Abstract
An attractive target for therapeutic intervention is constitutively activated, mutant FLT3, which is expressed in a subpopulation of patients with acute myelocyic leukemia (AML) and is generally a poor prognostic indicator in patients under the age of 65 years. PKC412 is one of several mutant FLT3 inhibitors that is undergoing clinical testing, and which is currently in late-stage clinical trials. However, the discovery of drug-resistant leukemic blast cells in PKC412-treated patients with AML has prompted the search for novel, structurally diverse FLT3 inhibitors that could be alternatively used to override drug resistance. Here, we report the potent and selective antiproliferative effects of the novel mutant FLT3 inhibitor NVP-AST487 on primary patient cells and cell lines expressing FLT3-ITD or FLT3 kinase domain point mutants. NVP-AST487, which selectively targets mutant FLT3 protein kinase activity, is also shown to override PKC412 resistance in vitro, and has significant antileukemic activity in an in vivo model of FLT3-ITD+ leukemia. Finally, the combination of NVP-AST487 with standard chemotherapeutic agents leads to enhanced inhibition of proliferation of mutant FLT3-expressing cells. Thus, we present a novel class of FLT3 inhibitors that displays high selectivity and potency toward FLT3 as a molecular target, and which could potentially be used to override drug resistance in AML.
Introduction
Acute myelocytic leukemia (AML) is a malignant disorder of hematopoietic cells with an incidence of around 10 000 new cases per year in the United States.1 The main features of AML are excessive proliferation of myeloid precursor cells and a block of cellular differentiation.1 The aberrant survival advantage of leukemic cells leads to infiltration of bone marrow and peripheral blood with immature leukemic myeloblasts resulting in bone marrow failure and such symptoms as anemia, bleeding and infection. Age, history of myelodysplasia, cytogenetics, and MDR1 expression are major prognostic determinants.2
Current therapies for AML often fail because of treatment-induced mortality or drug resistance.2 The use of conventional chemotherapeutic agents alone is associated with a high risk of relapse, but a low treatment-induced mortality.3 Allogeneic bone marrow transplantation (allo-BMT), a standard approach for the treatment of adults with AML, has a lower risk of relapse but a high treatment-induced mortality.3 Allo-BMT results in 25% to 30% 10-year survival for young patients; however, the outcome is poor for patients near the age of 60 years, and since the median age of AML patients is 64 years, the impact of current therapy on the majority of patients with this disease is small.4
The class III receptor tyrosine kinase FLT3 (Fms-like tyrosine kinase-3; STK-1 [human stem cell tyrosine kinase-1]; or FLK-2 [fetal liver kinase-2]),5 is constitutively activated by mutations occurring in approximately 30% of patients with AML and is regarded as an attractive target for therapy. The most common type of FLT3 mutation thus far identified is internal tandem duplications in the juxtamembrane (JM) domain (FLT3-ITD),6 observed in approximately 20% to 25% of patients with AML, but in fewer than 5% of patients with myelodysplastic syndrome (MDS).6-11 Another type of FLT3 mutation is point mutations within the “activation loop” of the kinase,12 which are believed to change the conformation of the domain, causing it to adopt an “activated” configuration. This mutation occurs in approximately 7% of patients with AML, most with a missense mutation in the aspartic acid residue at position 835. Less commonly, other point mutations in the kinase domain have been reported, including N841I13 and Y842C.14
FLT3-ITD is associated with decreased survival, while the prognostic impact of the D835Y mutation is less clear. Expression of each of these constitutively activated mutants in cells enhances viability, confers growth-factor–independent growth, and increases FLT3 autophosphorylation and tyrosine phosphorylation of other signaling factors.11,15 In addition, the transplantation of murine bone marrow cells infected with a retrovirus expressing a FLT3-ITD mutant leads to the development of a rapidly lethal myeloproliferative disease in mice.16
Several inhibitors of mutant FLT3 have been developed and are being tested as a novel therapeutic approach for AML, based on the prevalence of mutant forms of FLT3 in AML patients and the demonstrated enhancement of cellular proliferation, viability, and tyrosine phosphorylation by mutant FLT3. We have previously described the inhibitory effects of the protein tyrosine kinase inhibitor PKC412 (Novartis Pharma AG, Basel, Switzerland) on mutant FLT3-expressing cells in vitro and in vivo.17 Up to now, none of these inhibitors has achieved sustained cytogenic responses as a single agent in patients with AML, and combination therapy has emerged as the currently preferred therapeutic strategy. However, the detection of drug-resistant leukemic blast cells in PKC412-treated patients with AML has prompted us to search for novel, structurally diverse FLT3 inhibitors. These are expected to prevent development of drug resistance if applied in combination with antileukemic agents.
We report here initial characterization of NVP-AST487, a potent and selective inhibitor of mutant FLT3 protein kinase activity. We demonstrate that this compound selectively induces cell-cycle arrest and apoptosis of leukemic cells harboring mutant FLT3 with a potency approximately 50 times higher than that of the FLT3 inhibitor PKC412, with no apparent effect on cells expressing wild-type FLT3. Furthermore, we show that NVP-AST487 actively inhibits proliferation of patient blasts harboring the FLT3-ITD mutation and PKC412-resistant isoforms of FLT3-ITD. We also show that NVP-AST487 significantly extends the survival of mice with FLT3-ITD–induced leukemia. These results support the notion that FLT3 is a promising therapeutic target for AML, and demonstrates the emergence of a novel class of FLT3 inhibitors that display high selectivity and strong efficacy toward FLT3 as a molecular target.
Methods
Cell lines and cell culture
The IL-3–dependent murine hematopoietic cell line Ba/F3 was transduced with either FLT3-ITD or FLT3-D835Y–containing murine stem cell virus (MSCV) retroviruses harboring a neomycin selectable marker, and selected for resistance to neomycin.16 Mutant FLT3-transduced cells were selected for growth in G418 (1 mg/mL). PKC412-resistant Ba/F3 cell lines expressing FLT3 harboring mutations in the ATP-binding pocket were developed as described previously.18 All cell lines were cultured with 5% CO2 at 37°C, at a concentration of 2 × 105 to 5 × 105 in RPMI (Mediatech, Herndon, VA) with 10% fetal calf serum (FCS) and supplemented with 1% glutamine. Untransfected parental Ba/F3 cells were similarly cultured with 15% WEHI-conditioned medium as a source of IL-3. Mutant FLT3-expressing cells were cultured in media supplemented with G418 (1 mg/mL).
Chemical compounds and biologic reagents
NVP-AST487 and PKC412 were synthesized by Novartis Pharma AG and were dissolved in DMSO to obtain 10 mM stock solutions. Serial dilutions were then made to obtain final dilutions for cellular assays with a final concentration of DMSO not exceeding 0.1%. Cytosine β-D-arabinofuranoside (Ara-c; C1768) and doxorubicin hydrochloride (D1515) were purchased from Sigma-Aldrich (St Louis, MO).
Cell viability, cell cycle, and apoptosis analysis
The trypan blue exclusion assay has been previously described,17 and was used to determine proliferation of cells cultured in the presence and absence of NVP-AST487. Cell viability is reported as percentage of control (untreated) cells, and data are presented as the average of 2 independent experiments, except where indicated. Error bars represent the standard error of the mean for each data point. Apoptosis of drug-treated cells was measured using the Annexin-V–Fluos Staining Kit (Boehringer Mannheim, Indianapolis, IN), as previously described.17 Cell-cycle analysis was performed as previously described.17
Antibodies
Anti–p-Tyr (clone 4G10; Upstate Biotechnology, Lake Placid, NY) was used at 1:1000 for immunoblotting. Anti-FLT3/Flk-2 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:200 for immunoblotting. Anti–p-STAT5 (no. 9359; Cell Signaling Technology, Danvers, MA) and STAT5 (sc-835; Santa Cruz Biotechnology) were used at 1:10 000 for immunoblotting.
Immunoprecipitation
Protein lysis preparation and immunoprecipitation and immunoblotting were carried out as previously described.17
Drug combination studies
For drug combination studies, NVP-AST487 and Ara-C, doxorubicin, or PKC412 were added simultaneously at fixed ratios to FLT3-ITD-Ba/F3 cells. Cell viability was determined using the trypan blue exclusion assay, and expressed as the function of growth-affected (FA) drug-treated versus control cells; data were analyzed by Calcusyn software (Biosoft, Ferguson, MO and Cambridge, United Kingdom) using the Chou-Talalay method.19 The combination index = [D]1 [Dx]1 + [D]2/[Dx]2, where [D]1 and [D]2 are the concentrations required by each drug in combination to achieve the same effect as concentrations [Dx]1 and [Dx]2 of each drug alone. Values less than 1 indicate synergy, whereas values greater than 1 indicate antagonism.
Bone marrow colony assay
Normal human bone marrow cells were obtained from to-be-discarded bone marrow harvest collection bags, under approval of the Institutional Review Board. Cells were lysed in ammonium chloride buffer to remove erythrocytes and washed. Mononuclear cells were isolated from normal bone marrow by density gradient centrifugation through Ficoll-Plaque Plus (Amersham Pharmacia Biotech AB, Uppsala, Sweden) at 824g for 30 minutes, followed by 2 washes in 1× phosphate-buffered saline (PBS). Normal human bone marrow was analyzed in a colony assay: plates of 5 × 104 cells in “complete” methylcellulose medium containing recombinant cytokines (contents: fetal bovine serum, recombinant human stem cell factor [rhSCF], rh granulocyte macrophage colony-stimulating factor [GM-CSF], rhIL-3, bovine serum albumin [BSA], methylcellulose in Iscove modified Dulbecco medium [IMDM], 2-mercaptoethanol, rh erythropoietin, and L-glutamine; MethoCult GFH4434; StemCell Technologies, Vancouver, BC) were prepared. The plates also contained NVP-AST487 at the indicated concentrations. The plates were incubated at 37°C in 5% CO2 for more than 1 week, and then myeloid and erythroid colonies (early progenitors with erythroid and myeloid components: colony forming unit [CFU]–GM, CFU–erythrocyte [E], burst-forming unit [BFU]–E, and CFU–granulocyte-erythrocyte-macrophage-megakaryocyte [GEMM]) were counted on a Micromaster Inverted microscope (Fisher Scientific, Pittsburgh, PA).
AML patient cells
Frozen vials of peripheral blood and bone marrow samples from patients with AML identified as harboring nonmutated FLT3 or the FLT3-ITD mutation (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) were thawed prior to isolation of mononuclear cells (obtained as in “Bone marrow colony assay” using Ficoll-Plaque Plus). Mononuclear cells were then tested in liquid culture (IMDM supplemented with 20% FCS) in the presence of different concentrations of NVP-AST487. All blood and bone marrow samples from patients with AML were obtained under approval of the Dana Farber Cancer Institute Institutional Review Board.
Mouse studies
The FLT3-ITD-Ba/F3 cell line used in animal studies was free of Mycoplasma contamination and viral contamination. Cells were washed once with 1× Hanks balanced salt solution (HBSS; Mediatech) and then resuspended in 1× HBSS prior to administration to animals. Female nude mice (Nu/Nu; Charles River Laboratories, Wilmington, MA) initially weighing 12 to 15 g and aged 6 weeks at delivery, were administered cell suspensions containing 1 × 106 FLT3-ITD-Ba/F3 cells via tail-vein injection. After 3 days, 3 groups of 8 FLT3-ITD-Ba/F3–injected mice were treated by gavage with either vehicle (10% N-methylpyrrolodinone [NMP]–90% polyethyleneglycol [PEG300]) or NVP-AST487 (30 mg/kg/day or 50 mg/kg/day) once daily for 21 days. At the planned end of the study, body and spleen weights were recorded, and tissues were preserved in 10% formalin for histopathologic analysis. Studies involving mice were performed with Animal Care and Use Comittee protocols at Dana-Farber Cancer Institute.
Survival was measured as time from cell injection to morbidity, according to Institute protocols, at which time mice were killed. All starting animals were included in the statistical analysis. Survival analysis was performed using the method of Kaplan and Meier with statistical significance assessed using the log-rank test.
For the in vivo bioluminescence assay, FLT3-ITD-Ba/F3-luc+ cells free of Mycoplasma and viral contamination were resuspended in HBSS (Mediatech) prior to intravenous administration to mice. PKC412, synthesized by Novartis Pharma AG, was supplied as a microemulsion preconcentrate (5% wt/vol) and diluted with water to achieve the desired final concentrations. Diluted solutions were stored at 4°C until used for gavage treatment of mice. NVP-AST487 was prepared as described in the first paragraph of this section.
Male nude mice (Nu/Nu; Charles River Laboratories), 6 weeks of age at delivery, were administered a total of 8 × 106 FLT3-ITD-Ba/F3-luc+ cells by tail-vein injection. Mice were imaged and total body luminescence was quantified as previously described.20 Baseline imaging 1 day after tumor cell inoculation was used to establish treatment cohorts with matched tumor burden. Cohorts of mice were treated with oral administration of vehicle, 50 mg/kg per day, or 100 mg/kg per day PKC412 (formulated as in the first paragraph of this section), or 50 mg/kg NVP-AST487 (formulated as in the first paragraph of this section). Imaging was performed 6 days after intravenous injection of cells. Mice were then killed, and tissues were preserved in 10% formalin for histopathologic analysis.
Determination of drug concentration in mouse plasma
Female OF1 mice (n = 4) received a single oral dose of 15 mg/kg formulated in 10% NMP/90% PEG200 (vol/vol). At the allotted times, mice were killed and the plasma concentration of compound was determined by reverse-phase high-performance liquid chromatography (HPLC)/tandem mass spectrometry (MS-MS) analysis.
Results
NVP-AST487 is a potent FLT3 inhibitor
NVP-AST487 (structure; Figure 1A) was tested in biochemical assays for inhibition of FLT3 kinase activity. The Ki was determined to be 0.12 μM (Table S2, Figure S2A-C). Besides FLT3, NVP-AST487 inhibits RET, KDR, c-KIT, and c-ABL kinase with IC50 values below 1 μM.21,22
Inhibition of cellular proliferation of mutant FLT3–expressing cell lines by NVP-AST487. (A) Structure of PKC412 and NVP-AST487. (B) Three-day treatment of FLT3-ITD-Ba/F3 cells, in the presence and absence of IL-3, with NVP-AST487. (C) Three-day treatment of FLT3-D835Y-Ba/F3 cells, in the presence and absence of IL-3, with NVP-AST487. (D) Colony assay formation of human bone marrow progenitor cells in the presence of increasing concentrations of NVP-AST487. Experiments in panels B and C were performed in duplicate; error bars represent SEM.
Inhibition of cellular proliferation of mutant FLT3–expressing cell lines by NVP-AST487. (A) Structure of PKC412 and NVP-AST487. (B) Three-day treatment of FLT3-ITD-Ba/F3 cells, in the presence and absence of IL-3, with NVP-AST487. (C) Three-day treatment of FLT3-D835Y-Ba/F3 cells, in the presence and absence of IL-3, with NVP-AST487. (D) Colony assay formation of human bone marrow progenitor cells in the presence of increasing concentrations of NVP-AST487. Experiments in panels B and C were performed in duplicate; error bars represent SEM.
Inhibition of cellular proliferation of mutant FLT3–expressing cell lines and AML patient cells by NVP-AST487
Treatment of FLT3-ITD-Ba/F3 cells and D835Y-Ba/F3 cells with NVP-AST487 potently inhibited cellular proliferation (IC50 < .005 μM; Figure 1B,C). Supplementation of culture media with WEHI, used as a source of IL-3, led to rescue of the cells, suggesting that NVP-AST487 selectively inhibits FLT3-ITD and has no effect on IL-3 signaling. The antiproliferative activity of NVP-AST487 was not blunted by addition of human serum (Figure S3). Cells expressing the novel point mutant FLT3-N841I also showed sensitivity to NVP-AST487 (Figure S1).
Parental Ba/F3 cells were not affected by up to 0.1 μM NVP-AST487 (Figure 1B). Similarly, the results of a CFU-GM colony formation assay showed no toxicity of human bone marrow progenitor cells at concentrations up to 0.1 μM NVP-AST487 (Figure 1D).
Several AML patient samples, characterized as harboring the FLT3-ITD mutation (Table S1), were treated for 3 days with NVP-AST487 in parallel with FLT3-ITD-Ba/F3 cells (as a control; Figure 2). NVP-AST487 treatment of FLT3-ITD-Ba/F3 cells with 0.01 μM NVP-AST487 resulted in complete cell killing compared with approximately 50% killing of AML patient samples at the same concentration (Figure 2A). PKC412 and NVP-AST487 were equipotent in efficacy against AML patient samples harboring mutant FLT3 and wild-type FLT3 (Figure 2B-E; Table S1).
Inhibition of cellular proliferation of mutant-FLT3-expressing AML patient cells by NVP-AST487. (A) Three-day treatment of AML FLT3-ITD–expressing patient cells versus FLT3-ITD-Ba/F3 cells with NVP-AST487. Experiments were performed 1 time for each AML patient sample. Cell viability was determined by the Trypan blue exclusion. (B) Treatment of an AML peripheral blood sample with NVP-AST487 and PKC412, respectively. This sample tested positive for harboring both the FLT3-ITD mutation and the D835Y mutation. Cell viability was determined by Trypan blue exclusion. (C-E) Treatment of AML bone marrow samples with NVP-AST487 and PKC412, respectively. These samples tested negative for harboring the FLT3 mutation. Cell viability was determined by Trypan blue exclusion.
Inhibition of cellular proliferation of mutant-FLT3-expressing AML patient cells by NVP-AST487. (A) Three-day treatment of AML FLT3-ITD–expressing patient cells versus FLT3-ITD-Ba/F3 cells with NVP-AST487. Experiments were performed 1 time for each AML patient sample. Cell viability was determined by the Trypan blue exclusion. (B) Treatment of an AML peripheral blood sample with NVP-AST487 and PKC412, respectively. This sample tested positive for harboring both the FLT3-ITD mutation and the D835Y mutation. Cell viability was determined by Trypan blue exclusion. (C-E) Treatment of AML bone marrow samples with NVP-AST487 and PKC412, respectively. These samples tested negative for harboring the FLT3 mutation. Cell viability was determined by Trypan blue exclusion.
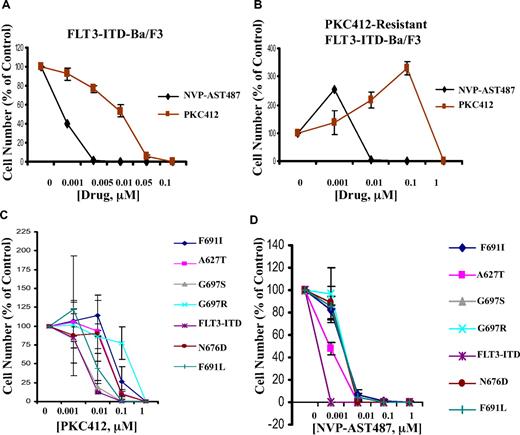
Inhibition of cellular proliferation of PKC412-sensitive and -resistant mutant FLT3-expressing cells by NVP-AST487
NVP-AST487 was tested in parallel with PKC412 against FLT3-ITD-Ba/F3 cells, and was found to exhibit significantly higher potency (10- to 50-fold) in inhibiting this cell line (Figure 3A). Similarly, as compared with PKC412, NVP-AST487 was approximately 100-fold more potent in inhibiting proliferation of PKC412-resistant FLT3-ITD-Ba/F3 cells that were previously generated17 by culturing FLT3-ITD-Ba/F3 cells in the presence of gradually increasing concentrations of PKC412 over a period of time (Figure 3B).
Inhibition of cellular proliferation of PKC412-sensitive and -resistant mutant FLT3-expressing cells by NVP-AST487. (A) Three-day treatment of FLT3-ITD-Ba/F3 cells with NVP-AST487 or PKC412. (B) Three-day treatment of PKC412-resistant FLT3-ITD-Ba/F3 cells with NVP-AST487 or PKC412 (n = 1 for NVP-AST487 treatment; n = 2 for PKC412 treatment). (C) Two-day treatment of FLT3-ITD-Ba/F3 cells and PKC412-resistant, mutant FLT3-expressing Ba/F3 cells with PKC412 (n = 2). (D) Two-day treatment of FLT3-ITD-Ba/F3 cells and PKC412-resistant, mutant FLT3-expressing Ba/F3 cells with NVP-AST487 (n = 2). Experiments were performed in duplicate; error bars represent SEM.
Inhibition of cellular proliferation of PKC412-sensitive and -resistant mutant FLT3-expressing cells by NVP-AST487. (A) Three-day treatment of FLT3-ITD-Ba/F3 cells with NVP-AST487 or PKC412. (B) Three-day treatment of PKC412-resistant FLT3-ITD-Ba/F3 cells with NVP-AST487 or PKC412 (n = 1 for NVP-AST487 treatment; n = 2 for PKC412 treatment). (C) Two-day treatment of FLT3-ITD-Ba/F3 cells and PKC412-resistant, mutant FLT3-expressing Ba/F3 cells with PKC412 (n = 2). (D) Two-day treatment of FLT3-ITD-Ba/F3 cells and PKC412-resistant, mutant FLT3-expressing Ba/F3 cells with NVP-AST487 (n = 2). Experiments were performed in duplicate; error bars represent SEM.
A panel of Ba/F3-derived cell lines that express FLT3-ITD harboring point mutations in the ATP-binding pocket of FLT3 were treated with either PKC412 or NVP-AST487; these mutations were previously shown to confer resistance to PKC412.18 There was an approximately 10-fold difference in potency of PKC412 against Ba/F3 cells expressing the FLT3-ITD mutation, compared with the majority of Ba/F3 cells harboring mutations in the ATP-binding pocket of FLT3 (Figure 3C). A similar shift in potency was observed in NVP-AST487–treated cells (Figure 3D). However, 0.01 μM NVP-AST487 already proved cytotoxic for the majority of mutant FLT3-expressing cells, whereas 0.1 μM PKC412 was necessary for killing the majority of mutant FLT3-expressing cells (Figure 3C,D). These results demonstrate an overall high potency of NVP-AST487 toward PKC412-resistant and nonresistant, mutant FLT3-expressing cells.
Induction of apoptosis and inhibition of cell-cycle progression of mutant FLT3-expressing cells by NVP-AST487
A dose-dependent increase of apoptotic cells was observed in FLT-ITD-Ba/F3 cells cultured in the presence of NVP-AST487 (at concentrations up to 0.1 μM; Figure 4A). Viability of cells cultured in the presence of the inhibitor in media supplemented with IL-3 was preserved following 3 days of treatment (Figure 4A). Induction of apoptosis was similarly observed in D835Y-Ba/F3 cells treated for 3 days in the presence of NVP-AST487 at concentrations of 0.01 μM and 0.1 μM (Figure 4B). There was no apparent induction of apoptosis of parental Ba/F3 cells cultured with IL-3 in the presence of NVP-AST487 for the same length of time (Figure 4C).
Induction of apoptosis and inhibition of cell-cycle progression of mutant FLT3-expressing cells by NVP-AST487. Effects of NVP-AST487 on viability of (A) FLT3-ITD-Ba/F3 cells, (B) FLT3-D835Y-Ba/F3 cells, and (C) parental Ba/F3 cells following 3 days of treatment. Effects of NVP-AST487 on cell-cycle progression of FLT3-ITD-Ba/F3 cells (D) following 36 hours of treatment.
Induction of apoptosis and inhibition of cell-cycle progression of mutant FLT3-expressing cells by NVP-AST487. Effects of NVP-AST487 on viability of (A) FLT3-ITD-Ba/F3 cells, (B) FLT3-D835Y-Ba/F3 cells, and (C) parental Ba/F3 cells following 3 days of treatment. Effects of NVP-AST487 on cell-cycle progression of FLT3-ITD-Ba/F3 cells (D) following 36 hours of treatment.
Treatment of FLT3-ITD-Ba/F3 cells with 0.01 μM NVP-AST487 for 36 hours resulted in G1 arrest of cells (Figure 4D). This suggests that the mechanism whereby these inhibitors inhibit cellular proliferation is via inhibition of cell=cycle progression as well as induction of programmed cell death.
Inhibition of autophosphorylation of FLT3 in mutant FLT3–expressing cells
Treatment of FLT3-ITD-Ba/F3 cells with 0.01 μM NVP-AST487 inhibited autophosphorylation of mutant FLT3 in these cells, with no apparent reduction in levels of the FLT3 protein (Figure 5A). Similar results were obtained with FLT3-N841I-Ba/F3 cells treated with 0.1 μM inhibitor (Figure 5B). A downstream effector of FLT3, STAT5, was also inhibited by both NVP-AST487 and PKC412 at 10 nM (Figure 5C,D). These results suggest that FLT3 kinase is a target of NVP-AST487, and inhibition of mutant FLT3 kinase activity leads to loss of growth factor independence and consequent cell death.
Inhibition of autophosphorylation of FLT3 and phosphorylation of STAT5 in mutant FLT3–expressing cells. (A) IP/Western: treatment of FLT3-ITD-Ba/F3 cells for 15 minutes with NVP-AST487 at 0.01 μM. A vertical line has been inserted to indicate a repositioned gel lane. (B) IP/Western: treatment of FLT3-N841I-Ba/F3 cells for 15 minutes with 0.01 or 0.1 μM NVP-AST487. (C) Immunoblot: treatment of FLT3-ITD-Ba/F3 cells for 2 hours with NVP-AST487 at 0 to 1000 nM. (D) Immunoblot: treatment of FLT3-ITD-Ba/F3 cells for 2 hours with PKC412 at 0 to 1000 nM.
Inhibition of autophosphorylation of FLT3 and phosphorylation of STAT5 in mutant FLT3–expressing cells. (A) IP/Western: treatment of FLT3-ITD-Ba/F3 cells for 15 minutes with NVP-AST487 at 0.01 μM. A vertical line has been inserted to indicate a repositioned gel lane. (B) IP/Western: treatment of FLT3-N841I-Ba/F3 cells for 15 minutes with 0.01 or 0.1 μM NVP-AST487. (C) Immunoblot: treatment of FLT3-ITD-Ba/F3 cells for 2 hours with NVP-AST487 at 0 to 1000 nM. (D) Immunoblot: treatment of FLT3-ITD-Ba/F3 cells for 2 hours with PKC412 at 0 to 1000 nM.
Plasma concentrations of NVP-AST487 in mice after oral administration
The bioavailability of NVP-AST487 was analyzed in mice. Mice received a single oral dose of 15 mg/kg NVP-AST487 formulated in 10% NMP/90% PEG300 by gavage. Plasma concentrations peaked after 1 hour (Cmax, 0.5 μM) and exceeded 0.1 μM for 6 hours (Table S2, Figure S2A-C). C24 hour was found to be more than 20 nM (Table S2, Figure S2A-C). Therefore, NVP-AST487 is orally bioavailable and reaches plasma concentrations expected to be antiproliferative based on cellular experiments.
Efficacy of NVP-AST487 in mice bearing mutant FLT3-expressing cells
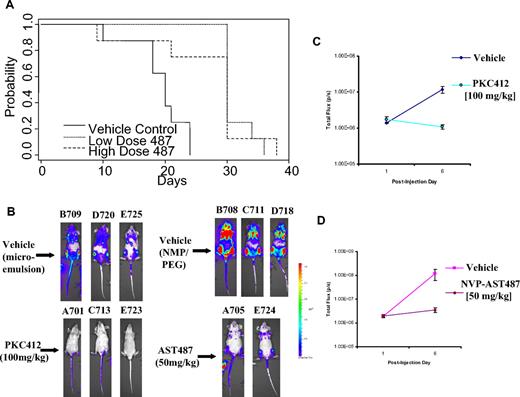
The ability of the inhibitor, NVP-AST487, to inhibit proliferation of mutant FLT3-expressing cells in vivo was investigated using athymic nude mice that had been inoculated with FLT3-ITD-Ba/F3 cells via tail-vein injection. Mice were orally administered vehicle (10% NMP-90% PEG300), 30 mg/kg NVP-AST487 (“low-dose” NVP-AST487), or 50 mg/kg NVP-AST487 (“high-dose” NVP-AST487) for a total of 21 days by gavage. Drug was not administered on weekends. All vehicle-treated mice died after 24 days following initial injection of the FLT3-ITD-Ba/F3 cells, whereas the majority of NVP-AST487–treated mice (at both doses) survived up to day 29 (Figure 6A). Median survival for vehicle control mice was 20 days; median for low- and high-dose mice was 30 days. The survival was different among the 3 groups (P < .001). Vehicle control mice died sooner than the low-dose-treated mice (P < .001) and sooner than the high-dose-treated mice (P = .005). There was no significant difference in survival between the low- and high-dose mice (P = .70).
Efficacy of NVP-AST487 in mice bearing mutant FLT3-expressing cells. (A) Time to onset of morbidity in mutant FLT3-Ba/F3–harboring athymic nude mice treated with vehicle or NVP-AST487 (30 mg/kg, or “low-dose”) or NVP-AST487 (50 mg/kg, or “high-dose”). (B) Mouse photos show bioluminescence of mice following 6 days after intravenous injection. B708, C711, and D718 are NMP/PEG300 vehicle controls for NVP-AST487–treated mice. B709, D720, and E725 are vehicle controls for PKC412-treated mice. (C,D) Bioluminescence values graphed for treatment groups. Error bars represent SEM.
Efficacy of NVP-AST487 in mice bearing mutant FLT3-expressing cells. (A) Time to onset of morbidity in mutant FLT3-Ba/F3–harboring athymic nude mice treated with vehicle or NVP-AST487 (30 mg/kg, or “low-dose”) or NVP-AST487 (50 mg/kg, or “high-dose”). (B) Mouse photos show bioluminescence of mice following 6 days after intravenous injection. B708, C711, and D718 are NMP/PEG300 vehicle controls for NVP-AST487–treated mice. B709, D720, and E725 are vehicle controls for PKC412-treated mice. (C,D) Bioluminescence values graphed for treatment groups. Error bars represent SEM.
At necropsy, all mice showed enlarged spleens, indicative of disease progression (Table S3). The P value for differences in total mouse weight was .52; for spleen, it was .37. Overall, these results demonstrate that NVP-AST487 prolongs the lifespan of mice harboring the FLT3-ITD mutation.
To confirm the in vivo antitumor efficacy of NVP-AST487 versus PKC412 as observed in the first in vivo study, we tested a mouse model of acute leukemia in which tumor burden was quantified by noninvasive imaging of luminescent tumor cells (Figure 6B,C). Taconic Line NCr nude mice were inoculated with FLT3-ITD-Ba/F3 cells engineered to stably express firefly luciferase. Noninvasive imaging was used to assess tumor burden, and mice with established leukemia were divided into cohorts with similar tumor burden. NVP-AST487 (50 mg/kg, 1 time daily) and PKC412 (100 mg/kg, 1 time daily) were then administered via oral gavage, as was vehicle. Both NVP-AST487 and PKC412 suppressed leukemia burden in mice compared with vehicle-treated controls (Figure 6B,C).
NVP-AST487 positively combines with Ara-c, doxorubicin, and PKC412
NVP-AST487 was tested in combination with Ara-C, doxorubicin, and PKC412 against FLT3-ITD-Ba/F3 cells. Calcusyn analysis of the combined effects of Ara-C and NVP-AST487 (Figure 7A) suggested slight to moderate synergism. Calcusyn analysis of a second, independent analysis of the combination of Ara-C and NVP-AST487 (Figure S4) suggested effects that ranged from nearly additive to moderately synergistic. Calcusyn analysis of the combined effects of doxorubicin and NVP-AST487 (Figure 7B) suggested slight synergy between the 2 agents. Calcusyn analysis of the combined effects of PKC412 and NVP-AST487 (Figure 7C) suggested effects that ranged from slight antagonism to slight synergism between the 2 agents.
NVP-AST487 positively combines with Ara-c, doxorubicin, and PKC412. (A) Proliferation study investigating the combination of NVP-AST487 and Ara-c. Results shown here are representative of 2 independent studies. Calcusyn analysis suggests slight to moderate synergism between the 2 agents (ED25: 0.84008; ED50: 0.77655; ED75: 0.73874; ED90: 0.72264). (B) Proliferation study investigating the combination of NVP-AST487 and doxorubicin, performed once. Calcusyn analysis suggests slight synergy between the 2 agents (ED25: 0.88419; ED50: 0.87274; ED75: 0.86262; ED90: 0.85377). (C) Proliferation study investigating the combination of NVP-AST487 and PKC412, performed once. Calcusyn analysis suggests effects that ranged from slight antagonism to slight synergism between the 2 agents (ED25: 0.88582; ED50: 0.95322; ED75: 1.02619; ED90: 1.10522).
NVP-AST487 positively combines with Ara-c, doxorubicin, and PKC412. (A) Proliferation study investigating the combination of NVP-AST487 and Ara-c. Results shown here are representative of 2 independent studies. Calcusyn analysis suggests slight to moderate synergism between the 2 agents (ED25: 0.84008; ED50: 0.77655; ED75: 0.73874; ED90: 0.72264). (B) Proliferation study investigating the combination of NVP-AST487 and doxorubicin, performed once. Calcusyn analysis suggests slight synergy between the 2 agents (ED25: 0.88419; ED50: 0.87274; ED75: 0.86262; ED90: 0.85377). (C) Proliferation study investigating the combination of NVP-AST487 and PKC412, performed once. Calcusyn analysis suggests effects that ranged from slight antagonism to slight synergism between the 2 agents (ED25: 0.88582; ED50: 0.95322; ED75: 1.02619; ED90: 1.10522).
NVP-AST487 was also tested for synergy with Ara-c and doxorubicin by administering the agents sequentially, in comparison to simultaneous treatment. The simultaneous administration of NVP-AST487 with Ara-c or doxorubicin led to the strongest positive combination effect, which was similar to and comparable with sequential administration of Ara-c or doxorubicin 24 hours prior to administration of NVP-AST487 (Figures S5A,B,E,F; S6A,B,E,F). However, the administration of NVP-AST487 24 hours prior to either Ara-c or doxorubicin resulted in a slightly weaker positive combination effect compared with the other regimens (Figures S5A-F, S6A-F).
Discussion
Constitutively activating mutations of FLT3 occur in a subset of patients with AML and are associated with a poor prognosis. Because FLT3 is an attractive molecular target for the treatment of AML, a variety of inhibitors of the FLT3 tyrosine kinase have been developed and are currently being investigated in early-phase clinical trials involving patients with AML refractory to standard chemotherapy.
The N-benzoylstaurosporine PKC412 (Novartis Pharma AG) is an orally bioavailable inhibitor of FLT3, PDGFR-β, c-KIT, and c-FMS that induces cell-cycle arrest and apoptosis of mutant FLT3–expressing cells via direct inhibition of FLT3.17 Results of a phase 2 clinical trial testing PKC412 as a single agent showed the drug to be generally well tolerated, and demonstrated inhibition of patient FLT3 phosphorylation and a decrease in peripheral blast counts in 35% of treated patients with relapsed/refractory AML with a median response duration of 13 weeks; PKC412 induces a hematologic response rate in patients with advanced AML comparable to what is observed in patients with chronic myelogenous leukemia (CML) in blast crisis treated with imatinib.23,24 The indolinone SU5416 (SUGEN; Pfizer, New York, NY), which also inhibits c-KIT, VEGFR1/2, and the SCF receptor, was demonstrated in a multicenter phase 2 clinical trial to inhibit phosphorylation of FLT3 in patients with refractory AML; partial responses were observed in a subset of patients lasting from 1 to 5 months.25 In another multicenter phase 2 study, SU5416 as a single agent had modest clinical activity in patients with refractory AML, with overall median survival of 12 weeks and grade 3 or 4 drug-related toxicities believed to be due to drug formulation.26 A phase 2 hematologic malignancy trial in the U.S. showed inhibition of FLT3 phosphorylation in SU5416-treated patients with refractory AML, although the vast majority of patients did not show a clinical response.27 Treatment of patients with mutant FLT3+ AML in phase 1 clinical trials with the orally administered indolinone SU11248 (SUGEN) was demonstrated to have anti-FLT3 activity in patients and resulted in morphologic or partial responses of short duration.28,29 The novel, orally administered FLT3 inhibitor CEP-701 (Cephalon, Frazer, PA) an indolocarbazole derivative, shows effectiveness against FLT3-ITD–expressing AML cells,30 and induced clinical responses of short duration in a phase 1/2 clinical trial in patients with relapsed or refractory disease.29 Complete inhibition of FLT3 autophosphorylation was observed in several patients with no accompanying clinical response.31 Several other patients showed a decrease in peripheral blood leukemic blasts to less than 5%; one patient showed a decrease in bone marrow blasts to a similar extent that was still apparent after 1 month.32 Also in early clinical trials involving patients with relapsed or refractory AML is the piperazonyl quinazonline MLN518 (CT53518; Millennium, Cambridge, MA).33 Recent reports of new FLT3 inhibitors in preclinical development include Ki23819, which has been shown to be effective against FLT3-ITD–expressing human cell lines.34
However, the FLT3 inhibitors tested thus far generally induce partial and transient responses in patients when used as single agents, suggesting a need for development of newer, possibly more efficacious/less toxic inhibitors of FLT3 that can be used effectively as single agents. It also suggests a need to investigate the potential of these compounds in combination with other therapeutics already in clinical use.
In our report, we present preclinical data for NVP-AST487 that show high potency and selectivity toward mutant FLT3 as a target, as evidenced by inhibition of cellular proliferation of FLT3-ITD– and D835Y-expressing Ba/F3 cells with an IC50 less than .005 μM, induction of apoptosis of mutant FLT3-expressing cells and inhibition of cell-cycle progression, and inhibition of FLT3 autophosphorylation in these cells. In addition, NVP-AST487 was observed to significantly reduce leukemia burden and prolong the survival of mice harboring mutant FLT3. Furthermore, NVP-AST487 was shown to potently kill a panel of PKC412-resistant Ba/F3 cell lines expressing FLT3 harboring mutations in the ATP-binding pocket. This observation is clinically relevant, as resistance to PKC412 in patients has been attributed to pre-existing or acquired mutations in the kinase domain of FLT3.35
A model of NVP-AST487 bound to the FLT3 kinase domain was created to rationalize our observations (supplementary data). The kinase was modeled in the inactive “DFG out” conformation based on a crystal structure of the Abl kinase in complex with a similar inhibitor.36 According to the model, as in the case of PKC412,18 the side chains of residues A627 and N676 do not have any contact with NVP-AST487. However, contrary to PKC412, NVP-AST487 does not make extensive contacts with residues G697 and F691, and mutations of these residues are not expected to dramatically alter the interaction of the inhibitor with the binding site. The observation that all the mutations investigated uniformly reduce the inhibitory activity of NVP-AST487 by approximately one order of magnitude, together with the previous modeling considerations, suggest an indirect effect of these mutations on the binding of the inhibitor by destabilization of the “DFG out” conformation.
As treatment with FLT3 inhibitors as single agents has demonstrated limited efficacy in patients with refractory AML, clinical outcome can potentially be improved by incorporation of novel FLT3 inhibitors into standard chemotherapeutic regimens. Various FLT3 inhibitors have been tested with standard AML chemotherapy drugs as a way to assess the overall efficacy of combined therapies. SU11248 was found, when combined with cytarabine or daunorubicin, to exhibit additive-to-synergistic inhibitory effects on mutant FLT3-ITD–expressing cells.37 The FLT3 inhibitor CEP-701 was demonstrated to be synergistic with cytarabine, daunorubicin, mitoxantrone, and etoposide, respectively, when administered simultaneously with the chemotherapy agents or immediately following their administration.38
The combination of NVP-AST487 with Ara-C, doxorubicin, or PKC412 generally resulted in an additive to the synergistic effects. This suggests that FLT3 inhibitors like NVP-AST487 could potentially be used in combination with standard chemotherapeutic agents currently in use for AML, and that the addition of such potent inhibitors of FLT3 to AML chemotherapy regimens could potentially result in improved treatment results.
The poor clinical outcome of standard chemotherapeutic agents presently in use for AML, and the limited utility of more promising approaches such as allo-BMT, point to a need for development of novel therapeutic strategies that could translate into higher overall drug responsiveness and a lower incidence of relapse. One approach is the use of 2 different FLT3 inhibitors that could potentially be used together if the mechanism whereby cells develop resistance to each is different. The development of novel agents such as NVP-AST487, with unique structures conferring higher potency and selectivity toward FLT3 as a target, represents a step toward overcoming some of the existing challenges and obstacles in the therapy of AML. It will be of great interest to determine whether or not this emerging new class of compounds has a beneficial therapeutic effect in patients with AML.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We wish to thank Dr Sophia Adamia for her insightful scientific discussions.
J.D.G is supported by National Institutes of Health (NIH) grant CA66996 and a Specialized Center of Research Award from the Leukemia & Lymphoma Society. J.D.G. is also supported by NIH grants CA36167 and DK50654.
National Institutes of Health
Authorship
Contribution: E.W. is responsible for generation of research findings reported here (design/performance of in vitro and in vivo imaging experiments), integrity and analysis of the data, and writing of the manuscript; J.R. is responsible for generation of research findings reported here (design/performance of in vitro experiments), integrity and analysis of the data, and writing of the manuscript; G.B. and P.F. assisted with preclinical characterization of NVP-AST487; J.J. assisted with technical aspects of the manuscript (specifically proliferation studies and immunoblotting associated with the N841I FLT3 mutant); J.C. developed the PKC412-resistant mutant FLT3-expressing cells screened with NVP-AST487; R.D.W. assisted with technical aspects of in vivo imaging experiments; E.N. and R.B. assisted with STAT5 immunoblotting; A.R. assisted with histopathologic analysis of mice used in in vivo imaging experiments and assisted with testing of AML patient samples; D.M. and E.H.-M. assisted with technical aspects of in vivo imaging experiments (specifically gavage); R.S. and I.G. assisted with patient sample acquisition and analysis; E.F. assisted with patient sample analysis; A.L.K. assisted with interpretation of research reported here and analysis of the data; J.F.D. and S.L.-K. assisted with flow cytometry; G.G. assisted with the development and provision of the PKC412-resistant mutant FLT3-expressing cells that were screened with NVP-AST487; and J.D.G. was responsible for conception of research reported here and integrity and analysis of the data.
Conflict-of-interest disclosure: J.R., G.B., and P.F. are employees of Novartis Pharma AG (Basel, Switzerland). R.S., A.L.K., and J.D.G. have a financial interest with Novartis Pharma AG. The remaining authors declare no competing financial interests.
Correspondence: James D. Griffin, Department of Adult Oncology, Dana-Farber Cancer Institute, 44 Binney Street, Boston, MA 02115; e-mail: james_griffin@dfci.harvard.edu.
References
Author notes
*E.W. and J.R. contributed equally to this work.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal