Abstract
Background: Improvements in supportive care and the use of reduced intensity conditioning regimens (RIC) have expanded the use of allogeneic stem cell transplantation (SCT) to older patients with myeloid leukemia (AML, MDS, CML or other myeloproliferative disorders). No large single or multi-institutional study looking at outcomes of patients over the age of 65 has been performed, and the results of allografting in septuagenarians (>70 years of age) have not been reported.
Rationale: To explore the long-term outcomes of allogeneic transplant in patients with hematologic malignancies over the age of 65 and determine the impact of age and other prognostic factors in this subset of patients.
Patients and Methods: From 1/96 to 12/07 a total of 96 patients over the age of 65 underwent an allogeneic SCT for a myeloid leukemia. Patient, treatment characteristics and outcomes are summarized in the accompanying table.
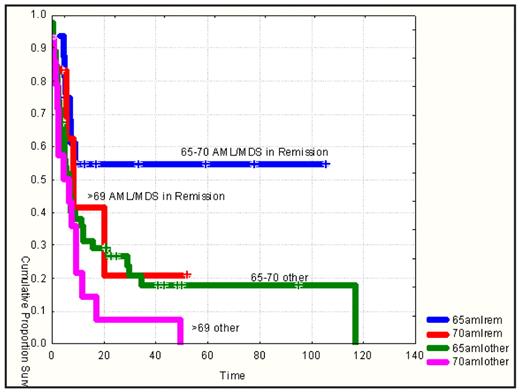
Outcomes: For AML/MDS patients in remission between 65 and 70 years of age the OS at 2 years is 54% (95%CI:27–75) and 21% (95%CI:1–59) for patients 70 years or older (p=0.3); for AML/MDS patients not in remission OS @ 2 years was 21% (95%CI:9–36) for patients between 65 and 70 years of age and 0% for patients 70 yrs or older (p=0.06).
Conclusion: Allogeneic SCT can result in long term disease control in selected patients over the age of 65. Disease status at the time of transplant remains the most important prognostic factor for outcomes in this patient population. Patients over the age of 70 with AML/MDS in remission or with MPD can undergo allografting safely and achieve long term benefits, patients over 70 with refractory disease should be treated primarily on clinical trials aimed at improving outcomes in this patient population.
| . | Less than 70 years of age . | 70 years of age or greater . |
|---|---|---|
| N | 71 | 25 |
| Median Age (range) | 66 (65–69) | 71 (70–76) |
| AML/MDS | 61 | 22 |
| CML/MPD | 10 | 3 |
| Disease Stage @ SCT | ||
| CR1/CR2/CP1 | 11/5/1 | 2/4/0 |
| Other | 54 | 19 |
| Regimen Type | ||
| Ablative | 10 | 1 |
| RIC | 48 | 14 |
| Non Ablative | 13 | 10 |
| % Matched Sibling Donor | 60 | 60 |
| % ATG in Conditioning | 39 | 40 |
| %OS @ 2 yrs (95%CI) | 35 (24–46) | 13 (3–30) p=0.08 |
| %PFS @ 2 yrs (95%CI) | 30 (19–42) | 13 (3–30) |
| %NRM @ 1 yr (95%CI) | 24 (16–37) | 44 (28–72) p=0.05 |
| %aGVHD 2 or greater | 28 (20–41) | 35 (20–61) |
| %cGVHD @ 2 yrs | 35 (25–50) | 26 (12–56) |
| . | Less than 70 years of age . | 70 years of age or greater . |
|---|---|---|
| N | 71 | 25 |
| Median Age (range) | 66 (65–69) | 71 (70–76) |
| AML/MDS | 61 | 22 |
| CML/MPD | 10 | 3 |
| Disease Stage @ SCT | ||
| CR1/CR2/CP1 | 11/5/1 | 2/4/0 |
| Other | 54 | 19 |
| Regimen Type | ||
| Ablative | 10 | 1 |
| RIC | 48 | 14 |
| Non Ablative | 13 | 10 |
| % Matched Sibling Donor | 60 | 60 |
| % ATG in Conditioning | 39 | 40 |
| %OS @ 2 yrs (95%CI) | 35 (24–46) | 13 (3–30) p=0.08 |
| %PFS @ 2 yrs (95%CI) | 30 (19–42) | 13 (3–30) |
| %NRM @ 1 yr (95%CI) | 24 (16–37) | 44 (28–72) p=0.05 |
| %aGVHD 2 or greater | 28 (20–41) | 35 (20–61) |
| %cGVHD @ 2 yrs | 35 (25–50) | 26 (12–56) |
Figure
Disclosures: Giralt:CELGENE: Consultancy, Research Funding, Speakers Bureau; GENZYME: Consultancy, Speakers Bureau; NOVARTIS: Consultancy, Research Funding, Speakers Bureau; MILLENIUM: Consultancy, Speakers Bureau. Off Label Use: MOST DRUGS USED IN STEM CELL TRANSPLANTATION ARE OFF LABEL.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal