–PE, and anti-IFNγ–PE were obtained from BD PharMingen (Basel, Switzerland). Anti-CD8–allophycocyanin was obtained from Biolegend (LuBioScience, Lucerne, Switzerland). Anti-CD62L–PE was obtained from ImmunoTools (Friesoythe, Germany). For flow cytometry, single-cell suspensions were generated from the indicated organs and 106 cells were incubated with the indicated mAb at 4°C for 20 minutes. For peripheral blood lymphocyte (PBL) samples, erythrocytes were lysed with fluorescence-activated cell sorter (FACS) Lysing Solution (BD PharMingen). Cells were analyzed with a FACScalibur flow cytometer using the CellQuest software (BD Biosciences). The cells were analyzed by flow cytometry, gating on viable leukocytes using 7-aminoactinomycin D (Sigma, Buchs, Switzerland).
Construction of tetrameric MHC class I peptide complexes and flow cytometry
MHC class I monomers complexed with β-gal (H-2Kb) or M45 peptides (H-2Db) were produced as previously described33 and tetramerized by addition of streptavidin-PE (Molecular Probes, Eugene, OR). At the indicated time points following infection, organs were removed and single-cell suspensions were prepared. Aliquots of 5 × 106 cells or 300 μL of blood were stained using 50 μL of a solution containing tetrameric class I peptide complexes at 37°C for 10 minutes, followed by staining with anti-CD8–FITC (BD PharMingen) at 4°C for 20 minutes. Absolute cell counts were determined by counting leukocytes in an improved Neubauer chamber (Sigma).
Chromium release assay
EL-4 cells pulsed with peptide or without peptide (negative control) were used as target cells in a standard 51Cr release assay. Cells were labeled with 7.4 MBq (200 μCi) 51Cr (EGT Chemie, Tägerig, Switzerland) for 1 hour at 37°C. A total of 104 target cells/well were incubated for 5 hours in 96-well round bottom plates with 3-fold serial dilutions of effector cells. Splenocytes from MCMV-LacZ–infected mice that were restimulated with the indicated peptides for 5 days were tested for their cytolytic activity. Spontaneous chromium release was always below 15%.
CFSE labeling of TCR-transgenic T cells and adoptive transfer
Single-cell suspensions from the spleens of Bg1 mice were subjected to hypotonic red blood cell lysis and stained with CFSE (Molecular Probes, Leiden, The Netherlands). A maximum concentration of 2.5 × 107 cells/mL were incubated in 5 μM CFSE in phosphate-buffered saline (PBS) for 10 minutes at 37°C. Cells were washed twice with ice-cold balanced salt solution (BSS) and resuspended in BSS at a concentration of 1.5 × 107 splenocytes/mL. Recipient B6, Tie2-LacZ, and the different subsets of chimeric mice were injected intravenously with 1.5 × 107 Bg1-Thy1.1+ splenocytes in 500 μL BSS.
Immunohistology
Freshly removed organs were immersed in Hanks BSS (HBSS) and snap-frozen in liquid nitrogen. Frozen tissue sections were cut in a cryostat and fixed in acetone for 10 minutes. Sections were incubated with antibodies against β-gal (MP Biomedicals, Irvine, CA) and CD8 (clone YTS169.4.2), followed by goat anti-rat Ig (Caltag Laboratories, Burlingame, CA) and alkaline phosphatase–labeled donkey anti-goat Ig (Jackson ImmunoResearch Laboratories, West Grove, PA). Alkaline phophatase was visualized by using AS-BI phosphate/new fuchsin; sections were counterstained with hemalum, and images were acquired using a Leica DM R microscope equipped with a Leica DC300 FX camera (Leica, Heerbrugg, Switzerland). Digital images were processed using Adobe Photoshop (Adobe, San Jose, CA).
Surgical procedure for liver transplantation
Donor procedure, back-table preparation, and recipient procedure were performed as described previously with minor modifications.34 Briefly, all vessels and ligaments of the liver were dissected in the donor after midline laparotomy. In situ perfusion of the liver was performed using cold (4°C) Ringer solution. Subsequently, the liver was separated from its retroperitoneal attachments and removed. The graft was stored in cold (4°C) Ringer solution for 60 minutes until implantation into the recipient. Following hepatectomy of the native liver in the recipient, the donor liver was implanted in an orthotopic position. The anhepatic time in the recipient was consistently kept below 20 minutes. The portal vein was reconstructed and the liver was reperfused after completing the anastomosis between the suprahepatic inferior vena cava of the recipient and donor. Arterial recirculation was established by an end-to-side anastomosis between the recipient aorta and an aortic segment attached to the hepatic artery of the graft. A single subcutaneous injection of 5 mg cefazolin provided antibiotic prophylaxis.
Heterotopic heart transplantation
Heterotopic vascularized cardiac transplantation was performed according to the method described by Corry et al.35 Donor hearts were explanted from either male Tie2-LacZ or male C57BL/6 mice. The donor heart was removed from the chest after intracaval injection of 1 mL heparin (100 U/mL), rinsed with NaCl 0.9%, and placed on ice. After isolation of the recipient's abdominal aorta and inferior vena cava, the donor ascending aorta and pulmonary artery were joined end-to-side to the recipient's aorta and vena cava, respectively, using 10-0 nylon running suture. The abdomen was closed with individual running sutures to musculofascial layer and skins. The recipient mouse was then warmed for a few hours during recovery from anesthesia and had free access to water and food. The function of the transplanted heart was assessed daily by abdominal palpation.
Statistical data analysis
To evaluate statistically significant differences, the unpaired 2-tailed Student test was used. P values smaller than .05 were considered statistically significant. Statistical data analysis was performed using GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego CA).
Results
CD8 T-cell tolerance in Tie2-LacZ mice
Currently, a number of transgenic mouse lines are available that exhibit EC-restricted transgene expression: von Willebrand factor–LacZ36 and thrombomodulin-LacZ37 mice, which both show patchy transgene distribution in some arteries; and tie2-H-2Kb mice,14 which express the H2-Kb molecule as a transgene. In this study, Tie2-LacZ mice26 backcrossed to the C57BL/6 background have been used because of the uniform β-gal Ag expression in ECs of all organs. It is noteworthy that the intensity of β-gal expression in Tie2-LacZ mice is most pronounced in small and large arteries, but clearly detectable in venous and capillary ECs (Figure 1A; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Furthermore, expression levels of β-gal mRNA in various organs were comparable (Figure S2), indicating that these mice are well suited to study EC–CD8+ T-cell interaction in vivo. Indeed, in a previous study, Rothermel et al38 used Tie2-LacZ mice on the FVB genetic background to assess CD4+ and CD8+ T-cell responsiveness under conditions of persisting Ag expression in ECs. Whereas this previous investigation suggested that Tie2-LacZ mice can mount CD8+ T-cell responses against the EC-restricted β-gal Ag,38 the results obtained in the present study indicate that CD8+ T cells in Tie2-LacZ mice are tolerant to the β-gal antigen. This is shown by the fact that Tie2-LacZ mice failed to mount β-gal–specific CTL responses following infection with β-gal–recombinant MCMV (MCMV-LacZ), whereas CD8+ T-cell responses against the viral M45 epitope were not influenced by the EC-specific transgene expression (Figure 1B,C).
CD8+ T-cell reactivity in Tie2-LacZ mice. (A) Heart and thymus sections of naive Tie2-LacZ mice were stained for β-gal and CD8. Images were acquired with a 25×/0.65 objective (magnification: ×162). (B,C) C57BL/6 (B6) and Tie2-LacZ (T2) mice were infected intravenously with 106 pfu MCMV-LacZ. (B) Tetramer analysis for the indicated β-gal– and MCMV-derived M45 epitopes was performed on day 6 after infection. Mean percentage of tetramer-positive cells within the CD8 compartment are indicated (± SEM; n = 3-4). (C) Lysis of peptide-pulsed EL-4 cells by MCMV-LacZ-induced CTLs. On day 6 after infection, splenocytes from the indicated mouse strains were restimulated in vitro for 5 days with β-gal497-504, β-gal96-103, or M45985-993 peptide and tested in a standard chromium release assay.
CD8+ T-cell reactivity in Tie2-LacZ mice. (A) Heart and thymus sections of naive Tie2-LacZ mice were stained for β-gal and CD8. Images were acquired with a 25×/0.65 objective (magnification: ×162). (B,C) C57BL/6 (B6) and Tie2-LacZ (T2) mice were infected intravenously with 106 pfu MCMV-LacZ. (B) Tetramer analysis for the indicated β-gal– and MCMV-derived M45 epitopes was performed on day 6 after infection. Mean percentage of tetramer-positive cells within the CD8 compartment are indicated (± SEM; n = 3-4). (C) Lysis of peptide-pulsed EL-4 cells by MCMV-LacZ-induced CTLs. On day 6 after infection, splenocytes from the indicated mouse strains were restimulated in vitro for 5 days with β-gal497-504, β-gal96-103, or M45985-993 peptide and tested in a standard chromium release assay.
We next addressed whether the apparent β-gal–specific CD8+ T-cell tolerance in Tie2-LacZ mice is mediated by thymic negative selection or by peripheral tolerizing mechanisms. To this end, Tie2-LacZ mice were crossed with TCR-transgenic Bg1 mice, which possess CD8+ T cells that recognize the H2-Kb–restricted β-gal96-103 epitope.30 A total of 60% to 70% of the Bg1 CD8+ T cells bind H2-Kb/β-gal96-103 tetramers (Figure 2), and Bg1 CD8+ T cells possess a high functional avidity for the β-gal96-103 epitope as shown by target cell recognition and proliferation assays (Figure S4). In the thymus of Tie2-LacZ × Bg1 mice, the numbers of transgenic Vβ7 chain–positive and tetramer-binding CD8+ cells were reduced to 40% (Figure 2), suggesting that central tolerance led to partial deletion of β-gal–specific T cells. Interestingly, in peripheral lymphoid organs such as the spleen, the numbers of Vβ7+ and β-gal96-103 tetramer-binding CD8+ T cells was further reduced from 60% to 70% in Bg1 to less than 10% in Tie2-LacZ × Bg1 mice. Thus, at a first glance, it appears that EC-specific Ag expression in Tie2-LacZ mice precipitated both central and peripheral tolerance.
CD8+ T cell tolerance in Tie2-LacZ mice. Tie2-LacZ mice were crossed with TCR-trangenic Bg1 mice. Thymocytes from Tie2-LacZ (T2; top row), Bg1 (middle row), and Bg1 × Tie2-LacZ mice (Bg1 × T2; bottom row) were stained for CD4 and CD8 expression. The expression of the transgenic Vβ7 chain and binding of the H2-Kb-β-gal96-103 tetramer was determined by gating on CD8+ T cells. Values in the top right quadrants indicate mean frequencies of CD4/CD8-positive cells in thymocytes or percentage of antigen-specific cells in single CD8+ thymocytes, respectively (T2, n = 2; Bg1, n = 3; Bg1 × T2, n = 7). Splenocytes were assessed for Vβ7 and CD8 expression. Percentage of β-gal96-103–specific cells was determined by Vβ7 and H2-Kb-β-gal96-103 tetramer staining, gating on CD8+ T cells (T2, n = 3; Bg1, n = 5; Bg1 × T2, n = 9).
CD8+ T cell tolerance in Tie2-LacZ mice. Tie2-LacZ mice were crossed with TCR-trangenic Bg1 mice. Thymocytes from Tie2-LacZ (T2; top row), Bg1 (middle row), and Bg1 × Tie2-LacZ mice (Bg1 × T2; bottom row) were stained for CD4 and CD8 expression. The expression of the transgenic Vβ7 chain and binding of the H2-Kb-β-gal96-103 tetramer was determined by gating on CD8+ T cells. Values in the top right quadrants indicate mean frequencies of CD4/CD8-positive cells in thymocytes or percentage of antigen-specific cells in single CD8+ thymocytes, respectively (T2, n = 2; Bg1, n = 3; Bg1 × T2, n = 7). Splenocytes were assessed for Vβ7 and CD8 expression. Percentage of β-gal96-103–specific cells was determined by Vβ7 and H2-Kb-β-gal96-103 tetramer staining, gating on CD8+ T cells (T2, n = 3; Bg1, n = 5; Bg1 × T2, n = 9).
EC-independent peripheral CD8+ T-cell tolerance
Peripheral CD8+ T-cell tolerance can be induced via different cell types, including circulating hematopoietic cells expressing mhAg,39,40 BM-derived APC cross-presenting antigen derived from parenchymal tissues,41,42 or particular subsets of ECs that also possess the ability to cross-present circulating antigens.8,14 In order to assess truly EC-mediated peripheral tolerance induction, we established first a highly sensitive in vivo restimulation assay to detect very low amounts of circulating Bg1 cells. To this end, graded numbers of sorted CD8+Thy1.1+ cells from naive Bg1 mice were transferred into Thy1.2+ Tie2-LacZ and C57BL/6 mice. At 6 days following adoptive transfer, mice were infected with MCMV-LacZ, and the expansion of Bg1 cells was assessed 6 days later. As shown in Figure 3A, β-gal–specific CD8+ T cells expanded in C57BL/6 but not in Tie2-LacZ mice, confirming that Bg1 cells encounter their antigen in Tie2-LacZ mice outside of the thymus, and that this interaction leads to their deletion. However, reconstituting Tie2-LacZ mice with C57BL/6 BM (B6 → T2) revealed that CD8+ T-cell tolerance in Tie2-LacZ mice was solely dependent on β-gal expression within the BM (Figure 3B,C). In addition, β-gal expression by ECs in B6 → T2 chimeric mice did not affect the pattern of responsiveness of adoptively transferred Bg1 cells following MCMV-LacZ infection (Figure S5). Taken together, these data indicate that expression of an mhAg by ECs alone is not sufficient to directly tolerize CD8+ T cells, nor is this antigen available to BM-derived APCs in a way that would lead to CD8+ T-cell tolerance.
Loss of adoptively transferred Bg1 CD8+ T cells in Tie2-LacZ mice is not dependent on β-gal expression by ECs. (A) Graded numbers of CD8+ Bg1 cells expressing the congenic marker Thy1.1 were adoptively transferred into Thy1.2+ C57BL/6 or Tie2-LacZ recipient mice. At 6 days later, mice were challenged with 2 × 106 pfu MCMV-LacZ, and the proliferation of Bg1 CD8+ T cells was determined on day 6 following immunization by staining for CD8, Thy1.1, and the transgenic Vβ7 chain. Representative data from one of 2 independent experiments are shown. (B,C) Adoptive transfer of Bg1 CD8+ T cells in BM chimeric mice. A total of 5 × 104 (B) or 105 (C) TCR transgenic Thy1.1+ Bg1 cells were adoptively transferred intravenously into the indicated Thy1.2+ BM chimeric mice. At 9 days (B) or 30 days (C) later, mice were challenged with 2 × 106 pfu MCMV-LacZ, and proliferation of Bg1 CD8+ T cells was determined on day 6 following MCMV-LacZ challenge in the indicated organs. Values represent mean percentage (± SEM) of Thy1.1+Vβ7+ cells within the CD8 T-cell compartment.
Loss of adoptively transferred Bg1 CD8+ T cells in Tie2-LacZ mice is not dependent on β-gal expression by ECs. (A) Graded numbers of CD8+ Bg1 cells expressing the congenic marker Thy1.1 were adoptively transferred into Thy1.2+ C57BL/6 or Tie2-LacZ recipient mice. At 6 days later, mice were challenged with 2 × 106 pfu MCMV-LacZ, and the proliferation of Bg1 CD8+ T cells was determined on day 6 following immunization by staining for CD8, Thy1.1, and the transgenic Vβ7 chain. Representative data from one of 2 independent experiments are shown. (B,C) Adoptive transfer of Bg1 CD8+ T cells in BM chimeric mice. A total of 5 × 104 (B) or 105 (C) TCR transgenic Thy1.1+ Bg1 cells were adoptively transferred intravenously into the indicated Thy1.2+ BM chimeric mice. At 9 days (B) or 30 days (C) later, mice were challenged with 2 × 106 pfu MCMV-LacZ, and proliferation of Bg1 CD8+ T cells was determined on day 6 following MCMV-LacZ challenge in the indicated organs. Values represent mean percentage (± SEM) of Thy1.1+Vβ7+ cells within the CD8 T-cell compartment.
ECs fail to directly activate naive CD8+ T cells in vivo
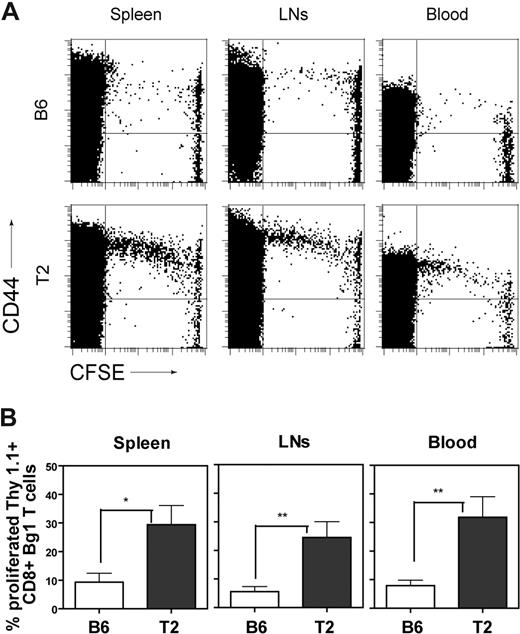
Deletional tolerization of CD8+ T cells via activation-induced cell death is usually associated with a transient period of T-cell activation and proliferation.24,43 Furthermore, it is possible that ECs in Tie2-LacZ mice might directly interact with CD8+ T cells in a way that leads to T-cell activation and/or proliferation. In order to assess a potential spontaneous T-cell activation by ECs in a GVHD-like model situation, 3 × 106 CFSE-labeled TCRtransgenic CD8+ T cells were adoptively transferred into Tie2-LacZ mice, and T-cell activation was monitored as CD44 up-regulation on proliferating Bg1 cells (Figure 4A). Furthermore, monitoring of CD44 up-regulation, expression of annexin V, and enumeration of the total numbers of Bg1 cells in spleens at later time points revealed that Bg1 T cells did not progress toward activation-induced cell death (Figure S6). Quantification of β-gal–dependent T-cell proliferation was achieved by adoptive transfer of CSFE-labeled, Thy1.1+ CD8+ Bg1 cells into either C57BL/6 or Tie2-LacZ mice (Figure 3B). This sensitive read-out system was then used to assess whether antigen presentation by ECs alone is sufficient to mediate CD8+ T-cell activation or whether BM-derived APCs, in particular DCs, contribute to the observed initial CD8+ T-cell triggering.
Activation of Bg1 CD8+ T cells in Tie2-LacZ mice. (A) A total of 1.5 × 107 CFSE-labeled splenocytes (corresponding to 3 × 106 CD8+ TCR-transgenic T cells) from Bg1 mice were adoptively transferred into C57BL/6 or Tie2-LacZ mice. Mice were killed on day 4 following transfer and cells from blood, spleen, and lymph nodes were analyzed by flow cytometry for CFSE dilution and CD44 up-regulation on CD8+ lymphocytes. Representative FACS plots from one representative of 2 independent experiments are shown. (B) Quantification of Bg1 T-cell proliferation. A total of 1.5 × 107 CFSE-labeled Bg1 Thy1.1+ splenocytes were injected into C57BL/6 or naive Tie2-LacZ mice. Mice were killed on day 4 following transfer and cells from blood, spleen, and lymph nodes were analyzed by flow cytometry. Values represent mean percentage (± SEM, n = 7; pooled data from 2 independent experiments) of proliferating CD8+Thy1.1+ cells (*P < .05; **P < .005; ***P < .001).
Activation of Bg1 CD8+ T cells in Tie2-LacZ mice. (A) A total of 1.5 × 107 CFSE-labeled splenocytes (corresponding to 3 × 106 CD8+ TCR-transgenic T cells) from Bg1 mice were adoptively transferred into C57BL/6 or Tie2-LacZ mice. Mice were killed on day 4 following transfer and cells from blood, spleen, and lymph nodes were analyzed by flow cytometry for CFSE dilution and CD44 up-regulation on CD8+ lymphocytes. Representative FACS plots from one representative of 2 independent experiments are shown. (B) Quantification of Bg1 T-cell proliferation. A total of 1.5 × 107 CFSE-labeled Bg1 Thy1.1+ splenocytes were injected into C57BL/6 or naive Tie2-LacZ mice. Mice were killed on day 4 following transfer and cells from blood, spleen, and lymph nodes were analyzed by flow cytometry. Values represent mean percentage (± SEM, n = 7; pooled data from 2 independent experiments) of proliferating CD8+Thy1.1+ cells (*P < .05; **P < .005; ***P < .001).
An array of BM chimeric mice was generated using different combinations between C57BL/6 (B6) and Tie2-LacZ (T2) controls, Tie2-LacZ mice on the C57BL/6bm1 background (T2bm1) exhibiting a mutated H2-Kb molecule that precludes H2-Kb-restricted presentation, and CD11c-DTR mice,27 which facilitate the specific ablation of CD11c+ DCs in lymphoid organs. As expected, Bg1 cells were not activated in B6 → B6 chimeras (Figure 5A), whereas transgenic CD8+ T cells proliferated in T2 → T2 chimeric mice (Figure 5B). Proliferation of Bg1 cells in B6 → T2 chimeras indicated that BM-derived nontransgenic APCs had activated the transgenic T cells (Figure 5C). This interpretation is supported by the fact that Bg1 proliferation was aborted in T2bm1 → T2 chimeras where direct and cross-presentation via BM-derived APCs is abolished, and only ECs can present the β-gal epitope (Figure 5D). Experiments with T2bm1 → T2bm1 chimeras confirmed that Bg1 cells do not proliferate in the absence of the appropriate H2 restriction element (Figure 5E). Bg1 activation could be restored in B6 → T2bm1 chimeras, confirming that BM-derived APCs are crucial in this setting (Figure 5F). Finally, reconstitution of T2bm1 mice with BM from CD11c-DTR mice together with diphteria toxin–mediated ablation of DCs showed that the proliferation of Bg1 cells depended strictly on the cross-presentation of β-gal Ag by DCs (Figure 5G). It is noteworthy that Bg1 activation in B6 → T2bm1 chimeras (Figure 5F) was somewhat reduced compared with the proliferative activity observed in B6 → T2 chimeras (Figure 5C), suggesting that ECs might to some extent contribute to the observed effects. Nevertheless, as a whole, the presented results indicate that β-gal–presenting vascular ECs remain immunologically ignored by CD8+ T cells, and that activation and proliferation of CD8+ T cells recognizing the mhAg in Tie2-LacZ mice is almost exclusively dependent on cross-presentation of Ag by BM-derived DCs.
In vivo proliferation of Bg1 CD8+ T cells in BM chimeric mice. A total of 1.5 × 107 CFSE-labeled splenocytes (corresponding to 3 × 106 CD8+ TCR-transgenic T cells) expressing the congenic marker Thy1.1 were adoptively transferred into the indicated Thy1.2+ bone marrow chimeras. (A) C57BL/6→C57BL/6 (B6 → B6). (B) Tie2-LacZ → Tie2LacZ (T2 → T2). (C) C57BL/6 → Tie2-LacZ (B6 → T2). (D) Tie2-LacZ × B6.C-H2bm1 → Tie2-LacZ (T2 × bm1 → T2). (E) Tie2-LacZ × B6.C-H2bm1 → Tie2-LacZ × B6.C-H2bm1 (T2 × bm1 → T2 × bm1). (F) C57BL/6 → Tie2-LacZ × B6.C-H2bm1 (B6 → T2 × bm1). (G) CD11c DTR → Tie2-LacZ × B6.C-H2bm1 (CD11cDTR → T2 × bm1). CD11c-DTR BM recipients had been injected intraperitoneally with 4 ng/g body weight diphteria toxin (DT), which led to a 95% to 98% depletion of CD11c+ cells for more than 48 hours. Mice were killed on day 4 following adoptive transfer and cells from blood and spleens were analyzed by flow cytometry. Values in the histograms represent mean percentages (± SEM, n = 5-7; pooled data from 3 independent experiments) of proliferating CD8+Thy1.1+ cells.
In vivo proliferation of Bg1 CD8+ T cells in BM chimeric mice. A total of 1.5 × 107 CFSE-labeled splenocytes (corresponding to 3 × 106 CD8+ TCR-transgenic T cells) expressing the congenic marker Thy1.1 were adoptively transferred into the indicated Thy1.2+ bone marrow chimeras. (A) C57BL/6→C57BL/6 (B6 → B6). (B) Tie2-LacZ → Tie2LacZ (T2 → T2). (C) C57BL/6 → Tie2-LacZ (B6 → T2). (D) Tie2-LacZ × B6.C-H2bm1 → Tie2-LacZ (T2 × bm1 → T2). (E) Tie2-LacZ × B6.C-H2bm1 → Tie2-LacZ × B6.C-H2bm1 (T2 × bm1 → T2 × bm1). (F) C57BL/6 → Tie2-LacZ × B6.C-H2bm1 (B6 → T2 × bm1). (G) CD11c DTR → Tie2-LacZ × B6.C-H2bm1 (CD11cDTR → T2 × bm1). CD11c-DTR BM recipients had been injected intraperitoneally with 4 ng/g body weight diphteria toxin (DT), which led to a 95% to 98% depletion of CD11c+ cells for more than 48 hours. Mice were killed on day 4 following adoptive transfer and cells from blood and spleens were analyzed by flow cytometry. Values in the histograms represent mean percentages (± SEM, n = 5-7; pooled data from 3 independent experiments) of proliferating CD8+Thy1.1+ cells.
Immunologic ignorance of antigen-expressing ECs in vascularized organ transplants
In order to assess whether these findings, obtained in a GVHD-like setting, also reflect EC-CTL interaction within vascularized organ grafts, a series of heart and liver transplantations were performed. Heterotopically transplanted Tie2-LacZ hearts were well-accepted in C57BL/6 recipients and spontaneous CD8+ T cells responses against both the β-gal497-503 (Figure 6A) and the β-gal96-103 epitope (not shown) could not be detected. To analyze with higher sensitivity, the effect of β-gal expression on vascular ECs, we used the adoptive transfer system of CSFE-labeled Bg1 cells. At 2 weeks following either heterotopic heart transplantation (Figure 6Bi,iii) or orthotopic liver transplantation (Figure 6Bii,iv), CFSE-labeled Bg1 CD8+ T cells were adoptively transferred either into recipients that had received C57BL/6 (Figure 6Bi,ii) or transgenic Tie2-LacZ organs (Figure 6Biii,iv). This analysis revealed no significant differences in Bg1 CD8+ T-cell activation between recipients of Tie2-LacZ and C57BL/6 control organs, indicating that ECs within the transplanted organs had not primed naive CD8+ T cells.
Lack of CD8+ cell activation in naive recipients of Tie2-LacZ vascularized organ grafts. (A) Spontaneous β-gal–specific CD8+ T-cell reactivity measured by tetramer analysis. C57BL/6 recipients received either C57BL/6 (B6 → B6) or Tie2-LacZ (T2 → B6) hearts, and the presence of β-gal497-504–specific CD8+ T cells in blood was assessed by flow cytometry on day 20 after transplantation. (B) Activation of CD8+ Bg1 T cells after adoptive transfer of 1.5 × 107 CFSE-labeled Bg1 splenocytes in C57BL/6 recipients on day 10 after transplantation. (i,iii) Heterotopic heart transplantation with donor organs from C57BL/6 (n = 7, i) and Tie2-LacZ (n = 8, iii) mice. Orthotopic liver transplantation with donor organs from C57BL/6 (n = 5, i) and Tie2-LacZ (n = 4, iii) mice. Mice were killed on day 4 following adoptive transfer, and cells from blood were analyzed by flow cytometry. Values in the histograms represent mean percentages (± SEM) of proliferating CD8+Thy1.1+ cells.
Lack of CD8+ cell activation in naive recipients of Tie2-LacZ vascularized organ grafts. (A) Spontaneous β-gal–specific CD8+ T-cell reactivity measured by tetramer analysis. C57BL/6 recipients received either C57BL/6 (B6 → B6) or Tie2-LacZ (T2 → B6) hearts, and the presence of β-gal497-504–specific CD8+ T cells in blood was assessed by flow cytometry on day 20 after transplantation. (B) Activation of CD8+ Bg1 T cells after adoptive transfer of 1.5 × 107 CFSE-labeled Bg1 splenocytes in C57BL/6 recipients on day 10 after transplantation. (i,iii) Heterotopic heart transplantation with donor organs from C57BL/6 (n = 7, i) and Tie2-LacZ (n = 8, iii) mice. Orthotopic liver transplantation with donor organs from C57BL/6 (n = 5, i) and Tie2-LacZ (n = 4, iii) mice. Mice were killed on day 4 following adoptive transfer, and cells from blood were analyzed by flow cytometry. Values in the histograms represent mean percentages (± SEM) of proliferating CD8+Thy1.1+ cells.
Discussion
In this study, we have used antigen-transgenic mice with uniform mhAg expression in ECs in combination with CD8+ T-cell TCR transgenics to demonstrate that mhAg presentation by ECs does neither precipitate T-cell activation nor tolerization. The lack of any tolerizing effect of prolonged EC–CD8+ T-cell interaction is unexpected because nonactivated, mhAg-presenting ECs in Tie2-LacZ provide “signal 1” (ie, antigen) in the absence of “signal 2” (ie, costimulation). Thus, the EC-associated antigen in Tie2-LacZ mice that is expressed in a widespread and easily accessible fashion should lead to CD8+ T-cell tolerance, in particular, in the absence of “danger signals.”44 One could argue that ECs possess an impaired capacity to present immunodominant peptides45 and therefore fail to interact with CD8+ T cells. However, ECs in transplanted Tie2-LacZ organs can become target cells of CTLs that had been primed with β-gal peptide-pulsed DCs. Such a treatment leads to vascular inflammatory disease with neointima formation and vascular occlusion, suggesting that it is the DC-mediated, prolonged presentation of mhAg within secondary lymphoid organs that drives activation of EC-specific CTLs and fosters thereby the development of chronic vascular rejection (D Engeler et al, submitted manuscript). Furthermore, it is unlikely that the antigen expression levels in ECs of Tie2-LacZ are too low to allow for productive EC–CD8+ T-cell interaction because sufficient EC-associated antigen is present in Tie2-LacZ mice for indirect (cross-) presentation by BM-derived DCs. It is therefore possible that studies describing activation and subsequent tolerization of CD8+ T cells, for example by particular subsets of EC such as liver sinusoidal ECs,8,14,15 may not have considered the contribution of professional APCs such as DCs and other BM-derived APCs.
Indeed, the complexity of the multicellular processes involved in EC-mediated antigen presentation in vivo requires careful consideration of possible confounding factors. Rothermel et al38 have suggested that immune recognition of ECs is context dependent, with antigen expressed in hearts of Tie2-LacZ mice being immunologically ignored, whereas ECs presenting β-gal antigen in skin are immunogenic and thus elicit T-cell responses capable of rejecting skin grafts. The results of our study clearly confirm the notion that direct presentation of mhAg by ECs is accompanied by immunologic ignorance. However, in the context of mhAg presentation in transplant vasculopathy and GVHD, DCs are probably the most important cell population that cross-presents the antigen in an immunogenic fashion.
Our study revealed further details that could confound the analysis of T-cell activation/tolerization in Tie2-LacZ mice: β-gal–specific CD8+ T cells were effectively tolerized in nonirradiated Tie2-LacZ and in T2 → T2 BM chimeric mice. We conclude from these findings that cells within the BM, but not professional APCs that descend from BM precursors, exert a tolerizing stimulus in Tie2-LacZ mice. Hematopoietic stem cells (HSCs) express the angiopoetin 1 receptor Tie2,46 and recent studies have shown that the tie2 promoter that has been used in Tie2-LacZ26 mice is active in HSCs during embryonic development.47 Likewise, in BM of adult Tie2-LacZ mice, LacZ transcripts were most abundant in HSCs (Figure S3). Thus, since circulating lymphocytes presenting mhAg efficiently tolerize naive CD8+ T cells,24,40 it is reasonable to assume that naive CD8+ T cells traveling through the BM can receive tolerizing stimuli within this compartment. Using Tie2-LacZ BM chimeras, it will be feasible to further characterize those cells within the BM that are highly efficient in inducing tolerance to mhAg.
Taken together, this study identifies the initial priming of mhAg-specific CD8+ T cells via DCs as a critical step in the generation of alloimmune responses. Therefore, it appears to be crucial that therapeutic intervention should aim at preventing or at least reducing the initial T-cell activation against mhAg. Indeed, blockade of essential costimulatory pathways such as CD40-CD15423,48 or CD28-CD80/8649 interaction during initial DC-mediated CD8+ T-cell stimulation bear a high potential for clinical application. It may well be that a combination of costimulatory blockade before and during priming of EC-specific CD8+ cell responses together with the induction of regulatory T cells49 will help to protect ECs from injury following transplantation of vascularized organs or during GVHD.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Silvia Behnke and Andre Fitsche for help with immunohistochemistry and Ton Rolink for help with FACS sorting of bone marrow cells.
This project received support from the Swiss National Science Foundation, the Velux Foundation (Zürich), and the Kanton of St Gallen.
Authorship
Contribution: B.L. designed the study and wrote the paper; B.B. performed research and wrote the paper; P.-A.C. designed the experimental procedure of liver transplantation; N.P.R. and D.C.P. provided mice; and E.S., P.K., D.E., Y.T., and S.M. performed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Burkhard Ludewig, Research Department, Kantonsspital St Gallen, Rorschacherstrasse 95, CH-9007 St Gallen, Switzerland; e-mail: burkhard.ludewig@kssg.ch.
References
Author notes
*B.B. and P.K. contributed equally to this work.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal