Abstract
Background: Iron overload has been suggested to be associated with various treatment-related complications of allogeneic stem cell transplantation. Since hepcidin, a peptide-hormone produced by the liver, plays a central role in the regulation of iron homeostasis, we analyzed the association between pre-transplant serum hepcidin-25 levels and early treatment-related complications within 100 days after transplantation.
Patients and methods: We studied 49 consecutive patients with a median age of 47 years (range, 23–64 years) who underwent allogeneic transplantations for hematologic malignancies at Kyoto University Hospital from 07/2006 to 08/2008. We excluded patients who had undergone any prior transplantation within one year or who had an active bacterial infection before transplantation. A total of 31 patients (63%) had myeloid malignancies; the remaining 18 (37%) had lymphoid malignancies. Twenty-seven patients (55%) received reduced-intensity conditioning regimens. The sources of stem cells were the bone marrow (n = 33), peripheral blood (n = 1), and cord blood (n = 15). Patients received fungal (fluconazole 400 mg/day) and viral (acyclovir 1000 mg/day) prophylaxes with a few exceptions. No bacterial prophylaxis was prescribed. Serum hepcidin-25 levels prior to the administration of conditioning regimen were measured by use of a liquid chromatography-tandem mass spectrometry-based assay system. The cumulative incidences of bacterial and fungal infections, hepatic veno-occlusive disease (VOD), and treatment-related mortality at day 100 were analyzed. The proportional-hazard model of sub-distribution functions in competing risks was used. The probability of overall survival at day 100 was also analyzed.
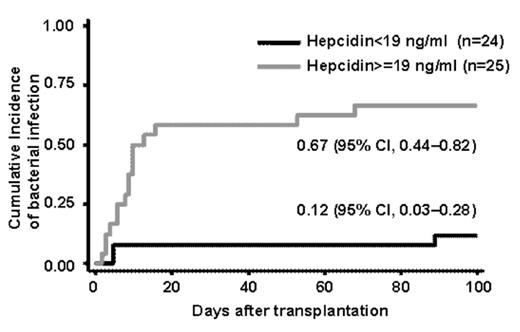
Results: The median pre-transplant serum hepcidin level among the patients was 18.7 ng/ml (range, 1.4–371 ng/ml), which was comparable to that among healthy volunteers (median, 19.1 ng/ml; range, 2.3–37 ng/ml, n = 17). The correlation between pre-transplant hepcidin and ferritin levels was weak among the patients (R2 = 0.2914). These patients were divided into two groups: the low hepcidin group (<19 ng/ml, n = 24) and high hepcidin group (≥19 ng/ml, n = 25). No significant difference was detected between the two groups with regard to the patient characteristics, except that serum ferritin levels were higher among the high hepcidin group (P = 0.039). The cumulative incidence of bacterial infection was significantly higher among the high hepcidin group than among the low hepcidin group [67% (95% confidence interval, 44–82%) vs. 12% (3–28%), P < 0.001] (Figure 1). This finding was confirmed in multivariate analysis adjusted for confounders, including the pre-transplant ferritin and C-reactive protein levels [hazard ratio for bacterial infection among the high hepcidin group as compared to low hepcidin group, 11.9; 95% confidence interval, 1.46–97.04; P = 0.021)]. No fungal infection was observed. VOD was observed in one patient with high hepcidin and high ferritin levels. We did not detect any association of the hepcidin levels with either the overall survival or the treatment-related mortality at day 100.
Conclusion: Pre-transplant serum hepcidin-25 levels were significantly associated with the incidence of early bacterial infection independent of the ferritin levels and might be useful for predicting the risk for post-transplant infectious complications. Larger prospective studies are warranted to confirm our findings.
Disclosures: Tomosugi:Medical Care Proteomics Biotechnology Co. Ltd.: Employment, Equity Ownership.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal