Abstract
Oncogenic activation of tyrosine kinase signaling pathway is recurrent in human leukemia. To gain insight into the oncogenic process leading to acute megakaryoblastic leukemia (AMKL), we performed sequence analyses of a subset of oncogenes known to be activated in human myeloid and myeloproliferative disorders. In a series of human AMKL samples from both Down syndrome and non–Down syndrome patients, mutations were identified within KIT, FLT3, JAK2, JAK3, and MPL genes, with a higher frequency in DS than in non-DS patients. The novel mutations were analyzed using BaF3 cells, showing that JAK3 mutations were activating mutations. Finally, we report a novel constitutively active MPL mutant, MPLT487A, observed in a non–Down syndrome childhood AMKL that induces a myeloproliferative disease in mouse bone marrow transplantation assay.
Introduction
Acute megakaryoblastic leukemia (AMKL) is a heterogeneous subtype of acute myeloid leukemia (AML) with diverse genetic and morphologic characteristics. The estimated frequency of AMKL ranges from 3% to 14% of AML, and is more frequent in children than in adults.1 Approximately 65% of AMKLs are associated with myelofibrosis.1,2 In adults, AMKL is frequently observed as secondary leukemia after chemotherapy or leukemic transformation of several chronic myeloproliferative disorders (MPDs) including chronic myelogenous leukemia (CML), polycythemia vera (PV), essential thrombocytosis (ET), and idiopathic myelofibrosis (IMF).3-5 Recently, point mutations have been described within the JAK2 gene in MPD and within the gene coding for the thrombopoietin receptor (MPL) in a subset of myelofibrosis and ET.6-9
Access to primary AMKL cells is extremely limited due in part to severe myelofibrosis in many cases that precludes bone marrow aspiration, and to its prevalence in pediatric populations. In childhood AMKL, 2 major subgroups have nevertheless been described that include patients with constitutional trisomy 21 associated with GATA1 mutations,10 and those with the t(1;22)(p13;q13) translocation encoding the OTT-MAL (RBM15-MKL1) fusion protein.11-13
Several observations suggest that these latter genetic mutations may not be sufficient to cause an AMKL phenotype. For example, although most Down syndrome (DS) patients with constitutional trisomy 21 and GATA1 mutations present with a transient myeloproliferative disorder (TMD) at or around birth, there is spontaneous remission of the TMD and absence of further malignant disease in most instances.14 In addition, in humans, patients with inherited GATA1 mutation (similar to those observed in TMD and AMKL) do not develop leukemia.15 Similarly, expression of a mutant GATA-1s protein in a knockin mouse model is able to induce a transient hyperproliferation of yolk sac and fetal liver megakaryocyte progenitors, but is not sufficient to induce AMKL per se.16 These observations indicate that there are multigenic contributions to the development of AMKL.
Constitutive tyrosine phosphorylation of STAT5 has been described in a significant proportion of cases of AML. In several instances, the molecular basis for the constitutive activation of STAT5 is known to be due to activating mutations in tyrosine kinases, including internal tandem duplication (ITD) and activation loop mutations in FLT3, as well as activating mutations in KIT.17,18 Although FLT3 mutations have been identified in a broad spectrum of AML, mutations in tyrosine kinases are rarely reported in AMKL.19-24
In search for activating mutations, we systematically analyzed the sequences coding for selected domains in proteins involved in the control of tyrosine kinase activity and known to be mutated in human AML and MPD.
Methods
Samples and sequencing
Patients with DS- and non–DS-AMKL have been described previously25 and are summarized in Table 1. No material was available from patients with the t(1;22) translocation. cDNAs were amplified using HotStar DNA polymerase (Qiagen, Courtaboeuf, France). Research was approved by the INSERM review board and conducted in accordance with the Declaration of Helsinki.
AMKL samples analyzed for KIT, FLT3, JAK2, JAK3, and MPLmutations
| Patient no. . | Age . | Sex . | % Blasts . | Karyotype . | GATA-1 mutations . | JAK2, JAK3, FLT3, KIT, and MPL changes . |
|---|---|---|---|---|---|---|
| Down syndrome | ||||||
| AMKL | ||||||
| 11 | 10m | F | 22 | Random aberration,+21c | + | − |
| 15 | 14m | M | 31 | 48,XY,+8,+21c | nd | FLT3 D835Y |
| 16 | 15m | F | 20 | na | nd | JAK3 V722I |
| 18 | 13m | F | 43 | 47,XX,+21c,del(6q) | + | − |
| 19 | 11m | F | 14 | 44∼46,XX,−4,−9,−16,+21c[cp7]/47,XX,+21c[7] | nd | FLT3 D835Y |
| 20 | 8m | F | 80 | Random aberration | + | JAK2 V617F |
| 30 | 18m | M | na | 47,XX,+21c/49,XX,inv(9)(p11;q12),+21c,+21c,+21c | + | − |
| Non-AMKL | ||||||
| 58 (TMD) | 2d | F | 14 | 47,XX,+21c | + | − |
| 85 (TMD) | 1m | M | na | 47,XY,+21[19]/46,XY[3] | + | − |
| 60 (AML) | 24m | M | 88 | 47,XY,+21c | + | − |
| 63 (AML) | 24m | F | 59 | 47,XX,+21c | + | − |
| Non–Down syndrome | ||||||
| Child AMKL | ||||||
| 69 | 1d | M | 90 | +21a | + | FLT3-ITD |
| 24 | 51m | M | 58 | Random aberration | nd | MPL T487A |
| 28 | 8m | F | 57 | 46, XX | nd | KIT D816V |
| 29 | 11y | M | 46 | 45,XY,inv(3)(q21q23),−7 | nd | − |
| 31 | 13m | M | na | 47XY,+2,+15,−19,t(7;8) | nd | JAK2 M535I |
| 33 | 16m | M | 31 | 46, XY | − | − |
| 34 | 33m | M | 40 | 46, XY | − | − |
| 35 | 20m | F | 73 | 47,XX,+3[4]/46,XX[21] | − | − |
| 36 | 30m | M | 80 | 46,XY,random aberration[7]/46,XY[15] | − | − |
| 37 | 36m | M | 74 | 46,XY,add(2)(q37),ins(3;?)(q21;?),del(5)(q13q22),−7,−9,add(16)(q24),+min,+mar | − | − |
| 38 | 24m | F | 60 | 46,XX,del(9)(p21)[1]/46,X,t(X;21)(q24;q21),del(9)(p21),+19[19] | − | − |
| 41 | 7m | M | 47 | 64-65,XY,t(1;8)(p32;q24),+2,+3,+4,+5,+6,+8,+9,+10,+19,−20,+22 | − | − |
| 44 | 8m | M | na | 6, XY[20] | − | − |
| 45 | 3m | M | 73 | 6, XY[20] | − | − |
| 46 | 26y | M | 15 | nd | − | − |
| 47 | 38m | M | 72 | nd | nd | − |
| 49 | 11m | M | 80 | nd | − | − |
| 66 | 14m | F | 83 | 46,XX,der(5),der(6),t(6;17)(p22;q22) del(6)(p22-pter)der(7)t(5;7)(?q;q31)del(7)(q31-qter),der(17)t(5;17)(?q;q22)[20] | − | − |
| 83 | 22m | M | 83 | nd | nd | − |
| 86 | 6y | M | 86 | 46,XY | nd | − |
| Adult AMKL | ||||||
| 48 | 62y | F | 62 | nd | − | − |
| 39 | 58y | M | 70 | 46,XY,−3,−5,del(7)(q21q33),der(20) t(3;20)(q23;q12),+21,+22[2]/88,XYY,−X,+Y,−4,−5,−5,+6,−7,+8,−9,−9,−11,del(12)(p12),+13,−14,−16,−17,−18,+19,+19,der(20)t(3;20)x2,+21,+21[18] | − | − |
| 54 | 40y | F | 42 | nd | nd | − |
| 71 | 67y | M | 50 | nd | nd | − |
| 72 | 47y | M | 90 | Complex aberrations | nd | − |
| 74 | 76y | F | 60 | 46,XX | nd | − |
| 82 | 45y | M | 71 | nd | − | − |
| 84 | 38y | M | na | nd | − | − |
| DS-AMKL | 20m | M | 40 | na | + | JAK2V617F |
| Cell lines | ||||||
| CMK | 10m | M | Complex hypotetraploid karyotype | + | JAK3A572V | |
| CMY | M | Complex hypotetraploid karyotype | + | JAK3A573V |
| Patient no. . | Age . | Sex . | % Blasts . | Karyotype . | GATA-1 mutations . | JAK2, JAK3, FLT3, KIT, and MPL changes . |
|---|---|---|---|---|---|---|
| Down syndrome | ||||||
| AMKL | ||||||
| 11 | 10m | F | 22 | Random aberration,+21c | + | − |
| 15 | 14m | M | 31 | 48,XY,+8,+21c | nd | FLT3 D835Y |
| 16 | 15m | F | 20 | na | nd | JAK3 V722I |
| 18 | 13m | F | 43 | 47,XX,+21c,del(6q) | + | − |
| 19 | 11m | F | 14 | 44∼46,XX,−4,−9,−16,+21c[cp7]/47,XX,+21c[7] | nd | FLT3 D835Y |
| 20 | 8m | F | 80 | Random aberration | + | JAK2 V617F |
| 30 | 18m | M | na | 47,XX,+21c/49,XX,inv(9)(p11;q12),+21c,+21c,+21c | + | − |
| Non-AMKL | ||||||
| 58 (TMD) | 2d | F | 14 | 47,XX,+21c | + | − |
| 85 (TMD) | 1m | M | na | 47,XY,+21[19]/46,XY[3] | + | − |
| 60 (AML) | 24m | M | 88 | 47,XY,+21c | + | − |
| 63 (AML) | 24m | F | 59 | 47,XX,+21c | + | − |
| Non–Down syndrome | ||||||
| Child AMKL | ||||||
| 69 | 1d | M | 90 | +21a | + | FLT3-ITD |
| 24 | 51m | M | 58 | Random aberration | nd | MPL T487A |
| 28 | 8m | F | 57 | 46, XX | nd | KIT D816V |
| 29 | 11y | M | 46 | 45,XY,inv(3)(q21q23),−7 | nd | − |
| 31 | 13m | M | na | 47XY,+2,+15,−19,t(7;8) | nd | JAK2 M535I |
| 33 | 16m | M | 31 | 46, XY | − | − |
| 34 | 33m | M | 40 | 46, XY | − | − |
| 35 | 20m | F | 73 | 47,XX,+3[4]/46,XX[21] | − | − |
| 36 | 30m | M | 80 | 46,XY,random aberration[7]/46,XY[15] | − | − |
| 37 | 36m | M | 74 | 46,XY,add(2)(q37),ins(3;?)(q21;?),del(5)(q13q22),−7,−9,add(16)(q24),+min,+mar | − | − |
| 38 | 24m | F | 60 | 46,XX,del(9)(p21)[1]/46,X,t(X;21)(q24;q21),del(9)(p21),+19[19] | − | − |
| 41 | 7m | M | 47 | 64-65,XY,t(1;8)(p32;q24),+2,+3,+4,+5,+6,+8,+9,+10,+19,−20,+22 | − | − |
| 44 | 8m | M | na | 6, XY[20] | − | − |
| 45 | 3m | M | 73 | 6, XY[20] | − | − |
| 46 | 26y | M | 15 | nd | − | − |
| 47 | 38m | M | 72 | nd | nd | − |
| 49 | 11m | M | 80 | nd | − | − |
| 66 | 14m | F | 83 | 46,XX,der(5),der(6),t(6;17)(p22;q22) del(6)(p22-pter)der(7)t(5;7)(?q;q31)del(7)(q31-qter),der(17)t(5;17)(?q;q22)[20] | − | − |
| 83 | 22m | M | 83 | nd | nd | − |
| 86 | 6y | M | 86 | 46,XY | nd | − |
| Adult AMKL | ||||||
| 48 | 62y | F | 62 | nd | − | − |
| 39 | 58y | M | 70 | 46,XY,−3,−5,del(7)(q21q33),der(20) t(3;20)(q23;q12),+21,+22[2]/88,XYY,−X,+Y,−4,−5,−5,+6,−7,+8,−9,−9,−11,del(12)(p12),+13,−14,−16,−17,−18,+19,+19,der(20)t(3;20)x2,+21,+21[18] | − | − |
| 54 | 40y | F | 42 | nd | nd | − |
| 71 | 67y | M | 50 | nd | nd | − |
| 72 | 47y | M | 90 | Complex aberrations | nd | − |
| 74 | 76y | F | 60 | 46,XX | nd | − |
| 82 | 45y | M | 71 | nd | − | − |
| 84 | 38y | M | na | nd | − | − |
| DS-AMKL | 20m | M | 40 | na | + | JAK2V617F |
| Cell lines | ||||||
| CMK | 10m | M | Complex hypotetraploid karyotype | + | JAK3A572V | |
| CMY | M | Complex hypotetraploid karyotype | + | JAK3A573V |
na indicates not available; and nd, not determined.
Primers were as follows: JH1-JAK2 I (TTCTTTCAGAGCCATCATACG; TATATTTCTCGTTGCCAGATCC); JH1-JAK2 II (TGCAAGGGTATGGAGTATCTTG; CTACTTTGGTCTCAGAATGAAGG); JH2-JAK2 I (GTCCCCCAAAGCCAAAAGATAAAT; TGTAATACTAATGCCAGGATCAC); JH2-JAK2 II (CTTATTCATGGGAATGTATGTGCC; TCTCTTCAAACTGTGTAGGATCC); SH2a-JAK2 (CCGAGTTGTAACTATCCATAAG; AGTAGGCCTCTGTAATGTTGG); JH2-JAK3 I (AATCCCAATACCAGCTGAG; GGATCCTGTCGGTGAGCAT); JH2-JAK3 II (GGAGCCCGCCCTTCATCA; TGCCGCTATGACCCGCTA); JH2-JAK3 III (CCTAAGGCAGGTCTGTGAGC; GAGGTGGGAAGAACAGCCTA); JH2-JAK3 IV (ACAGAGGTGCTGCTGAAGGT; CCACGTTTTCGCAGATACAT); SH2-JAK3 V (GCTACTTCCGGCTGACCAC; ACCACCTCATGGCGACAG); MPL I (TCGCTACCGTTTACAGCTGC; ACTTGGGGAGGATTTCAAGG); MPL II (GTTAGGGCGCTCTATCCTGTTG; AGTCCCTGGCGCAGGCGCTGT); KIT (GGATGACGAGTTGGCCCTAGA; GTAGAACTTAGAATCGACCGGCA); KIT-JM (AAACTCATCTGGGCCACCGTTT; CACTCGGCTTGAGCATCTTT); KIT-EC (ACCGAAGGAGGCACTTACAC; TACATTCAACCGTGCCATTG); FLT3 I (GCAATTTAGGTATGAAAGCCAGC; CTTTCAGCATTTTGACGGCAACC); FLT3 II (GAAGATCTTCTTTGCTTTGC; TATGACCAGACATCACTCTT); and FLT3 III (TTGCAATCATAAGCACCAGCC; AAGTACTCATTATCTGAGGAGCC).
Plasmids
The JAK2 expression vector has been previously described.26 The JAK3 and MPL sequences were polymerase chain reaction (PCR) amplified from a human wild-type cDNA template and subcloned in MSCV-IRES-GFP. Mutations were generated using the Quickchange kit (Stratagene, Amsterdam, The Netherlands). All constructions were verified by sequence analyses.
Cell culture
Murine interleukin-3 (IL-3)–dependent BaF3 cells were grown with 5% WEHI-conditioned medium (WCM) as a source of IL-3. Cells were electroporated and GFP-positive cells were sorted by flow cytometry after 6 days of culture. Cytokine-independent growth assays and serum starvation experiments were performed as described.26 Cell growth was monitored using a PCA-96 Easycyte (Guava Technologies, Hayward, CA).
Western blot analysis
Cells were lysed and separated by electrophoresis as described previously.26 All antibodies were from Cell Signaling (Ozyme, St Quentin-en-Yvelines, France) except the anti-MPL antibody (Millipore, St Quentin-en-Yvelines, France).
Bone marrow transplantation and mice analysis
This study received INSERM review board approval for the use of mice. Viral supernatants were obtained as described.27 Briefly, 6- to 8-week-old BALB/c donor mice were injected with fluorouracil (5-FU) 5 days prior to bone marrow collection. Primary bone marrow cells were obtained from femurs and tibiae. Suspensions were depleted of lineage marker–expressing cells (BD Biosciences, Le Pont de Claix, France) and cultured for 2 days in RPMI1640 supplemented with 10% fetal bovine serum in the presence of murine recombinant IL-3 (10 ng/mL), IL-6 (10 ng/mL), and SCF (100 ng/mL; all from PromoCell, Heidelberg, Germany).
Cells were mixed with viral supernatants on day 3 and day 4 and spinfected for 90 minutes at 1800g each time. After the second spinfection, 1.5 × 105 cells were injected into the retro orbital veins of lethally irradiated BALB/c recipients. At the time of injection, efficiency of retroviral transduction was assessed by GFP expression by flow cytometry and ranged from 40% to 50%. Eye bleeds were performed using EDTA or heparin-coated capillary tubes and blood counts were performed within 30 minutes on a MS9-5V (Melet Schloesing, Cergy-Pontoise, France). At the time of final analysis (30 days max after transplantation), the percentage of GFP-positive cells in the blood remained the same as at the time of injection for MPLWT (50%), but increased to 95% or more in MPLW515L and MPLT487A animals, suggesting positive selection. When possible, MPLT487A and MPLWT animals were analyzed simultaneously.
Staining for flow cytometry was performed in PBS. All antibodies were obtained from BD Biosciences, except the CD42b antibody obtained from Emfret (Eibelstadt, Germany). Acquisition of the data was performed on a CyanADP (Dako, Trappes, France) and analyzed with Summit software (Dako). For colony-forming assays, primary mouse bone marrow or spleen cells were collected and were subsequently treated with red blood cell lysis buffer. For myeloid colony assays, cells were plated in MethoCult 3434 or MethoCult 3231 (StemCell Technologies, Grenoble, France) and colonies were scored at day 7. For megakaryocytic progenitor colony assays, 2 × 105 bone marrow cells were mixed with MegaCult-C (StemCell Technologies) containing Tpo, IL-11, IL-3, and IL-6 and plated onto double-chamber culture slides; colonies were enumerated at day 7, after acetylcholinesterase staining.
Results
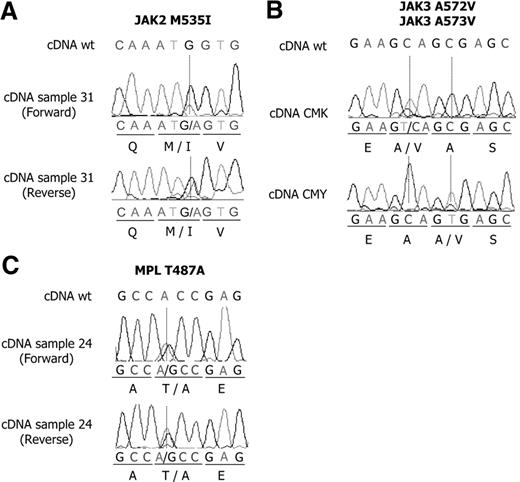
We investigated 11 DS and 28 non–DS-AMKL samples by nucleotide sequence analysis of cDNA. Because of the limited amount of material available, only selected exons were analyzed from PCR-amplified cDNA, including the sequences coding for the juxtamembrane regions and the activation loops of FLT3 and KIT; the regions encoding the JH1 and JH2 regions of JAK2 and the JH2 and SH2 regions of JAK3, where activating mutations have been described; and the MPL sequences coding for the protein segment spanning the membrane. The observed nucleotide sequence changes are shown in Figure 1 and Figure S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article) and are summarized Table 1.
Mutations of JAK2, JAK3, and MPL in AMKL samples and cell lines. Mutations of JAK2 M535I (A) and MPL T487A (C) were found in children with non–Down syndrome AMKL. Mutations of JAK3 A572V and JAK3 A573V were found in CMK and CMY cell lines, respectively (B).
Mutations of JAK2, JAK3, and MPL in AMKL samples and cell lines. Mutations of JAK2 M535I (A) and MPL T487A (C) were found in children with non–Down syndrome AMKL. Mutations of JAK3 A572V and JAK3 A573V were found in CMK and CMY cell lines, respectively (B).
No sequence changes were observed in non–DS AMKL samples. Three mutations were observed in FLT3: 1 ITD (patient 69) and 2 mutations leading to D to Y amino acid substitution at position 835 (D835Y; patients DS15 and DS19). One example of KIT mutation was observed in a non-DS patient (D816V; patient 28). Two changes in the JAK2 cDNA were also observed, resulting in M535I (patient 31) and V617F (patient DS20). A V722I was observed in JAK3 in patient DS16. Regarding MPL, no changes were observed within the codon coding W515 in our series. However a nonsynonymous nucleotide change was observed in a non-DS patient (patient 24), leading to a T to A substitution at amino acid 487. No remission material was available.
Taken together, 3 unambiguous mutations were observed in each subgroup (DS and non-DS patients). Although the number of patients is very small, the frequency of activating mutations within these genes seems higher in AMKL from DS patients (57%) with respect to non-DS patients (11%).
Three additional mutations have been identified. A JAK2V617F was observed in another DS-AMKL patient (Figure S1), unrelated to the above-mentioned series. Within JAK3, a A573V change was observed in the cell line CMY and a A572V change in CMK (Figure 1B). Sequence analyses showed an higher quantity of the mutated JAK3 allele, with respect to wild type, when starting from cDNA or from genomic DNA (Figure S2A). In keeping with these results, fluorescent in situ hybridization (FISH) analyses demonstrated the presence of 3 JAK3 loci in the cells (data not shown), suggesting the mutated allele has been duplicated. These 2 leukemic cell lines were established from DS-AMKL samples.28,29
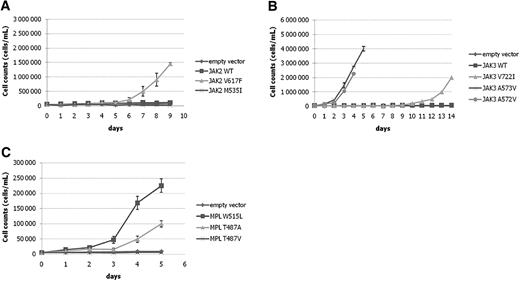
We next evaluated the newly identified amino acid changes functionally for their potential to confer cytokine-independent cell growth to BaF3 cells. MSCV-GFP constructs expressing JAK3A572V, JAK3A573V, JAK3V722I, JAK2M535I, JAK2V617F, and MPLT487A were transduced in the murine IL3-dependent BaF3 cell line and growth of sorted GFP-positive cells was tested in the absence of cytokine (Figure 2).
Analyses of the candidate mutations in BaF3 cells. (A) JAK2M535I does not induce the growth of BaF3 cells in the absence of IL-3. (B) JAK3A573V induces IL-3–independent growth of BaF3 cells. The growth of JAK3V722I cells is observed at later time points. (C) Expression of MPLT487A results in IL-3–independent growth of BaF3 cells. MPLT487A induces IL-3–independent growth of BaF3 cells slightly later than MPLW515L does; MPLT487V is not able to induce growth in these conditions. Two independent experiments, each in triplicate, were performed, and the mean plus or minus SD of 1 representative experiment is shown.
Analyses of the candidate mutations in BaF3 cells. (A) JAK2M535I does not induce the growth of BaF3 cells in the absence of IL-3. (B) JAK3A573V induces IL-3–independent growth of BaF3 cells. The growth of JAK3V722I cells is observed at later time points. (C) Expression of MPLT487A results in IL-3–independent growth of BaF3 cells. MPLT487A induces IL-3–independent growth of BaF3 cells slightly later than MPLW515L does; MPLT487V is not able to induce growth in these conditions. Two independent experiments, each in triplicate, were performed, and the mean plus or minus SD of 1 representative experiment is shown.
When tested in that assay, JAK2M535I was neither able to stimulate the growth of BaF3 in the absence of IL3, nor able to detectably activate signaling effectors (Figure 3A), suggesting that it is devoid of strong transforming properties.
Activation of signal transduction pathways by JAK2, JAK3, and MPL mutants. Studies of Stat5, Erk1/2, and Akt phosphorylation in the different BaF3 clones expressing the mutant JAK2 (A), JAK3 (B), and MPL (C) proteins.
Activation of signal transduction pathways by JAK2, JAK3, and MPL mutants. Studies of Stat5, Erk1/2, and Akt phosphorylation in the different BaF3 clones expressing the mutant JAK2 (A), JAK3 (B), and MPL (C) proteins.
Two of the JAK3 mutants are present in cell lines. Both cell lines harboring JAK3 mutations (CMK and CMY) exhibited constitutive STAT5 and JAK3 tyrosine phosphorylation and were sensitive to the JAK inhibitor I, indicating that JAK3A572V and JAK3A573V were bona fide activating mutations (Figure S2). All 3 mutations were expressed in BaF3 cells. JAK3A572V and JAK3A573V readily transformed BaF3 cells to IL3 independency, whereas JAK3V722I induced cell growth, albeit in a delayed fashion (Figure 2). Detailed analyses of JAK3A572V and JAK3V722I have been reported elsewhere,21 and JAK3A573V was assumed to behave similarly to JAK3A572V (Figure 3B).
Using this assay, MPLT487A was compared with MPLWT and MPLW515L. MPLT487A and MPLW515L were able to induce BaF3 growth in the absence of IL3. Accordingly, Stat5, Erk1/2, and Akt were constitutively phosphorylated in MPLT487A cells, as judged by Western blot analysis (Figure 3C). This result indicated that transformation by MPLT487A was associated with activation of the main signaling pathways normally activated by MPL.
The substitution of a threonine by an alanine resulted in both structural and polarity changes at this position. In several other species, such as mouse, a serine replaces this threonine. To test whether both structural and polar changes were important, we created an artificial mutant in which threonine was changed to valine, thereby maintaining the structure, but losing the polarity. When tested in BaF3 cells, the mutant MPLT487V was not able to induce BaF3 growth in the absence of IL3. This result indicates an important role of the beta-branched side chain structure (implying a certain degree of imposed rigidity) for the amino acid residue at this position for maintaining the unliganded human MPL inactive.
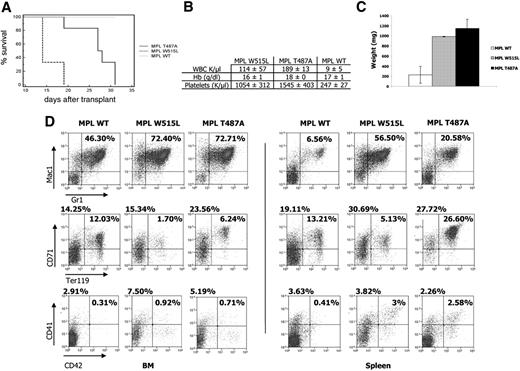
We next wanted to assess the in vivo effects of MPLT487A. Bone marrow cells from BALB/c mice were transduced with MSCV-GFP vectors containing MPLWT or MPLT487A and were engrafted in lethally irradiated recipients. The MPLW515L mutant was included for comparison purposes.
MPLW515L-transduced mice developed a myeloproliferative disease with a short latency (median survival: 14 days) as previously reported.6 As shown in Figure 4A, MPLT487A-transduced mice developed a similar disease, albeit with a longer latency (median survival: 27.5 days; P = .008). The disease was associated with thrombocytosis, leukocytosis, and splenomegaly (Figure 4B,C). Histopathology analysis was consistent with the presence of a MPD in MPLT487A- and MPLW515L-expressing mice, and staining of bone marrow evidenced an increased reticulin fiber network leading to a mild myelofibrosis in both cohorts of engrafted mice, but not in MPLTWT-transduced mice (Figure S3). Flow cytometric analyses of bone marrow and spleen cells of the transduced animals are shown Figure 4D. MPLT487A samples showed an increase of mature myeloid cells (Mac1+Gr1+) in both tissues, compared with MPLWT-transduced animals. Amplification of the immature erythroid compartment (CD71+Ter119−) was also observed in both bone marrow and spleen. A strong amplification of more mature erythroid cells (CD71+Ter119+) was observed specifically in the spleen of MPLT487A, with respect to MPLWT and MPLW515L. The percentage of CD41+/CD42+ cells was increased in bone marrow and spleen of MPLT487A, reflecting an amplification of the megakaryocytic lineage.
Comparison of BALB/c mice engrafted with bone marrow cells transduced with MPLT487A, MPLW515L, and MPLWT. (A) Kaplan-Meier survival plot of recipient BALB/c mice expressing MPLWT (n = 6), MPLW515L (n = 3), and MPLT487A (n = 6). MPLWT mice were killed without signs of disease. (B) Blood count analysis. MPLWT (n = 6), MPLW515L (n = 3), and MPLT487A (n = 6). (C) Splenomegaly in MPLW515L (n = 3) and MPLT487A (n = 5) mice. The spleens of 4 MPLWT mice were weighted for comparison purposes. Error bars denote SD. (D) Flow cytometric analysis of bone marrow cell suspensions (left panel) and spleen cell suspensions (right panel). It shows an increase of granulocytic cells (Mac1+Gr1+; first row) and of immature erythroid populations (CD71+/Ter119+; second row) and an amplification of the megakaryocytic lineage (CD41+/CD42+; third row), when MPLT487A-transduced cells are compared with the others. These analyses are representative of several: MPLWT (n = 3), MPLW515L (n = 1), and MPLT487A (n = 4).
Comparison of BALB/c mice engrafted with bone marrow cells transduced with MPLT487A, MPLW515L, and MPLWT. (A) Kaplan-Meier survival plot of recipient BALB/c mice expressing MPLWT (n = 6), MPLW515L (n = 3), and MPLT487A (n = 6). MPLWT mice were killed without signs of disease. (B) Blood count analysis. MPLWT (n = 6), MPLW515L (n = 3), and MPLT487A (n = 6). (C) Splenomegaly in MPLW515L (n = 3) and MPLT487A (n = 5) mice. The spleens of 4 MPLWT mice were weighted for comparison purposes. Error bars denote SD. (D) Flow cytometric analysis of bone marrow cell suspensions (left panel) and spleen cell suspensions (right panel). It shows an increase of granulocytic cells (Mac1+Gr1+; first row) and of immature erythroid populations (CD71+/Ter119+; second row) and an amplification of the megakaryocytic lineage (CD41+/CD42+; third row), when MPLT487A-transduced cells are compared with the others. These analyses are representative of several: MPLWT (n = 3), MPLW515L (n = 1), and MPLT487A (n = 4).
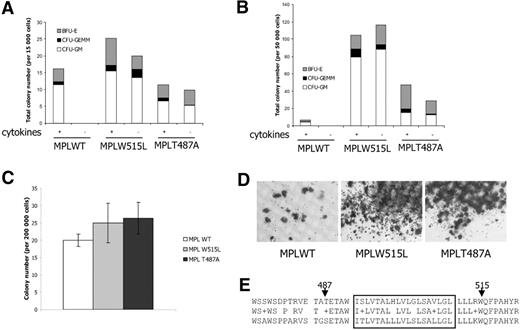
The MPLW515L is able to sustain cytokine-independent myeloid colony growth.6 To investigate this property in MPLT487A-expressing cells, cellular suspensions from the killed mice were tested for colony growth in vitro, either in the presence or in the absence of cytokines. When isolated from bone marrow or from spleen, cellular suspensions from MPLT487A-transduced mice were able to generate colonies in the presence of cytokines (Figure 5A,B). Similarly, MPLW515L samples generated myeloid colonies from bone marrow and spleen, whereas almost no colonies were observed from spleen of mice engrafted with MPLWT-transduced cells. In the absence of cytokine, only MPLT487A- and MPLW515L-expressing progenitors were able to generate colonies irrespective of their bone marrow or spleen origin.
In vitro analysis of MPLT487A cells. (A) Growth of myeloid colonies from bone marrow cells in the presence (+) or the absence (−) of cytokines. MPLW515L- and MPLT487A-transduced, but not MPLWT-transduced, cells are able to generate myeloid colonies in the absence of cytokines. Two MPLW515L, 5 MPLT487A, and 3 MPLWT animals were analyzed. (B) Growth of myeloid colonies from spleen cellular suspensions in the presence (+) or absence (−) of cytokines. Consistent with the observed splenomegaly, MPLW515L and MPLT487A mice generate myeloid colonies in the presence or absence of cytokines. Two MPLW515L, 5 MPLT487A, and 3 MPLWT animals were analyzed. (C) Megakaryocytic progenitor colony assays. Bone marrow samples of MPLT487A mice generated more megakaryocytic colonies than bone marrow from MPLWT mice (P = .01). Two MPLW515L, 5 MPLT487A, and 4 MPLWT animals were analyzed. Error bars denote SD. (D) Acetylcholine staining of the megakaryocytic colonies. As for MPLW515L, megakaryocytic colonies from MPLT487A mice were larger than those from MPLWT mice. (E) Alignment of human (top) and mouse (bottom) MPL proteins. Amino acids 474 to 522 (NP 005364) are shown for the human MPL protein and 465 to 513 (NP 034953) for the mouse MPL. The predicted transmembrane region is boxed. Arrowheads indicate the amino acids mutated in human hematologic malignancies. Immunohistochemistry-stained sections were examined under a Leica DMLB microscope (Leica, Heidelberg, Germany) equipped with a 10× eyepiece and a 10×/0.40 CS HC PL APO objective lens. Images were captured with a Sony Power HAD camera (Sony France, Paris, France) and imaging was performed with the version 1.4 software TRIBVN-ICS (Image Communication Software, Chatillon, France) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
In vitro analysis of MPLT487A cells. (A) Growth of myeloid colonies from bone marrow cells in the presence (+) or the absence (−) of cytokines. MPLW515L- and MPLT487A-transduced, but not MPLWT-transduced, cells are able to generate myeloid colonies in the absence of cytokines. Two MPLW515L, 5 MPLT487A, and 3 MPLWT animals were analyzed. (B) Growth of myeloid colonies from spleen cellular suspensions in the presence (+) or absence (−) of cytokines. Consistent with the observed splenomegaly, MPLW515L and MPLT487A mice generate myeloid colonies in the presence or absence of cytokines. Two MPLW515L, 5 MPLT487A, and 3 MPLWT animals were analyzed. (C) Megakaryocytic progenitor colony assays. Bone marrow samples of MPLT487A mice generated more megakaryocytic colonies than bone marrow from MPLWT mice (P = .01). Two MPLW515L, 5 MPLT487A, and 4 MPLWT animals were analyzed. Error bars denote SD. (D) Acetylcholine staining of the megakaryocytic colonies. As for MPLW515L, megakaryocytic colonies from MPLT487A mice were larger than those from MPLWT mice. (E) Alignment of human (top) and mouse (bottom) MPL proteins. Amino acids 474 to 522 (NP 005364) are shown for the human MPL protein and 465 to 513 (NP 034953) for the mouse MPL. The predicted transmembrane region is boxed. Arrowheads indicate the amino acids mutated in human hematologic malignancies. Immunohistochemistry-stained sections were examined under a Leica DMLB microscope (Leica, Heidelberg, Germany) equipped with a 10× eyepiece and a 10×/0.40 CS HC PL APO objective lens. Images were captured with a Sony Power HAD camera (Sony France, Paris, France) and imaging was performed with the version 1.4 software TRIBVN-ICS (Image Communication Software, Chatillon, France) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA).
To perform a specific analysis of megakaryocytic progenitors, bone marrow suspensions were seeded in specific megakaryocytic conditions (Figure 5C). In these conditions, the number of colonies was higher in MPLT487A than MPLWT animals. In addition, the size of these colonies was much larger compared with MPLWT (Figure 5D).
Taken together, our results show that, similarly to MPLW515L, MPLT487A induces a myeloproliferative disorder in the mice and is able to sustain cytokine-independent growth of myeloid cells in vitro, thereby establishing that MPLT487A is a bona fide activating mutation.
Discussion
AMKL can develop either de novo or as a transformation of MPD. Transformation of megakaryoblastic progenitor is a multistep process that remained poorly understood because of the very limited access to primary cells. We investigated the status of a set of genes mutated in human AML and MPD and encoding molecules involved in tyrosine kinase signaling in a series of human AMKL samples, from DS and non-DS patients. In addition to oncogenic mutations within JAK2, FLT3, and KIT, we identified nonsynonymous nucleotide changes in JAK2, JAK3, and MPL, which were not readily attributable to known polymorphisms.
It seems from the sequence data that the JAK2V617F mutation is present at low level, suggesting its presence in a minor clone. The shortage of material prevented any accurate quantification of the mutation in our samples. However, this observation suggests that JAK2V617F only weakly cooperates with + 21 and GATA1s.
We observed a JAK3V722I in a DS-AMKL patient, a nonsynonymous nucleotide change repeatedly reported in similar patients. As previously reported,21 the mutant protein transforms BaF3 cells in a delayed fashion, compared with the other mutations. The exact role of this mutation in transformation remains elusive.
The JAK3A573V possesses hallmarks of a gain-of-function mutation: the protein is constitutively tyrosine phosphorylated; supports the growth of CMY, the cell line endogenously expressing JAK3A573V; is sensitive to JAK tyrosine kinase inhibitors; and induces IL3-independent growth of BaF3. Two independent descriptions of the same mutation in primary cells of DS-AMKL were recently reported,30,31 indicating that this is not a cell line artifact. Taken together, our data strongly support the recurrent participation of JAK activating mutations in DS leukemogenesis.21,26,32
Examination of patient 69, the unique non–DS-AMKL patient with an FLT3 mutation, showed that he harbored an additional chromosome 21 and a mutated GATA1 gene.25 Therefore, our results strengthen the proposed oncogenic cooperation between tyrosine kinase signaling and GATA1s in a + 21 context.33 Such a cooperation may be related to the fact that GATA1s induces an increased proliferation of fetal but not of adult MK progenitors.16 Therefore, acquisition of a tyrosine kinase mutation might be required in the transformation of GATA1s cells because a switch from fetal to adult hematopoiesis seems to occur during the first year of life.
We report a novel mutation with the MPL cytokine receptor in a non–DS AMKL patient. This mutation was not observed in a small series of 30 AML samples (data not shown and Table S1), indicating it might be specific for megakaryoblastic diseases. Study of the MPLT487A mutant showed that it was able to induce a myeloproliferative disease in mice, underscoring a link between MPD and AMKL.
The exact mechanism by which MPLT487A induces MPL signaling will need to be investigated. The targeted threonine is predicted to be situated in the extracellular domain of MPL, close to the membrane, in a position symmetrically opposite to W515, the amino acid mutated in MPD6,8,9,34 (Figure 5E). It has been shown that W515 mutations affect an amphipathic alpha-helix and induce a change in receptor conformation and constitutive activation.34 The 2 types of mutations, T487A and W515L/K, affecting residues flanking the MPL transmembrane domain, might abolish mechanisms that maintain the unliganded MPL inactive, possibly by allowing productive dimerization of MPL and activation of JAK2. The myeloproliferative diseases induced by MPLT487A and W515L appear, however, slightly different. The MPLT487A-induced disease had milder progression with the presence of an important erythroid participation. This suggests subtle differences in signaling between the 2 mutants. Finally, our findings suggest that a more expansive genomic screen for involvement of other components of the JAK-STAT signaling pathway is warranted in AMKL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the service commun de sequencage (Institut Cochin, Paris V, Paris, France), Gérard Pivert (service d'anatomie pathologique, Hôpital Necker-Enfants Malades, Paris, France) for technical assistance with histologic experiments, Gaelle Elain for reticulin staining, and Julie Piquet (LEAT, Faculté de Médecine Necker-Enfants Malades, Paris) for mice nursing. We thank Isabelle Radford (Hôpital Necker) for FISH experiments. We also thank S. H. Orkin (Dana-Farber Cancer Institute, Boston, MA) for the generous gift of the RNA samples.
This work was supported by grants from Association pour la Recherche sur le Cancer (ARC) and Institut National du Cancer (INCa); and grants to S.N.C. from Fonds National de la Recherche Scientifique, Fondation contre le cancer, Fondation Salus Sanguinis, Action de Recherche Concertée MEXP31C1 of the University catholique de Louvain, Brussels, the Fondation contre le Cancer, Brussels, the PAI Program BCHM61B5, Belgium, and the Atlantic Philantropies/Ludwig Institute for Cancer Research Clinical Discoveries Program.
S.M. was a recipient of successive Fondation pour la Recherche Médicale and Société Française d'Hematologie fellowships.
Authorship
Contribution: S.M. and C.R. designed research, performed research, and analyzed data; V.D.-V., C.P., and D.P. performed research; D.R., N.D., J.L.-P., L.M., A.B., and J.-P.B. provided vital reagents; W.V., J.-P.B., and S.N.C. analyzed data and revised the paper; V.P.-L. and O.A.B. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virginie Penard-Lacronique, E0210 INSERM, Tour Pasteur-Hôpital Necker, 149 Rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: virginie.penard-lacronique@inserm.fr; or Olivier Bernard, E0210 INSERM, Tour Pasteur-Hôpital Necker, 149 Rue de Sèvres, 75743 Paris Cedex 15, France; e-mail: olivier.bernard@inserm.fr.
References
Author notes
*S.M. and C.R. contributed equally to this work.