Abstract
Idiopathic CD4+ lymphocytopenia (ICL) is a rare non–HIV-related syndrome with unclear natural history and prognosis. This prospective natural history cohort study describes the clinical course, CD4 T lymphocyte kinetics, outcome, and prognostic factors of ICL. Thirty-nine patients (17 men, 22 women) 25 to 85 years old with ICL were evaluated between 1992 and 2006, and 36 were followed for a median of 49.5 months. Cryptococcal and nontuberculous mycobacterial infections were the major presenting opportunistic infections. Seven patients presented with no infection. In 32, CD4 T-cell counts remained less than 300/mm3 throughout the study period and in 7 normalized after an average of 31 months. Overall, 15 (41.6%) developed an opportunistic infection in follow-up, 5 (13.8%) of which were “AIDS-defining clinical conditions,” and 4 (11.1%) developed autoimmune diseases. Seven patients died, 4 from ICL-related opportunistic infections, within 42 months after diagnosis. Immunologic analyses revealed increased activation and turnover in CD4 but not CD8 T lymphocytes. CD8 T lymphocytopenia (< 180/mm3) and the degree of CD4 T cell activation (measured by HLA-DR expression) at presentation were associated with adverse outcome (opportunistic infection-related death; P = .003 and .02, respectively). This trial is registered at http://clinicaltrials.gov as #NCT00001319.
Introduction
Idiopathic CD4 lymphocytopenia (ICL) is a syndrome first defined in 1992 by the Centers for Disease Control and Prevention (CDC)1 as “a documented absolute CD4 T lymphocyte count of less than 300 cells per cubic millimeter or of less than 20% of total T cells on more than one occasion, no evidence of infection on HIV testing, and the absence of any defined immunodeficiency or therapy associated with depressed levels of CD4 T cells.” One year later, 47 patients were reported in a CDC-coordinated effort to describe the epidemiologic, clinical, immunologic, and virologic characteristics of this new syndrome.2-5 Since then, it is widely accepted that ICL is a rare, heterogeneous syndrome not caused by HIV-1, HIV-2, HTLV-I, or HTLV-II and not appearing to be caused by any transmissible agent.6 ICL is usually detected after the occurrence of an opportunistic infection in a person without known immunodeficiency or immunosuppression. The clinical course, immunologic characteristics, CD4 T-cell kinetics, long-term outcome, and prognosis of this syndrome remain poorly defined. In 1992, the Laboratory of Immunoregulation, National Institute of Allergy and Infectious Diseases, National Institutes of Health initiated a prospective study of ICL to evaluate the natural history of ICL. This report details the results of this study.
Methods
Patients were recruited between 1992 and 2006 nationwide by published requests for referrals, targeted mailings, and spontaneous referrals. Patients sought were to have at least 2 confirmed CD4 T-cell counts of less than 300 cell/mm3 or less than 20% of lymphocytes, no serologic evidence of HIV-1 infection, no coexisting condition thought to be a likely cause of lymphocytopenia, and be capable of providing informed consent. Patients with common variable immunodeficiency were considered to have a coexisting condition associated with lymphocytopenia and were excluded. The protocol was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Patients were seen by a physician at the Warren G. Magnuson National Institutes of Health Clinical Center (Bethesda, MD) for history taking, physical examination, routine hematologic and chemistry blood panels, and immunologic and virologic assessment.
Patients were invited to return for follow-up approximately every year after their initial assessment. Patients were seen more than once a year if directed by their clinical condition and/or opportunistic infection. Those unable or unwilling to return were asked to respond to a health questionnaire, to respond to questions about their health by telephone, and to release medical records in the case of an interim hospitalization. They were asked to mail in blood for immunophenotyping (performed within 48 hours of phlebotomy) or, if unwilling to mail a sample, to provide written documentation of the last CD4 T-cell count obtained by their physician.
This work spanned several years and led to previous publications, which have included a portion of this cohort as case reports,7,8 a control population,9,10 or a distinct immunologic investigation.11 This report summarizes the clinical and laboratory information obtained from a prolonged follow-up in this population.
All immunologic laboratory parameters were compared with a group of 10 healthy volunteers obtained under a separate National Institutes of Health research protocol. Thresholds for low CD8, natural killer (NK), and B-cell counts were defined as less than the lower 2.5% of counts of a cohort of 435 healthy volunteers (≥ 18 years of age, weight ≥ 50 kg and seronegative for HIV-1, p24, HIV-2, HTLV-1, HTLV-2, HBsAg, and HCV).
In every ICL patient visit, lymphocyte phenotyping and antibodies to HIV-1 and HIV-2 were assessed by enzyme-linked immunosorbent assay, and Western blot (HIV-1). Patient serum was also examined for the presence of HIV-1 p24 antigen by enzyme-linked immunosorbent assay and polymerase chain reactions were performed in peripheral blood mononuclear cells as previously described.12
Immunologic assessment included immunophenotyping of viable cryopreserved peripheral blood mononuclear cells and ex vivo bromodeoxyuridine (BrdU) staining of peripheral blood for the evaluation of lymphocyte proliferation (patients enrolled after 1998) and were performed as previously described.7,13-16 Naive T cells were defined as CD45RO+CD27− and Treg cells were identified by FoxP3 and CD25 expression. The following antibodies were used for immunophenotyping in this study: anti-CD3 fluorescein isothiocyanate (FITC; clone SK7), anti-CD4 peridinin chlorophyll protein (PerCP) or allophycocyanin (APC; clone SK3), anti-CD19 (clone 4G7), anti-CD16 (clone B73), anti-CD56 (clone MY31), anti-CD8 PerCP (clone SK1), anti-CD45RO APC (clone UCHL-1), anti-CD25 PE (clone M-A251), anti-Ki-67 phycoerythrin (PE) or FITC (clone B56), anti-CD27 FITC (clone M-T271; all from BD Biosciences, San Jose, CA), anti-Forkhead Box Protein P3 (FoxP3) PE (clone 206D) from BioLegend (San Diego, CA), and anti-CD127 PE (Beckman Coulter clone R34.34, Fullerton, CA). Immunoglobulins were also measured in all visits.
Statistical analysis
ICL patients were compared with controls with respect to several immunologic factors, including cell counts for CD4, CD8, NK, and B cells; percent naive lymphocytes; and expression of HLA-DR, Ki-67, FoxP3, and CD127. The Mann-Whitney test was used to compare the distribution of expression levels for the 2 patient groups. The correlation between 2 factors was assessed using Spearman rank correlation coefficient. Immunologic factors were also included in an exploratory analysis to determine whether these or other clinical factors were significantly associated with the disease course as measured by time to death and time to opportunistic infection-related death. Immunologic factors considered for the survival analysis were: CD8, CD4, B- and NK-cell counts, and percent of CD4 T cells expressing HLA-DR. Binary variables for low levels of an immunologic factor were also examined for: CD8, CD4, B and NK cells, where the cutoff was determined by the lower 2.5 percentile of the healthy controls. Other clinical factors considered were sex, age over 50 years, and history of autoimmune disease. The survival time was measured as time since diagnosis, and patients were censored at the time of the last follow-up visit. Kaplan-Meier survival curves were calculated and comparisons of survival curves between groups of ICL patients were done using the exact log-rank test. Cox proportional hazards models were used to assess the association between continuous-valued covariates and survival time. Because of the small number of deaths, significance in the Cox model was determined by a permutation test. For all tests, a P value less than .05 was considered significant.
To analyze the longitudinal CD4 measurements, a linear mixed-effects model with a fixed effect of time and random intercept and slope coefficients was fitted to the data from all patients.17 This mixed-effects model assumes that the CD4 T-cell counts for an individual follow a line over time and allows for a subject-specific baseline (intercept) and velocity (slope) for the CD4 measurements. The model relies on the assumption that enough data were observed for each patient and that the reasons for missing CD4 measurements after baseline were not directly related to some unobserved change in their CD4 pattern. To consider the impact of only a few subjects having extended follow-up, a second model was fitted using only data from the first 2 years for comparison.
Statistical analysis was performed in R (version 2.2.1, Vienna, Austria), and figures were obtained by R and GraphPad Prism 5.0 (San Diego, CA).
Results
Study population
Fifty-seven persons were screened for ICL. Ten who did not have a CD4 T-cell count less than 300/mm3 and one who was HIV positive were excluded. Five additional patients were excluded because of alternative diagnoses at initial evaluation (non-Hodgkin lymphoma, lymphomatoid granulomatosis, common variable immunodeficiency, transient apheresis-related lymphocytopenia, chronic myelomonocytic leukemia). Two parents of ICL patients were screened, but no laboratory abnormalities were found. Thirty-nine patients fulfilling the CDC criteria for the definition of ICL1,4 were identified. Three of these patients had only a baseline evaluation and no follow-up. The clinical characteristics of the final analysis cohort (N = 39) are presented in Table 1.
Characteristics of 39 patients with idiopathic CD4 lymphocytopenia
| Characteristic/category . | No (%) . |
|---|---|
| Sex | |
| Male | 17 (43.6) |
| Female | 22 (56.4) |
| Race | |
| White | 35 (89.7) |
| Black | 2 (5.1) |
| Asian | 2 (5.1) |
| Age, y (range) | |
| 20 to 30 | 2 (5.1) |
| 30 to 40 | 12 (30.8) |
| 40 to 50 | 17 (43.6) |
| 50 to 60 | 4 (10.3) |
| 60 to 70 | 2 (5.1) |
| 70 to 80 | 0 |
| 80 to 90 | 2 (5.1) |
| OI at diagnosis | |
| Cryptococcosis | 13 (33.3) |
| Human papillomavirus infection | 8 (20.5) |
| Nontuberculous mycobacteria | 4 (10.3) |
| Histoplasmosis | 2 (5.1) |
| Mucosal candidiasis | 2 (5.1) |
| Dermatomal varicella zoster virus infection | 1 (2.6) |
| Pneumocystis jirovecii pneumonia | 1 (2.6) |
| Cytomegalovirus pneumonia | 1 (2.6) |
| No infection | 7 (17.9) |
| OI (episodes) in follow-up | |
| Human papillomavirus infection | 6 (15.4) |
| Mucosal candidiasis | 4 (10.3) |
| Dermatomal varicella zoster virus infection | 4 (10.3) |
| Nontuberculous mycobacteria | 2 (5.1) |
| P jirovecii pneumonia | 1 (2.6) |
| EBV lymphoproliferative disease | 1 (2.6) |
| Molluscum contagiosum | 1 (2.6) |
| Progressive multifocal leucoencephalopathy | 1 (2.6) |
| Any infection in follow-up | 15 (38.5) |
| No infection in follow-up | 21 (53.8) |
| No follow-up | 3 (7.7) |
| Median follow-up, mo (range) | 49.5 (0-204) |
| Characteristic/category . | No (%) . |
|---|---|
| Sex | |
| Male | 17 (43.6) |
| Female | 22 (56.4) |
| Race | |
| White | 35 (89.7) |
| Black | 2 (5.1) |
| Asian | 2 (5.1) |
| Age, y (range) | |
| 20 to 30 | 2 (5.1) |
| 30 to 40 | 12 (30.8) |
| 40 to 50 | 17 (43.6) |
| 50 to 60 | 4 (10.3) |
| 60 to 70 | 2 (5.1) |
| 70 to 80 | 0 |
| 80 to 90 | 2 (5.1) |
| OI at diagnosis | |
| Cryptococcosis | 13 (33.3) |
| Human papillomavirus infection | 8 (20.5) |
| Nontuberculous mycobacteria | 4 (10.3) |
| Histoplasmosis | 2 (5.1) |
| Mucosal candidiasis | 2 (5.1) |
| Dermatomal varicella zoster virus infection | 1 (2.6) |
| Pneumocystis jirovecii pneumonia | 1 (2.6) |
| Cytomegalovirus pneumonia | 1 (2.6) |
| No infection | 7 (17.9) |
| OI (episodes) in follow-up | |
| Human papillomavirus infection | 6 (15.4) |
| Mucosal candidiasis | 4 (10.3) |
| Dermatomal varicella zoster virus infection | 4 (10.3) |
| Nontuberculous mycobacteria | 2 (5.1) |
| P jirovecii pneumonia | 1 (2.6) |
| EBV lymphoproliferative disease | 1 (2.6) |
| Molluscum contagiosum | 1 (2.6) |
| Progressive multifocal leucoencephalopathy | 1 (2.6) |
| Any infection in follow-up | 15 (38.5) |
| No infection in follow-up | 21 (53.8) |
| No follow-up | 3 (7.7) |
| Median follow-up, mo (range) | 49.5 (0-204) |
OI indicates opportunistic infection; and EBV, Epstein-Barr virus.
Infections at presentation
The ICL-related infections at case presentation and the spectrum of infections during the follow-up time are also summarized in Table 1. Notably, the 3 most common infections were cryptococcal disease, persistent genital human papilloma virus (HPV) infection, and nontuberculous mycobacterial infection. Cryptococcal disease was meningitis in 10 patients, osteomyelitis in 2, and pulmonary infection in one. All the patients with HPV infection had treatment persistent and locally spread genital infection with or without perianal and/or hand/plantar disease (8 patients). In 3 of these patients, cervical intraepithelial neoplasia was also present. Patients diagnosed with non tuberculous mycobacterial infection included 2 with disseminated Mycobacterium avium complex (MAC): one with pulmonary MAC and one with Mycobacterium chelonae. Two patients had disseminated histoplasmosis and 2 had esophageal and persistent vaginal candidiasis. One had pulmonary histoplasmosis, one Pneumocystis jirovecii pneumonia (PCP), and one dermatomal zoster (VZV).
Seven patients (17.9%) had no reported opportunistic infections, and ICL was an incidental finding during evaluation for persistent lymphocytopenia. None of the 7 patients received any immunosuppressive or immunomodulatory treatment during ICL identification except one treated with interferon-α for widespread genital HPV infection. Six patients (15.4%) had evidence of persistent lymphocytopenia long before they were diagnosed with ICL, with a range from 9 months to more than 10 years in the past.
Lymphocyte subsets at presentation
Although the majority of patients (84.6%) had inverted CD4/CD8 ratio at time of diagnosis, a normal CD4/CD8 ratio in others reflected a concomitant severe depletion of CD8 T-cell numbers (Table 2). Indeed, 15 ICL patients had CD8 T-cell counts less than the lower 2.5% of CD8 T-cell counts for the control population (or < 180/mm3) and 23 patients had CD8 T-cell counts more than 180/mm3, thus effectively dividing our cohort in 2 subgroups in terms of CD8 T lymphocyte levels (one patient had no measurement at presentation). With a similar definition, 13 patients also had low absolute B lymphocyte counts (or < 99/mm3) and 18 had low absolute NK lymphocyte counts (or < 76/mm3) of 36 with available data at presentation (Table 2). Nine had low B and NK T lymphocytes and 5 had low CD8, B, and NK cells. Immunoglobulins were within normal limits in all persons.
Lymphocyte subset counts in 39 idiopathic CD4 lymphocytopenia patients and controls
| . | Idiopathic CD4 lymphocytopenia . | Controls . |
|---|---|---|
| At diagnosis | ||
| CD4 T cells/mm3 | 139 (4-290) | 861 (361-2299) |
| CD8 T cells/mm3 | 241 (22-2120) | 434 (108-1202) |
| B cells/mm3 | 141 (4-601) | 216 (40-973) |
| NK cells/mm3 | 79 (4-246) | 194 (38-682) |
| At end of study, CD4 T cells/mm3 | 123 (7-892) | 861 (361-2299) |
| . | Idiopathic CD4 lymphocytopenia . | Controls . |
|---|---|---|
| At diagnosis | ||
| CD4 T cells/mm3 | 139 (4-290) | 861 (361-2299) |
| CD8 T cells/mm3 | 241 (22-2120) | 434 (108-1202) |
| B cells/mm3 | 141 (4-601) | 216 (40-973) |
| NK cells/mm3 | 79 (4-246) | 194 (38-682) |
| At end of study, CD4 T cells/mm3 | 123 (7-892) | 861 (361-2299) |
Values are medians (range).
Virologic studies
No subjects had HIV-1 DNA or p24 antigen detected, and none of the patients developed antibodies to HIV-1 or HIV-2 during follow-up. No subjects were found to have evidence of retroviral infection by lymphocyte cocultivation.
Immunophenotype results
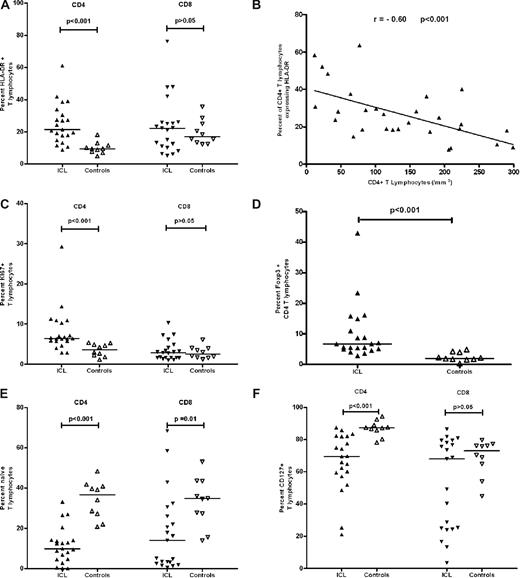
Immunophenotypic analysis revealed a significantly higher proportion of activated (HLA-DR+) CD4 (P < .001), but not CD8, T cells in ICL patients compared with controls that correlated with the degree of CD4 T cell lymphocytopenia (R = −0.60, P < .001) as shown in Figure 1A,B. The absolute number of HLA-DR+ CD4 cells correlated with the absolute number of CD4 cells (R = 0.58, P < .001; data not shown). The CD4 T-cell turnover as measured by Ki-67 was higher in CD4 (P < .001, Figure 1C) but not CD8 T cells of ICL patients compared with controls. Similar findings were observed in 10 ICL patients compared with another set of healthy controls (N = 1503) with ex vivo BrdU labeling, which specifically identifies cells in S phase (data not shown). Intracellular Foxp3 expression, which is considered indicative of the CD4 T regulatory cell subset, was higher as a percentage in ICL patients (P < .001) (Figure 1D) but lower as absolute number compared with controls (P = .017) (data not shown). Foxp3-positive cells had also high expression of CD25 (CD25high/Foxp3). In addition, a significantly lower proportion of CD4 T cells in ICL patients was of naive phenotype or expressed CD127 (the alpha chain of the IL-7 receptor) as shown in Figure 1E,F (P < .001).
Immunologic investigation of ICL patients. The percentage of HLA-DR+ (A), Ki-67+ (C), and Foxp3+ (D) expression within CD4 T lymphocytes is higher in 21 ICL patients compared with 10 controls. Naive (E) and CD127 + (F) cells are lower in CD4 T lymphocytes of ICL patients compared with controls. There is no difference in HLA-DR+ (A), Ki-67+ (C), and CD127+ (F) expression within the CD8T lymphocyte subset in ICL patients and controls. Naive (E) cells are lower in CD8 T lymphocytes of ICL patients compared with controls. Finally, there is a negative correlation between expression of HLA-DR on CD4 T cells and the CD4 T-cell count in 28 ICL patients (B). Horizontal lines represent median values.
Immunologic investigation of ICL patients. The percentage of HLA-DR+ (A), Ki-67+ (C), and Foxp3+ (D) expression within CD4 T lymphocytes is higher in 21 ICL patients compared with 10 controls. Naive (E) and CD127 + (F) cells are lower in CD4 T lymphocytes of ICL patients compared with controls. There is no difference in HLA-DR+ (A), Ki-67+ (C), and CD127+ (F) expression within the CD8T lymphocyte subset in ICL patients and controls. Naive (E) cells are lower in CD8 T lymphocytes of ICL patients compared with controls. Finally, there is a negative correlation between expression of HLA-DR on CD4 T cells and the CD4 T-cell count in 28 ICL patients (B). Horizontal lines represent median values.
Clinical follow-up
Patients in this cohort were followed for a median of 49.5 months (range, 0-204 months) and the median number of visits was 4 (range, 0-23).
Overall, 15 (41.6%) patients were diagnosed with infections potentially related to ICL during follow-up, most of them during the first 3 years and mostly HPV, dermatomal varicella zoster virus (VZV), and mucosal candidiasis (Table 1). In one patient, VZV infection was multidermatomal.
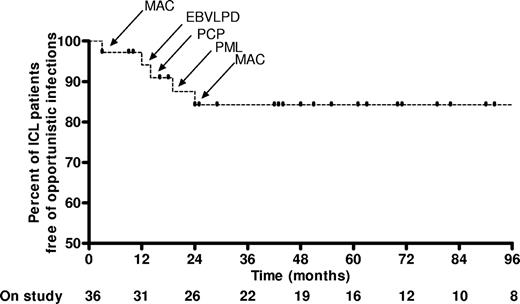
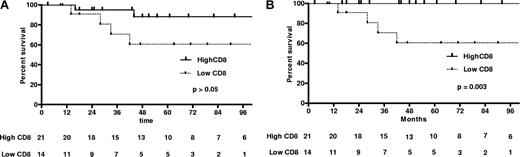
Five patients (13.8%) developed “AIDS defining clinical conditions” (category C HIV infections)18 during the first 24 months of follow-up (Figure 2): 2 episodes of MAC (CD4, 64 and 160 cells/mm3), 1 episode of PCP (CD4, 22 cells/mm3), 1 episode of progressive multifocal leukoencephalopathy (PML; CD4, 7 cells/mm3), and 1 Epstein-Barr virus–related lymphoproliferative disease, which evolved into B-cell lymphoma (CD4, 25 cells/mm3). “AIDS defining clinical conditions” and death were interrelated and 3 of these patients eventually died (from MAC, PML, and B-cell lymphoma, respectively). One additional patient died of an opportunistic infection–related condition. This was a 40-year-old man with severe and persistent genital HPV infection who died of metastatic squamous cell carcinoma originating from his penis 42 months after initial diagnosis. All 4 “opportunistic infection–related deaths” were among the 15 patients who had low CD8 T-cell counts (Figure 3). There were 3 nonopportunistic infection–related deaths during follow-up, all in the 21 patients who presented with normal CD8 T cell numbers. Two died of microbial pneumonia: one developed hospital-acquired pneumonia while on high-dose steroids for lupus nephritis; the other died approximately 6 years after his CD4 T-cell count had returned to normal, during which period he remained free of any symptoms or illness. Another patient died of metastatic prostate cancer at the age of 84 years. These 3 patients died because of conditions not clearly associated with CD4 lymphocytopenia or an opportunistic infection and were thus not considered “opportunistic infection–related deaths” in terms of analysis.
Kaplan-Meier curve for the occurrence of “AIDS-defining clinical conditions” during follow-up. Five “AIDS-defining” opportunistic infections occurred during the follow-up of 36 patients with ICL within the first 24 months of follow-up. Bottom line represents the number of ICL patients still on follow-up at 12-month intervals. Vertical lines represent the time at which patients were censored (end of follow-up or occurrence of an “AIDS-defining clinical condition”). MAC indicates M avium complex; EBVLPD, Epstein-Barr virus lymphoproliferative disease; PCP, P jirovecii pneumonia; PML, progressive multifocal leucoencephalopathy.
Kaplan-Meier curve for the occurrence of “AIDS-defining clinical conditions” during follow-up. Five “AIDS-defining” opportunistic infections occurred during the follow-up of 36 patients with ICL within the first 24 months of follow-up. Bottom line represents the number of ICL patients still on follow-up at 12-month intervals. Vertical lines represent the time at which patients were censored (end of follow-up or occurrence of an “AIDS-defining clinical condition”). MAC indicates M avium complex; EBVLPD, Epstein-Barr virus lymphoproliferative disease; PCP, P jirovecii pneumonia; PML, progressive multifocal leucoencephalopathy.
Kaplan-Meier curves of patients with low and high CD8 T-cell counts at diagnosis (< 180/mm3 or > 180/mm3, respectively). (A) All-cause survival. (B) Opportunistic infection (AIDS-defining illness)–related survival. The P value for the exact log rank test is also provided. Bottom lines represent the number of ICL patients still in follow-up at 12-month intervals. Vertical lines represent the time at which patients were censored: (A) at end of follow-up and (B) at end of follow-up or non-ICL death. In Figure 1A, one patient with high CD8 T-cell count died of a nonopportunistic infection after 96 months (at 114 months).
Kaplan-Meier curves of patients with low and high CD8 T-cell counts at diagnosis (< 180/mm3 or > 180/mm3, respectively). (A) All-cause survival. (B) Opportunistic infection (AIDS-defining illness)–related survival. The P value for the exact log rank test is also provided. Bottom lines represent the number of ICL patients still in follow-up at 12-month intervals. Vertical lines represent the time at which patients were censored: (A) at end of follow-up and (B) at end of follow-up or non-ICL death. In Figure 1A, one patient with high CD8 T-cell count died of a nonopportunistic infection after 96 months (at 114 months).
Among the 7 patients who were incidentally identified with ICL and had no opportunistic infections, none developed an “AIDS defining clinical condition” in follow-up and none died.
A comparison of survival curves with an exact log-rank test identified a low CD8 T-cell count at presentation (< 180/mm3) and high expression of HLA-DR (as a percentage of CD4 T cells) as predictors of an adverse outcome (opportunistic infection–related death; P = .003 and .02, respectively). A low B lymphocyte count at diagnosis was also associated with an adverse outcome, but the association was not as strong as with low CD8 T-cell counts (P = .02). Moreover, those with low B cells who had an adverse outcome had also low CD8 T-cell counts. Kaplan-Meier curves for high versus low CD8 T lymphocyte counts are shown in Figure 3. For all-cause mortality, only age was significantly associated (negatively) with survival (P = .02).
Four patients (11.1%) received prophylactic treatment for both PCP and MAC. Thirty-two (88.9%) received no prophylactic treatment for a cumulative follow-up period of 164 patient years.
CD4 T lymphocytes during follow-up
The CD4 T-cell count at the last available measurement, at a median of 40 months after diagnosis was 123/mm3 (median, range 7-892/mm3; Table 2). Only 7 patients of 36 with follow-up (19.4%) had CD4 T-cell counts more than 300/mm3 at the end of follow-up. In these patients, the CD4 T-cell count returned to normal after an average of 31 months after initial National Institutes of Health assessment. The number of CD4 T-cell count measurements per patient ranged from 2 to 23. Transient CD4 T-cell counts greater than 300/mm3 early in the follow-up period were noted in 3 patients.
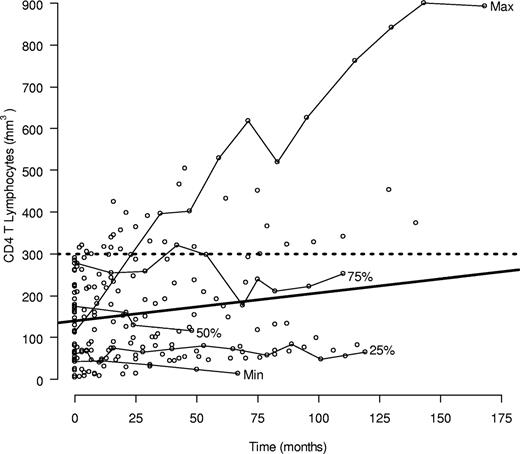
Figure 4 displays the CD4 T-cell counts for all patients along with the averaged trend line for the individual CD4 trajectories fitted to the linear mixed model. The CD4 velocity was estimated at 0.66/month (95% confidence interval, −0.20-1.54) and was not significantly different from 0 (P = .13). Although the average within person slope was not significant, some patients showed clear improvement over time, whereas others showed none or a slight decline. To understand the possible influence of subjects with extreme CD4 velocities or subjects with greater numbers of CD4 measurements, the mixed model was refitted on certain subgroups. The average CD4 profile without 2 subjects whose slopes were more than 2 SD greater than the overall model was only approximately one-third the slope based on all the data but was still not significantly different from 0. The linear mixed model was also fitted using measurements just from the first 2 years of follow-up, to dampen the influence of patients with longer CD4 profiles. The resulting fitted line was similar to one based on all the data. In summary, both a slight increase over time and no increase are consistent with the observed data. A progressive fall in CD4 counts, as might be expected in untreated HIV infection, can thus be excluded from these data.
CD4 T-cell counts from 39 ICL subjects during follow-up. Individual CD4 trajectories are shown for 5 subjects whose median CD4 T-cell count among those with at least 2 years of follow-up and 4 measurements was the minimum, maximum, or an innerquartile trajectory. The average individual trend fitted from the linear mixed model is also shown (bold solid line). The slope (SD) of the estimated trend is 0.66/mm3 per month (95% confidence interval, −0.20-1.54).
CD4 T-cell counts from 39 ICL subjects during follow-up. Individual CD4 trajectories are shown for 5 subjects whose median CD4 T-cell count among those with at least 2 years of follow-up and 4 measurements was the minimum, maximum, or an innerquartile trajectory. The average individual trend fitted from the linear mixed model is also shown (bold solid line). The slope (SD) of the estimated trend is 0.66/mm3 per month (95% confidence interval, −0.20-1.54).
Autoimmune phenomena
Autoimmunity was common in this cohort, and 9 patients or 23.1% were diagnosed with autoimmune diseases either before ICL was identified (5 patients, 13.8%) or in the follow-up period (4 patients, 11.1%; Table 3). Patient 1 has been previously described7 and fulfilled criteria for the diagnosis of antiphospholipid antibody syndrome (APS) after a deep venous thrombosis and pulmonary embolism with IgG- and IgM-positive anticardiolipin antibodies at the age of 47 years, 1 year before the diagnosis of disseminated MAC. Patient 2 was also diagnosed with APS at the age of 46 years (6 years after the diagnosis of ICL) because of a right cephalic vein thrombosis with positive IgG and IgM anticardiolipin antibodies, anti-β2 glycoprotein 1 antibodies, and lupus anticoagulant. The same patient had been diagnosed several years earlier with psoriasis and autoimmune thyroiditis. Patient 3 had systemic lupus erythematosus (SLE) with arthritis and facial erythema that she developed 18 years before an episode of CMV pneumonitis and CD4 lymphocytopenia. Patient 4 developed bilateral uveitis, arthritis, and facial erythema, which led to the diagnosis of SLE at the age of 42 years, 3 years before further evaluation identified persistently low CD4 T-cell counts and ICL. Patient 5 developed lupus nephritis 3 years after the diagnosis of ICL at the age of 37 years. Patient 6 developed autoimmune hemolytic anemia in the absence of immunoproliferative disease at the age of 41 years, almost concurrently with the diagnosis of ICL. Patient 7 had ulcerative colitis several years before ICL diagnosis and patient 8 had vitiligo. Finally, patient 9 developed Graves disease 13 years after the diagnosis of ICL. None of these patients received corticosteroid treatment during or immediately before diagnosed with ICL. Foxp3 expression, activation markers, and CD8+ T lymphocyte counts were not different from the rest of the ICL cohort.
Autoimmune diseases in the idiopathic CD4 lymphocytopenia patients
| Autoimmune disease . | No. . |
|---|---|
| Systemic lupus erythematosus | 3 |
| Antiphospholipid antibody syndrome | 2* |
| Graves disease | 1 |
| Autoimmune hemolytic anemia | 1 |
| Ulcerative colitis | 1 |
| Psoriasis | 1* |
| Vitiligo | 1 |
| Autoimmune thyroiditis | 1* |
| Autoimmune disease . | No. . |
|---|---|
| Systemic lupus erythematosus | 3 |
| Antiphospholipid antibody syndrome | 2* |
| Graves disease | 1 |
| Autoimmune hemolytic anemia | 1 |
| Ulcerative colitis | 1 |
| Psoriasis | 1* |
| Vitiligo | 1 |
| Autoimmune thyroiditis | 1* |
One patient had antiphospholipid antibody syndrome, psoriasis, and autoimmune thyroiditis.
The temporal relationship of autoimmune disease and ICL and the exact nature of correlation of the 2 diagnoses could not adequately addressed and investigated from this study. Furthermore, autoimmunity could not be used as an endpoint because of the variability of autoimmune manifestations (APS, SLE, vitiligo, Graves disease), ambiguity about the exact time of diagnosis in relation to diagnosis of ICL, and the presence of multiple autoimmune phenomena in a single patient (patient 2).
Discussion
ICL is a heterogeneous condition diagnosed typically in middle age, usually after an opportunistic infection, although it can also be an incidental laboratory finding. In a few patients in our study, lymphocytopenia in routine complete blood counts had been largely overlooked for several months or years before eventually ICL was diagnosed resulting from an opportunistic infection. This raises the possibility that CD4 T lymphocytopenia existed long before an opportunistic infection occurred in those patients. Data obtained from blood banks confirm the rare existence of a small population of otherwise healthy persons with low CD4 T-cell counts (0.25%-0.5% of blood donors),20,21 although it is not clear if this represented a transient or persistent low count.
The spectrum of opportunistic infections in ICL seems to overlap with that found in HIV-positive patients with similar CD4 T-cell counts.22 Cryptococcosis and nontuberculous mycobacterial infections were the most frequent in our ICL cohort, but the effects of referral bias are unknown. Certainly, appearance of these 2 infections in an HIV-negative patient should lead to an investigation for the possibility of ICL. Manifestations of HPV infection and of dermatomal zoster were common in our patients, and the possibility of this diagnosis should be considered, especially in an HIV-negative patient with disseminated and recalcitrant condylomata acuminata and warts or multidermatotomal VZV infection. Although ICL patients are susceptible to PCP and several cases have been previously described,23,24 only one patient presented with PCP in our population. Investigation toward alternative diagnoses at disease presentation should always include lymphoproliferative diseases or lymphomas and other forms of immunodeficiency, such as common variable immunodeficiency.
There appear to be at least 2 subtypes of ICL in terms of the presence or absence of CD8 T lymphocytopenia. This observation precludes the use of the CD4/CD8 ratio for diagnostic purposes in ICL and supports that it is a heterogeneous syndrome that could be further accompanied by B-cell and/or NK-cell lymphocytopenia.
The CD4 T-cell counts in our patients remained less than 300/mm3 for several years, demonstrating absence of progression of lymphocytopenia over time with only one-fifth of the patients resolving their lymphocytopenia within 3 years of diagnosis. As a result, it is reasonable to consider following ICL patients more closely during the first 3 years after diagnosis, not only because some of them are in danger of serious infections but also because some may return to normal CD4 T-cell counts, allowing discontinuation of any prophylaxis if initially given. Although the need for PCP or MAC prophylaxis of ICL patients could not be readily addressed in this cohort, only one episode of PCP and 2 of MAC were diagnosed during 164 patient-years of follow-up. Prophylaxis should be considered for a subset of ICL patients with the worst prognosis, such as those with low CD8 counts or patients presenting with an “AIDS defining condition.” It remains unclear which opportunistic infection should be targeted by such prophylaxis or what CD4 count might prompt it, although the HIV approach might be appropriate.
Although the clinical follow-up of patients with ICL was dominated by HPV, dermatomal VZV, and mucosal candidiasis, more serious and potentially lethal subsequent opportunistic infections did occur. The fact that most of these infections occurred within 2 years of ICL identification underlines the necessity of close follow-up of these patients early in diagnosis. Moreover, a second “AIDS defining condition” during follow-up heralds an ominous prognosis because 3 of 5 such patients eventually died of this complication. An exception to this rule may be patients in whom ICL was not diagnosed in the context of some opportunistic infection. These patients may represent a disease subset with a more benign course as all 7 remained alive and well during a long follow-up period. Low CD8 T-cell counts at diagnosis represent a subset of ICL with a worse prognosis and increased risk for a serious opportunistic infection or death. Because of the small number of deaths and limited follow-up for several of the ICL patients, only simple, univariate analyses for association were considered. A more robust statistical approach with multivariate analysis and taking into account the time-varying nature of many of these prognostic factors could only be done in a study with longer, more consistent follow-up, and ideally an even larger group of subjects. This is an important consideration for future studies of these patients.
In addition to opportunistic infections, autoimmune diseases were common in this cohort as has been described in animal models of lymphocytopenia.25 This break of tolerance could be related to the increased T-cell turnover and/or a dysregulated immune response to a specific pathogen. The high frequency of CD4 T cells expressing Foxp3 argues against the lack of regulatory T cells as the culprit, although these cells could represent activated T cells with only weak suppressive function.26 Functional studies looking at their suppressive potential will be necessary to investigate this further.
In all ICL patients in our cohort, increased CD4 T-cell activation and turnover were observed, as indicated by increased HLA-DR, Ki-67 expression, and ex vivo BrdU labeling. Prior reports of increased apoptosis in CD4 T cells in ICL may also reflect increased turnover.27,28 Loss of naive T cells, decreased CD127 (IL-7 receptor) expression, together with a report of increased serum IL-7,11 may reflect an inefficient homeostatic response to a low circulating pool of CD4 T cells that was the result of a transient lymphocytopenia-inducing insult, such as a viral infection. Alternatively, a pathogen-specific immune response of CD4 T cells may be continuously driving CD4 T-cell activation and turnover, leading to a stable depletion of the CD4 T-cell reservoir. Either way, the mechanisms maintaining normal T-cell homeostasis are severely perturbed in these patients. Serum antibody to CD4 T cells has been reported in some patients with ICL, although the functional consequence of this antibody is unknown.29 Despite striking similarities, there are significant differences between the immunologic profile of ICL and HIV-infected patients. In HIV, increased activation and turnover, loss of naive cells, and decreased CD127 expression occur in both CD4 and CD8 T cells.15 Untreated HIV-positive patients have progressive depletion of their T-cell reservoir, which highlights the effect of direct and indirect HIV pathogenicity that leads to continuous losses of an already depleted CD4 T-cell pool.30
In conclusion, ICL is a heterogeneous yet distinctive condition that is quite different clinically and immunologically from infection with HIV. Experimental cytokine therapies, such as IL-731 or IL-2,32-34 that could improve the survival and lead to CD4 (and CD8) T-cell expansions could be contemplated for future studies, but the risk-to-benefit ratio should be carefully considered. Patients at risk for progression but without active opportunistic infections could be potential candidates for such experimental approaches.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and clinic staff who participated in this study.
This work was supported in part with federal funds from the National Cancer Institute, National Institutes of Health (contract N01-CO-12400). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
National Institutes of Health
Authorship
Contribution: D.I.Z. analyzed and interpreted data, performed statistical analysis, and drafted the manuscript; J.F. designed research, performed research, collected data, and drafted the manuscript; J.E.B. analyzed and interpreted data and helped draft the manuscript; P.A.S. performed statistical analysis and drafted the manuscript; D.C. and J.A.M. performed research and collected data; M.W.B., J.W.A., and S.J.K. performed research and analyzed data; M.A.P., J.A.K., R.T.D., H.C.L., and H.M. designed and performed research and helped draft the manuscript; I.S. analyzed and interpreted the data and helped draft the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Irini Sereti, 9000 Rockville Pike, Building 10, Room 11C103, Bethesda, MD 20892; e-mail: isereti@mail.nih.gov; or Dimitrios I. Zonios, Spiliadou 6-8, 48100, Preveza, Greece; e-mail: dzonios@yahoo.com.