Abstract
Cell-cell contact–dependent mechanisms that modulate proliferation and/or differentiation in the context of hematopoiesis include mechanisms characteristic of the interactions between members of the Notch family of signal transduction molecules and their ligands. Whereas Notch family members and their ligands clearly modulate T lymphopoietic decisions, evidence for their participation in modulating myelopoiesis is much less clear, and roles for posttranslational control of Notch-dependent signal transduction in myelopoiesis are unexplored. We report here that a myeloproliferative phenotype in FX−/− mice, which are conditionally deficient in cellular fucosylation, is consequent to loss of Notch-dependent signal transduction on myeloid progenitor cells. In the context of a wild-type fucosylation phenotype, we find that the Notch ligands suppress myeloid differentiation of progenitor cells and enhance expression of Notch target genes. By contrast, fucosylation-deficient myeloid progenitors are insensitive to the suppressive effects of Notch ligands on myelopoiesis, do not transcribe Notch1 target genes when cocultured with Notch ligands, and have lost the wild-type Notch ligand-binding phenotype. Considered together, these observations indicate that Notch-dependent signaling controls myelopoiesis in vivo and in vitro and identifies a requirement for Notch fucosylation in the expression of Notch ligand binding activity and Notch signaling efficiency in myeloid progenitors.
Introduction
The number of circulating neutrophils in mammals is maintained within a rather narrow range in health and increases dramatically in the context of inflammation and other pathogenic circumstances.1-3 According to one proposed hierarchical scheme for hematopoiesis, hematopoietic multipotent progenitors differentiate to give rise to the earliest common myeloid progenitors (CMPs) and common lymphoid progenitors (CLPs).4,5 Granulocyte and macrophage progenitors (GMPs) derived from CMPs differentiate into mature macrophages/monocytes and granulocytes through myelopoiesis. Instructive commitment of hematopoietic stem cells (HSCs) to a myeloid fate, and terminal differentiation of myeloid progenitors requires growth and differentiation factors and intimate interaction with the marrow stroma.6,7 These types of extracellular cues control expression of transcription factors, such as C/EBPα and PU.1, which are essential for myeloid development.6,8-10
Adhesion of HSCs and progenitors to the extracellular matrix and stromal cells within the bone marrow contributes to stem cell renewal, myeloid lineage commitment, differentiation, and survival.11-13 Such interactions are dependent in part on a variety of cell adhesion molecules (CAMs) expressed on HSCs and progenitors. These CAMs include members of the integrin family and the selectin family. Notch family members and their ligands are also implicated in the control of HSC biology by influencing the numbers of osteoblastic cells that are essential components of the bone marrow niche.14
Four Notch receptors in humans and mice are assigned important roles in cell-fate specification in hematopoietic progenitors.15,16 For example, activated Notch1 is observed to enhance self-renewal of HSCs.17,18 By contrast, in lymphopoiesis, Notch1 is essential for T-cell versus B-cell fate specification and T-cell development.16,19-21 However, studies exploring the role of Notch signaling in myelopoiesis are not fully informative, and whether or how Notch receptors contribute to myelopoiesis thus remains unclear and controversial. Some in vitro studies indicate that Notch1 activation inhibits differentiation of myeloid precursors,22-26 whereas others indicate that Notch1 activation promotes myeloid differentiation.27,28 There are few in vivo studies suggesting that Notch signaling does indeed contribute to the control of myelopoiesis. For example, bone marrow cells expressing the constitutively active Notch1 intracellular domain exhibit preferential lymphoid over myeloid reconstitution, accompanied by increased lymphoid precursors and inhibition of myeloid differentiation.18 The embryonic lethal phenotype of mice homozygously deficient in Notch1 or Notch2 has precluded the use of these mice to study the role of Notch1 or Notch2 in myelopoiesis.29-31 Conditional inactivation of Notch1 in the bone marrow of mice is not accompanied by aberrances in dendritic and Langerhans cells, although the consequences of this maneuver on myelopoiesis have not been reported.32 Taken together, the available experimental evidence thus suggests that Notch signaling may modulate myelopoeisis, but definitive in vivo, physiologic evidence to support this hypothesis remains to be developed.
Notch receptors and their ligands are single-transmembrane proteins characterized by extracellular domains that contain numerous tandem epidermal growth factor (EGF)–like repeats. Some of these EGF repeats are modified by fucose linked to specific serine or threonine residues.33,34 O-fucose moieties are attached to the EGF domains in the endoplasmic reticulum, by protein O-fucosyltransferase 1 (pofut1). O-fucose on EGF repeats can be further elongated by members of the Fringe family of fucose-specific β1,3N-acetylglucosaminyltransferases.35-37 O-fucosylation can strongly modulate Notch signaling in mammalian cell lines and in Drosophila.35,38-41 Fringe-mediated extension of O-fucose has been shown to differentially influence Notch signaling in a cell type–specific manner by inhibiting Jagged1-mediated Notch signaling and potentiating Delta-mediated Notch signaling.35,39,42,43 However, roles for these modifications in controlling Notch signaling in hematopoiesis are not yet understood.
We have previously reported an extreme neutrophilia in the FX−/− mouse (30 × 109/L in FX−/− vs 1.6 × 109/L in wild-type (WT) mice), which is conditionally deficient in guanosine diphosphate (GDP)-fucose, the donor substrate for Pofut1 and other fucosyltransferases.44 The FX−/− mouse is deficient in the FX protein that converts GDP-mannose to GDP-fucose in the de novo fucose synthesis pathway.45 The FX−/− mouse exhibits shortened survival of as yet uncertain cause, general deficiency of glycan fucosylation, and thymic hypoplasia (Y.M. and J.B.L., unpublished data). Deficiency of glycan fucosylation and the peripheral neutrophilia in the FX−/− mouse is reversed by exogenous fucose supplementation, through a salvage pathway for GDP-fucose synthesis. Fucosylation-dependent neutrophilia in the FX−/− mouse is accompanied by marrow myeloproliferation, characterized by an increased myeloid to erythroid ratio and increased numbers of granulocyte-macrophage colony-stimulating factor. These observations suggested that fucosylation deficiency in the FX−/− mouse is accompanied by the loss of control mechanisms that account for homeostasis of marrow myelopoiesis. Given evidence that Notch signaling requires O-fucosylation of Notch, and because Notch and its receptors are expressed on hematopoietic stem cells and marrow stromal cells and are involved in multiple stage of hematopoiesis, we sought to determine whether, in the FX−/− mouse, loss of fucosylation on Notch accounts for some or all of the myeloproliferation. Using bone marrow transplant approaches, we find that FX−/− myeloid progenitors maintain a myeloproliferative phenotype in a WT stromal environment. To define the molecular basis for this progenitor-autonomous myeloproliferation, we examined myeloid progenitors in Notch ligand–dependent in vitro assays for myelopoiesis. These studies disclose that Notch suppresses myelopoiesis in a fucosylation-dependent, Notch ligand–dependent manner. We also observed that control of myelopoiesis in these contexts is accompanied by fucosylation-dependent modulation of the strength of Notch signal transduction in myeloid progenitors and correlates with fucosylation-dependent binding of Notch ligands to myeloid progenitors. Considered together, these observations indicate that Notch signaling tonically suppresses myelopoiesis, and indicate that O-fucosylation modulates these Notch-dependent processes by controlling the quality of the interactions between Notch and its ligands.
Methods
The animal research related to this manuscript was approved by Case Western Reserve University Institutional Animal Care and Use Committee.
Animals
Mice used include 12- to 18-week-old WT and FX−/− mice reared on fucose-supplemented chow since weaning.46 In experiments where fucose-deficient FX−/− mice were used, mice were reared on a fucose-supplemented diet (0.25% fucose) until 12 weeks, and then on standard chow for 4 weeks before use.
Purification of CMPs and LSKs
Total bone marrow-nucleated cells obtained from 2 femurs and 2 tibias were stained with a cocktail of biotinylated lineage antibodies. Lineage-positive cells were depleted with rat antimouse IgG-magnetic beads (Miltenyi Biotec, Auburn, CA). The cells were further stained with streptavidin-APC-Cy7, FITC-antiSca-1, and APC-anti-c-kit to obtain LSK populations (Lin−Sca-1+c-kit+). When isolating CMPs (Lin−c-kit+Sca-1−IL7R−CD34+FcγRIIlow), biotinylated anti-Sca-1 and anti-IL7 receptor were included in the antibody cocktails, and cells were stained with FITC-anti-CD34, APC-anti-c-kit, and phycoerythrin (PE)–anti-FcγRII. Four-color analysis was performed on a FACSAria (BD Biosciences, San Jose, CA).
Differentiation of CMP in vitro and OP9 coculture studies
OP9 cells transduced with the Ret10 vector (control), or with each of the 5 Notch ligands were constructed as described.47,48 CMP cells from WT and FX−/− mice were cultured for 11 to 14 days on OP9-control and OP9-Notch ligand–expressing cells in the presence of rmSCF (50 ng/mL), rmIL-3 (5 ng/mL), rmFlt3L (10 ng/mL), and rmGM-CSF (2.5 ng/mL). Cells were transferred to fresh OP9 cells every 5 to 7 days. The number of cells was enumerated during cell transfer and at the end of culture. The extent of myelopoietic differentiation was determined by anti-Gr-1 followed by fluorescence-activated cell sorting (FACS) analysis.
The γ-secretase inhibitor (L-685,458; Sigma-Aldrich, St Louis, MO) was resuspended in dimethyl sulfoxide and used at a concentration of 10 μM.49 The same volume of dimethyl sulfoxide was added to control culture.
Bone marrow repopulation assays
Bone marrow transplant recipient mice (Ly5.1) were lethally irradiated (9.5 Gy), then tail vein-injected with donor-derived bone marrow cells (Ly5.2; 1.8 × 106 total/each mouse). Mice were analyzed 1 to 6 months after transplantation. Three independent groups of transplanted mice were analyzed separately, and the results were pooled.
Binding of recombinant mouse Notch ligand chimera with CMPs
Chimera of the extracellular domain of the Notch ligands (Dll1, 3, and 4) fused with human IgG Fc were constructed as described.50 Chimeras were prepared from 293 T cells transfected with hIgG-Dll1ext, Dll4ext, Dll3ext, or the vector, and quantified by ELISA. Approximately 105 freshly isolated CMPs were cultured on OP9 (rmSCF 10 ng/mL) for 60 hours, followed by incubation with Notch ligand chimera in Hank balanced salt solution supplemented with Ca2+ and Mg2+ for 30 minutes at room temperature, and analyzed by FACS using PE-anti–human IgG Fc.
Peripheral blood and bone marrow analysis
After transplantation, the proportion of donor-derived myeloid and lymphoid cells was analyzed by FITC-anti-Ly5, PE-anti-Gr-1, APC-anti-B220, and PE-Cy7-anti-CD3ϵ. For cell-cycle analysis, 1 month after transplantation, mice were injected with bromodeoxyuridine (BrdU; 1 mg/6 g of mouse weight) and maintained with BrdU-containing water (1 mg/mL, Sigma-Aldrich) for 3 days before analysis. Bone marrow-nucleated cells were stained with FITC-anti-BrdU and 7-amino-actinomycin D (BD Biosciences), PE-anti-CD34, and APC-anti-c-kit after lineage depletion.
For imaging capture, we used Spot CCD camera (Diagnostic Instruments. Sterling Heights, MI) with Spot Basic Imaging manager (version 3.5.4) through Leica DMRB microscope (Wetzlar, Germany), using a 40×/1.0 oil objective.
Quantitative RT-PCR analysis
Total RNA was prepared from OP9-control and OP9-Dll1 culture cells, or freshly isolated LSK and CMP cells. RNA digested with DNAse I was used for reverse transcription. Primer sequences used for quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis are available on request.
Statistical analysis
Data are presented as means plus or minus SD, unless otherwise stated. Statistical significance was assessed by Student t test.
Results
FX−/− mice exhibit a myeloproliferative phenotype
We have previously observed that FX−/− mice develop a peripheral neutrophilia and marrow myeloproliferation that is reversible on restoration of glycan fucosylation through the salvage pathway for GDP-fucose synthesis.
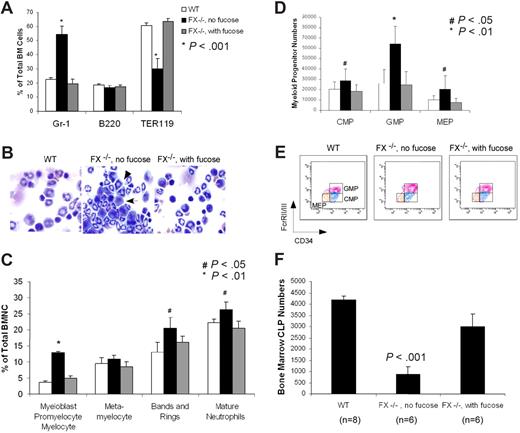
To define the cellular and molecular basis for this phenotype, we sought to comprehensively characterize the myeloid lineage cells in these mice. Flow cytometric analysis and cytologic examination confirmed an increase of nearly 2-fold in Gr-1–positive granulocytes and approximately 40% decrease of erythroid cells in the marrow (Figure 1A,B). Differential counts on cytospins of marrow cells disclosed that all myeloid progenitors were present in increased numbers (Figure 1C). Further examination revealed a slight increase in cells within the CMP and MEP compartments and a profound increase in cells in the GMP compartment (Figure 1D,E). However, lymphoid progenitors (CLPs) were significantly decreased (20% of WT; Figure 1F), suggesting a skewed myeloid progenitor populations in the marrow of these mice. By contrast, FX−/− mice reared on a fucose-supplemented diet did not exhibit any evidence of marrow myeloid hyperplasia, indicating that aberrance in the numbers of cells in these hematopoietic compartments is fucosylation-dependent. Examination of other cell lineages revealed no abnormalities in the numbers of marrow megakaryocytes and platelets in the periphery, and the numbers of marrow LSK cells in the FX−/− mice were not different from WT marrows (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The FX−/− mice have a 2-fold increase in cellularity in the spleen, accounted for largely by increased numbers of Gr-1–positive cells. The absolute number of mature B cells and B precursors in the spleen was not changed. However, we observed a mild decrease in the number of B progenitors (pre-proB) and mature B cells in the marrow, of as yet uncertain significance. The peripheral lymph nodes are small and lymphopenic, accounted for by absence of L-selectin ligand activity on peripheral node high endothelial venules (B.P. and J.B.L., unpublished data) as also observed in Fuc-TIV−/−/Fuc-TVII−/− mice.51
FX−/− mice develop a myeloproliferative disorder. (A) FACS analysis of the percentage of granulocytes (Gr-1+), B lymphocytes (B220+), and erythrocytes (TER119+) in the bone marrow in 3- to 4-month-old control mice (WT; n = 8), FX−/− mice reared on fucose-supplemented chow until 12 weeks of age, and then on standard chow for at least 4 weeks (n = 8; FX−/−, no fucose), and FX−/− mice reared on fucose-supplemented chow until use (n = 8; FX−/−, with fucose). (B) May-Grünwald-Giemsa–stained cytospins of marrow cells showing increased segmented neutrophils ( ) and myeloid progenitor cells (
) and myeloid progenitor cells ( ) in the bone marrow of FX−/− mice (no fucose). (C) Differential blood counts on May-Grünwald-Giemsa–stained cytospins of marrow cells showing percentage of myeloid progenitor cells at various developmental stage and mature neutrophils (WT, n = 8; FX−/−, no fucose, n = 9; FX−/−, with fucose, n = 9). (D,E) FACS analysis of bone marrow myeloid progenitors (CMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRIIlow; GMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRII+; MEP: Lin−c-kit+Sca-1−IL7R−CD34lowFcγRIIlow). (F) FACS analysis of CLP compartment (Lin−c-kitlowSca-1lowIL7R+). Bar graphs represent the average total number of CLP cells (± SD) from 2 tibias and 2 femurs of each mouse.
) in the bone marrow of FX−/− mice (no fucose). (C) Differential blood counts on May-Grünwald-Giemsa–stained cytospins of marrow cells showing percentage of myeloid progenitor cells at various developmental stage and mature neutrophils (WT, n = 8; FX−/−, no fucose, n = 9; FX−/−, with fucose, n = 9). (D,E) FACS analysis of bone marrow myeloid progenitors (CMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRIIlow; GMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRII+; MEP: Lin−c-kit+Sca-1−IL7R−CD34lowFcγRIIlow). (F) FACS analysis of CLP compartment (Lin−c-kitlowSca-1lowIL7R+). Bar graphs represent the average total number of CLP cells (± SD) from 2 tibias and 2 femurs of each mouse.
FX−/− mice develop a myeloproliferative disorder. (A) FACS analysis of the percentage of granulocytes (Gr-1+), B lymphocytes (B220+), and erythrocytes (TER119+) in the bone marrow in 3- to 4-month-old control mice (WT; n = 8), FX−/− mice reared on fucose-supplemented chow until 12 weeks of age, and then on standard chow for at least 4 weeks (n = 8; FX−/−, no fucose), and FX−/− mice reared on fucose-supplemented chow until use (n = 8; FX−/−, with fucose). (B) May-Grünwald-Giemsa–stained cytospins of marrow cells showing increased segmented neutrophils ( ) and myeloid progenitor cells (
) and myeloid progenitor cells ( ) in the bone marrow of FX−/− mice (no fucose). (C) Differential blood counts on May-Grünwald-Giemsa–stained cytospins of marrow cells showing percentage of myeloid progenitor cells at various developmental stage and mature neutrophils (WT, n = 8; FX−/−, no fucose, n = 9; FX−/−, with fucose, n = 9). (D,E) FACS analysis of bone marrow myeloid progenitors (CMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRIIlow; GMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRII+; MEP: Lin−c-kit+Sca-1−IL7R−CD34lowFcγRIIlow). (F) FACS analysis of CLP compartment (Lin−c-kitlowSca-1lowIL7R+). Bar graphs represent the average total number of CLP cells (± SD) from 2 tibias and 2 femurs of each mouse.
) in the bone marrow of FX−/− mice (no fucose). (C) Differential blood counts on May-Grünwald-Giemsa–stained cytospins of marrow cells showing percentage of myeloid progenitor cells at various developmental stage and mature neutrophils (WT, n = 8; FX−/−, no fucose, n = 9; FX−/−, with fucose, n = 9). (D,E) FACS analysis of bone marrow myeloid progenitors (CMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRIIlow; GMP: Lin−c-kit+Sca-1−IL7R−CD34+FcγRII+; MEP: Lin−c-kit+Sca-1−IL7R−CD34lowFcγRIIlow). (F) FACS analysis of CLP compartment (Lin−c-kitlowSca-1lowIL7R+). Bar graphs represent the average total number of CLP cells (± SD) from 2 tibias and 2 femurs of each mouse.
Myeloproliferation in the FX−/− mice is both progenitor cell–autonomous and marrow environment–dependent
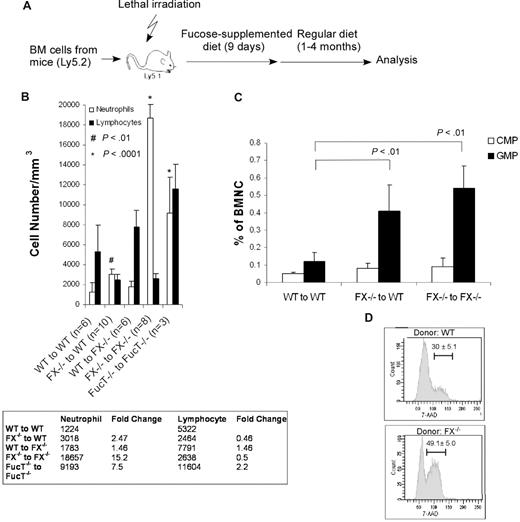
To determine whether the myeloproliferation observed in FX−/− mice is marrow progenitor-autonomous or stromal environment-dependent, or a combination of the 2, we performed marrow reconstitution experiments using WT or FX−/− marrow cells as donors and lethally irradiated FX−/− or WT mice as recipients (Figure 2A). To ensure equal subpopulation compartments that serve as donors and equal homing of donor cells to the recipient marrow, FX−/− mice, when used as donors, were maintained on fucose-supplemented diet before use. After transplantation, all mice were maintained on fucose-supplemented diet for 7 to 9 days, and then on standard chow until analysis (Figure 2A). After transplantation, peripheral blood was collected and analyzed to define neutrophil reconstitution. Marrow CMP and GMP cells were enumerated to define the degree to which these progenitors were expanding as a function of the genotype of the progenitors, and the genotype of the stromal environment. Cell-cycle analysis was also completed on cells within the combined CMP and GMP compartments (Lin−c-Kit+Scal-1−IL7R−CD34+).
In vivo reconstitution of myeloid and lymphoid lineages using marrow cells from WT or FX−/− mice injected into FX−/− and WT recipients, and marrow cells from Fuc-T−/− mice injected into Fuc-T−/− recipients. (A) Schematic representation of the transplantation protocol. (B,C) Analysis of peripheral neutrophils (Gr-1+) and lymphocytes (B220+ and CD3ϵ+), and bone marrow myeloid progenitor cells by FACS 4 months after transplantation. Data are means plus or minus SD. Student t test was performed to compare the neutrophil or lymphocyte numbers in each transplant setting with those of WT to WT. P > .5 unless otherwise indicated. (D) One month after transplantation, WT recipient mice receiving donor cells from WT or FX−/− mice were injected intraperitoneally with BrdU and fed with water containing BrdU for 3 days. The percentage of CMP and GMP cells (Lin−c-kit+Sca-1−IL7R−CD34+; numbers on graphs) in S-G2/M phase of the cell cycle was analyzed by anti-BrdU and 7-amino-actinomycin D.
In vivo reconstitution of myeloid and lymphoid lineages using marrow cells from WT or FX−/− mice injected into FX−/− and WT recipients, and marrow cells from Fuc-T−/− mice injected into Fuc-T−/− recipients. (A) Schematic representation of the transplantation protocol. (B,C) Analysis of peripheral neutrophils (Gr-1+) and lymphocytes (B220+ and CD3ϵ+), and bone marrow myeloid progenitor cells by FACS 4 months after transplantation. Data are means plus or minus SD. Student t test was performed to compare the neutrophil or lymphocyte numbers in each transplant setting with those of WT to WT. P > .5 unless otherwise indicated. (D) One month after transplantation, WT recipient mice receiving donor cells from WT or FX−/− mice were injected intraperitoneally with BrdU and fed with water containing BrdU for 3 days. The percentage of CMP and GMP cells (Lin−c-kit+Sca-1−IL7R−CD34+; numbers on graphs) in S-G2/M phase of the cell cycle was analyzed by anti-BrdU and 7-amino-actinomycin D.
Neutrophil counts were not elevated in FX−/− recipients receiving WT marrow cells but were elevated approximately 2.5-fold in WT recipients receiving FX−/− cells (Figure 2B). In the FX−/− donor/WT recipient mice, neutrophilia was accompanied by approximately 3-fold increases in the numbers of donor-derived GMPs, relative to WT donor/WT recipient mice (Figure 2C). The increased number of GMPs is accompanied by increased numbers of myeloid progenitors actively engaged in cell division, as 49% of the pooled GMPs and CMPs in the FX−/− donor/WT recipients were found in the S and G2/M phases, whereas 30% of these cells of the control WT donor/WT recipients were in the S and G2/M phases (Figure 2D). Considered together, these observations are consistent with the hypothesis that myeloproliferation in the FX−/− mouse is characterized by progenitor-autonomous accumulation of cells within the GMP compartment, in part because of active division of cells within the CMP and/or GMP compartments.
Peripheral neutrophils were increased to extreme levels in FX−/− recipients receiving FX−/− marrow cells (in alignment with our prior studies).46 Nonetheless, these mice maintained the same approximately 3-fold increase in GMPs that characterizes the FX−/− donor/WT recipients. The discrepancy between the mild neutrophilia in the FX−/− donor/WT recipients and the profound neutrophilia in FX−/− donor/ FX−/− recipients, in the face of similar GMP expansion, implies that the stromal environment also plays a fucosylation-dependent role in controlling the numbers of circulating neutrophils. Such stromal-dependent control may be in part the result of subtle reconstitution of the salvage pathway for GDP-fucose synthesis in the FX−/− donor cells, through fucose or fucosylated glycans elaborated by the WT stromal cells. Evidence for a contribution by this “cross-feeding” mechanism comes from the observation that marrow progenitors and peripheral neutrophils derived from transplanted FX−/− donor express low levels of fucosylated glycans when present in WT recipients (Figure S2).
In the FX−/− mice, the relative contribution of loss of selectin counter-receptor activity to their neutrophilia is estimated to be approximately 40% to 50%, on the basis of 2 sets of observations. First, the number of neutrophils in fucose-deficient FX−/− mice is roughly double the neutrophil counts in Fuc-TIV−/−/Fuc-TVII−/− (Fuc-T−/−) mice.46 Second, the neutrophil number in FX−/− recipients transplanted with FX−/− marrow is roughly double the neutrophil counts in Fuc-T−/− recipients transplanted with Fuc-T−/− marrow (Figure 2B) and is roughly double the neutrophil counts in WT recipients transplanted with Fuc-T−/− marrow, a circumstance where “cross-feeding”–dependent reconstitution of selectin counter-receptor activities cannot occur as it may with FX−/− donor cells in WT recipients (data not shown).
In vitro suppression of myelopoiesis by stromal cells expressing Notch ligands
Because previous reports suggest that Notch can inhibit myelopoiesis from hematopoietic progenitors,49 and O-fucosylation of Notch is implicated in the control of Notch signaling,35,38-41 we sought to determine whether the myeloproliferation in fucose-depleted FX−/− progenitors is a consequence of aberrant Notch signaling. We first determined whether Notch ligands do indeed modulate myelopoiesis of WT hematopoietic progenitors, by using an in vitro system for assessing Notch ligands in directing differentiation and proliferation of myeloid progenitors. In this system, OP9 mouse stromal cell lines expressing different Notch ligands or none (Ret10)47 are cocultured with freshly isolated CMP cells, under conditions that lead to myeloid differentiation of HSCs (Figure 3A).52 Myeloid differentiation is monitored by assessing the number of Gr-1–positive cells. WT CMPs cocultured for 11 to 14 days with OP9 cells that do not express a Notch ligand yield 34.2 × 104 Gr-1–positive cells. WT CMPs cocultured with OP9 cells bearing Dll3, a Notch ligand not implicated in hematopoiesis,53 yield a similar number of Gr-1–positive cells. By contrast, WT CMPs cocultured with OP9 cells expressing Dll1, Dll4, Jagged1, or Jagged2 yield Gr-1–positive cells that are reduced by nearly half relative to the numbers observed when CMPs are cocultured with OP9-control (Figure 3B). The Notch ligands Dll1 and Dll4 also imparted a significant expansion in the total number of cells derived from CMPs (68.6 × 104 and 60.7 × 104 total cells, relative to 39.2 × 104 in OP9-control). Thus, suppression of Gr-1–positive cells by these 2 ligands, as a fraction of the total number of cells (28.2% and 23.0%, respectively), implied that Dll1 and Dll4 promote the expansion of immature myeloid cells while also suppressing the formation of Gr-1–positive cells. These immature myeloid cells maintained a myeloid phenotype that is confirmed by the absence of lymphoid markers CD4 and CD8 (data not shown), and by cytospins showing immature myeloid progenitors develop from CMPs cultured with OP9-Jagged1 or OP9-Dll1 (Figure 3C).
Stromal cells expressing Notch ligand block myelopoiesis in vitro. (A) Schematic representation of the in vitro coculture of CMP cells with OP9 cells expressing Notch ligands with cytokine cocktails. (B) Day 14 culture of bone marrow–derived CMP cells, cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligands, were analyzed for Gr-1 expression. Data are mean values of at least 4 determinations. The bar graph represents the percentage of Gr-1+ and Gr-1− cells among total viable cells. (C) May-Grünwald-Giemsa–stained cytospins of day 14 cultured cells with OP9-control, OP9-Jagged1, and OP9-Dll1. (D) Short-term culture (60 hours) of CMP cells with SCF was analyzed for the percentages of GMP and MEP generated from CMP (numbers shown to right of graphs). Data are representative of at least 3 independent experiments. (E) Myelopoiesis transcription factors (PU.1 and C/EBPα) were differentially expressed in OP9-Dll1 versus OP9-control cultures. Results (means ± SD; n = 4) have been standardized for GAPDH levels and were expressed as percentage of expression relative to the levels detected in OP9-control (set at 1). aTotal number of cells present at the end of culture with 500 cells input grown on OP9 stromal cells with SCF (50 ng/mL), IL-3 (5 ng/mL), Flt3L (10 ng/mL), and GM-CSF (2.5 ng/mL). Data are means plus or minus SD of 6 independent experiments (*P < .05, #P < .01).
Stromal cells expressing Notch ligand block myelopoiesis in vitro. (A) Schematic representation of the in vitro coculture of CMP cells with OP9 cells expressing Notch ligands with cytokine cocktails. (B) Day 14 culture of bone marrow–derived CMP cells, cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligands, were analyzed for Gr-1 expression. Data are mean values of at least 4 determinations. The bar graph represents the percentage of Gr-1+ and Gr-1− cells among total viable cells. (C) May-Grünwald-Giemsa–stained cytospins of day 14 cultured cells with OP9-control, OP9-Jagged1, and OP9-Dll1. (D) Short-term culture (60 hours) of CMP cells with SCF was analyzed for the percentages of GMP and MEP generated from CMP (numbers shown to right of graphs). Data are representative of at least 3 independent experiments. (E) Myelopoiesis transcription factors (PU.1 and C/EBPα) were differentially expressed in OP9-Dll1 versus OP9-control cultures. Results (means ± SD; n = 4) have been standardized for GAPDH levels and were expressed as percentage of expression relative to the levels detected in OP9-control (set at 1). aTotal number of cells present at the end of culture with 500 cells input grown on OP9 stromal cells with SCF (50 ng/mL), IL-3 (5 ng/mL), Flt3L (10 ng/mL), and GM-CSF (2.5 ng/mL). Data are means plus or minus SD of 6 independent experiments (*P < .05, #P < .01).
To address whether the decreased number of mature granulocytes in these coculture experiments is the result of a block in the differentiation of the input CMPs, instead of a consequence of Notch ligand-dependent cell death, we used a short-term assay described by Akashi et al,5 in which marrow CMPs were cultured with OP9 cells in the presence of SCF, to specifically assess the formation of GMPs, the earliest progeny of the CMPs. After 60 hours of culture on OP9-control, CMPs (CD34+FcγRIIlow) gave rise to GMPs (CD34+FcγRIIhigh) and MEPs (CD34−FcγRIIlow). The percentage of GMPs derived from CMPs cultured on OP9-Dll1 (Figure 3D), as in coculture with OP9 cells bearing other ligands (Figure S3), however, decreased significantly compared with those on OP9-control. By contrast, CMPs increased in these experiments by up to 62%. These experiments also indicate that cell apoptosis does not change as a function of exposure to Notch ligands, in the mature granulocyte or in the immature myeloid progenitors (data not shown). Taken together, these results indicate that Notch ligand–expressing OP9 suppress conversion of CMP to GMP in vitro, in consonance with the decreased numbers and/or percentages of mature granulocytes generated from the CMPs.
To develop confirmatory molecular correlates for the cell surface marker and morphologic phenotypes reported herein, we examined the expression of 2 transcription factors, PU.1 and C/EBPα, known to be up-regulated during myeloid differentiation. After 40-hour coculture of CMPs with OP9-Dll1, the level of PU.1 in the cells was 55% of the level observed in CMPs cocultured with OP9-control. Similarly, CMPs had only 37% level of C/EBPα expression after incubation with OP9-Dll1 compared with OP9-control (Figure 3E). These observations indicate that the transcriptional programs associated with myeloid differentiation are down-regulated in association with exposure of CMPs to the Notch ligand Dll1 and thus support the conclusion that the blocked development of CMP to mature granulocytes is mediated through suppression of myeloid differentiation. The Notch-driven lymphoid transcriptional activation is not observed in the WT CMPs, presumably because these cells are committed to the myeloid lineage, but does occur when LSKs are cultured with OP9-Dll1 and OP9-Dll448 (Y.M. and J.B.L., unpublished data).
Suppression of myeloid differentiation by OP9 cells bearing Notch ligands is mediated via Notch activation
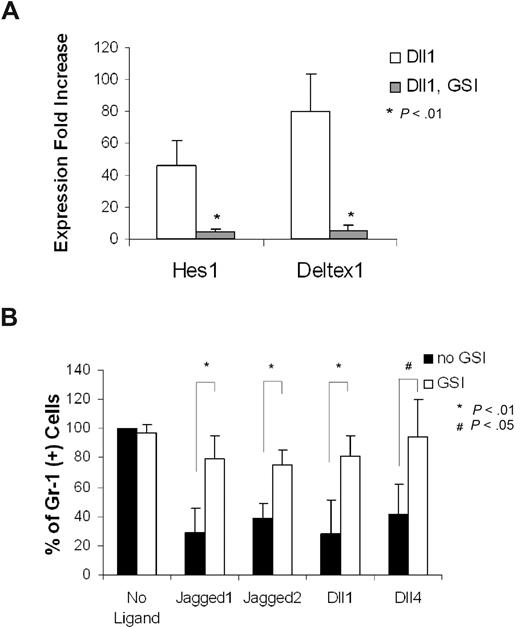
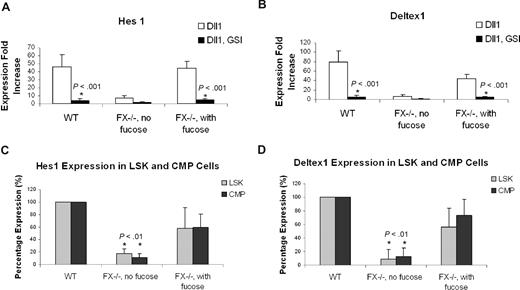
Notch ligand–dependent activation of the canonical Notch signaling requires γ-secretase activity.54 To confirm that Notch activation accounts for the suppression of granulopoiesis from CMPs cocultured with OP9 cells bearing Notch ligands, we asked if the gamma-secretase inhibitor (GSI) would reverse this process.49 In the presence of GSI, CMPs cocultured with OP9 bearing Notch ligands (Dll1, Dll4, Jagged1, and Jagged2) exhibited a myeloid differentiation phenotype essentially identical to CMPs cocultured with OP9-control (Figure 4B). Restoration of myeloid differentiation was accompanied by suppression of the Notch signaling targets Hes1 and Deltex1. To address the signaling in close temporal proximity to the initial Notch activation, we examined expression of Hes1 and Deltex1 4 days after WT CMPs were cocultured with OP9 cells. In CMPs cocultured with OP9-Dll1, expression of Hes1 was increased 46.1-fold, relative to Hes1 expression in CMPs cocultured with OP9-control. In the presence of GSI, the Dll1-dependent up-regulation of Hes1 was nearly completely suppressed. Similarly, a Dll1-dependent increase in Deltex1 expression was also suppressed by GSI (Figure 4A). These observations indicate that coculture of CMPs with Notch ligand–expressing stromal cells activates the canonical Notch pathway in the CMPs, and implicate this pathway in suppression of myeloid differentiation.
GSI reverses the Notch target gene expression and blocked myeloid differentiation induced by Notch ligand in OP9 culture. (A) Expression of Hes1 and Deltex1 was analyzed by quantitative RT-PCR from 4-day CMP culture on OP9-Dll1 cells relative to the levels detected in OP9-control (set at 1) in the absence (Dll1) or the presence of 10 μM GSI (Dll1, GSI). (B) Day 14 culture of bone marrow–derived CMP cells, cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligand, in the absence or presence of 10 μM GSI, was analyzed for Gr-1 expression. Data are mean values (± SD) of at least 4 determinations and expressed as percentage of Gr-1+ cells relative to that from WT cells grown on OP9-control (no GSI; set at 100%).
GSI reverses the Notch target gene expression and blocked myeloid differentiation induced by Notch ligand in OP9 culture. (A) Expression of Hes1 and Deltex1 was analyzed by quantitative RT-PCR from 4-day CMP culture on OP9-Dll1 cells relative to the levels detected in OP9-control (set at 1) in the absence (Dll1) or the presence of 10 μM GSI (Dll1, GSI). (B) Day 14 culture of bone marrow–derived CMP cells, cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligand, in the absence or presence of 10 μM GSI, was analyzed for Gr-1 expression. Data are mean values (± SD) of at least 4 determinations and expressed as percentage of Gr-1+ cells relative to that from WT cells grown on OP9-control (no GSI; set at 100%).
Suppression of myelopoiesis by Notch is fucosylation-dependent
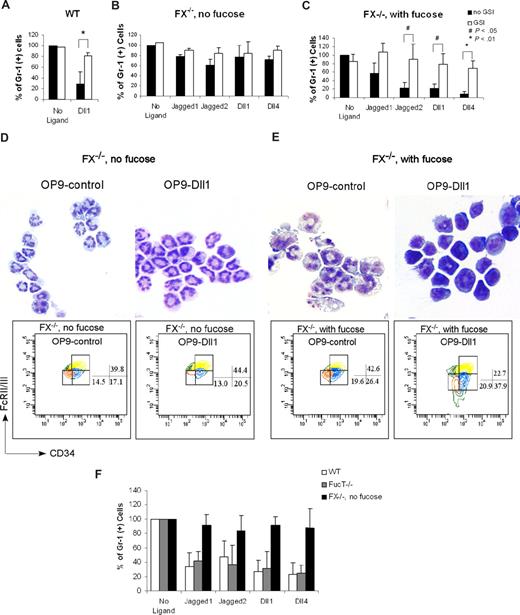
FX−/− mice reared on fucose-deficient diet develop peripheral neutrophilia and marrow myeloproliferation, coincident with a deficiency of leukocyte cell surface fucosylation. By contrast, FX−/− mice reared on a fucose-supplemented diet have normal white cell counts and myeloid progenitor subpopulations, and normal levels of leukocyte cell surface fucosylation.46 These observations, and the data reported above, combined with evidence that O-fucosylation of Notch is required for Notch signaling,39 suggest that myeloproliferation in fucose-deficient FX−/− mice is consequent to loss of normal Notch-dependent control of myelopoiesis. To examine this hypothesis, we assessed myeloid differentiation of CMPs from FX−/− mice as a function of fucosylation. CMPs obtained from FX−/− mice reared on a fucose-supplement diet and grown in the medium containing fucose (FX−/− with fucose) exhibited Notch ligand–dependent, GSI-reversible suppression of myeloid differentiation essentially identical to that observed with WT CMPs (Figure 5A,C). By contrast, CMPs isolated from fucose-depleted FX−/− mice (FX−/− without fucose) did not exhibit Notch ligand suppression of myelopoiesis (Figure 5B). Fucosylation-dependent suppression of myeloid differentiation in these experiments is paralleled by fucosylation-dependent modulation of Hes1 and Deltex1 expression (Figure 6A,B). In parallel, we observed that expression of Hes1 and Deltex1 was suppressed in marrow LSK and CMP cells isolated from fucose-depleted FX−/− mice, relative to its expression in WT marrow progenitors, or in progenitors from FX−/− mice with fucose supplementation (Figure 6C).
Notch pathway–induced myeloid development block is fucosylation dependent. Expression of Gr-1 from day 14 culture CMP cells derived from FX−/− mice maintained on standard chow (FX−/−, no fucose; B) or maintained on fucose-supplemented chow at least 4 weeks and with 1 mM fucose in the culture medium (C), was compared with WT CMP culture on OP9-control and OP9-Dll1 (A), in the absence or presence of 10 μM GSI. Data are mean values (± SD) of at least 4 determinations and expressed as percentages of Gr-1+ cells relative to that on OP9-control (no GSI; set at 100%). Cells from panels B and C cultured on OP9-control and OP9-Dll1 were examined by May-Grünwald-Giemsa staining and FACS analysis of CMP to GMP conversion (D,E). Numbers on plots are percentages of the indicated cell types. (F) Day 14 culture of marrow CMP cells derived from WT, FX−/− mice maintained on standard chow (FX−/−, no fucose), and mice with double deficiency for the 2 alpha1,3fucosyltransferases Fuc-TIV−/−Fuc-TVII−/− (Fuc-T−/−), cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligands, was analyzed for Gr-1 expression. Data are mean values (± SD) of at least 4 determinations and expressed as percentage of Gr-1+ cells relative to that from cells grown on OP9-control (set at 100%). Student t test was performed to compare the parameters obtained with GSI to those without GSI (A-C) or parameters obtained from Fuc-T−/− and FX−/− mice to those from WT mice (F).
Notch pathway–induced myeloid development block is fucosylation dependent. Expression of Gr-1 from day 14 culture CMP cells derived from FX−/− mice maintained on standard chow (FX−/−, no fucose; B) or maintained on fucose-supplemented chow at least 4 weeks and with 1 mM fucose in the culture medium (C), was compared with WT CMP culture on OP9-control and OP9-Dll1 (A), in the absence or presence of 10 μM GSI. Data are mean values (± SD) of at least 4 determinations and expressed as percentages of Gr-1+ cells relative to that on OP9-control (no GSI; set at 100%). Cells from panels B and C cultured on OP9-control and OP9-Dll1 were examined by May-Grünwald-Giemsa staining and FACS analysis of CMP to GMP conversion (D,E). Numbers on plots are percentages of the indicated cell types. (F) Day 14 culture of marrow CMP cells derived from WT, FX−/− mice maintained on standard chow (FX−/−, no fucose), and mice with double deficiency for the 2 alpha1,3fucosyltransferases Fuc-TIV−/−Fuc-TVII−/− (Fuc-T−/−), cocultured with OP9-control cells (no ligand) or OP9 cells expressing Notch ligands, was analyzed for Gr-1 expression. Data are mean values (± SD) of at least 4 determinations and expressed as percentage of Gr-1+ cells relative to that from cells grown on OP9-control (set at 100%). Student t test was performed to compare the parameters obtained with GSI to those without GSI (A-C) or parameters obtained from Fuc-T−/− and FX−/− mice to those from WT mice (F).
Expression of Notch target genes in FX−/− mice marrow cells in vitro and in vivo. Expression of Hes1 (A) and Deltex1 (B) from 4-day CMP (WT; FX−/−, no fucose; FX−/−, with fucose) cultured on OP9-Dll1 was compared with OP9-control cells (set at 1) in the absence or the presence of 10 μM GSI, expressed as fold increase. (C,D) Hes1 and Deltex1 expression was analyzed from freshly isolated LSK and CMP cells from WT, FX−/− mice maintained on standard chow (FX−/−, no fucose) and FX−/− mice maintained on fucose-supplement chow (FX−/−, with fucose). Data are means plus or minus SD of 4 determinations. Student t test was performed to compare the parameters obtained with GSI to those without GSI (A,B) or values obtained from FX−/− mice to those from WT mice (C,D).
Expression of Notch target genes in FX−/− mice marrow cells in vitro and in vivo. Expression of Hes1 (A) and Deltex1 (B) from 4-day CMP (WT; FX−/−, no fucose; FX−/−, with fucose) cultured on OP9-Dll1 was compared with OP9-control cells (set at 1) in the absence or the presence of 10 μM GSI, expressed as fold increase. (C,D) Hes1 and Deltex1 expression was analyzed from freshly isolated LSK and CMP cells from WT, FX−/− mice maintained on standard chow (FX−/−, no fucose) and FX−/− mice maintained on fucose-supplement chow (FX−/−, with fucose). Data are means plus or minus SD of 4 determinations. Student t test was performed to compare the parameters obtained with GSI to those without GSI (A,B) or values obtained from FX−/− mice to those from WT mice (C,D).
The requirement for fucosylation in Notch ligand–dependent suppression of myelopoiesis was further assessed by examining the generation of GMPs from CMPs. When CMPs obtained from FX−/− mice with fucose supplementation were cultured on OP9-Dll1 and other ligands, the percentage of GMPs decreased compared with that on OP9-control. This is essentially the same as observed in WT cells. However, when CMPs obtained from fucose-depleted FX−/− mice were cultured with OP9-Dll1 and OP9-control, we observed a similar percentage of GMPs derived from CMPs (Figures 5D,E and S3).
Fucosylation-dependent suppression of myeloid differentiation is not mediated through selectin ligand-dependent processes because we observe a WT GMP/CMP ratio (data not shown) and myeloid differentiation block of CMPs in Fuc-T−/− mice doubly deficient for the 2 alpha1,3-fucosyltransferases required for E- and P-selectin ligand activity51 (Figure 5F). Considered together, these observations are consistent with the conclusion that the fucosylation-dependent myeloproliferation in FX−/− mice is consequent to loss of control of myelopoiesis normally dictated by signal transduction engendered by Notch ligands in the marrow which act on Notch in early myeloid progenitors.
Fucosylation-dependent binding of Notch ligands to myeloid progenitors
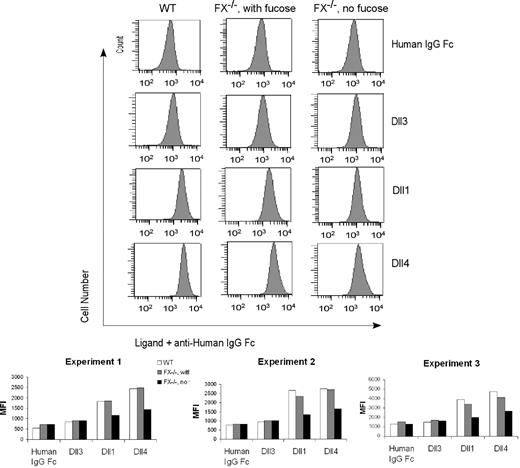
To begin to define the mechanism(s) accounting for loss of canonical Notch signaling events observed in fucosylation-deficient myeloid progenitors, we sought to determine whether fucosylation modulates binding of Notch ligands to the progenitors by probing CMPs using flow cytometry and recombinant Notch ligands. In one example of 3 similar experiments, we observed that WT CMP cells bound the recombinant Dll4 and Dll1 chimeras with mean fluorescence intensity (MFI) of 2442 and 1829, respectively, compared with a reduced background binding of a control IgG Fc chimera (MFI 538) or of a nonactivating Notch ligand Dll3 construct (MFI 852). CMP cells isolated from FX−/− mice with fucose supplementation exhibited a WT binding phenotype. By contrast, we observed an approximately 40% reduction in the binding of the Dll1 and Dll4 chimeras to CMP cells isolated from fucose-depleted FX−/− mice (Figure 7). Considered together, these observations are consistent with the conclusion that loss of fucosylation-dependent control of Notch signaling in fucose-deficient FX−/− progenitors is accounted for, in part, by loss of effective binding of Notch ligands to these cells, via mechanisms involving Notch O-fucosylation.
Fucosylation-dependent binding of Notch ligands to FX−/− mice myeloid progenitors. Flow cytometric analysis of binding of recombinant Notch ligands Dll1 (1.3 μg/mL), Dll4 (0.5 μg/mL), Dll3 (0.3 μg/mL), and control (human IgG1 Fc, 1 μg/mL) to CMP cells isolated from WT mice, from FX−/− mice reared in the presence of fucose, or from FX−/− mice reared in the absence of fucose. Chelation of Ca2+ with ethylenediaminetetraacetic acid abolished binding (data not shown). Data are the flow cytometric histogram of experiment 1 (top panel) and mean fluorescence intensity (MFI) of the binding of CMPs with control and Notch ligands in 3 independent experiments (bottom panel).
Fucosylation-dependent binding of Notch ligands to FX−/− mice myeloid progenitors. Flow cytometric analysis of binding of recombinant Notch ligands Dll1 (1.3 μg/mL), Dll4 (0.5 μg/mL), Dll3 (0.3 μg/mL), and control (human IgG1 Fc, 1 μg/mL) to CMP cells isolated from WT mice, from FX−/− mice reared in the presence of fucose, or from FX−/− mice reared in the absence of fucose. Chelation of Ca2+ with ethylenediaminetetraacetic acid abolished binding (data not shown). Data are the flow cytometric histogram of experiment 1 (top panel) and mean fluorescence intensity (MFI) of the binding of CMPs with control and Notch ligands in 3 independent experiments (bottom panel).
Discussion
In prior studies, we sought to identify novel biologic functions for fucose, a monosaccharide that posttranslationally modifies N-glycans, O-glycans, and some serines and threonines,44,55 by engineering a strain of mice with a conditional deficiency in the synthetic pathway for GDP-fucose, the substrate required for all known murine fucosyltransferases. These mice exhibit a myeloproliferative phenotype characterized by an extreme granulocytosis and marrow myeloid hyperplasia. Our studies imply that loss of fucosylation-dependent Notch signaling accounts for this fucosylation-dependent myeloproliferative phenotype.
Examination of the marrows in these mice in the fucosylation-deficient state discloses that the numbers of progenitors within the lymphoid lineage are suppressed, whereas the numbers of cells within the GMP compartment are expanded. Because the numbers of CMPs, the precursors to GMPs, remain essentially unchanged, these observations suggested the possibility that normal fucosylation processes are required for tonic control of the CMP to GMP differentiation process. Although some studies suggest that adhesion between selectins and their fucosylated counter-receptors can modulate hematopoietic progenitor cell growth, differentiation, or apoptosis,56 the considerations are not likely to be operative in the FX−/− mice because we observed a WT GMP/CMP ratio in Fuc-T−/− mice, which are deficient in all selectin ligand activity.51
In principle, the myeloproliferation could have been consequent to defects in the progenitor cells or in the stromal compartment, or a combination of both, and/or to mechanisms involving cells, molecules, and events external to the marrow. However, bone marrow transplantation experiments indicate that a significant component of the myeloproliferative phenotype tracks with the hematopoietic progenitors, and in vitro studies confirm that fucosylation deficiency confers a myeloproliferative phenotype on the progenitor compartment. However, the combination of an FX−/− stromal environment and FX−/− hematopoietic progenitors yields a more pronounced myeloproliferation than when the progenitor compartment alone is fucosylation-deficient. These observations suggest that the influences on myelopoiesis imparted by the stromal environment are also modulated by fucosylation. This could include stromal cell–borne Notch family members, or Notch ligands, which are themselves modified by O-fucose present on EGF repeats, and/or other proteins with fucose-modified EGF repeats.57-59 Thrombospondin repeats are also modified by O-fucose in some instances (properidin and thrombospondin1,60-62 and members of the ADAMTS superfamily of proteases63,64 ). These proteins are also candidates for contributing to fucosylation-dependent control of myelopoiesis by the stromal compartment.
Loss of influences that such stromal-borne molecules may normally impart in the context of fucosylation deficiency is not evident when WT progenitors differentiate within an FX−/− stromal environment. Although mechanisms to account for this observation remain to be defined, it is possible that FX−/− stromal cells acquire modest fucose or GDP-fucose from the WT progenitors, perhaps through gap junctions,65,66 or via acquisition of fucose from the WT plasma. These processes could restore GDP-fucose synthesis via the salvage pathway, with partial restoration of the fucosylation-dependent influences that stromal cells impart on progenitors, as apparently occurs with FX−/− progenitors embedded in a WT stromal environment (Figure S2). Future studies will be necessary to determine the extent to which these processes may diminish fucosylation-dependent, progenitor cell–autonomous or stromal cell–autonomous control of myelopoiesis.
The progenitor cell–autonomous myeloproliferation suggested the possibility that loss of fucosylation engendered a disruption in control of myelopoiesis normally mediated by contact between myeloid progenitors and the bone marrow stroma.6 Such cell-cell contact–dependent mechanisms could include those interactions between members of the Notch receptors and their ligands.17,67 A possible role for Notch and its ligands in the myeloproliferative phenotype of the FX−/− progenitors was suggested by prior observations linking protein O-fucosylation with Notch function.33,39,40 Although Notch receptors and their ligands clearly modulate T lymphopoiesis, evidence for a role for such interactions in modulating myelopoiesis is much less consistent. More recent studies indicate that the Notch ligand Dll1 suppresses the expression of GATA-2, a Notch target required for progenitor cell commitment to the myeloid cell fate,49 and lend support to the hypothesis that Notch receptors and their ligands exert significant control over myelopoietic differentiation and proliferation. Our studies provide additional clear support for this hypothesis, as we observed that the myeloproliferative phenotype in FX−/− mice is consequent to loss of Notch-dependent signal transduction on myeloid progenitor cells, in vivo and in vitro. Specifically, we find that the Notch ligands suppress myeloid differentiation of myeloid progenitors in the context of a WT fucosylation phenotype. Suppression is associated with robust activation of Notch target genes and strong binding of these Notch ligands to the progenitor cells.
Importantly, we observed that fucosylation-deficient myeloid progenitors are insensitive to the suppressive effects of Notch ligands on myeloid differentiation and do not transcribe Notch target genes. Considered together, these observations indicate that Notch-dependent signaling controls myelopoiesis and suggest the hypothesis that myeloid differentiation is the default lineage commitment program experienced by cells within the multipotent progenitor compartment in the absence of Notch signaling, whereas Notch activation drives lymphoid specification at the expense of myeloid specification.
Our observations also identify a requirement for Notch fucosylation in the expression of Notch ligand binding “activity” in myeloid progenitors. However, it remains to be determined if diminished binding accounts fully for the loss of signaling in these cells,42 or if loss of Notch ligand binding is consequent to diminished cell-surface Notch expression as reported in other contexts,41 and/or to other mechanisms.
We observe robust Notch ligand–dependent suppression of myeloid differentiation of CMPs, in contrast to de Pooter et al,49 who observed that Dll1 suppresses CMP differentiation to a modest extent only. This apparent discrepancy may be the result of differences in the type and concentrations of cytokines used in our coculture assay and theirs. Additional in vivo studies will be needed to resolve this apparent discrepancy and other questions about the role of Notch family members and their ligands in myelopoiesis.
In particular, the identity of the Notch receptor(s) and Notch ligand(s) that modulate myelopoiesis, in vivo, remains mysterious, as do the contributions of each such receptor/counter-receptor pair to different stages of myelopoiesis. Gene deletion approaches to address these issues and the general role of Notch family members and their ligands in myelopoiesis have not yet been fully informative. In mice with genetic deficiency of Notch receptors and ligands,31,68-72 or in mice where the Notch1 or the Notch2 locus has been inducibly deleted, the apparently normal myelopoietic phenotype may be consequent to functional redundancies of the Notch family members in myelopoiesis (see review by Maillard et al73 ). The absence, to date, of compelling in vivo studies that address the role of Notch family members and their ligands in myelopoiesis is thus reflective of the complexity and genetic redundancy of the Notch-Notch ligand family, contrasting with the strength of the in vitro evidence supporting this receptor-counter receptor axis in modulating myeloid differentiation, and calls for the application of additional in vivo approaches to address how Notch family members and their ligands contribute to homeostasis in the myeloid lineage.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Blood Foundation Research Grant and the Ohio Cancer Research Grant (L.Z.) and National Institutes of Health grant 1P01CA71932 (J.B.L.).
National Institutes of Health
Authorship
Contribution: L.Z., L.W.L., Q.Y., and C.S. performed experiments; L.Z. analyzed results and made the figures; B.P., Y.M., J.S., and S.C. contributed vital new reagents and analytical tools; L.Z. and J.B.L. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lan Zhou, Department of Pathology, Case Western Reserve University, 6527 Wolstein Research Building, 2103 Cornell Road, Cleveland, OH 44106; e-mail: lan.zhou@case.edu.