Abstract
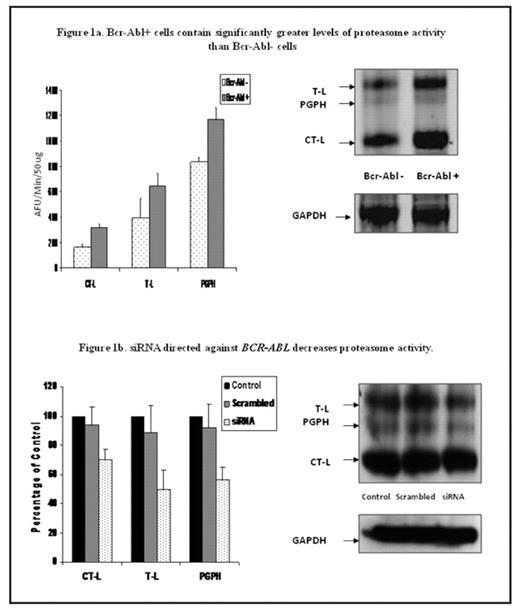
Chronic myeloid leukemia (CML) is a malignant disorder of the hematopoietic stem cell, characterised by the constitutively active tyrosine kinase BCR-ABL. The current first-line therapy for CML is the tyrosine kinase inhibitor imatinib. Although imatinib induces durable responses, a number of patients develop resistance to this treatment, highlighting the need to identify new molecular targets in this disease. Proteasome inhibition has recently emerged as a novel anti-cancer therapy. There is evidence to suggest that the proteasome is a valid target in CML. We have previously reported that proteasome activity is higher in bone marrow from patients with CML than normal controls. Furthermore, we demonstrated using a cell line model that Bcr-Abl positive cells were more sensitive to induction of apoptosis by proteasome inhibition than Bcr-Abl negative cells. The present study investigates the relationship between Bcr-Abl expression and proteasome activity and the effect of proteasome inhibition on primary human CML cells. Conventional fluorogenic substrate assays for all three catalytic activities of the proteasome [chymotrypsin-like (CT-L), trypsin-like (T-L), post glutamyl peptide hydrolysing (PGPH)] and proteasome activesite label DansylAhx3L3VS were used to profile proteasome activity levels in ts-Bcr-Abl FDCP-Mix cells and mock transfected FDCP-Mix cells. Both methods confirmed that Bcr-Abl positive cells have higher levels of proteasome activity than Bcr-Abl negative cells (p ≤ 0.04; Figure 1a). Conversely, downregulation of BCR-ABL using si-RNA was associated with a significant decrease in proteasome activity (p < 0.05; Figure 1b). The ability of the proteasome inhibitor BzLLLCOCHO to induce apoptosis in primary human CML cells was evaluated using Mitosensor™ and Hoescht/Propidium Iodide staining. Treatment with BzLLLCOCHO (1μM), selectively induced apoptosis in primary CML cells compared to normal mononuclear cells (39 ± 9.6 % vs 18.1 ± 4.02 %, 72 hrs, p = 0.01). Drug combination experiments were performed with BzLLLCOCHO (1 μM) and imatinib (1 μM) in ts-Bcr-Abl FDCP-mix cells and primary CML cells. Using Calcusyn software to generate the median effect of Chou-Talalay, the sequential addition of imatinib followed by BzLLLCOCHO was found to synergistically enhance the induction of apoptosis in ts-Bcr-Abl FDCP-Mix cells and resulted in additive effects in primary CML cells (n=4). The effect of the compounds on Bcr-Abl kinase activity was assessed by immunoblotting for phosphorylated Crkl. No effect on Bcr-Abl activity was seen following treatment of ts-Bcr-Abl FDCP-Mix cells and primary CML cells with BzLLLCOCHO alone, however, the combination of BzLLLCOCHO and imatinib resulted in a greater reduction of Bcr- Abl activity (59.8 ± 9.9 %) than imatinib alone (34.1 ± 9.6 %). Finally, we investigated the effect of BzLLLCOCHO on two human CML cell lines which are resistant to imatinib (KCL22-r, LAMA84-r). Imatinib resistant cells were found to be equally as sensitive to induction of apoptosis by BzLLLCOCHO as their imatinib sensitive counterparts (KCL22-s 30.7 ± 5.7 % vs KCL22-r 32 ± 1.7 %; LAMA84-s 56.3 ± 3.2 % vs Lama84-r 57.2 ± 7.9 %; 72 hrs). The present findings suggest that higher proteasome activity in Bcr- Abl positive cells may render these cells more susceptible to induction of apoptosis by proteasome inhibition and provide a rational basis to examine the potential of proteasome inhibitors as a therapeutic target in CML, particularly in imatinib resistant disease.
Bcr-Abl+ cells contain significantly greater levels of proteasome activity than Bcr-Abl cells
siRNA directed against BCR-ABL decreases proteasome activity.
Bcr-Abl+ cells contain significantly greater levels of proteasome activity than Bcr-Abl cells
siRNA directed against BCR-ABL decreases proteasome activity.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal