Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is no longer the first treatment option for patients with chronic myelogenous leukemia (CML) but there is a considerable debate about its use as a second line therapy. When used in this indication the second-generation tyrosine kinase inhibitors (2G-TKI) induce complete cytogenetic responses (CCyR) in 40–50% of patients in chronic phase but those without CCyR are unlikely to benefit in long term. It is therefore important to identify groups of patients with a good outcome after transplantation so that this may be offered as second line therapy where appropriate. The outcome of allo-SCT has improved over time so we restricted our analysis to the most recent 8 years to coincide with the introduction of imatinib into clinical practice. 131 patients received myeloablative transplants from January 2000 till December 2007. 67 patients were transplanted in chronic phase (14 in second and 2 in third chronic phase), 46 in accelerated phase and 2 in blastic phase. Forty-nine patients received imatinib at some point prior to transplantation and 30 of these experienced failure of imatinib therapy (as defined by European LeukemiaNet criteria). Conditioning consisted of cyclophosphamide and total body irradiation for 51 recipients of sibling stem cells. In addition in vivo T cell depletion with anti CD52 antibody (Campath 1H) was used for 80 unrelated donor transplants. The median age of the patients was 33.4 (15 to 56) years and the median disease duration at transplant was 13 (2 to 105) months.
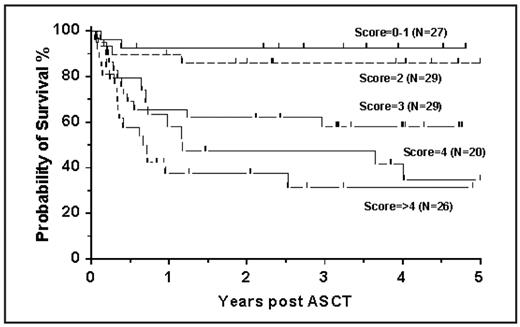
The probability of overall survival (OS) at 3 and 5 years was 64.8% and 62.6% respectively. We confirmed the prognostic value of the EBMT risk assessment score (Gratwohl) and pretransplant level of the C-reactive protein (CRP) and developed a combined additive pretransplant scoring system based on these predictive factors (EBMT risk assessment score plus 0 for CRP <2 mg/L, 1 for CRP from 2 to 10 mg/L, and 2 for CRP >10 mg/L). This identified 5 prognostic groups (Figure 1) with 3yr probabilities of survival of 92.6% (N= 27, score 0–1), 86.2% (N=29, score 2), 58.2% (N=29, score 3), 47.5% (N=20, score 4) and 30.8% (N=26, score 5 or more). The patients who failed imatinib (N=30) had significantly higher prognostic scores on the above described pre-transplant scoring system compared to the rest of patients transplanted (p=0.001). However, in a multivariate analysis adjusted for prognostic scores, their OS was significantly better (p=0.032).
The OS in the best prognostic group is comparable with that of unselected patients treated with imatinib and it is possible that their long-term survival might be better. Allogeneic transplantation is unlikely to be preferred as the first line therapy even in selected patients due to its higher early mortality but our data support its use as second line therapy in patients in chronic phase who failed imatinib and have poor pre-2G-TKI predictive factors for CCyR as determined previously at our institution (namely Sokal risk score at diagnosis, the best cytogenetic response obtained on imatinib, G-CSF requirement during imatinib therapy and time from detection of imatinib failure to onset of 2G-TKI therapy) but achieved good score on the pre-transplant scoring system. It should also be used for those whose disease is more advanced where the 2G-TKI do not offer durable remissions.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal