Abstract
Down syndrome (DS) children have a high frequency of acute megakaryoblastic leukemia (AMKL) in early childhood. At least 2 in utero genetic events are required, although not sufficient, for DS-AMKL: trisomy 21 (T21) and N-terminal–truncating GATA1 mutations. To investigate the role of T21 in DS-AMKL, we compared second trimester hemopoiesis in DS without GATA1 mutations to gestation-matched normal controls. In all DS fetal livers (FLs), but not marrows, megakaryocyte-erythroid progenitor frequency was increased (55.9% ± 4% vs 17.1% ± 3%, CD34+CD38+ cells; P < .001) with common myeloid progenitors (19.6% ± 2% vs 44.0% ± 7%) and granulocyte-monocyte (GM) progenitors (15.8% ± 4% vs 34.5% ± 9%) commensurately reduced. Clonogenicity of DS-FL versus normal FL CD34+ cells was markedly increased (78% ± 7% vs 15% ± 3%) affecting megakaryocyte-erythroid (∼ 7-fold higher) and GM and colony-forming unit–granulocyte, erythrocyte macrophage, megakaryocyte (CFU-GEMM) progenitors. Replating efficiency of CFU-GEMM was also markedly increased. These data indicate that T21 itself profoundly disturbs FL hemopoiesis and they provide a testable hypothesis to explain the increased susceptibility to GATA1 mutations in DS-AMKL and DS-associated transient myeloproliferative disorder.
Introduction
Children with Down syndrome (DS) have a uniquely high frequency of acute megakaryoblastic leukemia (AMKL) in early childhood.1-3 The leukemic cells acquire mutations in utero in the critical megakaryocyte transcription factor GATA1.3-10 In many DS children, the first manifestation is neonatal transient myeloproliferative disorder (TMD),1,2 which evolves to AMKL in 20% to 30% of infants.3 TMD and AMKL represent 2 distinct steps in the pathogenesis of DS-AMKL. However, an additional necessary leukemogenic event (most probably the initiating event) is trisomy 21 (T21). T21 is essential for GATA1-associated TMD and AMKL5,11,12 ; truncating GATA1 mutations in the absence of T21 are not leukemogenic.13 GATA1 mutations also occur at high frequency in T21 (∼ 5% of all DS neonates14 ) and 25% of DS-associated AMKL patients have multiple, independent clones with GATA1 mutations.6 These data suggest that GATA1 mutations and T21 specifically synergize to generate preleukemic TMD. The cellular and molecular mechanisms by which this occur are unclear.
That a putative leukemia-initiating cell is present in T21 fetal liver (FL) is suggested both by the natural history of TMD (origin in utero, frequent liver involvement, and spontaneous resolution as FL hemopoiesis ceases11,15 ) and by analysis of germline N-terminal mutant Gata1 phenotypes in mouse and humans.16 To investigate the impact of T21, independent of GATA1 mutations, on human hemopoiesis, we studied myeloid progenitors from second trimester T21 FL and bone marrow.
Methods
Second-trimester FLs and marrow collected during elective surgical termination of pregnancy were processed immediately as previously described.17 The study was approved by Hammersmith and Queen Charlotte's Hospitals Research Ethics Committee; written informed consent was obtained in accordance with the Declaration of Helsinki. CD34+ cell separation, flow cytometry, clonogenic assays, liquid cultures, GATA1 mutation analysis, and gene expression were performed on freshly isolated cells using standard methods (Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
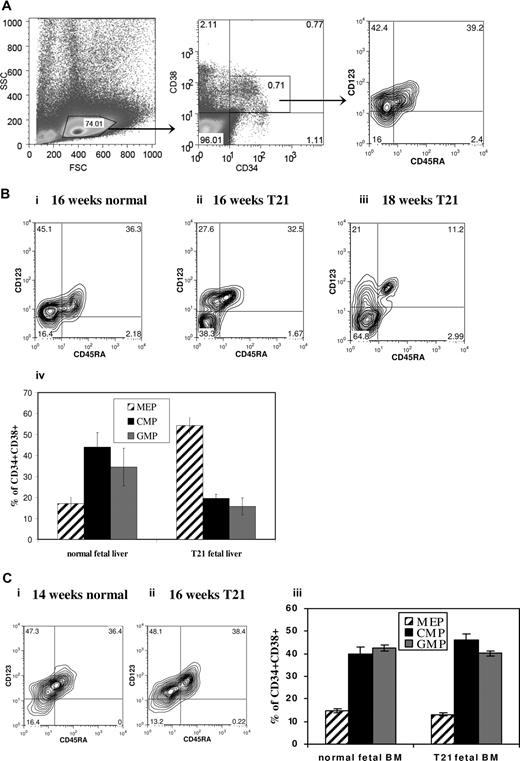
To investigate whether fetal hemopoiesis was perturbed in DS, we analyzed the myeloid progenitor compartment in freshly isolated FL mononuclear cells (MNCs) with T21 with no GATA1 mutations. There was no significant difference in percentage of CD34+ cells in DS (n = 10; median gestation, 15 weeks) versus normal FL (n = 5; median gestation, 16 weeks) MNC samples (mean ± SEM, 3.1% ± 0.8% vs 4.9% ± 0.9%; P = .2). However, the CD34+ CD38+ compartment showed a strikingly increased frequency of megakaryocyte-erythroid progenitors (MEPs) in DS-FL compared with normal FL (55.9% ± 4% vs 17.1% ± 3% of CD34+CD38+ cells; P < .001), whereas common myeloid progenitors (CMPs; 19.6% ± 2% vs 44.0% ± 7%; P = .002) and granulocyte-monocyte progenitors (GMPs; 15.8% ± 4% vs 34.5% ± 9%; P = .025) were correspondingly lower (Figure 1A,B). To determine whether these differences were specific to FL, we analyzed marrow from DS fetuses (n = 4) without GATA1 mutations, which showed normal MEP frequency (13.2% ± 0.1%) compared with gestation-matched controls (14.7% ± 2%; n = 4; Figure 1C), although it is possible that differences between DS and normal marrow may not become apparent until later gestations.
Myeloid progenitors in second trimester liver and bone marrow from DS and normal gestation-matched fetuses. MEPs, CMPs, and GMPs were analyzed by flow cytometry of freshly isolated fetal liver (FL) mononuclear cells (MNCs) (n = 10 with DS and n = 5 normal) and fetal bone marrow MNCs (n = 4 with DS and n = 4 normal). The gating strategy used to identify the MEP compartment, defined as CD34+CD38+CD123−CD45RA−, is shown in panel A, representative plots show the proportion of MEPs in DS-FL cells (Bii,iii), gestation-matched normal FL (Bi), DS fetal bone marrow (Cii), and gestation-matched normal fetal bone marrow (Ci). The frequency of MEP was significantly higher in DS-FL compared with normal FL (P < .001), whereas the proportions of CMPs (P = .002) and GMPs (P = .025) were significantly reduced (Biv). There were no significant differences between the frequency of MEPs, CMPs, and GMPs between DS and normal fetal bone marrow (Ciii). Data are means plus or minus SEM.
Myeloid progenitors in second trimester liver and bone marrow from DS and normal gestation-matched fetuses. MEPs, CMPs, and GMPs were analyzed by flow cytometry of freshly isolated fetal liver (FL) mononuclear cells (MNCs) (n = 10 with DS and n = 5 normal) and fetal bone marrow MNCs (n = 4 with DS and n = 4 normal). The gating strategy used to identify the MEP compartment, defined as CD34+CD38+CD123−CD45RA−, is shown in panel A, representative plots show the proportion of MEPs in DS-FL cells (Bii,iii), gestation-matched normal FL (Bi), DS fetal bone marrow (Cii), and gestation-matched normal fetal bone marrow (Ci). The frequency of MEP was significantly higher in DS-FL compared with normal FL (P < .001), whereas the proportions of CMPs (P = .002) and GMPs (P = .025) were significantly reduced (Biv). There were no significant differences between the frequency of MEPs, CMPs, and GMPs between DS and normal fetal bone marrow (Ciii). Data are means plus or minus SEM.
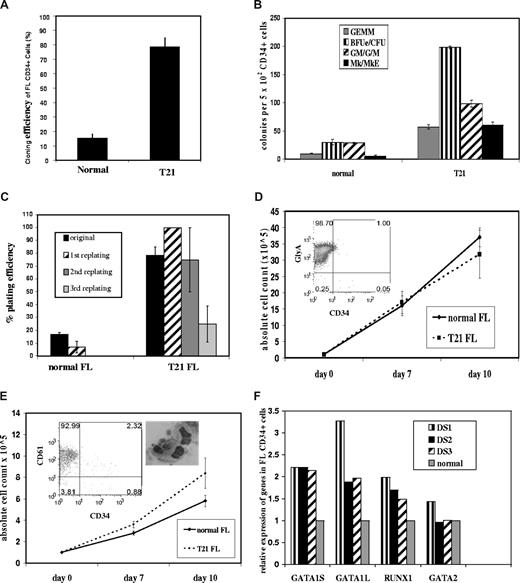
Therefore, we investigated whether FL megakaryocyte-erythroid compartment expansion was cell-intrinsic, rather than secondary to DS-FL microenvironment differences, by performing clonogenic assays on purified CD34+ cells. Clonogenicity of DS-FL CD34+ cells was markedly increased compared with normal FL (78% ± 7% vs 15% ± 3%; Figure 2A), affecting not only megakaryocyte, megakaryocyte-erythroid, and erythroid progenitors, which together were approximately 7-fold higher in DS-FL versus normal FL, but also CFU-GEMM and G/GM/M progenitors, consistent with cell-intrinsic abnormalities of the DS-FL CD34+ compartment (Figure 2B). This was supported by markedly increased replating efficiency of individual CFU-GEMM from DS-FL versus normal FL CFU-GEMM (Figure 2C) indicative of increased self-renewal.
Clonogenic assays, liquid cultures, and gene expression of CD34+ cells from DS and normal FL. (A-C) CD34+ cells purified from freshly isolated MNCs from DS (n = 5) and gestation-matched normal (n = 4) FLs were plated at 500 to 1000 cells/well with interleukin-3 (IL-3), IL-6, IL-11, stem cell factor, Flt3 ligand, granulocyte-macrophage colony-stimulating factor, Tpo, and Epo and counted after 12 to 14 days. (A) Cloning efficiency of DS-FL CD34+ cells was higher than normal FL CD34+ cells (78 ± 7 vs 15 ± 3). (B) Absolute numbers of all myeloid progenitors were increased in DS-FL compared with normal FL: erythroid (198 + 2 vs 30 + 5 colonies/5 × 102 cells; P < .001), megakaryocyte/megakaryocyte-erythroid (60 + 6 vs 5 + 2.6 colonies/5 × 102 CD34+ cells; P < .001), CFU-GEMM (57 + 4 vs 10 + 0.5; P < .001), and CFU-G/GM/M (98 + 6 vs 29 + 1; P < .001). (C) Serial replating of individual CFU-GEMM from DS-FL (n = 3) showed markedly increased replating efficiency compared with normal FL CFU-GEMM (n = 3): secondary replating of normal FL CFU-GEMM produced only occasional CFU-e with no tertiary replating ability, whereas secondary replating of DS-FL CFU-GEMM produced tertiary CFU-GEMM and erythroid colonies (BFU-e and CFU-e) and tertiary replating of DS-FL secondary CFU-GEMM gave rise to CFU-e, which had no replating ability. (D) Erythroid liquid cultures: MACS-purified DS-FL (n = 5) and normal gestation-matched FL CD34+ cells (n = 3) cultured with Epo, stem cell factor, Flt3 ligand, and IL-3 generated similar numbers of GlyA+ cells after 7 and 10 days of culture (inset: flow cytometry of total cells after 10 days of culture). (E) Megakaryocyte liquid cultures: MACS-purified DS-FL CD34+ cells (n = 5) cultured with Tpo generated a slightly higher total number of cells compared with normal FL CD34+ cells (n = 3) after 10 days (P = .07) with a similar proportion of CD61+ cells and morphologically normal megakaryocytes (inset: flow cytometry of total cells and cytospin of megakaryocytes after 10 days of culture). (F) Expression of full-length GATA1, truncated GATA1 (GATA1s), RUNX1, and GATA2 transcripts by FL CD34+ cells in T21 (n = 3) and normal (n = 3) FL was measured by Taqman quantitative reverse-transcribed polymerase chain reaction in 3 independent experiments, each comparing a T21 with a normal sample. Expression levels in each of the T21 samples normalized against a normal FL sample is shown.
Clonogenic assays, liquid cultures, and gene expression of CD34+ cells from DS and normal FL. (A-C) CD34+ cells purified from freshly isolated MNCs from DS (n = 5) and gestation-matched normal (n = 4) FLs were plated at 500 to 1000 cells/well with interleukin-3 (IL-3), IL-6, IL-11, stem cell factor, Flt3 ligand, granulocyte-macrophage colony-stimulating factor, Tpo, and Epo and counted after 12 to 14 days. (A) Cloning efficiency of DS-FL CD34+ cells was higher than normal FL CD34+ cells (78 ± 7 vs 15 ± 3). (B) Absolute numbers of all myeloid progenitors were increased in DS-FL compared with normal FL: erythroid (198 + 2 vs 30 + 5 colonies/5 × 102 cells; P < .001), megakaryocyte/megakaryocyte-erythroid (60 + 6 vs 5 + 2.6 colonies/5 × 102 CD34+ cells; P < .001), CFU-GEMM (57 + 4 vs 10 + 0.5; P < .001), and CFU-G/GM/M (98 + 6 vs 29 + 1; P < .001). (C) Serial replating of individual CFU-GEMM from DS-FL (n = 3) showed markedly increased replating efficiency compared with normal FL CFU-GEMM (n = 3): secondary replating of normal FL CFU-GEMM produced only occasional CFU-e with no tertiary replating ability, whereas secondary replating of DS-FL CFU-GEMM produced tertiary CFU-GEMM and erythroid colonies (BFU-e and CFU-e) and tertiary replating of DS-FL secondary CFU-GEMM gave rise to CFU-e, which had no replating ability. (D) Erythroid liquid cultures: MACS-purified DS-FL (n = 5) and normal gestation-matched FL CD34+ cells (n = 3) cultured with Epo, stem cell factor, Flt3 ligand, and IL-3 generated similar numbers of GlyA+ cells after 7 and 10 days of culture (inset: flow cytometry of total cells after 10 days of culture). (E) Megakaryocyte liquid cultures: MACS-purified DS-FL CD34+ cells (n = 5) cultured with Tpo generated a slightly higher total number of cells compared with normal FL CD34+ cells (n = 3) after 10 days (P = .07) with a similar proportion of CD61+ cells and morphologically normal megakaryocytes (inset: flow cytometry of total cells and cytospin of megakaryocytes after 10 days of culture). (F) Expression of full-length GATA1, truncated GATA1 (GATA1s), RUNX1, and GATA2 transcripts by FL CD34+ cells in T21 (n = 3) and normal (n = 3) FL was measured by Taqman quantitative reverse-transcribed polymerase chain reaction in 3 independent experiments, each comparing a T21 with a normal sample. Expression levels in each of the T21 samples normalized against a normal FL sample is shown.
To further investigate growth and maturation of DS-FL progenitors, DS-FL (n = 5) and gestation-matched normal FL CD34+ cells (n = 3) were cultured in erythroid and megakaryocyte liquid cultures. DS-FL CD34+ cells proliferated and differentiated normally with erythropoietin (Epo) (similar numbers of GlyA+ cells) (Figure 2D). In thrombopoietin (Tpo)–containing cultures, DS-FL CD34+ cells generated slightly higher total and CD61+ cell numbers compared with normal FL CD34+ cells (P = .07), but cultured megakaryocytes appeared morphologically normal (Figure 2E) and no gross differences in proplatelet formation were noted on light microscopy (data not shown). Nevertheless, defects in late megakaryocyte maturation may be present because, despite increased megakaryocyte progenitors, DS fetal blood (n = 4) showed low/low normal platelets vs normal fetuses, and abnormal/giant platelets were seen on all DS fetal blood smears (Figure S1) consistent with results in DS neonates.18,19 Thus, further studies are warranted.
To exclude expansion of occult mutant GATA1 clones in DS-FL, GATA1 mutational analysis was performed on DNA from DS-FL CD34+ cells from all samples, from individual hemopoietic colonies and DS-FL CD34+ cells in megakaryocyte and erythroid liquid cultures. No GATA1 mutations were detected. In addition, CD34+ cells from DS-FL and normal FL expressed both full-length GATA1 and GATA1s mRNA (Figure 2F), consistent with the hypothesis that perturbation of FL hemopoiesis is directly the result of T21 rather than occult mutant GATA1 clones. This is supported by the observation that hemopoietic abnormalities were seen in every DS-FL rather than a subset. The abnormal FL CD34+ compartment, and increased megakaryocyte-erythroid progenitors in particular, probably provides a T21-dependent cellular substrate uniquely sensitive to the growth-promoting effects of GATA1 mutation(s), which would not be leukemogenic in the absence of T21.13 The absence of a similarly abnormal progenitor population in DS fetal marrow provides one possible explanation for a fetal-specific window for initiation of TMD and DS-AMKL. Further work is needed to investigate the role of FL and marrow microenvironments and the nature of any intrinsic difference in DS FL versus marrow CD34+ cells.
The mechanism(s) by which T21 perturbs FL hemopoiesis is (are) not clear. Several chromosome 21 candidate genes involved in megakaryocyte differentiation and/or leukemogenesis have been investigated in DS-AMKL and TMD, including ERG and RUNX1.20-22 Because RUNX1 is crucial in embryonic/fetal hemopoiesis and megakaryocyte maturation and RUNX1 mutations are frequent in non-DS myeloid leukemias,23 we measured RUNX1 expression in DS-FL CD34+ cells (n = 3) by quantitative reverse-transcribed polymerase chain reaction and found a 1.5- to 2-fold increase in expression compared with normal FL CD34+ cells (Figure 2F), consistent with gene-dosage effect in T21 and in contrast to DS-AMKL where RUNX1 expression is reduced.20 Further work on purified progenitors and functional studies will determine whether altered RUNX1 is relevant to abnormal FL hemopoiesis in DS. We also found a modest (∼ 2-fold) increase in GATA1 expression in DS-FL CD34+ cells (Figure 2F), similar to TMD and DS-AMKL.20 Changes in these megakaryocyte transcription factors could also simply reflect the increased megakaryocyte-erythroid compartment, although GATA2 expression was comparable with normal FL cells (Figure 2F). These and other data about T21 in other cell types suggest that the role of T21 in DS-AMKL is probably complex and the result of changes in expression of many genes on chromosome 21 and/or indirect effects on other disomic genes rather than a single gene or very limited set of genes.24
In conclusion, for the first time, this study shows that perturbation of the FL myeloid progenitor compartment in human DS precedes the acquisition of GATA1 mutations and is characterized by relative expansion of megakaryocyte-erythroid progenitors and enhanced myeloid lineage clonogenicity, strongly suggesting that a “leukemia-initiating” progenitor population is present within FL in all DS fetuses. This has important implications because it provides a testable hypothesis that DS T21 specifically expands an FL-derived hemopoietic progenitor compartment, thereby creating a substrate on which GATA1 mutations confer a further selective advantage.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Vaskar Saha and Georgina Hall for helpful discussions and David Roper for expert assistance with photomicroscopy.
This work was supported by grants from the Kay Kendall Leukaemia Fund and the Medical Research Council. I.R. and A.K. were supported in part by the Imperial College London Comprehensive Biomedical Research Centre, and P.V. was supported in part by the Oxford Partnership Comprehensive Biomedical Research Centre, both of which received funding from the Department of Health's NIHR Biomedical Research Centres funding scheme.
Authorship
Contribution: O.T.-P. and A.R. designed and performed the research, analyzed the data, and wrote the paper; J.d.l.F. and A.N. performed research; N.M.F. and P.B. contributed to the study design and writing of the paper; and P.V., A.K., and I.R. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irene Roberts, Department of Haematology, Imperial College London and Imperial Healthcare NHS Trust, Du Cane Road, London W12 0NN, United Kingdom; e-mail: irene.roberts@imperial.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal