Abstract
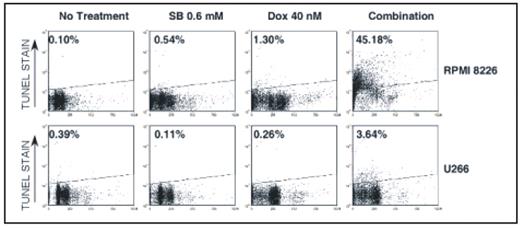
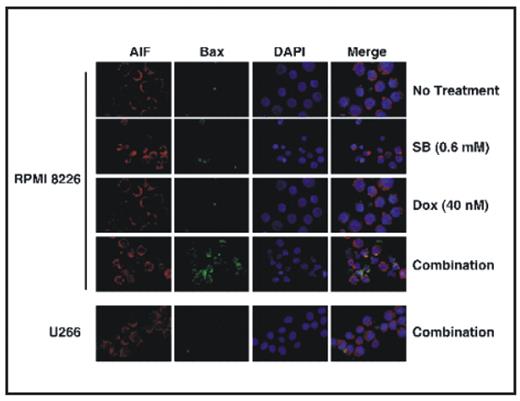
Combining drugs that inhibit different antiapoptotic pathways will be useful in overcoming drug resistance in multiple myeloma (MM). Since histone deacetylase inhibitors (HDACi) affect multiple pathways of cancer progression by relaxing chromatin; their ability to reverse apoptosis resistance in MM in combination with a DNA damaging agent (doxorubicin) was investigated. Sodium butyrate (SB) (0.15 mM, 0.3 mM and 0.6 mM) and vorinostat (0.3 uM and 0.6 μM) were used as HDACi and doxorubicin (10 nM, 20 nM and 40 nM) was used as a DNA damaging agent. Percent survival of fresh MM cells was assessed with flow cytometry and viability of MM cell lines (NCI H929, RPMI 8226 and U266) was measured with alamar blue. Molecular mechanisms of synergy between HDACi and doxorubicin were characterized in MM cell lines using TUNEL assay for apoptosis, immunofluorescence microscopy for apoptosis-inducing factor (AIF) release and Bax conformational change. Cathepsin B (Cat B) and calpain activity were measured using kits from Biovision Inc. Gene expression array studies were carried out using Human V6 Illumina chip. Treatment of mononuclear cells containing stromal and plasma cells (CD138+) from bone marrow aspirates of 22 patients with SB (0.6 mM), doxorubicin (40 nM) or with their combinations for 72 hr markedly decreased the percentage survival of CD138+ cells. One way repeated measures of ANOVA showed a significant (P<0.0001) reduction in percentage of CD138+ survival by combination treatment with mean survival 47.9+6.6% vs. 73.6+5.3% (SB) or 83.8+4.3% (doxorubicin) as single agents. Similarly, vorinostat, a clinically relevant HDACi in combination with doxorubicin also markedly reduced the percentage survival of fresh MM cells further suggesting the clinical relevance of the drug combinations evaluated. Although combinations of SB and doxorubicin synergistically (with a combination index of <1 in Chou and Talalay analysis) reduced the viability of all MM cell lines, combination treatment induced marked apoptosis only in 3 out of 5 MM cell lines tested (Figure 1). Studies undertaken to understand the molecular basis of differential apoptosis sensitivity revealed that SB plus doxorubicin induced apoptosis in a caspase independent manner and differential release of AIF and Endonuclease G from mitochondria correlated with differential sensitivity. Among various proapoptotic factors analyzed, activation and mitochondrial translocation of Bax was found to be associated with AIF release in co-localization studies (Figure 2). SB plus doxorubicin induced Bax activation in a caspase independent manner by synergistically increasing the activity of Cat B. Thioredoxin an inhibitor of Cathepsin is overexpressed in tumor cells including MM. Studies have shown that Thioredoxin and its inhibitory protein thioredoxin interacting protein (TXIN) were induced by doxorubicin and HDACi respectively. Consistent with this, in gene expression array studies, relative to NCI H929 and U266 cells, mRNA levels of TXIN were 2X higher in apoptosis sensitive RPMI 8226 cells and were further increased another 3X by vorinostat. In apoptosis resistant NCI H929 and U266 cells vorinostat failed to upregulate TXIN. These results support a model for HDACi plus doxorubicin induced caspase independent apoptosis in MM by an increase in expression of Cathepsin and TXIN with a concurrent decrease in thioredoxin. Under this condition the activity calpain, a lysosomal enzyme that is also capable of activating Bax remained unchanged. More importantly, in apoptosis resistant cell lines (NCI H929 and U266) HDACi plus doxorubicin combination failed to increase Cat B activity, conformational change of Bax and mitochondrial release of AIF. A block in the pathway defined here may lead to development of resistance in MM to HDAC inhibitors or doxorubicin.
Differential sensitivity of MM cells to HDACi-doxorubicin combination
Differential sensitivity of MM cells to HDACi-doxorubicin combination
Differential activation of Bax and AIF release by HDACi-doxorubicin in MM cells
Differential activation of Bax and AIF release by HDACi-doxorubicin in MM cells
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal