Abstract
Type 2 diabetes is associated with altered immune and hemostatic responses. We investigated the selective effects of hyperglycemia and hyperinsulinemia on innate immune, coagulation, and fibrinolytic responses during systemic inflammation. Twenty-four healthy humans were studied for 8 hours during clamp experiments in which either plasma glucose, insulin, both, or none was increased, depending on randomization. Target plasma concentrations were 5 versus 12 mM for glucose, and 100 versus 400 pmol/L for insulin. After 3 hours, 4 ng/kg Escherichia coli endotoxin was injected intravenously to induce a systemic inflammatory and procoagulant response. Endotoxin administration induced cytokine release, activation of neutrophils, endothelium and coagulation, and inhibition of fibrinolysis. Hyperglycemia reduced neutrophil degranulation (plasma elastase levels, P < .001) and exaggerated coagulation (plasma concentrations of thrombin-antithrombin complexes and soluble tissue factor, both P < .001). Hyperinsulinemia attenuated fibrinolytic activity due to elevated plasminogen activator-inhibitor-1 levels (P < .001). Endothelial cell activation markers and cytokine concentrations did not differ between clamps. We conclude that in humans with systemic inflammation induced by intravenous endotoxin administration hyperglycemia impairs neutrophil degranulation and potentiates coagulation, whereas hyperinsulinemia inhibits fibrinolysis. These data suggest that type 2 diabetes patients may be especially vulnerable to prothrombotic events during inflammatory states.
Introduction
Type 2 diabetes is associated with an increased risk for thrombotic complications.1 It has been estimated that 80% of diabetic patients die from acute arterial thrombosis, such as myocardial infarction and ischemic cerebrovascular events.2 Apart from the accelerated development of atherosclerosis in patients with diabetes, these patients were also found to have an increased risk of thrombotic events, explained by an increased procoagulant activity combined with a decreased fibrinolytic capacity.1 In addition, diabetic patients are prone to develop infectious diseases and more frequently die from infections than nondiabetic controls.3 The enhanced infection risk is, at least in part, related to an impaired innate immune system. Concentrations of cytokines, molecules that orchestrate the innate immune response, are altered in diabetic patients,4,5 and several functions of neutrophils, specialized in the killing of invading bacteria, are suppressed.6
A prominent feature of type 2 diabetes is the concurrent existence of hyperglycemia and hyperinsulinemia. We recently demonstrated that hyperglycemia and hyperinsulinemia have differential and selective effects on the hemostatic balance in healthy humans.7 In a strictly controlled setting, we showed that acute hyperglycemia activates coagulation independent of insulin levels, whereas hyperinsulinemia inhibits fibrinolysis irrespective of plasma glucose levels.7 Inflammation and coagulation are tightly intermingled in a variety of conditions, and bidirectional interactions exist between several inflammatory pathways on the one side and coagulation and fibrinolysis on the other.8 Although diabetes and inflammatory diseases are procoagulant conditions by themselves, knowledge of the impact of diabetes on activation of coagulation during infection and inflammation is limited. Moreover, the influence of hyperglycemia and/or hyperinsulinemia on the hemostatic and immune response during systemic inflammation is not known. Such knowledge is of additional interest considering the recent trials in critically ill patients showing a survival benefit in those in whom plasma glucose concentrations were maintained within the normal range by intensive insulin treatment.9,10
The primary objective of the current study was to determine the distinct effects of hyperglycemia and hyperinsulinemia on immune and hemostatic responses during systemic inflammation. We used the well-established model of human endotoxemia to elicit a reproducible inflammatory and procoagulant response in healthy subjects.11 These subjects were challenged with intravenous lipopolysaccharide (LPS) in the presence of one of the following: (1) normal glucose and insulin concentrations, (2) hyperglycemia (targeted at 12 mM) with low insulin levels, (3) hyperinsulinemia (targeted at 400 pmol/L) with normal glucose levels, or (4) combined hyperglycemia and hyperinsulinemia.
Methods
Subjects
Twenty-four healthy, nonsmoking, male volunteers (age [mean ± SEM]: 22.8 ± 0.5 years; weight: 74.4 ± 1.4 kg; body mass index: 22.2 ± 0.3) were studied. None of them used medication or had a positive family history of diabetes. All volunteers had normal plasma values of fasting glucose, insulin, erythrocyte sedimentation rate, complete blood count, lipid profile, and renal and hepatic function and all had a normal oral glucose tolerance test. The study was approved by the Medical Ethical Committee of the Academic Medical Center in Amsterdam, and written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki.
Study protocol
The clamps were performed essentially as described before.7 Each volunteer was studied during one of the following clamps: a lower insulinemic euglycemic (LinsuEgluc) clamp (target insulin level: 100 pmol/L, target glucose level: 5 mM), a lower insulinemic hyperglycemic (LinsuHgluc) clamp (insulin: 100 pmol/L, glucose: 12 mM), a hyperinsulinemic euglycemic (HinsuEgluc) clamp (insulin: 400 pmol/L, glucose: 5 mM), or a hyperinsulinemic hyperglycemic (HinsuHgluc) clamp (insulin: 400 pmol/L, glucose: 12 mM). For 3 days prior to the study, all volunteers consumed a weight-maintaining diet containing at least 250 g carbohydrates. After an overnight fast, the subjects were admitted to the clinical research unit and confined to bed. The study started at 7:30am with insertion of a catheter into an antecubital vein for infusion of insulin, somatostatin, glucagon, and glucose 10% or 20%. Another catheter was inserted retrogradely into a contralateral hand vein kept in a thermoregulated (60°C) Plexiglas box for sampling of arterialized venous blood. Saline (NaCl 0.9%) was infused with a slow drip to keep the catheters patent. Figure 1 shows all infusions and measurements that were performed during the study. At T = −3 (8am), infusions of somatostatin (250 μg/h, Somatostatine-ucb; UCB Pharma BV, Breda, The Netherlands), to suppress endogenous insulin and glucagon secretion, and glucagon (1 ng·kg−1·min−1, Glucagen; Novo Nordisk, Alphen aan den Rijn, The Netherlands), to replace endogenous glucagon concentrations, were started; concurrently, infusions of insulin (Actrapid; Novo Nordisk) at a rate of 10 or 40 mU·m−2 body surface area (BSA)·min−1 (lower or hyperinsulinemic clamp, respectively) and glucose 10% or 20% at a variable rate to obtain euglycemia or hyperglycemia were started. Glucose 20% was used during the LinsuEgluc clamp; in the other clamps, glucose 10% was used to prevent the possibility of phlebitis induced by the high infusion rates that were required. The HinsuEgluc clamp had a slightly different insulin infusion regime after an interim analysis of glucose, and insulin in 3 subjects had indicated that during the hyperglycemic clamps, somatostatin infusions appeared insufficient to achieve complete suppression of endogenous insulin production between 2 and 4 hours after LPS administration (data not shown and not included in further analyses). To keep distinct lower and high insulin concentrations from T = 2 hours onward, we included 6 new subjects in whom insulin infusion in the HinsuEgluc clamp was increased at this time point to 100 mU·m−2 BSA·min−1. All infusions were administered by calibrated syringe pumps (Perfusor fm; Braun, Melsungen, Germany). To clamp glucose at 5 or 12 mM from T = −3 until T = 5, every 5 minutes bedside plasma glucose concentration was measured on a Beckman glucose analyzer 2 (Beckman, Palo Alto, CA). For determination of plasma insulin concentration, blood samples were drawn at T = −3 and hourly from T = 0 until T = 5. From T = 1:20 till T = 2:00 and from T = 4:40 till T = 5:00 every 10 minutes blood samples were drawn for determination of the concentration of plasma insulin. In “Glucose and insulin,” the mean values of these measurements are presented for T = 2 and T = 5. At T = 0, LPS (Escherichia coli lipopolysaccharide, United States Pharmacopeial Convention, Rockville, MD) was administered as a bolus intravenous injection at a dose of 4 ng/kg body weight. Oral temperature, blood pressure, heart rate, and oxygen saturation were measured at half-hour intervals. For all measurements except plasma glucose and insulin concentrations (this paragraph), blood was collected before the initiation of the infusions (T = −3), directly before LPS administration (T = 0) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, and 24 hours thereafter. All blood samples, except those for the determination of leukocyte counts and differentials, were centrifuged at 1500g for 10 minutes at 4°C, and plasma was stored at −20°C until assays were performed.
Study design. Twenty-four subjects were studied (4 groups of 6). Somatostatin and glucagon were administered to all subjects using the same doses regardless of clamp type. Glucose was infused at variable rates to achieve plasma concentrations of 5 mM (euglycemic clamps) or 12 mM (hyperglycemic clamps). Insulin was infused at 10 mU·m−2 BSA·min−1 (lower insulinemic clamps) or 40 mU·m−2 BSA·min−1 (high insulinemic clamps). In the HinsuEgluc clamp, insulin infusion was increased to 100 mU·m−2 BSA·min−1 at T = 2 hours (“Study protocol”). LPS was infused at T = 0 hours. Measurements were performed at indicated time points.
Study design. Twenty-four subjects were studied (4 groups of 6). Somatostatin and glucagon were administered to all subjects using the same doses regardless of clamp type. Glucose was infused at variable rates to achieve plasma concentrations of 5 mM (euglycemic clamps) or 12 mM (hyperglycemic clamps). Insulin was infused at 10 mU·m−2 BSA·min−1 (lower insulinemic clamps) or 40 mU·m−2 BSA·min−1 (high insulinemic clamps). In the HinsuEgluc clamp, insulin infusion was increased to 100 mU·m−2 BSA·min−1 at T = 2 hours (“Study protocol”). LPS was infused at T = 0 hours. Measurements were performed at indicated time points.
Assays
Insulin was determined by a chemiluminescent immunometric assay (Immulite; Diagnostic Products, Los Angeles, CA), using heparin-anticoagulated plasma. Coagulation and fibrinolysis assays were done using citrated plasma, with all other assays using EDTA plasma. Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-8, and IL-10 were measured by cytometric beads array (CBA) multiplex assay (BD Biosciences, San Jose, CA). Soluble forms of E-selectin, intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), von Willebrand factor, elastase12 and myeloperoxidase (MPO),13 thrombin-antithrombin complexes (TATc's), soluble tissue factor, plasminogen activator inhibitor type 1 (PAI-1), tissue-type plasminogen activator (tPA), and plasmin-a2-antiplasmin complexes (PAPc's) were measured using enzyme-linked immunosorbent assays (ELISAs; E-selectin, ICAM-1, and VCAM-1: Eli-pair [Diaclone, Besançon, France]; von Willebrand factor [Dako, Glostrup, Denmark]; TATc and PAPc [Dade Behring, Marburg, Germany]; soluble tissue factor [American Diagnostics, Greenwich, CT]; PAI-1: TintElize PAI-1 [Biopool, Umea, Sweden]; and tPA: Asserachrom tPA [Diagnostica Stago, Asnieres-sur-Seine, France]. PAI-1 activity and plasminogen activator activity (PA activity) were measured by amidolytical assays.14,15
Statistical analysis
All variables, except cytokines TNF-α, IL-6, IL-8, and IL-10, were normally distributed and are presented as means (± SEM). Cytokines are presented as medians and interquartile ranges (IQRs). All variables were rank transformed for subsequent analysis. To analyze the effect of hyperinsulinemia and/or hyperglycemia, their interactions, and the effect of time, results of the 4 clamps were compared using a repeated measures analysis of variance (ANOVA). In case of the existence of an overall effect, differences between clamps on separate time points were analyzed using an ANOVA followed by LSD posthoc analyses. Probability values less than .05 were considered statistically significant. SPSS statistical software version 12.0.1 (SPSS, Chicago, IL) was used to analyze the data.
Results
Glucose and insulin
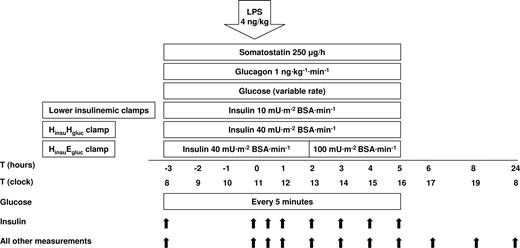
The plasma glucose and insulin levels during all clamps are depicted in Figure 2. In the 2 hyperglycemic clamps, plasma glucose levels rapidly increased during the first hour, and from −2 hours onward the extent of hyperglycemia was similar in the LinsuHgluc and HinsuHgluc clamps: plasma glucose levels were approximately 12 mM, except for a temporary small drop between 2 and 4 hours after LPS. Throughout the euglycemic clamps, plasma glucose levels remained at approximately 5 mM (both P < .001 for the difference with either of the 2 hyperglycemic clamps).
Plasma glucose and insulin concentrations. Mean (± SE) plasma levels of glucose (A) and insulin (B), after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). Glucose was measured every 5 minutes; insulin was measured at time points as described in “Study protocol.”
Plasma glucose and insulin concentrations. Mean (± SE) plasma levels of glucose (A) and insulin (B), after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). Glucose was measured every 5 minutes; insulin was measured at time points as described in “Study protocol.”
During clamping, insulin concentrations were statistically different between the lower insulinemic and hyperinsulinemic clamps, except between 2 and 4 hours after LPS injection when insulin concentrations increased in all clamps except the LinsuEgluc clamp (P < .001 for all time points except from T = 2 until T = 4 hours).
Neutrophil activation
LPS administration led to degranulation of neutrophils, as reflected by increased plasma concentrations of elastase and MPO (Figure 3). Hyperglycemia attenuated the LPS-induced increases in elastase (Figure 3A; overall P < .001) and MPO (Figure 3B; overall P = .009) levels irrespective of insulin concentrations. For elastase, these differences became apparent at 2 hours after LPS and were maximal at T = 4 hours, when peak elastase levels were measured. Increases in MPO concentrations were more gradual, with peak levels achieved during our latest measurement (T = 24 hours). The observed effect of hyperglycemia on MPO concentrations became apparent at T = 5 hours. LPS injection induced a neutrophilic leukocytosis, which was not influenced by either glucose or insulin concentrations (Figure 3C; P = .232 and P = .230, respectively). Therefore, acute hyperglycemia reduced LPS-induced neutrophil degranulation irrespective of insulin concentrations.
Neutrophil functions. Mean (± SE) plasma levels of elastase (A) and MPO (B), and whole blood neutrophil numbers (C) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). *P < .05, **P < .01, and ***P < .001 hyperglycemic versus euglycemic clamps.
Neutrophil functions. Mean (± SE) plasma levels of elastase (A) and MPO (B), and whole blood neutrophil numbers (C) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). *P < .05, **P < .01, and ***P < .001 hyperglycemic versus euglycemic clamps.
Cytokine concentrations
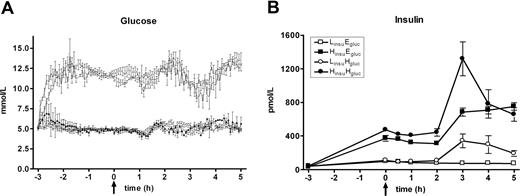
As expected, LPS administration was followed by transient rises in plasma cytokine concentrations (Figure 4). Throughout the experiment, neither hyperinsulinemia nor hyperglycemia influenced concentrations of TNF-α (P = .405 and P = .768, respectively), IL-6 (P = .279 and P = .818, respectively), or IL-8 (P = .255 and P = .167, respectively). Although IL-10 levels tended to be higher in the HinsuEgluc clamp, the difference with other clamps was explained by one subject with very high IL-10 concentrations (peak level: 1392 pg/mL), and no differences in IL-10 levels were found upon exclusion of this one subject.
Cytokine concentrations. Median (± IQR) plasma levels of TNF-α (A), IL-6 (B), IL-8 (C), and IL-10 (D) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). P = NS.
Cytokine concentrations. Median (± IQR) plasma levels of TNF-α (A), IL-6 (B), IL-8 (C), and IL-10 (D) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). P = NS.
Endothelial cell activation
To assess the influence of hyperinsulinemia and hyperglycemia on endothelial cell activation during inflammation, we measured the plasma levels of soluble E-selectin, soluble ICAM-1, soluble VCAM-1, and von Willebrand factor. As anticipated, LPS administration elicited profound rises in the plasma levels of these proteins, which were not influenced by either hyperinsulinemia (P ≥ .289 for all parameters) or hyperglycemia (P ≥ .201 for all parameters; shown for soluble E-selectin and von Willebrand factor in Figure 5).
Endothelial cell activation. Mean (± SE) plasma levels of soluble E-selectin (A) and von Willebrand factor (B) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). P = NS.
Endothelial cell activation. Mean (± SE) plasma levels of soluble E-selectin (A) and von Willebrand factor (B) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). P = NS.
Coagulation
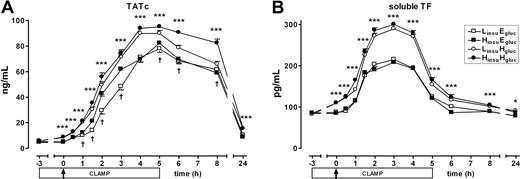
To investigate the influence of hyperinsulinemia and hyperglycemia on the coagulation system, we measured TATc and soluble TF (Figure 6). Hyperglycemia strongly elevated levels of TATc and soluble TF (both P < .001). This was already found after 3 hours of hyperglycemia, just before LPS administration (both P < .001). As expected, LPS administration was followed by sharp increases of TATc and soluble TF. Remarkably, hyperglycemia led to even higher levels of both parameters compared with normoglycemia (P < .001 for overall glucose effect). Besides hyperglycemia, hyperinsulinemia also potentiated the LPS-induced rises in TATc (P < .001 for overall insulin effect), albeit to a much lesser extent; soluble TF was not affected by hyperinsulinemia. Thus, hyperglycemia induced coagulation and aggravated LPS-induced coagulation, whereas hyperinsulinemia modestly enhanced the LPS-induced rise in TATc concentrations without influencing soluble TF levels.
Coagulation markers. Mean (± SE) plasma levels of TATc (A) and soluble TF (B) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). *P < .05 and ***P < .001 hyperglycemic versus euglycemic clamps; †P < .05 hyperinsulinemic versus lower insulinemic clamps.
Coagulation markers. Mean (± SE) plasma levels of TATc (A) and soluble TF (B) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). *P < .05 and ***P < .001 hyperglycemic versus euglycemic clamps; †P < .05 hyperinsulinemic versus lower insulinemic clamps.
Fibrinolysis activation
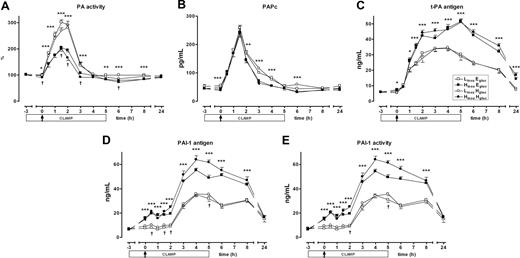
To investigate the influence of hyperinsulinemia and hyperglycemia on inflammation-induced activation of fibrinolysis, we measured markers of the fibrinolytic system (Figure 7). Hyperinsulinemia strongly influenced all measured parameters (P < .001 for overall insulin effect for all fibrinolytic parameters). LPS injection was followed by a rapid and transient increase in PA activity. Hyperinsulinemia was associated with a reduced PA activity, especially in the first 3 hours after LPS administration. Analysis of PAPc levels revealed a similar insulin effect. Although peak levels were not significantly different, hyperinsulinemia was accompanied by lower PAPc levels, especially from 2 until 6 hours after LPS. Antigen concentrations of t-PA and PAI-1, and PAI-1 activity were more increased in both hyperinsulinemic clamps compared with the lower insulinemic clamps. The influence of hyperinsulinemia was most profound on PAI-1 antigen and activity. Already before LPS injection, hyperinsulinemia almost doubled plasma levels and activity, whereas the differences in peak levels and activity (at T = 4 hours) were almost of the same magnitude. Thus, hyperinsulinemia increased both activation and inhibition of fibrinolysis during endotoxemia; the net effect of hyperinsulinemia was a reduced LPS-induced activation.
Fibrinolysis markers. Mean (± SE) plasma levels of PA activity (A), PAPc (B), t-PA antigen (C), PAI-1 antigen (D), and PAI-1 activity (E) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). *P < .05, **P < .01, and ***P < .001 hyperinsulinemic versus lower insulinemic clamps; †P < .05 hyperglycemic versus euglycemic clamps.
Fibrinolysis markers. Mean (± SE) plasma levels of PA activity (A), PAPc (B), t-PA antigen (C), PAI-1 antigen (D), and PAI-1 activity (E) after LPS administration (4 ng/kg intravenously, T = 0 hours), during one of the following conditions: an LinsuEgluc clamp (□), an LinsuHgluc clamp (○), an HinsuEgluc clamp (■), and an HinsuHgluc clamp (●). *P < .05, **P < .01, and ***P < .001 hyperinsulinemic versus lower insulinemic clamps; †P < .05 hyperglycemic versus euglycemic clamps.
Besides the insulin effects on fibrinolysis, an overall glucose effect appeared to be present with regard to LPS-induced PA activity (inhibitory effect; P < .001) and PAI-1 release (stimulatory effect; P = .008). However, this effect was visible at fewer separate time points compared with the hyperinsulinemia effect. In addition, differences between average euglycemic and hyperglycemic plasma levels of PA activity and PAI-1 were not as big as differences between average lower and high insulin plasma levels.
Discussion
This study demonstrates for the first time that, during a systemic inflammatory response induced by an intravenous injection of endotoxin, acute hyperglycemia and/or hyperinsulinemia have differential effects on several aspects of innate immunity, coagulation, and fibrinolysis. We show that hyperglycemia attenuates inflammation-induced neutrophil activation and aggravates coagulation, whereas hyperinsulinemia strongly reduced fibrinolytic activity induced by inflammation, due to an exaggerated PAI-1 induction and despite higher t-PA concentrations. Hyperglycemia and hyperinsulinemia did not influence cytokine release or endothelial activation in this model of human endotoxemia.
Several studies investigated the effects of hyperglycemia and/or hyperinsulinemia on innate immune responses. Not only in diabetes,6 but also during acute hyperglycemia,16 leukocyte functions are decreased. These studies did not investigate separate effects of hyperglycemia and/or hyperinsulinemia, and none assessed leukocyte functions in an in vivo model of enhanced leukocyte activity (ie, under inflammatory conditions). Our finding that hyperglycemia impaired LPS-induced elastase and MPO release suggests that an acute elevation of circulating glucose concentrations under systemic inflammatory conditions diminishes the capacity of neutrophils to secrete protease contained within their (azurophilic) granules. Indeed, proteases within azurophilic granules are important for the antimicrobial action of neutrophils, and proteases secreted from neutrophils can form neutrophil extracellular traps with chromatin that facilitate the elimination of bacteria.17 The decreased elastase and MPO concentrations during acute hyperglycemia could not be explained by neutrophil counts, as clamping did not influence these cell numbers. Some evidence exists that diabetic patients have higher neutrophil counts compared with nondiabetic subjects18 and that total leukocyte and neutrophil counts are correlated to insulin levels in diabetic patients.19 Of note, hyperinsulinemia tended to increase the neutrophilic leukocytosis at 24 hours after LPS injection (not significant).
Diabetes is regarded as a proinflammatory state based on findings of mildly elevated basal plasma cytokine levels. In a noninflammatory state, concentrations of TNF-α and IL-6 are usually below 5 pg/mL, whereas concentrations in diabetic patients are increased up to 3-fold.4,5 However, during acute inflammation, concentrations of these cytokines can temporarily increase into the nanogram/milliliter range. We here sought to reproduce an acute inflammatory state in a metabolic background of hyperinsulinemia and/or hyperglycemia by intravenous injection of LPS. The assessment of cytokine concentrations during combined states of inflammation and metabolic disturbances that occur during diabetes has been the subject of previous research, however with variable results. In rats, acute hyperglycemia increased IL-6 and TNF-α levels after exposure to a low dose of intravenous LPS compared with normoglycemia, but cytokine increases were equal after a high LPS dose.20 In human volunteers who received LPS intravenously, IL-6 concentrations were augmented during hyperinsulinemia21 and during combined hyperglycemia and hyperinsulinemia,22 while TNF-α concentrations were unaffected by clamping. Of note, the effect of separate hyperglycemia was not evaluated in these human studies, and the insulin levels were outside the physiological range and much higher (800-1200 pmol/L) than in our current investigation (around 400 pmol/L for the major part of the clamps), which may explain at least in part why we did not find an insulin effect on cytokine levels.
The influence of hyperglycemia and hyperinsulinemia on endothelial cells and markers of endothelial cell activation has been investigated previously. Endothelial cells in hyperglycemic culture medium expressed increased ICAM-1, VCAM-1, and E-selectin.23 In type 2 diabetic patients, the concentrations of soluble adhesion molecules correlated with fasting plasma glucose and HbA1c values, indicating that endothelial cell activation is present during chronic hyperglycemia.24 The authors also showed that soluble E-selectin levels correlated with fasting insulin concentrations. The effect of acute hyperglycemia is less clear-cut, as an oral glucose load either increased25 or decreased26 endothelial cell activation markers in type 2 diabetes patients. Endotoxemia27 and sepsis28 are known to induce soluble E-selectin release. To our knowledge, we are the first to investigate endothelial cell activation under combined inflammatory and acute hyperglycemic and/or hyperinsulinemic conditions; in this study setup, neither hyperglycemia nor hyperinsulinemia contributed further to the inflammation-induced activation of the vascular endothelium.
The influence of selective hyperinsulinemia on hemostasis in vivo has been investigated earlier. Samad et al demonstrated that insulin increased PAI-1 concentrations in mice, and that PAI-1 gene expression and glucose transport are mediated by different insulin-signaling pathways.29 Indeed, elevated levels of fasting insulin are associated with increased circulating PAI-1 (and t-PA antigen) levels in humans, even in subjects with normal glucose tolerance.30 Some investigators who infused exogenous insulin under euglycemic and hyperglycemic conditions did not measure altered plasma PAI-1 activity.31,32 In our previous clamp study, we demonstrated a time-dependent increase in PAI-1 activity during up to 6 hours of selective hyperinsulinemia.7 Importantly, this latter study is the only one in which glucagon secretion was suppressed by somatostatin, which is of relevance considering that glucagon itself may influence PAI-1 activity.33 Our current investigation not only reproduced the acute effect of insulin on PAI-1 antigen levels and activity in the absence of an inflammatory stimulus (ie, during the first 3 hours of the clamps, directly before LPS was administered), but also shows that the insulin effect is of the same magnitude during systemic inflammation, a condition known to increase PAI-1 activity by itself.34,35 The fact that hyperinsulinemia induced more t-PA during endotoxemia could not prevent that net fibrinolytic activity, as measured by PA activity, was relatively inhibited under hyperinsulinemic conditions. Therefore, it is likely that during inflammatory states, the presence of hyperinsulinemia leads to a decreased fibrinolytic activity, independent of glucose concentrations. Research on selective hyperglycemia on hemostasis is scarce, as induction of hyperglycemia almost immediately leads to concurrent insulin secretion. In our previous study, we also used somatostatin to suppress endogenous insulin secretion during hyperglycemia; we demonstrated that hyperglycemia induces TATc and soluble TF irrespective of insulin levels.7 We here confirmed that acute hyperglycemia enhances coagulation in the absence of an inflammatory stimulus and in addition show that combined states of inflammation and hyperglycemia have additive effects on the coagulation system. This seemed particularly the case for soluble TF. More downstream in the coagulation cascade, TATc concentrations were also more increased by hyperglycemia, although we also measured extra TATc induction by hyperinsulinemia (albeit this effect was quite weak). Therefore we cannot exclude that hyperinsulinemia may also be capable of somewhat potentiating coagulation during a systemic inflammatory response. Of note, hyperinsulinemia did not have an effect on coagulation in the absence of an inflammatory stimulus (this study and Van den Berghe et al10 ). Activation of coagulation in this model of human endotoxemia is mediated by TF.34 As such, it would have been of interest to measure TF activity in blood. Aras et al demonstrated an increase in TF activity associated with microparticles and whole blood after LPS administration to healthy humans.36 Samples for these measurements were not collected during our study. It should be noted, however, that at least part of TF activity generated upon intravenous LPS injection is likely to be produced by cellular sources not present in blood drawn by venipuncture (eg, monocytes attached to activated endothelium, endothelial cells, and/or tissue macrophages). Together our data indicate that the combination of systemic inflammation and acute elevations in plasma glucose and/or insulin in vivo induces excess coagulation (hyperglycemia and to a lesser extent hyperinsulinemia) and reduced fibrinolytic activity due to excess PAI-1 activity (hyperinsulinemia and to a lesser extent hyperglycemia).
What is the clinical relevance of our findings and can our results be extrapolated to certain patient populations? The tightly controlled study design that we used for this investigation makes it logistically impossible to study distinct effects of hyperglycemia and hyperinsulinemia for longer periods of time in humans in vivo. Therefore, it is difficult to explain immune defects and hemostatic derangements of patients with type 2 diabetes or insulin resistance syndromes by extrapolation of our findings, also because these patients frequently have other metabolic disturbances such as hyperlipidemia. Nevertheless, our results are in line with the decreased neutrophil function and hemostatic imbalance that are found in diabetic patients.1,6 In addition, our results can be viewed upon in the context of acute hyperglycemia and acute insulin resistance, which are very common in various patient populations. In consecutive hospitalized patients, hyperglycemia was present in 38% and one-third of these patients had no history of diabetes before hospital admission. Importantly, mortality and morbidity of acute hyperglycemic patients are consistently higher compared with those with normoglycemia.37 Furthermore, correction of acute hyperglycemia improves the outcome of critically ill patients.9,10 Although in our study, performed in healthy humans, some LPS-induced responses were relatively modestly influenced by hyperinsulinemia and/or hyperglycemia, the data obtained in patients with concurrent diseases and/or other metabolic disturbances1,6,9,10,37 combined with our present findings suggest that hyperglycemia and hyperinsulinemia can worsen the hypercoagulable state and immune defects that occur during severe illness.
Although we used somatostatin to suppress endogenous insulin and glucagon production during all clamps, we did not achieve complete suppression: from 2 until 4 hours after LPS administration, somatostatin concentrations did not completely block hyperglycemia-driven insulin production in the hyperglycemic clamps. Importantly, before and after this time window somatostatin suppression seemed adequate, as indicated by a clear separation between high and low glucose and insulin levels. Apparently, factors in the early inflammation phase interfered with the suppressive effect of somatostatin. Using a higher dose of somatostatin would have been difficult because this can have nausea as a side effect. Using the current dose, the combination with endotoxin already induced nausea in 19 of 24 volunteers. Therefore and considering the fact that in the human endotoxemia model the first 2 hours after LPS administration are most important for immune and coagulant responses to develop,11 we chose to adjust the protocol for the HinsuEgluc clamp to keep sufficient distinction between glucose and insulin levels in the 4 different clamps.
In conclusion, our results provide evidence that during systemic inflammation, hyperglycemia and hyperinsulinemia exert acute and differential effects on innate immune and hemostatic responses. Hyperglycemia diminishes inflammation-induced neutrophil degranulation and exacerbates procoagulant responses, whereas hyperinsulinemia inhibits fibrinolysis during the early inflammatory reaction due to extra stimulation of PAI-1 activity.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. T. Ackermans, A. F. C. Ruiter, B. C. E. Voermans, and the staff of the Clinical Research Unit of our hospital for their indispensable help during the experiments.
This work was supported by a grant from the Dutch Diabetes Research Foundation (grant number 2002.00.008) to M.E.S.
Authorship
Contribution: M.E.S. and S.N.C. designed and performed the research, and collected and interpreted data; M.E.S. also analyzed all immunologic data and drafted the paper; R.M.E.B. performed the research, and collected and interpreted data; M.L. and J.C.M.M. analyzed and interpreted coagulation and fibrinolysis data; M.J.S. supervised the research and interpreted data; M.W.T.T. performed statistical analysis; H.P.S. and T.P. designed and supervised the research, interpreted data, and took part in writing the paper; all authors reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michiel E. Stegenga, Center for Experimental and Molecular Medicine (CEMM), Academic Medical Center, University of Amsterdam, Room F0-117, Meibergdreef 9, 1105 AZ Amsterdam, The Netherlands; e-mail: m.e.stegenga@amc.uva.nl.