Abstract
Children with Down syndrome exhibit 2 related hematopoietic diseases: transient myeloproliferative disorder (TMD) and acute megakaryoblastic leukemia (AMKL). Both exhibit clonal expansion of blasts with biphenotypic erythroid and megakaryocytic features and contain somatic GATA1 mutations. While altered GATA1 inhibits erythro-megakaryocytic development, less is known about how trisomy 21 impacts blood formation, particularly in the human fetus where TMD and AMKL originate. We used in vitro and mouse transplantation assays to study hematopoiesis in trisomy 21 fetal livers with normal GATA1 alleles. Remarkably, trisomy 21 progenitors exhibited enhanced production of erythroid and megakaryocytic cells that proliferated excessively. Our findings indicate that trisomy 21 itself is associated with cell-autonomous expansion of erythro-megakaryocytic progenitors. This may predispose to TMD and AMKL by increasing the pool of cells susceptible to malignant transformation through acquired mutations in GATA1 and other cooperating genes.
Introduction
Transient myeloproliferative disorder (TMD) occurs in 10% to 20% of newborns with Down syndrome (DS) and usually resolves after birth. However, approximately 30% of TMD patients develop acute megakaryoblastic leukemia (AMKL) within 4 years, suggesting that TMD is a premalignant disorder and that both diseases originate in the fetus.1-4 DS TMD and AMKL blasts harbor somatic mutations of GATA1, which encodes an essential hematopoietic transcription factor.5-7 These mutations occur in exon 2 and result in exclusive expression of an amino-truncated protein, termed GATA-1 short (GATA-1s).
Presumably, GATA-1s cooperates with trisomy 21 (T21) to induce a preleukemic state that progresses to frank malignancy through additional mutations. TMD and AMKL blasts exhibit erythro-megakaryocytic features8-10 and GATA-1 regulates the maturation, survival, and proliferation of these lineages.11 In contrast, little is known about how T21 itself alters hematopoiesis, particularly in the fetus, where TMD/AMKL-initiating events occur. We analyzed fetal liver hematopoiesis in T21 and control abortuses. In vitro and in vivo transplantation studies show that T21 is associated with enhanced erythroid and megakaryocytic precursor expansion, independent of GATA1 mutations. These findings provide insight into the hematopoietic abnormalities of DS and indicate how T21 might synergize with GATA-1s to promote TMD and AMKL.
Methods
Fetal livers were obtained from pathology specimens of week 13 to 23 abortuses. Institutional Review Board–approval was obtained from The Children's Hospital of Philadelphia and The University of Pennsylvania. Informed consent was obtained in accordance with the Declaration of Helsinki. T21 was confirmed by karyotype analysis of fetal tissue. DNA sequencing, hematopoietic assays, and gene expression analysis were performed using standard methods described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). Approval from the Institutional Animal Care and Use Committee at the University of Pennsylvania was obtained for mouse studies.
Results and discussion
DS-associated TMD and AMKL initiate in utero, as evidenced clinically1,3 and by GATA1 mutational analysis.7,12,13 We analyzed fetal liver specimens from DS and control abortuses at 13 to 23 weeks' gestation. Histologically, T21 fetal livers were indistinguishable from controls (not shown). We isolated fetal liver hematopoietic mononuclear cells (MNCs), amplified GATA1 exon 2 by polymerase chain reaction (PCR), and subcloned the fragments. No mutations in 31 T21 or 10 control fetal liver MNC samples were detected by direct sequencing of the PCR product and 24 independent clones from each fetal liver, consistent with the relatively low incidence of GATA1 mutations (3.8%) identified by screening 585 DS newborns.13 The absence of GATA1 mutations in our specimens allowed us to study the hematopoietic effects of T21 in isolation.
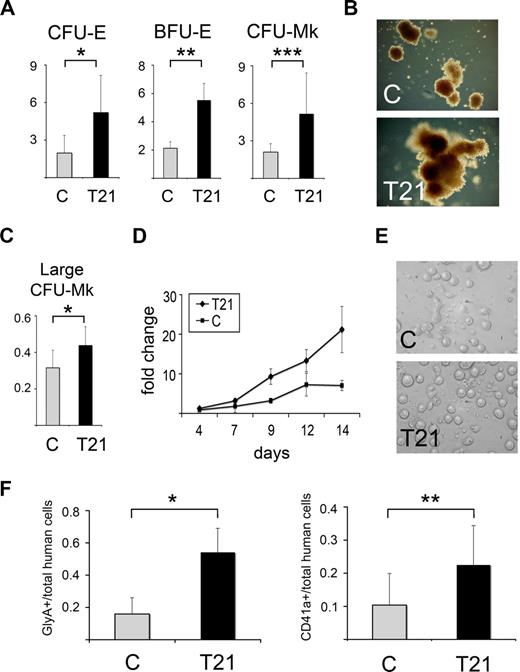
We analyzed T21 and control fetal liver MNCs in methylcellulose progenitor assays and liquid megakaryocyte cultures. The absolute number of colonies generated per MNC varied between different fetal livers. This was independent of gestational age and presumably due to inherent interindividual differences or variability in specimen processing before MNC isolation (Table S2). However, T21 MNCs consistently generated increased proportions of erythroid (CFU-E and BFU-E) and megakaryocyte (CFU-Mk) colonies relative to granulocyte-macrophage (CFU-GM) colonies (Figure 1A; Table S2). Moreover, the majority of T21 erythroid and megakaryocytic colonies were approximately twice the size of control colonies (Figure 1B,C). T21 fetal liver cells also gave rise to an increased proportion of large CFU-Mks (> 50 GPIIb/IIIa + cells/colony, Figure 1C). T21 megakaryocyte progenitors proliferated more rapidly in liquid culture (Figure 1D), but were indistinguishable from controls with respect to morphology, lineage marker expression (CD41a and CD42), and visual inspection for proplatelet formation (Figure 1E and not shown), indicating that cellular maturation was not affected. We performed erythropoietin (EPO) and thrombopoietin (TPO) dose response studies and found no hypersensitivity to these cytokines (not shown) or cytokine-independent colony formation. In addition, we found no difference in Epo or Tpo mRNA expression in whole fetal liver from DS samples compared with controls (not shown).
Increased erythroid and megakaryocytic potential of trisomy 21 fetal hematopoietic cells. (A) Methylcellulose colony assays of mononuclear cells (MNCs) from trisomy 21 (T21, n = 8) and control (C, n = 8) fetal livers. The numbers of burst-forming unit–erythroid (BFU-E) colonies are normalized to the numbers of colony-forming unit–granulocyte macrophage (CFU-GM) colonies obtained from the same culture dishes. The numbers of colony-forming unit–erythroid (CFU-E) and colony-forming unit–megakaryocyte (CFU-Mk) colonies are normalized to the numbers of CFU-GM obtained from the same number of cells plated in parallel GM cultures (y-axis, CFU-E, BFU-E, or CFU-Mk:CFU-GM ratio). CFU-Mk formation was assessed in semisolid cultures that were subsequently dehydrated, fixed, and stained with anti-GPIIb/IIIa antibody; CFU-Mk were scored based on GPIIb/IIIa positively staining cells. Colony assays were performed in triplicate. Results are shown as mean values plus or minus SD. *P = .014, **P < .005, ***P = .021. (B) Representative examples of BFU-E colony morphology from T21 and C fetal liver cells. T21 BFU-E colonies tended to be larger, with the majority approximately twice the size of control colonies. Original magnification, 10X. Photographs were taken using a Carl Zeiss Axiovert 25 microscope (Carl Zeiss, Thornwood, NY) equipped with a digital color camera (Canon Powershot A640; Canon, Lake Success, NY) and Axiovision acquisition software (Zeiss). (C) Large CFU-Mk, defined as more than 50 cells per colony, generated from T21 (n = 8) and C (n = 8) fetal liver MNCs. The y-axis shows the ratio of large CFU-Mk normalized to total CFU-Mk colonies generated by the same fetal liver specimen. Results are shown as mean values plus or minus SD. *P = .028. (D) Growth of megakaryocytes in liquid culture. T21 (n = 4) and C (n = 3) fetal liver MNCs were seeded into serum-free growth medium supplemented with thrombopoietin (Tpo). The y-axis shows relative numbers of megakaryocytes, as defined by expression of the lineage marker CD41a. By 14 days, approximately 90% of the cells expressed megakaryocyte markers CD41a and CD42 (not shown). Results are indicated as mean values plus or minus SD. (E) Representative examples of proplatelet formation in T21 and C fetal liver liquid megakaryocyte cultures on day 13. Proplatelet formation appeared on day 8 of culture and increased with each subsequent day in both T21 and C liquid cultures. By visual inspection, there were no differences in the extent of proplatelet formation between T21 and C samples. Original magnification, 20X. Photographs were taken using an Olympus IX70 microscope (Olympus, Center Valley, PA) equipped with a Photometrics Coolsnap camera (Photometrics, Pleasanton, CA) and Openlab version 5.5 acquisition software (Improvision, Waltham, MA). (F) Hematopoietic development of T21 (n = 11) and C (n = 12) fetal liver MNCs 5 to 10 weeks after transplantation into NOD/SCID/IL-2Rγcnull mice. Mice that received T21 MNCs exhibited an increased fraction of erythroid cells (Glycophorin A+, left panel) and megakaryocytes (CD41a+, right panel) within the human hematopoietic compartment in bone marrow of chimeric mice. Results are shown as the mean values plus or minus SD. *P < .005, **P = .007.
Increased erythroid and megakaryocytic potential of trisomy 21 fetal hematopoietic cells. (A) Methylcellulose colony assays of mononuclear cells (MNCs) from trisomy 21 (T21, n = 8) and control (C, n = 8) fetal livers. The numbers of burst-forming unit–erythroid (BFU-E) colonies are normalized to the numbers of colony-forming unit–granulocyte macrophage (CFU-GM) colonies obtained from the same culture dishes. The numbers of colony-forming unit–erythroid (CFU-E) and colony-forming unit–megakaryocyte (CFU-Mk) colonies are normalized to the numbers of CFU-GM obtained from the same number of cells plated in parallel GM cultures (y-axis, CFU-E, BFU-E, or CFU-Mk:CFU-GM ratio). CFU-Mk formation was assessed in semisolid cultures that were subsequently dehydrated, fixed, and stained with anti-GPIIb/IIIa antibody; CFU-Mk were scored based on GPIIb/IIIa positively staining cells. Colony assays were performed in triplicate. Results are shown as mean values plus or minus SD. *P = .014, **P < .005, ***P = .021. (B) Representative examples of BFU-E colony morphology from T21 and C fetal liver cells. T21 BFU-E colonies tended to be larger, with the majority approximately twice the size of control colonies. Original magnification, 10X. Photographs were taken using a Carl Zeiss Axiovert 25 microscope (Carl Zeiss, Thornwood, NY) equipped with a digital color camera (Canon Powershot A640; Canon, Lake Success, NY) and Axiovision acquisition software (Zeiss). (C) Large CFU-Mk, defined as more than 50 cells per colony, generated from T21 (n = 8) and C (n = 8) fetal liver MNCs. The y-axis shows the ratio of large CFU-Mk normalized to total CFU-Mk colonies generated by the same fetal liver specimen. Results are shown as mean values plus or minus SD. *P = .028. (D) Growth of megakaryocytes in liquid culture. T21 (n = 4) and C (n = 3) fetal liver MNCs were seeded into serum-free growth medium supplemented with thrombopoietin (Tpo). The y-axis shows relative numbers of megakaryocytes, as defined by expression of the lineage marker CD41a. By 14 days, approximately 90% of the cells expressed megakaryocyte markers CD41a and CD42 (not shown). Results are indicated as mean values plus or minus SD. (E) Representative examples of proplatelet formation in T21 and C fetal liver liquid megakaryocyte cultures on day 13. Proplatelet formation appeared on day 8 of culture and increased with each subsequent day in both T21 and C liquid cultures. By visual inspection, there were no differences in the extent of proplatelet formation between T21 and C samples. Original magnification, 20X. Photographs were taken using an Olympus IX70 microscope (Olympus, Center Valley, PA) equipped with a Photometrics Coolsnap camera (Photometrics, Pleasanton, CA) and Openlab version 5.5 acquisition software (Improvision, Waltham, MA). (F) Hematopoietic development of T21 (n = 11) and C (n = 12) fetal liver MNCs 5 to 10 weeks after transplantation into NOD/SCID/IL-2Rγcnull mice. Mice that received T21 MNCs exhibited an increased fraction of erythroid cells (Glycophorin A+, left panel) and megakaryocytes (CD41a+, right panel) within the human hematopoietic compartment in bone marrow of chimeric mice. Results are shown as the mean values plus or minus SD. *P < .005, **P = .007.
To further examine the effects of T21 on hematopoiesis, we transplanted fetal liver MNCs into sublethally irradiated NOD/SCID/IL-2Rγcnull (NOG) mice. Five to 10 weeks after transplantation, we used human-specific antibodies to assess the progeny of a range of progenitors including B cell, macrophage, granulocyte, erythroid, and megakaryocyte (Figure S1). Trisomy 21 fetal liver cells exhibited enhanced erythroid differentiation in vivo, as evidenced by increased proportions of engrafted donor cells expressing glycophorin A (Figure 1F left panel). Transplanted T21 fetal liver cells also generated increased proportions of megakaryocytes (Figure 1F right panel), although human megakaryocytic development occurred at relatively low levels, as reported by others.14 These findings are consistent with our in vitro cultures linking T21 to enhanced differentiation and proliferation of erythro-megakaryocytic lineages. TMD typically resolves spontaneously after birth, suggesting that in DS, specific properties of fetal hematopoietic progenitors and/or the fetal liver microenvironment are necessary for expansion of TMD blasts. Our transplantation experiments demonstrate that T21 hematopoietic progenitors exhibit unique abnormalities in euploid adult mice. Hence, at least some of these properties are cell-intrinsic and independent of the trisomic fetal environment or artifacts related to in vitro culture.
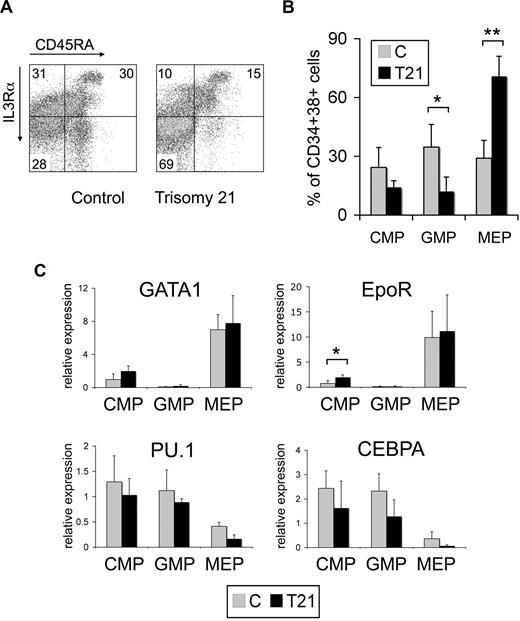
To better localize the hematopoietic defects of DS, we analyzed multipotential progenitor compartments within the fetal liver using flow cytometry.15 The population of CD34+CD38+ progenitors was distinctly different in T21 fetal livers compared with controls (Figure 2A,B). Specifically, in T21 there was a marked increase in the proportion of megakaryocyte erythroid progenitors (MEPs, IL-3Rα−/CD45RA–). The proportion of common myeloid progenitors (CMPs, IL-3Rαlo/CD45RA−) and granulocyte macrophage progenitors (GMPs, IL-3Rαlo/CD45RA+) were commensurately reduced. Hematopoietic colony assays performed with sorted cell populations verified that these progenitors were enriched by our fractionation strategy (Figure S2). Of note, both T21 CMPs and MEPs gave rise to increased proportions of erythroid and megakaryocyte colonies relative to controls (Figure S2). Moreover, T21 erythroid colonies generated from purified fetal liver CMPs and MEPs were relatively enlarged, similar to what we observed in colony assays from unfractionated MNCs (not shown). As expected, mRNAs encoding the myeloid genes PU.1 and CEBPα were reduced in purified MEPs. Also, the erythro-megakaryocytic genes GATA1 and EpoR were barely expressed in GMPs (Figure 2C). These data further verify the enrichment for specific multilineage progenitor populations. Of note, there was a trend toward increased GATA1 and EpoR expression and decreased PU.1 and CEBPa expression within T21 CMPs, consistent with the enhanced erythro-megakaryocytic differentiation that we observed in colony assays.
The megakaryocyte-erythroid progenitor (MEP) compartment is expanded in trisomy 21 fetal hematopoiesis. (A) Representative flow cytometric analysis of control (C) and trisomy 21 (T21) fetal liver MNCs. CD34+38+ cells were fractionated according to IL-3Rα and CD45RA expression to delineate different progenitor compartments: common myeloid progenitor (CMP, IL-3Rαlo/CD45RA−, top left panel), granulocyte macrophage progenitor (GMP, IL-3Rαlo/CD45RA+, top right panel), and megakaryocyte erythroid progenitor (MEP, IL-3Rα−/CD45RA−, bottom left panel). The numbers indicate percentages of each population within the CD34+CD38+ fraction. (B) Distribution of multipotential progenitors in T21 (n = 4) and C (n = 5) fetal liver MNCs. Data are plotted as percent of the CD34+CD38+ population. Bars represent the mean plus or minus SD. *P = .01, **P = .0003. (C) Relative expression of selected chromosome 21–encoded hematopoietic transcription factors in purified T21 (n = 3) and C (n = 4) fetal liver CMPs. Results shown are the mean plus or minus SD. *P = .03.
The megakaryocyte-erythroid progenitor (MEP) compartment is expanded in trisomy 21 fetal hematopoiesis. (A) Representative flow cytometric analysis of control (C) and trisomy 21 (T21) fetal liver MNCs. CD34+38+ cells were fractionated according to IL-3Rα and CD45RA expression to delineate different progenitor compartments: common myeloid progenitor (CMP, IL-3Rαlo/CD45RA−, top left panel), granulocyte macrophage progenitor (GMP, IL-3Rαlo/CD45RA+, top right panel), and megakaryocyte erythroid progenitor (MEP, IL-3Rα−/CD45RA−, bottom left panel). The numbers indicate percentages of each population within the CD34+CD38+ fraction. (B) Distribution of multipotential progenitors in T21 (n = 4) and C (n = 5) fetal liver MNCs. Data are plotted as percent of the CD34+CD38+ population. Bars represent the mean plus or minus SD. *P = .01, **P = .0003. (C) Relative expression of selected chromosome 21–encoded hematopoietic transcription factors in purified T21 (n = 3) and C (n = 4) fetal liver CMPs. Results shown are the mean plus or minus SD. *P = .03.
Together, progenitor subfractionation indicates significant expansion of MEPs and increased tendency toward erythro-megakaryocytic development in the CMP and MEP compartments of T21 fetal liver. Trisomy 21 CMPs themselves may be inherently altered to enhance erythro-megakaryocytic production, possibly by giving rise to increased MEPs rather than GMPs. Alternatively, T21 MEPs may exhibit enhanced self-renewal. Either way, the data map the T21 hematopoietic phenotype to one or more population(s) of enriched multipotential progenitors. DS presumably alters blood development through complex mechanisms involving relatively small changes in the expression of multiple chromosome 21 genes. We examined several chromosome 21–encoded transcription factors known to influence hematopoietic fates, including ERG, RUNX1, ETS2, and SON in a hematopoietic stem cell–enriched population (CD34+CD38−) from fetal liver. While expression of these mRNAs was relatively consistent in control HSCs, there was considerable variation in T21 HSCs (Figure S3). Of note, none of these candidate chromosome 21 genes were significantly over-expressed in DS fetal liver HSCs or myeloid progenitor compartments (not shown), consistent with a model in which subtle alterations of multiple chromosome 21 genes contribute to the pathogenesis of DS phenotypes, including TMD/AMKL.
Malignant transformation is a multistep process caused by mutations that synergize to promote proliferation, survival and maturation arrest of immature progenitors. Down Syndrome–associated AMKL is an interesting example in which germline T21 cooperates with somatic GATA1 mutations to induce TMD, a preleukemic disorder characterized by clonal expansion of blasts with erythroid and megakaryocytic characteristics. Presumably additional, unknown mutations are required for progression to AMKL. Isolated mutations in GATA1 are known to block erythro-megakaryocytic maturation and promote the expansion of megakaryocytes and MEP-like precursors.11,16-18 Here we provide the first evidence that erythroid and megakaryocytic precursors are also expanded in T21. These abnormalities occur in multilineage fetal progenitors and are at least partly cell-intrinsic. Accordingly, it is possible that T21 predisposes to AMKL by causing polyclonal expansion of a progenitor pool that is susceptible to additional transforming mutations involving GATA1 and other genes.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Department of Pathology at Pennsylvania Hospital for processing fetal liver specimens and are grateful to the Flow Cytometry Core at the University of Pennsylvania for advice on cell sorting and to the Stem Cell and Xenograft Core at the University of Pennsylvania for assistance with the in vivo experiments. They thank Paul Gadue for help with flow cytometry.
This work was supported by the Hope Street Kids Foundation (Alexandria, VA; S.T.C.), the American Society of Clinical Oncology (Alexandria, VA; S.T.C.), the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; M.J.W.), and The Clinical & Translational Research Center (CTRC) at The Children's Hospital of Philadelphia (grant no. UL1-RR-024134 from the National Center for Research Resources, Bethesda, MD). M.J.W. is a Leukemia & Lymphoma Society (White Plains, NY) Scholar. S.T.C. is a Leukemia & Lymphoma Society Fellow.
National Institutes of Health
Authorship
Contribution: S.T.C designed and conducted research, analyzed data, and wrote the paper; J.O., Y.Y., M.F, and A.K. designed and conducted research and analyzed data; J.S.B., J.K.C, A.M.G., G.D., and R.N. designed research and analyzed data; and M.J.W. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mitchell J. Weiss, MD, PhD, Division of Hematology, Abramson Research Center, Room 316B, 3615 Civic Center Boulevard, Philadelphia, PA 19104; e-mail: weissmi@email.chop.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal