Abstract
Introduction: The Bone Marrow Transplant Program at Mayo Clinic (Rochester, Minnesota) developed a multidisciplinary approach (involving physicians, nurses, pharmacists, dietitians, and financial specialists) for outpatient management of patients undergoing stem cell transplantation. This approach uses an electronic ordering system for diagnostic tests and chemotherapy to minimize medical errors.
Table. Patient Characteristics (N=716)
| Characteristic . | Value . |
|---|---|
| Male, no. of patients (%) | 426 (59) |
| Age, median (IQR), y | 59 (53–65) |
| Creatinine, median (IQR), mg/dL | 1.0 (0.9–1.2) |
| b2-Microglobulin, median (IQR), mcg/mL | 2.5 (1.9–3.7) |
| Gammopathies, no. of patients (%) | |
| Measured by serum immunofixation or immunoelectrophoresis | |
| IgA | 148 (21) |
| IgG | 414 (58) |
| IgM | 6 (1) |
| IgD | 12 (2) |
| Light chain | 83 (12) |
| Biclonal | 6 (1) |
| None | 47 (7) |
| Measured by urine immunofixation or immunoelectrophoresis | |
| k light chain | 402 (56) |
| l light chain | 226 (32) |
| Biclonal | 1 (0) |
| None | 79 (11) |
| Not performed (anuric) | 8 (1) |
| Status at stem cell transplant, no. of patients (%) | |
| Untreated | 4 (1) |
| Plateau | 436 (61) |
| Primary refractory | 113 (16) |
| Relapse after therapy | 93 (13) |
| Relapse during therapy | 70 (10) |
| Characteristic | Value |
| CD34+ cells, median (IQR), ×106/kg | |
| Collected, no. | 10.0 (7.6–12.6) |
| Infused, no. | 4.7 (3.9–6.2) |
| Sessions of apheresis, median (IQR) | 3 (2–5) |
| Engraftment, median (IQR), days after transplantation | |
| Neutrophils, 0.5×109/L | 13 (12–15) |
| Platelets, 50×109/L | 16 (14–22) |
| Duration of hospitalization, median (IQR), d | 4 (0–10) |
| Characteristic . | Value . |
|---|---|
| Male, no. of patients (%) | 426 (59) |
| Age, median (IQR), y | 59 (53–65) |
| Creatinine, median (IQR), mg/dL | 1.0 (0.9–1.2) |
| b2-Microglobulin, median (IQR), mcg/mL | 2.5 (1.9–3.7) |
| Gammopathies, no. of patients (%) | |
| Measured by serum immunofixation or immunoelectrophoresis | |
| IgA | 148 (21) |
| IgG | 414 (58) |
| IgM | 6 (1) |
| IgD | 12 (2) |
| Light chain | 83 (12) |
| Biclonal | 6 (1) |
| None | 47 (7) |
| Measured by urine immunofixation or immunoelectrophoresis | |
| k light chain | 402 (56) |
| l light chain | 226 (32) |
| Biclonal | 1 (0) |
| None | 79 (11) |
| Not performed (anuric) | 8 (1) |
| Status at stem cell transplant, no. of patients (%) | |
| Untreated | 4 (1) |
| Plateau | 436 (61) |
| Primary refractory | 113 (16) |
| Relapse after therapy | 93 (13) |
| Relapse during therapy | 70 (10) |
| Characteristic | Value |
| CD34+ cells, median (IQR), ×106/kg | |
| Collected, no. | 10.0 (7.6–12.6) |
| Infused, no. | 4.7 (3.9–6.2) |
| Sessions of apheresis, median (IQR) | 3 (2–5) |
| Engraftment, median (IQR), days after transplantation | |
| Neutrophils, 0.5×109/L | 13 (12–15) |
| Platelets, 50×109/L | 16 (14–22) |
| Duration of hospitalization, median (IQR), d | 4 (0–10) |
Abbreviation: IQR, interquartile range.
Results: During a 45-month period after implementation of the program (2005-Sept 2007), the day-100 survival rate was 99.5% for low-risk myeloma patients (transplantation during first plateau; n=202) and 97% for high-risk myeloma patients (refractory, relapsing or second or greater plateau; n=71). The overall day-100 survival rate was 99%(270/273). Analysis of hospitalization trends since inception of the program showed that 39% of patients completed the transplant procedure as outpatients. The median duration of hospitalization for all patients was 4 days; age and serum creatinine levels were predictive of the need for and duration of hospitalization
Conclusion: Outpatient stem cell transplantation is feasible for patients with multiple myeloma with a therapy mortality of 1%. Nearly 40% of our patients completed the procedure without hospitalization. Age and serum creatinine levels predicted a higher likelihood of hospitalization. Implementation of out patient transplant requires affordable housing in the community since many third party payers do not support hotel costs despite the reduction of hospital days. The hospital physical plant and patient registration process has to support use of designated hospital rooms for purely out patient practice. The transplant team must have process owners to standardize across all disease categories collection protocols, conditioning regimens, infusion SOP’s and post infusion supportive care guidelines for standard antibiotics, narcotics, laboratory work and transfusion thresholds. A care team meeting between physicians, nursing, dietary, pharmacy, business office, data base and appointment coordinators is essential to coordinate all the multidisciplinary efforts.
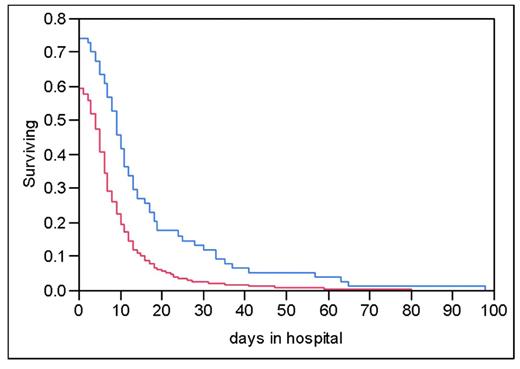
Kaplan-Meier curve shows the percentage of patients remaining in the hospital through time t, stratified on the basis of age greater than or less than or equal to 65 years
Kaplan-Meier curve shows the percentage of patients remaining in the hospital through time t, stratified on the basis of age greater than or less than or equal to 65 years
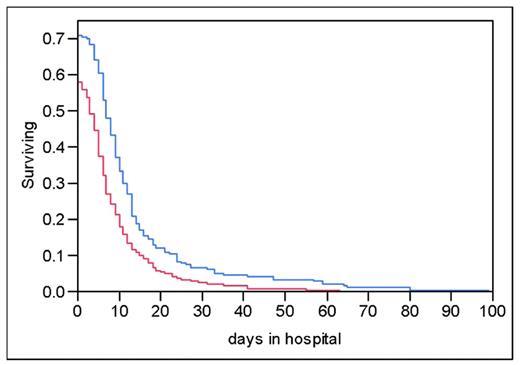
Kaplan-Meier curve shows the percentage of patients remaining in the hospital through time t, stratified on the basis of creatinine greater than or equal to or less than 1.5mg/dL
Kaplan-Meier curve shows the percentage of patients remaining in the hospital through time t, stratified on the basis of creatinine greater than or equal to or less than 1.5mg/dL
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal