Abstract
Polymorphonuclear leukocyte (PMN)–derived microparticles display inhibitory properties on target cells as assessed in vitro; since PMNs contain abundant amounts of the endogenous anti-inflammatory protein annexin 1 (AnxA1), we tested here whether biologically active AnxA1 could be present in PMN-derived microparticles. PMN adhesion to human umbilical vein endothelial cell (HUVEC) monolayers led to the generation of microparticles that contained AnxA1, as detected by Western blotting, flow cytometry, and mass spectrometry analyses. Addition of these microparticles to recipient PMNs prior to flow over HUVEC monolayers significantly inhibited cell adhesion, an effect abrogated by a neutralizing anti-AnxA1 antibody, or an antibody raised against the AnxA1 receptor, that is termed lipoxin A4 receptor or ALX. Intravenous delivery of human PMN–derived microparticles markedly inhibited PMN recruitment to an air pouch inflamed with IL-1β. This anti-inflammatory effect was also dependent on endogenous AnxA1, since injection of microparticles produced from wild-type PMNs (bone marrow derived), but not from AnxA1-null PMNs, inhibited IL-1β–induced leukocyte trafficking. In conclusion, PMN-derived microparticles contain functionally active AnxA1 that confers them anti-inflammatory properties; generation of these microparticles in the microcirculation could promote inflammatory resolution by time-dependent dampening of cell recruitment.
Introduction
Microparticles were originally identified from activated platelets prior to appreciating their generation, upon activation, by several other cell types. Initial general wisdom was that microparticles were inert, and hence were cellular debris or bypass products of cell activation1 ; however, several recent studies have now described biologic functions for these small particles that might therefore have a potential for impacting on pathophysiological processes, including ability to transfer packed information and promoting cell-to-cell cross talk.1-3
Microparticles are heterogeneous in their nature, even when produced from a single cell source, varying both in terms of content and size. When generated from cells entering in apoptosis (for a review, see Distler et al1 ) or from activated leukocytes and platelets, microparticles are right-side-out oriented, expressing phosphatidylserine, and specific patterns of membrane proteins that have been used to identify their cellular origin4 ; for example, CD14, CD3, CD41a, and CD146 are markers for monocyte-, lymphocyte-, platelet-, and endothelial cell–derived microparticles, respectively.3 The marker of polymorphonuclear leukocyte (PMN)–derived microparticles is cell surface L-selectin (CD62L) and other proteins, including CD11b, whereas intracellular proteins detected in these microparticles include myeloperoxidase, elastase, and other proteolytic enzymes.3
The release of microparticles from activated cells can be triggered by different stimuli; in all the different cases, microparticle generation is the end point of an activation cascade requiring increases in intracellular calcium concentration, rearrangement of the cytoskeleton, and the budding of these vesicles from the plasma membrane.1
The present project stemmed from the notion that PMN-derived microparticles produce inhibitory effects when coincubated with human monocyte-derived macrophages for up to 24 hours.1,5 Because human PMNs contain high levels of the anti-inflammatory protein annexin 1 (AnxA16 ; representing between 2% and 4% of total cytosolic proteins7 ), we questioned whether presence of AnxA1 could underlie some of the inhibitory effects of PMN-derived microparticles. In fact, AnxA1 (37 kDa) is one of the effectors of endogenous anti-inflammation,8 hence it contributes to the complex series of mechanisms activated in the host to keep under control cell activation and trafficking in the resolution of inflammation.9
There is ample experimental evidence that exogenously added AnxA1, as well as the endogenous protein, would inhibit PMN chemotaxis, activation, and migration.10-13 Equally important, a large proportion of AnxA1 is contained in granules and vesicles,14,15 such that the protein is rapidly mobilized onto the PMN membrane compartment first, then PMN cell surface (and eventually released into the incubation medium) upon cell activation and adhesion.10,12,13 On this basis, we reasoned there was adequate rationale to test whether PMN-derived microparticles would contain AnxA1, and if so, whether this mediator would contribute, at least in part, to their anti-inflammatory/inhibitory properties.
Methods
Unless otherwise specified, materials were obtained from Sigma-Aldrich (Poole, United Kingdom). Human cells were prepared according to a protocol approved by the East London & the City Local Research Ethics Committee (no. 06/Q605/40; P/00/029 ELCHA, London, United Kingdom). Animal work was performed according to Home Office regulations (Guidance on the Operation of Animals, Scientific Procedures Act, 1986) and was approved by the Queen Mary University of London Ethics Committee (London, United Kingdom).
Human PMN microparticle preparation
Human PMN–derived microparticles were prepared as reported by Gasser et al.4 Briefly, 107 PMNs prepared by gradient separation10 were added to unstimulated human umbilical vein endothelial cell (HUVEC) monolayers in 6-well plates, routinely in the presence of 1 μM fMLP for 1 hour at 37°C. In some cases, platelet-activating factor (0.1 μM) was used, with no different results (data not shown). In another protocol, freshly prepared PMNs were not added to HUVECs, but rather kept in suspension for 1 hour at 37°C. In all cases, supernatants were collected and centrifuged at 4000g for 5 minutes prior to further ultracentrifugation at 100 000g for 1 hour. Microparticle pellets were washed once and resuspended in PBS.
AnxA1 detection in microparticles
Western blotting.
Microparticle extracts subjected to standard SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto PVDF membranes (Millipore, Watford, United Kingdom). These were incubated with mAb 1B (1 μg/mL) and HRP-conjugated goat anti–mouse IgG (Dako, Cambridge, United Kingdom). Proteins were detected using enhanced chemiluminescence (ECL) detection kit and visualized on Hyperfilm (GE Healthcare, Little Chalfont, United Kingdom).
Mass spectrometry.
Proteins were separated by SDS-PAGE and the resulting gel was stained with Coomassie blue dye: the 37-kDa band was excised and in-gel reduction, alkylation, and digestion with trypsin were performed prior to analysis.16 The mass spectral data were processed into peak lists and searched against the Swiss Prot17 and National Center for Biotechnology (NCBI) nonredundant database18 using MASCOT software (Matrix Science, London, United Kingdom).
FACS analysis.
Microparticles were stained with the following mAb at 10 μg/mL: anti-AnxA1 (clone 1B19 ); anti-CD62L (clone FMC46; Serotec, Oxford, United Kingdom); anti-CD62P (clone MCA796; Serotec); anti-CD14 (clone MCA1568; Serotec), or isotype control (MCA928 and MCA691; Serotec) for 1 hour at 4°C; a final staining with a rabbit anti–mouse IgG (STAR9B; Serotec) was conducted. In some cases FITC-labeled annexin V was added (10 μL of manufacturer's solution; Serotec) to monitor exposure of phosphatidylserine on the outer side of the microparticles. Fluorescence-activated cell sorting (FACS) analysis was conducted using a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA) using CellQuest software (Becton Dickinson). Beads (1-μm each; Becton Dickinson) were also run, in selected samples, to control for microparticles size.
In some experiments microparticles were washed once in PBS supplemented with EDTA (0.1 mM; 10 minutes) prior to immunostaining and FACS analysis.
Flow chamber assay
The assay was performed as recently reported.20 Microparticles were added to PMNs (8 minutes) prior to the actual flow experiment whereby 5 × 106 PMNs (in 1 mL) were flown over HUVECs activated with TNF (10 ng/mL; 4 hours); in either case, the total number of interacting cells was then quantified to determine the degree of cell capture, rolling, and adhesion.20 In some cases, PMNs or microparticles were preincubated with neutralizing antibodies (5 minutes at 37°C), using a mouse anti–human ALX (5 μg/mL; clone 6C7-3) or anti-AnxA1 (10 μg/mL; mAb 1B), respectively; clone MCA928 (10 μg/mL; Serotec) was used as isotype control.
Mouse PMN–derived microparticles
Murine PMNs were prepared from wild-type and AnxA1-null mice21 (backcrossed 10 times onto a Balb/c background) as described.22 Briefly, bone marrows were extracted by flushing the tibias and femurs of 7 mice per group. Following extraction, and a PBS wash, cells were extracted using a Percoll gradient.22 Microparticles were produced by adding 5 × 106 PMNs onto monolayers of murine endothelial cells (prepared by magnetic selection from wild-type mouse lung23 ) and stimulated with 10 μM fMLP for 1 hour at 37°C. Microparticles were harvested and isolated following the same procedure described for the human ones. For flow cytometry, mouse microparticles were stained for 1 hour at 4°C with rat antimouse CD62L (MEL-14 clone, at 5 μg/mL; generous gift of Dr N. Gozzard, UCB-Celltech, Slough, United Kingdom) or with FITC-labeled annexin V (Serotec). The remainder of the analysis was conducted as described for the human PMN microparticles.
Air pouch model for in vivo PMN migration
Air (2.5 mL) was injected subcutaneously on day 0 and day 3. On day 6, microparticles (at the reported doses) or human recombinant AnxA1 (20 μg mouse, equivalent to 0.54 nmol) were given intravenously in 200 μL volume, 5 minutes prior to the local injection of 10 ng mouse IL-1β.24 Air pouches were washed 4 hours later and migrated PMNs counted with light microscopy and stained with Gr1 (clone rb6-8c5; eBioscience, Wembley, United Kingdom) as described.21
Data handing and statistical analysis
In vitro experiments result from 4 independent experiments; in vivo PMN migration was performed in 5 mice per group. In all cases, data are reported as mean plus or minus SEM. Statistical differences between groups were determined by one-way ANOVA and, if significant, by Student Newman-Keuls test, taking a probability value P less than .05 as significant.
Results
Presence of AnxA1 in PMN-derived microparticles
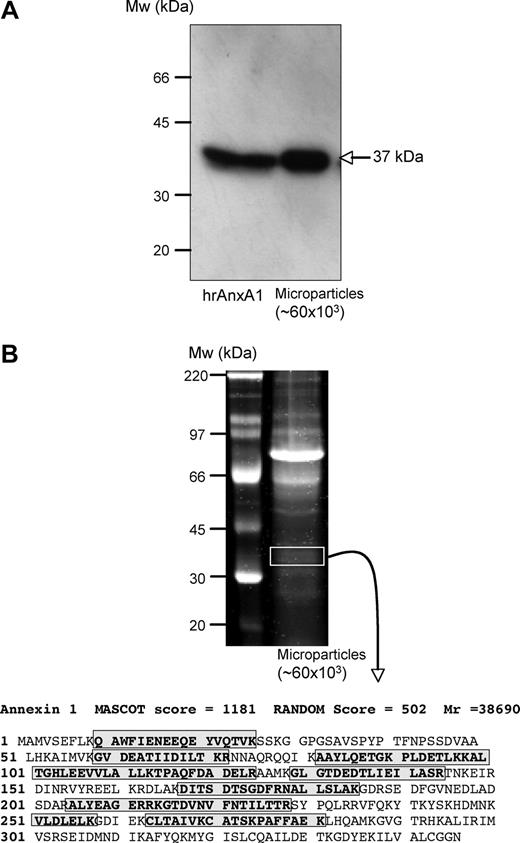
PMN-derived microparticles were prepared by promoting their adhesion to HUVECs, a procedure that provokes remarkable PMN activation and rapid mobilization of AnxA1.10 Western blotting analysis of microparticles recovered from the incubation medium revealed presence of an AnxA1-like immunoreactivity (37-kDa band; Figure 1A). Coomassie blue staining of microparticle extracts indicated the presence of quite a few bands (Figure 1B). We then conducted liquid chromatography mass spectrometry/mass spectrometry (LC-MS/MS) analysis of the bands excised from the approximate 37-kDa area: peptide sequences in red in Figure 1 highlight the large presence of positive hits, many of them with a high MASCOT score (Figure 1B).
Identification of annexin 1 in human PMN–derived microparticles. Use of multiple detection approaches revealed annexin 1 (AnxA1; 37 kDa) presence in PMN-derived microparticles. (A) Western blot analysis comparing human recombinant AnxA1 (0.1 ng) and extracts from approximately 60 × 103 microparticles; arrow indicates AnxA1 band. (B) Protein gel electrophoresis revealing multiple bands in PMN-derived microparticles. Bands in the approximately 37-kDa region were excised, digested, and subjected to LC/MS/MS where both the MASCOT scores and the RANDOM database scores confirm the presence of this protein; boxed amino acid sequences indicate the fragments identified from this analysis. Data are representative of 4 distinct analyses.
Identification of annexin 1 in human PMN–derived microparticles. Use of multiple detection approaches revealed annexin 1 (AnxA1; 37 kDa) presence in PMN-derived microparticles. (A) Western blot analysis comparing human recombinant AnxA1 (0.1 ng) and extracts from approximately 60 × 103 microparticles; arrow indicates AnxA1 band. (B) Protein gel electrophoresis revealing multiple bands in PMN-derived microparticles. Bands in the approximately 37-kDa region were excised, digested, and subjected to LC/MS/MS where both the MASCOT scores and the RANDOM database scores confirm the presence of this protein; boxed amino acid sequences indicate the fragments identified from this analysis. Data are representative of 4 distinct analyses.
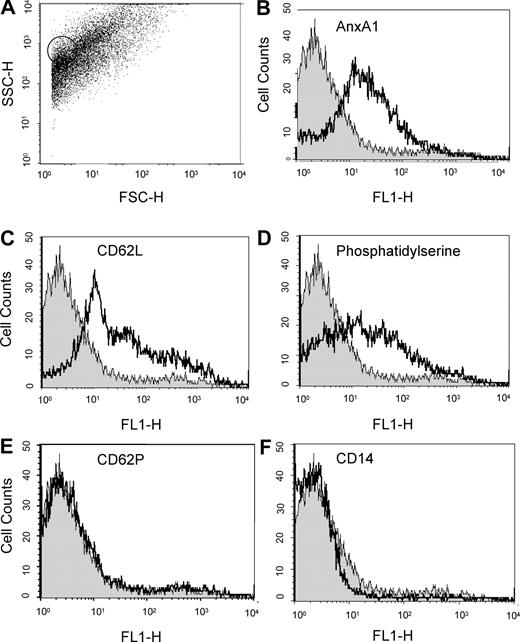
FACS analysis of the microparticle preparations revealed the expected heterogeneous population,4 with a size varying approximately between 0.1 and 2 μm (Figure 2A), as verified with control 1-μm beads (circled dots in Figure 2A). The AnxA1 immunoreactivity revealed by Western blot and LC/MS/MS was accessible in intact microparticles with a specific mAb (Figure 2B); in addition, PMN-derived microparticles stained for CD62L, used as an internal control4 (Figure 2C), and bound to annexin 5, indicating exposure of phosphatidylserine (Figure 2D). PMN-derived microparticles were negative for CD62P (Figure 2E), CD14 (Figure 2F), and CD62E (not shown), indicating no contamination by microparticles from platelets, monocytes, or endothelial cells, respectively.
FACS analysis on microparticles derived from adherent PMNs. Flow cytometric analysis of adherent PMN (to HUVEC monolayers)–derived microparticles, with acquisition of 5 to 10 000 events per sample. (A) Dot plot showing the heterogeneity in microparticle sizes; circled dots refer to 1-μm beads run in parallel as internal control. (B-F) Histograms demonstrating detection of AnxA1, L-selectin (CD62L), and phosphatidylserine (using FITC-labeled annexin V) in PMN-derived microparticles, with no signal observed for CD62P and CD14. Isotype control staining (gray histograms) is also shown. Data are representative from 3 or more independent microparticle preparations.
FACS analysis on microparticles derived from adherent PMNs. Flow cytometric analysis of adherent PMN (to HUVEC monolayers)–derived microparticles, with acquisition of 5 to 10 000 events per sample. (A) Dot plot showing the heterogeneity in microparticle sizes; circled dots refer to 1-μm beads run in parallel as internal control. (B-F) Histograms demonstrating detection of AnxA1, L-selectin (CD62L), and phosphatidylserine (using FITC-labeled annexin V) in PMN-derived microparticles, with no signal observed for CD62P and CD14. Isotype control staining (gray histograms) is also shown. Data are representative from 3 or more independent microparticle preparations.
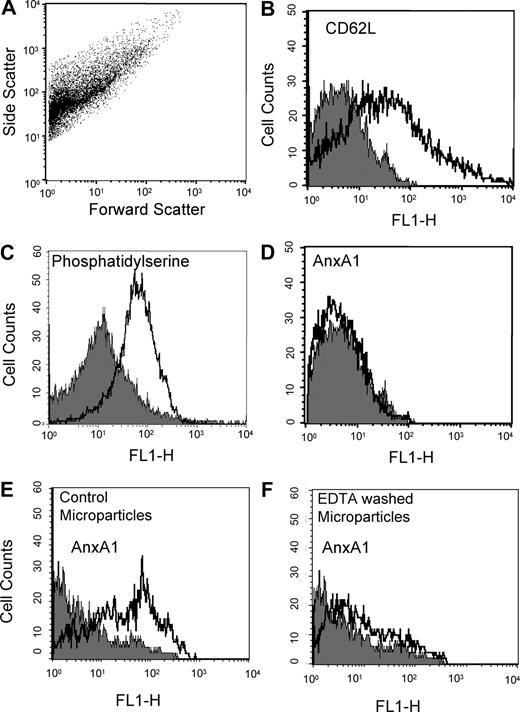
Analysis of resting PMN supernatants revealed presence of microparticles (Figure 3). These microparticles were positive for CD62L (Figure 3B) and bound to annexin 5 (indicating exposure of phosphatidylserine; Figure 3C) but did not immunostain for AnxA1 (Figure 3D).
Comparison of annexin 1 expression, and localization, in microparticles derived from resting or adherent PMNs. Flow cytometry of resting PMN (20 minutes at 37°C in suspension)–derived microparticles, with acquisition of 5 to 10 000 events per sample. (A) Dot plot showing the heterogeneity in microparticle sizes. (B-D) Histograms demonstrating detection of L-selectin (CD62L), phosphatidylserine (using FITC-labeled annexin V), but not AnxA1 in microparticles prepared from resting PMNs. (E,F) EDTA wash of microparticles prepared from adherent PMNs markedly reduces AnxA1 immunostaining. Left panel shows histogram of intact microparticles; right panel, histogram of microparticles washed with EDTA. Data are representative from 3 independent microparticle preparations.
Comparison of annexin 1 expression, and localization, in microparticles derived from resting or adherent PMNs. Flow cytometry of resting PMN (20 minutes at 37°C in suspension)–derived microparticles, with acquisition of 5 to 10 000 events per sample. (A) Dot plot showing the heterogeneity in microparticle sizes. (B-D) Histograms demonstrating detection of L-selectin (CD62L), phosphatidylserine (using FITC-labeled annexin V), but not AnxA1 in microparticles prepared from resting PMNs. (E,F) EDTA wash of microparticles prepared from adherent PMNs markedly reduces AnxA1 immunostaining. Left panel shows histogram of intact microparticles; right panel, histogram of microparticles washed with EDTA. Data are representative from 3 independent microparticle preparations.
These initial characterizations were completed by assessing the localization of AnxA1 in the microparticles. Washing them with PBS plus EDTA removed more than 80% of immunoreactivity for the protein, as shown by comparison of Figure 3E with Figure 3F. Therefore, the large majority of AnxA1 is exposed on the microparticles surface, and it is not integral in the phospholipids bilayer.
PMN-derived microparticles inhibit PMN/HUVEC interaction under flow
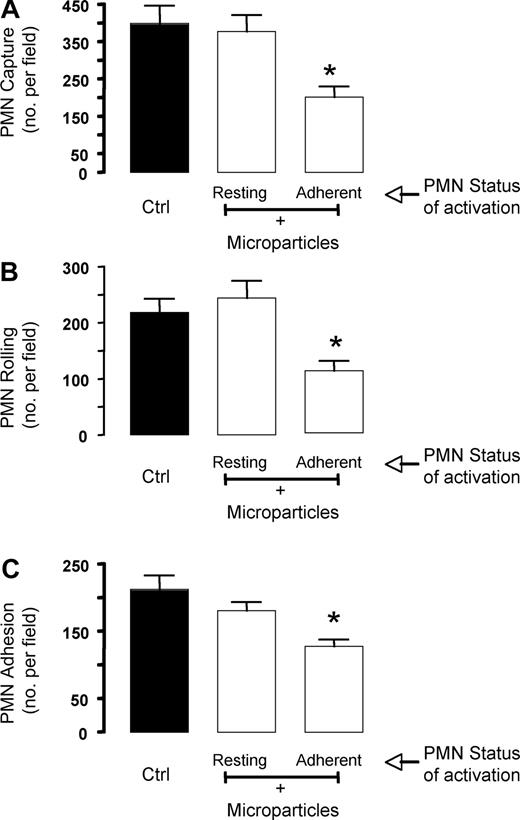
We have recently reported that nanomolar concentrations of AnxA1 inhibit PMN/HUVEC interactions under flow,20 hence in an in vitro experimental setting that resembles the events regulating PMN trafficking in an inflamed microvascular bed. Preincubation of human PMNs for 5 to 15 minutes with microparticles obtained from adherent PMNs (Figures 1,2) prior to flow over HUVEC monolayers inhibited the extent of leukocyte interaction, with an optimal effect with an 8-minute preincubation time (data not shown). This short incubation with microparticles yielded significant attenuation of PMN capture, rolling, and adhesion (Figure 4). Importantly, microparticles obtained from resting PMNs—which did contain AnxA1—were not able to significantly affect any of the parameters of PMN/HUVEC interaction under flow (Figure 4).
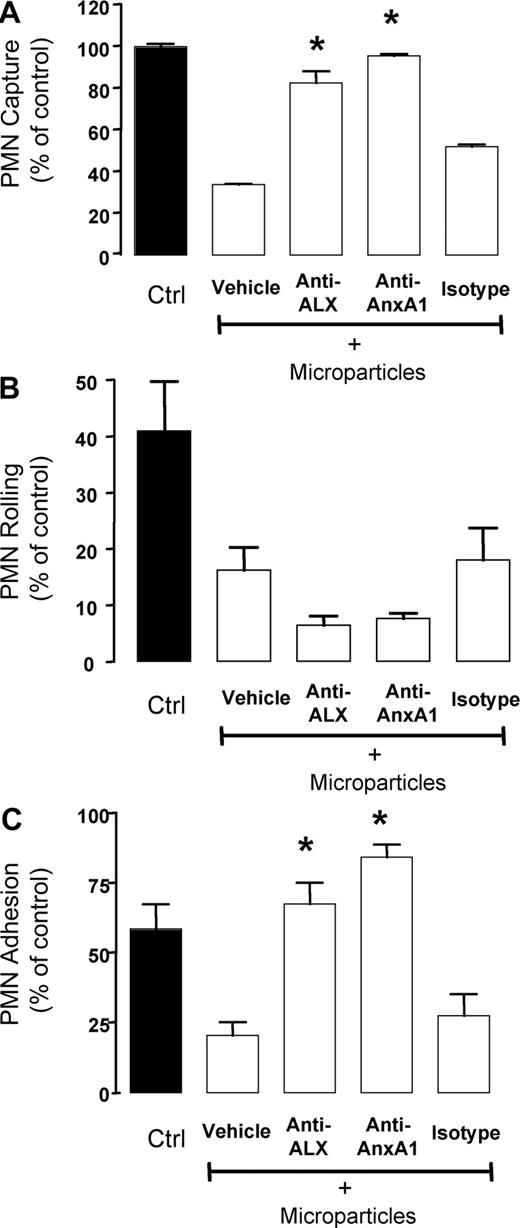
Annexin 1–rich microparticles inhibit PMN/HUVEC interaction under flow. Microparticles (between 30 and 50 × 103) were generated from resting or adherent PMNs and added to fresh PMNs for 8 minutes prior to the flow experiment. PMNs (5 × 106) were then flown for 15 minutes at 1 dyne/cm2, prior to quantifying the degree of PMN interaction with the HUVECs, as (A) PMN capture, (B) PMN rolling, or (C) PMN adhesion. Data are mean plus or minus SEM of from 3 or more independent experiments (with distinct microparticles, PMN, and HUVEC preparations). *P < .05 versus respective control group.
Annexin 1–rich microparticles inhibit PMN/HUVEC interaction under flow. Microparticles (between 30 and 50 × 103) were generated from resting or adherent PMNs and added to fresh PMNs for 8 minutes prior to the flow experiment. PMNs (5 × 106) were then flown for 15 minutes at 1 dyne/cm2, prior to quantifying the degree of PMN interaction with the HUVECs, as (A) PMN capture, (B) PMN rolling, or (C) PMN adhesion. Data are mean plus or minus SEM of from 3 or more independent experiments (with distinct microparticles, PMN, and HUVEC preparations). *P < .05 versus respective control group.
The indication that AnxA1 presence might be associated with the efficacy of adherent PMN-derived microparticles to inhibit PMN/HUVEC interactions under flow required functional support. Addition of a neutralizing anti-AnxA1 mAb to this experimental system reverted the inhibitory effect displayed by the microparticles on PMN capture (Figure 5A), resulting from an antagonism of microparticle-mediated inhibition of PMN adhesion (Figure 5C) but not rolling (Figure 5B).
Role for annexin 1 in the inhibitory property of PMN-derived microparticles. PMNs (5 × 106) were incubated with microparticles (between 30 and 50 × 103) for 8 minutes at 37°C. In some tubes, isotype control Ab, or neutralizing anti-AnxA1 or anti-ALX mAb was also added. PMNs (5 × 106) were then flown for 15 minutes at 1 dyne/cm2, prior to quantifying the degree of PMN interaction with the HUVECs, as (A) PMN capture, (B) PMN rolling, or (C) PMN adhesion. Data are mean plus or minus SEM of from 4 or more independent experiments (with distinct microparticles, PMN, and HUVEC preparations). *P < .05 versus microparticle vehicle group.
Role for annexin 1 in the inhibitory property of PMN-derived microparticles. PMNs (5 × 106) were incubated with microparticles (between 30 and 50 × 103) for 8 minutes at 37°C. In some tubes, isotype control Ab, or neutralizing anti-AnxA1 or anti-ALX mAb was also added. PMNs (5 × 106) were then flown for 15 minutes at 1 dyne/cm2, prior to quantifying the degree of PMN interaction with the HUVECs, as (A) PMN capture, (B) PMN rolling, or (C) PMN adhesion. Data are mean plus or minus SEM of from 4 or more independent experiments (with distinct microparticles, PMN, and HUVEC preparations). *P < .05 versus microparticle vehicle group.
Since AnxA1 inhibitory actions on the PMNs are brought about by activation of the anti-inflammatory receptor termed ALX,20,25,26 the effects of a neutralizing anti-ALX mAb was tested: this tool was also effective in abrogating the inhibitory effects of microparticles on PMN capture and adhesion (Figure 5).
Anti-inflammatory actions of PMN-derived microparticles
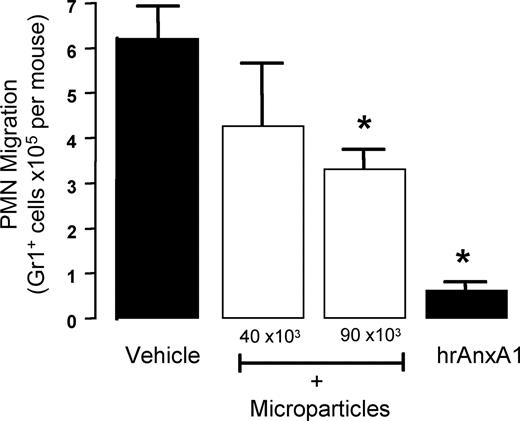
In the last section of the study, we tested—for the first time—whether PMN-derived microparticles would produce anti-inflammatory actions in vivo. Data in Figure 6 demonstrated an inhibitory effect of human PMN–derived microparticles, tested at 2 doses of 40 and 90 × 103 per mouse, with approximate 25% and 50% reduction in PMN recruitment elicited by IL-1β, respectively. Human AnxA1, tested as positive control at the dose of 10 μg (0.27 nmol), provoked a 90% reduction (Figure 6).
Anti-inflammatory effects of human PMN–derived microparticles. Annexin 1 (AnxA1)–rich microparticles are anti-inflammatory. Mice received 200 μL saline intravenously or 2 doses of human PMN–derived microparticles, or human recombinant AnxA1 (10 μg; positive control), immediately before the local injection of mouse IL-1β into 6-day-old air pouches. The extent of cell migration was determined 4 hours later, following air pouch washing and staining of migrated cells with Gr1 marker. Data are mean plus or minus SEM of 5 mice per group. *P < .05 versus vehicle group.
Anti-inflammatory effects of human PMN–derived microparticles. Annexin 1 (AnxA1)–rich microparticles are anti-inflammatory. Mice received 200 μL saline intravenously or 2 doses of human PMN–derived microparticles, or human recombinant AnxA1 (10 μg; positive control), immediately before the local injection of mouse IL-1β into 6-day-old air pouches. The extent of cell migration was determined 4 hours later, following air pouch washing and staining of migrated cells with Gr1 marker. Data are mean plus or minus SEM of 5 mice per group. *P < .05 versus vehicle group.
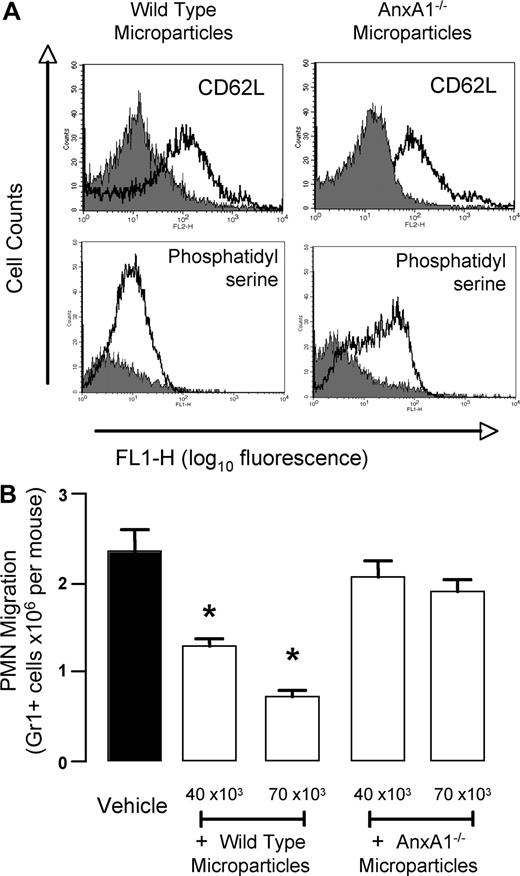
The mechanistic role of AnxA1 in this in vivo property of PMN-derived microparticles was determined by preparing them from AnxA1-null PMNs. Bone marrow–derived PMNs were prepared and stimulated by 1-hour adhesion to mouse endothelial cells (from wild-type mice) to generated microparticles: these particles were analyzed by flow cytometry, displaying the expected size range (not shown), and being positively stained for CD62L and annexin V (Figure 7A). When prepared from wild-type PMNs, murine microparticles were highly effective in inhibiting PMN trafficking promoted by IL-1β, with approximate inhibitions of 40% and 65% at doses of 40 × 103 and 70 × 103 microparticles per mouse, respectively (Figure 7B). Microparticles prepared from AnxA1-null PMNs11,27 were totally ineffective, giving values for PMN migration to the inflamed air pouches similar to those produced in vehicle-treated mice (Figure 7B).
Annexin 1 is responsible for the anti-inflammatory effects of murine PMN–derived microparticles. Bone marrow–derived PMNs were prepared from wild-type and AnxA1−/− mice, hence microparticles were produced with a protocol very similar to the one used to generated human ones (“Human PMN microparticle preparation”). (A) Validation of murine microparticles by flow cytometry: staining with anti-CD62L or for annexin V binding is shown for both wild-type and AnxA1−/− microparticles. (B) Two doses of mouse PMN–derived microparticles were injected intravenously immediately before the local injection of mouse IL-1β into 6-day-old air pouches. Air pouches were washed 4 hours later, quantifying the extent of Gr1+ cell migration. Data are mean plus or minus SEM of 5 mice per group. *P < .05 versus vehicle group.
Annexin 1 is responsible for the anti-inflammatory effects of murine PMN–derived microparticles. Bone marrow–derived PMNs were prepared from wild-type and AnxA1−/− mice, hence microparticles were produced with a protocol very similar to the one used to generated human ones (“Human PMN microparticle preparation”). (A) Validation of murine microparticles by flow cytometry: staining with anti-CD62L or for annexin V binding is shown for both wild-type and AnxA1−/− microparticles. (B) Two doses of mouse PMN–derived microparticles were injected intravenously immediately before the local injection of mouse IL-1β into 6-day-old air pouches. Air pouches were washed 4 hours later, quantifying the extent of Gr1+ cell migration. Data are mean plus or minus SEM of 5 mice per group. *P < .05 versus vehicle group.
Discussion
We report here that PMN-derived microparticles contain the anti-inflammatory protein AnxA1, and that this mediator of endogenous anti-inflammation confers them inhibitory properties on the process of leukocyte/endothelium interaction under flow; in addition, administration to mice of these microparticles reduced PMN trafficking in response to local cytokine application, and this effect was reliant on AnxA1, as determined by testing microparticles from AnxA1-null PMNs.
Initial reports of microparticles indicated their likely “bystander” nature, that is, that they were generated by cells entering into apoptosis as a sort of terminal act, safely releasing potentially toxic molecules in enclosed particles (reviewed in Distler et al1 ). However, the observation that microparticles were generated from activated cells, even in the absence of programmed cell death, was the first indication that they might solve specific biologic function(s). The last few years have witnessed a proliferation of studies investigating the components of microparticles1,4 ; their cell-specific peculiarities3 ; their potential role in cell-to-cell cross talk1,28 ; and their detection in the plasma of patients suffering from inflammatory diseases.2,3 Therefore, it is possible we have so far just identified very few of the potential biologic properties of microparticles; this may be particularly true when we consider their variety in sizes and components, as well as variety in relation to their cell source. Here we addressed our attention to PMN-derived microparticles.
In the context of experimental inflammation, we have pioneered the focus on anti-inflammation, addressing in particular the role played by the homeostatic mediator AnxA1. Human and rodent neutrophils have high cytoplasmic levels of this protein, which is augmented in response to both glucocorticoid and cytokines.27 During the initial phases of the leukocyte migration response, AnxA1 translocates to the plasma membrane10 and interacts with a specific G-protein–coupled receptor25 to control the extent and rate of emigration of the PMN into the subendothelial tissue space, toward the inflammatory site.11,27 Therefore, the “annexin 1 pathway” as a whole becomes operative within the microenvironment of an adherent PMN. Disruption of this system, achieved with the global AnxA1−/− mouse colony, leads to altered pathology as determined in relevant experimental models, including stroke and multiple organ failure after endotoxemia.27,29 In addition, blood neutrophils taken from these mice display higher degrees of activation in response to classical leukocyte stimulants (eg, PAF or fMLP).11
AnxA1 lacks a signal peptide,30 yet is nonetheless abundantly released from activated PMNs.10,12,31 We proposed that the rapid mobilization of AnxA1 observed in activated PMNs was consequent to intracellular granule mobilization, since a good proportion of the protein is contained in gelatinase granules,14 a finding first confuted,32 but then confirmed by proteomic analyses.15 The data here presented indicate that AnxA1 could be released from activated PMN via the “microparticle pathway,” so that multiple ways of mobilizing the protein from this cell type might exist. AnxA1 will not be a unique protein or mediator to follow multiple manners of release, since also CD62L, for instance, can be shed as soluble protein or also embedded in phosphatidylserine-positive microparticles.4 Such a dichotomy of secretion (soluble and microparticle associated) mechanism has also been proposed for the short-lived lipid platelet-activating factor.33 Another analogy for this mode of secretion of AnxA1 would be with IL-1β34 : like AnxA1, this cytokine lacks a signal peptide35 ; nonetheless, it is released from activated cells, and microparticles are the route identified in monocytic cells.1,34 We would propose that release of pivotal proteins from activated cells via microparticle formation could be a relatively more general process in inflammatory cell pathobiology. Since apoptotic cells release microparticles, we would predict that AnxA1 release via this pathway could be responsible for its augmenting property of the phagocytosis of apoptotic PMNs by macrophages.36 In any case, by analogy to the studies where the inhibitory and anti-inflammatory properties of human recombinant AnxA1 have been dissected (eg, see Perretti and Flower6 ), the presence of AnxA1 PMN–derived microparticles led us to hypothesize that these microparticles could exert inhibitory properties on the PMN extravasation process.
Then, we tested the functionality of PMN-derived microparticles using the flow chamber assays. Static assays have established that human recombinant AnxA1 can inhibit PMN chemotaxis,12 and adhesion to13 and transmigration through endothelial monolayers.10 In addition, the protein would inhibit PMN/HUVEC adhesion under flow, an effect reliant on activation of the specific AnxA1 receptor, termed ALX.20 Incubation of PMN-derived microparticles exerted a marked inhibition on PMN capture onto the HUVEC monolayer, this resulting from an inhibition of both PMN rolling and adhesion. Of interest, neutralization strategies against endogenous AnxA1 or its receptor ALX produced a selective abrogation of the inhibitory effect of microparticles on PMN adhesion, with no effect on rolling. This is strikingly reminiscent of the selective inhibition produced by human recombinant AnxA1, able to inhibit PMN adhesion, but not rolling, when coincubated with PMNs prior to flow.20 Therefore, AnxA1 expressed or exposed by PMN-derived microparticles is biologically active, replicating the properties described for exogenous addition of the protein. Moreover, the model that emerges from these studies is the one where AnxA1, presented by the microparticles, would activate ALX expressed on the recipient PMNs, diminishing its reactivity with the HUVEC monolayer. These data identify the AnxA1/ALX pathway as a major checkpoint mechanism, devoted to the rapid control of the extent of PMN/HUVEC interaction.
Equally convincingly, these data indicate that microparticle effect upon PMN rolling is not AnxA1 mediated. It is possible that CD62L—expressed by all PMN-derived microparticles4 —might be responsible for this action, since blockade of this selectin affects PMN rolling, as determined in similar experimental conditions.20 However, it is more likely that other mediators are causing this inhibitory effect, since microparticles derived from resting PMNs did contain CD62L, yet were not able to significantly attenuate the extent PMN rolling. Proteomic profiling of microparticles derived from differently activated PMNs will shed light on these puzzling data.
These data produced with the flow chamber assay are in apparent contrast with an earlier report,37 where overnight incubation of PMN-derived microparticles produced an activating phenotype of the HUVECs. Gasser and Schifferli have commented on this discrepancy,5 and we would agree that differences in time frame (minutes vs hours), preincubation procedures, and experimental protocols (flow vs static conditions) would give potential explanation for these different outcomes. Future studies may address this point, possibly linking it to the heterogeneity of PMN-derived microparticle population in function of the stimuli applied.1,4
Finally, we tested whether PMN-derived microparticles could produce anti-inflammatory actions on the process of in vivo PMN trafficking. The mouse air pouch model inflamed with IL-1β was chosen, since it is exquisitely sensitive to the inhibitory actions of human recombinant AnxA1 and its bioactive peptides24 ; in addition, agonism at the murine orthologue of ALX produced potent inhibitory effects on the extent of PMN migration.25,38 Injection into the air pouches of IL-1β produced the expected influx of PMNs reliably assessed at the 4-hour time point.24 Administration to mice of doses ranging from 40 to 90 × 103 microparticles inhibited PMN trafficking, with the murine ones more effective than the ones generated from human PMNs; however, the 2 “species” of microparticles did not display qualitatively different properties, both able to significantly attenuate PMN influx in response to cytokine injection. To assess the potential role of microparticle-associated AnxA1 in these actions, we resorted to the use of microparticles produced from AnxA1-null PMNs: the data obtained strongly indicated a functional role for these endogenous effectors of anti-inflammation in bringing about the antimigratory effect of microparticles. We did not dissect, here, which specific step of the leukocyte recruitment process is chiefly affected by the microparticles; future studies will rely on the technique of intravital microscopy, with the use of protocols successfully used to assess the actions of exogenous and endogenous AnxA1,11,39 to address this specific question.
In conclusion, we report several novel features of PMN microparticles, describing for the first time their expression of AnxA1; this homeostatic protein, likely interacting with its receptor ALX, is functionally active in modulating human PMN reactivity with activated HUVECs. In addition, PMN-derived microparticles, delivered by intravenous administration, display potent antimigratory properties. Again, such action is relying on their AnxA1 content as demonstrated using microparticles prepared from AnxA1-null mice. These results prompt us to speculate that generation of AnxA1-rich microparticle production within the inflamed microcirculation, and their potential layering onto the vessel wall, could be another way by which the endogenous anti-inflammatory axis centered on AnxA1 and ALX could operate to bring about inflammatory resolution, hence dampening PMN recruitment in the late phase of the innate immune response.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr O. Gasser (University Hospital, Basel, Switzerland) for his kind guidance on microparticle isolation. We thank Dr D. Henderson (AstraZeneca, Macclesfield, United Kingdom) for the supply of anti-ALX mAb, and Drs Nourshargh and Benoit-Voisin (Barts and The London Medical School, London, United Kingdom) for guidance in bone marrow–derived PMN preparation.
This work was supported by the Arthritis Research Campaign UK (Chesterfield, United Kingdom; fellowship 15775 to M.P.), the William Harvey Research Foundation (London, United Kingdom), and the Research Advisory Board of Barts and The London Medical School (grants MRes/PhD03).
Authorship
Contribution: J.D. performed research and collected, analyzed, and interpreted data; L.V.N. performed research and collected data; D.R. performed research; D.C. analyzed and interpreted data; K.-Y.L. performed experiments and analyzed and interpreted data; and M.P. designed research, analyzed and interpreted data, and drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mauro Perretti, William Harvey Research Institute, Barts and The London Medical School, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: m.perretti@qmul.ac.uk.