Abstract
It remains unresolved how different BCR-ABL transcripts differentially drive lymphoid and myeloid proliferation in Philadelphia chromosome–positive (Ph+) leukemias. We compared BCR-ABL transcript type and level with kinase domain (KD) mutation status, genotype, and phenotype in 1855 Ph+ leukemias. Compared with e1a2/p190 BCR-ABL cases, de novo e13-e14a2/p210 Ph+ lymphoid leukemia more frequently showed CML-type background, had higher blast-normalized BCR-ABL transcript levels, and more frequent persistent BCR-ABL transcript in the absence of detectable lymphoblasts. Secondary lymphoid blast transformation of CML was exclusively due to e13/e14a2/p210 BCR-ABL but was associated, at a much higher level than p210 myeloid transformation, with acquisition of new KD mutations and/or Ph genomic amplification. In contrast, myeloid blast transformation was more frequently accompanied by new acquisition of acute myeloid leukemia-type chromosomal aberrations, particularly involving the EVI1 and RUNX1 loci. Therefore, higher kinase activity by mutation, transcriptional up-regulation or gene amplification appears required for lymphoid transformation by p210 BCR-ABL.
Introduction
An unresolved question in the biology of the BCR-ABL chimeric kinase is the preferential association of different fusion proteins with Philadelphia chromosome–positive (Ph+) acute lymphoid leukemia (ALL) and chronic myelogenous leukemia (CML).1 The major breakpoint cluster region (BCR) chromosomal rearrangement seen in CML is associated with production of the e13a2 (b2a2) and/or e14a2 (b3a2) fusion transcript and the p210 BCR-ABL protein. In contrast, the p190 protein arising from the minor BCR rearrangement producing the e1a2 fusion transcript is seen in the majority of cases of Ph+ ALL. However, expression of e13a2 and/or e14a2 fusion transcript are noted in ALL, especially in adult patients.2 Cases of CML associated with the e1a2 transcript have also been occasionally reported.3,4 The biology is further complicated by transformation of CML to lymphoid blast phase (LBP), including cases that present as acute leukemia, with chronic-phase CML emerging only after initial therapy.5
The workup of leukemias has progressed substantially since the original studies on transcript association with CML and ALL were published, including use of minimal residual disease (MRD) flow cytometric (FCM) profiling for ALL and the use of highly sensitive reverse transcription quantitative polymerase chain reaction (RQ-PCR) to track transcript levels.6,7 Here we compare genotype, phenotype, BCR-ABL transcript levels, and treatment response patterns associated with blast transformation in p190 versus p210 Ph+ leukemias.
Methods
All cases of fully characterized Ph+ leukemias seen at the University of Texas M. D. Anderson Cancer Center between the start of BCR-ABL RQ-PCR on July 17, 2001, and January 1, 2008, were included. A protocol under the first author (D.J.) for laboratory studies to perform molecular and laboratory studies to detect prognostic factors in leukemia was approved by the M. D. Anderson Cancer Center Institutional Review Board in accordance with the Declaration of Helsinki. Cases were diagnosed according to the criteria of the revised World Health Organization criteria,8 except that a 30% blast cutoff was used for secondary blast phase transformation of CML. Only acute leukemias with FCM characterization of the blasts were included. Nearly all patients presenting with Ph+ acute leukemias during this period received intensive multiagent chemotherapy and a tyrosine kinase inhibitor (usually imatinib mesylate and, more recently, dasatinib).9 Myeloid and lymphoid blasts were enumerated in posttreatment samples by 4-color flow cytometry (FCM), by comparison with the phenotype of normal marrow precursors using a standard MRD protocol assessing 2 × to 5 × 105 cells with a panel with lymphoid, myeloid, and monocytic markers.10
BCR-ABL RQ-PCR, kinase domain mutation DNA sequencing, BCR-ABL fluorescence in situ hybridization (FISH), and G-banded karyotyping were done as previously described.11 The RQ-PCR assay detects e1a2, e13a2, and e14a2 transcripts in a single tube and is normalized to ABL1, with BCR-ABL transcript type(s) determined by subsequent capillary electrophoretic separation of the fluorochrome-labeled products.12 This assay detects residual leukemia with up to 4- to 5-log decrease from baseline (newly diagnosed) levels. We note that 10% to 15% of e13a2/e14a2-expressing leukemias also express very low levels of the e1a2 transcript.13,14 False-negative results in diagnostic samples were extremely rare in this RQ-PCR assay, seen in only 11 of 1855 (0.5%) cases in which PCR was negative but the BCR-ABL fusion was detected by karyotype and/or BCR-ABL FISH. As determined by sequencing of the BCR-ABL transcript using a separate long-range nested PCR assay,15 these included leukemias with e1a3 (2 cases), e14a3 (3 cases), and e19a2/p230 (2 cases) transcripts, and 4 cases in which the BCR-ABL transcript could not be identified.
BCR-ABL RQ-PCR level and blast enumeration by FCM were compared and cases were considered discordant if FCM identified no residual tumor blast population but BCR-ABL transcripts were detectable in at least 2 separate samples, or vice versa. Given the differing sensitivity of the RQ-PCR and FCM monitoring techniques, levels of BCR-ABL transcript that were more than 3 logs below baseline level (roughly 1 in 1000 Ph+ cells or fewer) were not considered as discordances. Discordances were also recorded if the level of lymphoid tumor blasts identified by FCM was more than 10-fold lower than that predicted by the BCR-ABL transcript level based on reduction from baseline levels.
Results and discussion
Features distinguishing p190 and p210 Ph+ acute leukemias
The p190/e1a2 BCR-ABL transcript was expressed in 127 of 168 (76%) Ph+ lymphoid leukemias, in 4 of 9 (44%) biphenotypic/bilineal acute leukemias and in 2 of 17 (12%) Ph+ AML. Mean presenting BCR-ABL levels normalized to blast counts were higher in e13/e14a2 ALL than in e1a2 cases (3751 vs 2313; P = .015, t test). Nineteen of the 41 (46%) e13/e14a2-expressing de novo ALL had features suggesting transformation from underlying CML, including background myeloid hyperplasia, abnormal megakaryocytic proliferation and/or basophilia, compared with only 3 of 120 (3%) e1a2-expressing cases (P < .001). At diagnosis or relapse, basophilia was seen in only 1 of 120 e1a2 lymphoid leukemias, compared with 10 of 41 e13/e14a2 cases. Results are summarized in Table 1.
Association of BCR-ABL transcript types and levels with pattern of Ph+ leukemia
| Pattern of disease . | e1a2 (p190) . | e13a2/e14a2 (p210) . | P . |
|---|---|---|---|
| Ph+ ALL at presentation* | 127† | 41‡ | |
| Normalized BCR-ABL transcript level (copies per μg RNA/blast count) | 2313 (37-41 743) | 3751 (224-57 569) | .015 (t test) |
| FCM/qPCR results match on FU | 84/87 (97%) | 15/28 (54%) | <.001 |
| BCR-ABL transcript detectable, 3 months | 49/80 (61%) | 27/30 (90%) | <.005 |
| BCR-ABL transcript detectable, 6 months | 29/64 (45%) | 24/30 (80%) | <.002 |
| Bilineal/biphenotypic Ph+ acute leukemia§ | 4 | 5 | |
| Ph+ AML at presentation | 2‖ | 15¶ | |
| CML at presentation# | 5** | 1645 | |
| Progression to lymphoid blast crisis | 0 | 26 | |
| Progression to myeloid blast crisis | 2 | 69 |
| Pattern of disease . | e1a2 (p190) . | e13a2/e14a2 (p210) . | P . |
|---|---|---|---|
| Ph+ ALL at presentation* | 127† | 41‡ | |
| Normalized BCR-ABL transcript level (copies per μg RNA/blast count) | 2313 (37-41 743) | 3751 (224-57 569) | .015 (t test) |
| FCM/qPCR results match on FU | 84/87 (97%) | 15/28 (54%) | <.001 |
| BCR-ABL transcript detectable, 3 months | 49/80 (61%) | 27/30 (90%) | <.005 |
| BCR-ABL transcript detectable, 6 months | 29/64 (45%) | 24/30 (80%) | <.002 |
| Bilineal/biphenotypic Ph+ acute leukemia§ | 4 | 5 | |
| Ph+ AML at presentation | 2‖ | 15¶ | |
| CML at presentation# | 5** | 1645 | |
| Progression to lymphoid blast crisis | 0 | 26 | |
| Progression to myeloid blast crisis | 2 | 69 |
ALL indicates acute lymphoid leukemia; AML, acute myeloid leukemia; CML, chronic myelogenous leukemia; FCM, flow cytometry; FU, follow-up sample after treatment; and qPCR, BCR-ABL quantitative PCR.
Two cases had cryptic BCR-ABL and are not included in column totals.
Including 24 with one relapse, 20 with 2 or more relapses, 20 with persistent disease not initially responsive to therapy, and 6 who died during or before induction therapy.
Including 9 with one relapse, 9 with 2 or more relapses, 6 with persistent disease not initially responsive to therapy, and 2 who died during or before induction therapy.
Of these, 7 cases were biphenotypic by FCM (5 myeloid/B-cell, 2 myeloid/T-cell phenotype), and 2 cases had apparent bilineal lymphoid and myeloid blasts.
Blasts had monocytic and minimally differentiated phenotype, respectively.
Blasts were minimally differentiated in 2, myeloid in 12, myelomonocytic in 1, and megakaryocytic in 2.
Two cases had cryptic BCR-ABL translocations not detected by PCR assay, 2 had e1a3 fusions, 2 had e19a2/p230, and 3 cases had e14a3 type. Translocations not detected by the quantitative PCR assay are not included in the column totals.
Two cases had an altered transcript with truncation of the e1 exon, identified by DNA sequencing.
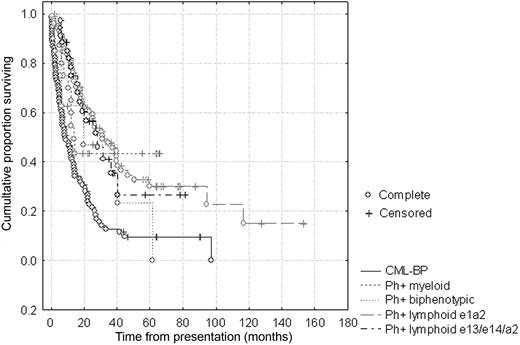
Leukemic blast percentages quantified by MRD FCM and BCR-ABL transcript levels were compared after treatment among all Ph+ lymphoid leukemias that had at least 3 sequential samples. Overall, cases with e13/e14a2 transcripts were more likely to have residual BCR-ABL transcript detectable at 3 months (90% vs 61%; P < .005) and 6 months (80% vs 45%; P < .002) after therapy initiation. FCM/RQ-PCR discordances were rare (3/87, 3%) in e1a2-expressing lymphoid leukemias, but common in e13/e14a2-expressing cases (13/28, 46%; P < .001). There were no differences in overall survival in e1a2 versus e13/e14a2 lymphoid leukemias (Figure 1), similar to some16 but not other studies which showed inferior17 or superior survival2 in different settings.
Outcome of de novo Ph+ acute leukemias versus CML-BP. Kaplan-Meier analysis shows no difference in overall survival comparing e1a2-expressing and e13/e14a2-expressing lymphoid or biphenotypic acute leukemias, but slightly superior survival compared with CML blast phase (CML-BP) assessed from the time of transformation. For the e1a2-expressing leukemias, median overall survival (OS) was 20.3 months (range, 0.5-170.5 months), and median follow-up was 18.2 months (range, 0.5-170.5 months). For e13/e14a2 lymphoid leukemias, OS was 19.3 months (range, 7.1-61.9 months) and median follow-up was 17.3 months (range, 6.0-81.2 months).
Outcome of de novo Ph+ acute leukemias versus CML-BP. Kaplan-Meier analysis shows no difference in overall survival comparing e1a2-expressing and e13/e14a2-expressing lymphoid or biphenotypic acute leukemias, but slightly superior survival compared with CML blast phase (CML-BP) assessed from the time of transformation. For the e1a2-expressing leukemias, median overall survival (OS) was 20.3 months (range, 0.5-170.5 months), and median follow-up was 18.2 months (range, 0.5-170.5 months). For e13/e14a2 lymphoid leukemias, OS was 19.3 months (range, 7.1-61.9 months) and median follow-up was 17.3 months (range, 6.0-81.2 months).
Features differentially associated with myeloid versus lymphoid blast transformation of p210-expressing CML
Expression of the e1a2 transcript characterized only 5 of 1659 (0.3%) cases presenting as chronic- or accelerated-phase CML, including 2 cases in which DNA sequencing demonstrated an altered e1a2 fusion transcript with partial in-frame deletions in the e1 exon. All of the 26 cases of CML that underwent secondary LBP and the 5 that underwent mixed myeloid/lymphoid bilineal/biphenotypic transformation expressed e13/e14a2 transcripts. Two CMLs that rapidly underwent myeloid blast transformation (MBP) with a monocytic blast phenotype expressed e1a2, whereas all other cases with MBP expressed e13/e14a2.
Factors differentially associated with LBP and MBP in p210 CML were sought by comparing FISH for BCR-ABL gene copy number, frequency of BCR-ABL kinase domain mutation (KDM), and cytogenetic changes acquired at time of transformation. For MBP, 6 of 34 cases (18%) acquired a new KDM, 15 of 51 (29%) showed BCR-ABL amplification, and 60 of 68 (88%) had new karyotypic abnormalities, mostly frequently additional AML-type reciprocal chromosomal translocations [inv(3)(q21;q26) including the EVI1 locus in 13, t(3;21)(q26;q22) including the RUNX1 locus in 12, and inv(16)(p13;q22)/t(16;16) including the CBFB locus in 5] or chromosomal losses/gains, particularly del17p13/monosomy 17/isochromosome 17q, seen in 39 (57%) cases. With LBP, 17 of 21 (81%) cases acquired a new KDM (6 T315I, 4 Y253H, 2 F317L, and 1 each with F359C, F359V, E255K, M244V, and Q252H), 8 of 21 (38%) showed BCR-ABL amplification, and 7 of 24 (29%) had new clonal evolution, most frequently whole chromosome copy number changes (aneuploidy).
We conclude that BCR-ABL transcript type and levels in association with pattern of secondary genetic changes can largely predict blast phenotype, with e1a2/p190 BCR-ABL expression nearly always exclusively driving lymphoid transformation, as supported by experimental studies.18-20 LBP of CML is unrelated to p190 BCR-ABL, but is highly associated with BCR-ABL genomic amplification and acquisition of highly active ABL mutations similar to relapsed ALL,20 whereas MBP of CML is much more often associated with specific AML-type cytogenetic changes often related to dominant-negative myeloid transcription factor changes. Patients presenting with lymphoid blasts and the e13a2 and/or e14a2 transcript often showed low-level disease persistence not obviously related to the lymphoid blast populations, suggesting that the p210 BCR-ABL drives a component of myeloid disease in these patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This investigation was supported by a developmental grant from the Leukemia SPORE (P50 CA100632) awarded by the National Cancer Institute, Department of Health and Human Services.
National Institutes of Health
Authorship
Contribution: D.J. wrote the manuscript and analyzed data; R.L., J.C., D.T., S.O., M.L., and H.K. analyzed data; C.B-R., S.H., and J.L.J. performed studies and analyzed data; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dan Jones, MD, PhD, Department of Hematopathology, Box 72, 1515 Holcombe Blvd, Houston TX 77030; e-mail: dajones@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal