Abstract
Chronic graft-versus-host disease (cGVHD) is a major limitation of successful hematopoietic cell transplantation. The safety and efficacy of extracorporeal photopheresis (ECP) for 12 to 24 weeks together with standard therapy was compared with standard therapy alone in patients with cutaneous manifestations of cGVHD that could not be adequately controlled by corticosteroid treatment. The primary efficacy end point was a blinded quantitative comparison of percent change from baseline in Total Skin Score (TSS) of 10 body regions at week 12. Ninety-five patients were randomized to either ECP and standard therapy (n = 48) or standard therapy alone (n = 47). The median percentage improvement in TSS at week 12 was 14.5% for the ECP arm and 8.5% for the control arm (P = .48). The proportion of patients who had at least a 50% reduction in steroid dose and at least a 25% decrease from baseline in TSS was 8.3% in the ECP arm at week 12 and 0% in the control arm (P = .04). The nonblinded investigator assessment of skin complete or partial responses revealed a significant improvement in favor of ECP (P < .001). ECP was generally well tolerated. These results suggest that ECP may have a steroid-sparing effect in the treatment of cGVHD. Clinical trials registered at www.ClinicalTrials.gov as NCT00054613.
Introduction
Chronic graft-versus-host disease (cGVHD) occurs in approximately 50% of patients after allogeneic hematopoietic cell transplantation (HCT) and frequently requires long-term systemic immunosuppressive treatment.1 The clinical management of patients with extensive cGVHD is difficult because of the wide variability of disease manifestations, clinical course, infectious complications, and treatment related toxicity.2 cGVHD is initially treated with immunosuppressive medications, including corticosteroids as first-line therapy,3-5 followed by other agents, such as mycophenolate mofetil.6-8 Despite the many treatment options, no single class of immunosuppressive agents has consistently produced a steroid-sparing effect in patients with cGVHD.5 A recent pilot open label trial of rituximab has demonstrated steroid-sparing effects in patients with cGVHD.9
Extracorporeal photopheresis (ECP) therapy represents a potential therapeutic approach for treatment of chronic GVHD.10,11 ECP induces apoptosis of leukocytes, and infusion of these cells has been hypothesized to generate a tolerogenic response and modulation of cytokine production.12-14 Since the initial report of the first case of cGVHD successfully treated with ECP by Owisanowski and colleagues in 1994,15 several additional reports and observational studies of ECP treatment in patients with cGVHD have demonstrated that this approach may be feasible, well tolerated, and associated with beneficial treatment effects in multiple organ systems, including sclerotic forms of the cutaneous manifestation.16-24 In a recently published retrospective case series, Couriel25 reported a 59% response rate in cutaneous manifestations of cGVHD, and 42% of these responses were observed in patients with sclerodermatous manifestations of the disease.
We undertook a prospective randomized controlled trial of extracorporeal photopheresis to evaluate the effects of ECP treatment on the cutaneous and extracutaneous manifestations of cGVHD and to assess the potential for a steroid-sparing effect.
Methods
Patient characteristics
The study included patients with a prior bone marrow or mobilized blood stem cell transplant from a related or unrelated donor who developed histologically confirmed cGVHD with cutaneous manifestations at 100 days or more after HCT. After individual case review, the sponsor also permitted selected patients to be enrolled who had well-defined clinical and histologic evidence of cGVHD but developing before 100 days after HCT. All patients had cGVHD that was corticosteroid-refractory (defined as lack of response or disease progression after administration of at least 1 mg/kg of methylprednisolone equivalent) or corticosteroid-dependent (requiring more than 10 mg methylprednisolone equivalent to control skin manifestations) or had corticosteroid intolerance (including avascular necrosis, severe myopathy, uncontrolled diabetes mellitus, systemic viral or fungal infections). To be eligible, patients had to be receiving a stable corticosteroid dose for at least 2 weeks before randomization. Patients could also be receiving FK-506, cyclosporine A, or mycophenolate mofetil at stable doses at the discretion of the investigator for 4 weeks before randomization. Discontinuation of these agents was not permitted during the course of the study unless absolutely required for safety reasons. Other eligibility criteria included a total leukocyte count of 1000/mm3 or higher, platelet count of 25 000/mm3 or higher, and a Karnofsky Performance Score of 30% or higher at the time of study entry. Patients were excluded from the study if they had received prior ECP; had an intolerance to methoxsalen, heparin, or citrate products; were pregnant or lactating; or had received any other treatments for cGVHD within 14 days before study entry. Conventional anti-infective prophylaxis and supportive care was governed by institutional guidelines. Patients were not permitted to use topical steroids. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki, and the protocol was approved by the institutional review board of each participating institution. Patients were aware that they could be randomized to a treatment arm (control group) that had already been demonstrated to be ineffective or poorly tolerated. However, investigators were free to remove patients in the control arm from the trial at any time if standard therapy continued to be poorly tolerated or if GVHD progressed.
Study design and end points
This randomized, single-blind, multicenter study was conducted at 23 transplantation centers in North and South America, Europe, and Australia ( Appendix). The study was sponsored by Therakos (Exton, PA). Enrollment in the study commenced in June 2002 and was completed in April 2005. Patients were randomized in a 1:1 ratio to receive 12 weeks of ECP treatments in addition to conventional treatment (ECP arm) or to receive conventional treatment alone (control arm). A block randomization scheme was used. The primary end point was the median percentage change in the Total Skin Score (TSS) after 12 weeks of treatment compared with the baseline (pretreatment) value using a validated ordinal 50-point whole body scoring system.26 Although the theoretical maximum TSS is 50, patients infrequently have values above 30 (H.T.G., personal communication, December 2007). Use of the TSS has been reported in 1 prior observational study by Greinix and collaborators26 and requires validation in other prospective controlled clinical trials.
The TSS was assessed by a medical professional who was trained in the skin evaluation scoring system, not otherwise involved with the care of the patient, and not informed of the patient's study arm assignment. The fraction of each of 10 topographic areas involved with 1 or more of 5 types of skin lesions was estimated and recorded as follows: 0 = normal; 1 = discolored (hyperpigmentation, hypopigmentation, erythematous) or alopecia; 2 = lichenoid plaque, thickening, able to move; 3 = thickened, able to move and pinch; 4 = hidebound, unable to move or pinch (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). For each area with a score of 3 or 4, the assessor also determined the concurrent fractional involvement of the area exhibiting overlying erythema (see example in Figure S1B). For each of the 10 areas, a regional score was calculated by multiplying the grade scores (0-4) by the respective proportions of affected surface within the area. The skin score for each regional area could range from 0 to 5 (Figure S1B). Finally, the scores of all 10 regional areas were added to arrive at the total skin score, which could range from 0 to 50. Skin assessments were performed at study weeks 2, 4, 6, 8, 10, 12, 16, 20, and 24.
For patients in the ECP arm, ECP treatment was administered 3 times during week 1, and then twice weekly on consecutive days during weeks 2 through 12. Responding patients in the ECP group could continue 2 ECP treatments every 4 weeks until week 24. Patients in the control group were permitted to receive ECP treatments before week 12 if they exhibited progressive skin disease (defined as greater than 25% worsening of the TSS from baseline) or after week 12 if they had an inadequate response of skin GVHD to treatment (defined as less than 15% improvement from baseline in TSS or a less than 25% reduction in corticosteroid dose). Only the first 12 weeks of treatment are considered in the analyses presented in this paper. The ECP procedure was performed using the UVAR photophoresis system (Therakos) as previously described.27
The primary objective of the study was to determine the effect of ECP treatment on the cutaneous manifestations of cGVHD as assessed by the TSS at week 12. Secondary study objectives included the proportion of patients in each treatment group who had the following: at least 25% improvement in TSS at weeks 12 and 24; a 50% or greater reduction in daily steroid dose compared with the baseline dose; steroid-sparing in conjunction with an improvement of at least 25% in the TSS; a change in oral mucosa, lungs, eyes, joints, liver, and gastrointestinal tract, assessed by the investigator as resolved, improved, stable, or worsened; complete or partial (improvement of > 50% of body surface area involved) resolution of skin disease by the unblinded investigator, and a change in a patient self-report questionnaire assessing quality of life, as described below.
Investigators were asked to maintain a stable dose of corticosteroids during the first 6 weeks of the study except in cases of unacceptable toxicity requiring dose reduction. After 6 weeks of participation in the study, corticosteroid tapering was allowed when, in the opinion of the investigator, cutaneous GVHD had improved. If chronic GVHD worsened during corticosteroid tapering, the corticosteroid dose could be increased up to but no higher than the original corticosteroid dose. No standardized steroid tapering protocol was furnished to the investigators, and steroid tapering was performed at the investigators discretion based on the overall status of each individual patient's GVHD.
A Targeted Symptoms Assessment (TSA) was completed by the patient at baseline and at weeks 4, 8, 12, 16, 20, and 24. The TSA consisted of 12 questions addressing the impact of skin, eye, and oral disease on the patient's quality of life, including tiredness, depression, sleeping, discomfort from dry or itchy eyes, oral sores, interference with normal activities of school or work, sexual activities, effect on other family members, self-consciousness, and mobility. The TSA responses use a 5-point scale measurement as follows: 0, never; 1, rarely; 2 sometimes; 3, often; 4, all the time. The maximum possible TSA score was 48. For each question, “never” represented the best and “all the time” represented the worst score.
Safety assessments included adverse events, vital signs, and standard hematologic and chemistry tests.
Statistical methods
The safety dataset consisted of all patients who were randomized into the study and signed an informed consent. The efficacy (modified intent-to-treat [MITT]) dataset consisted of all patients who had at least 1 postrandomization TSS determination and, for the ECP arm, also had at least 1 postrandomization ECP treatment. All efficacy determinations at weeks 12 and 24 in both treatment arms were based on the observed data or, in the case of missing data, on the last observation carried forward to the analysis time point. Statistical comparisons are limited to the first 12 weeks of the study; week 24 data are represented only by descriptive statistics.
Continuous variables were summarized by the median and range. Categorical variables were summarized by the number and percentage of patients in each category. For the primary end point, the Wilcoxon rank sum test was used. A log-rank test was used to compare cumulative incidence of complete or partial skin response between the 2 groups.
Patient disposition
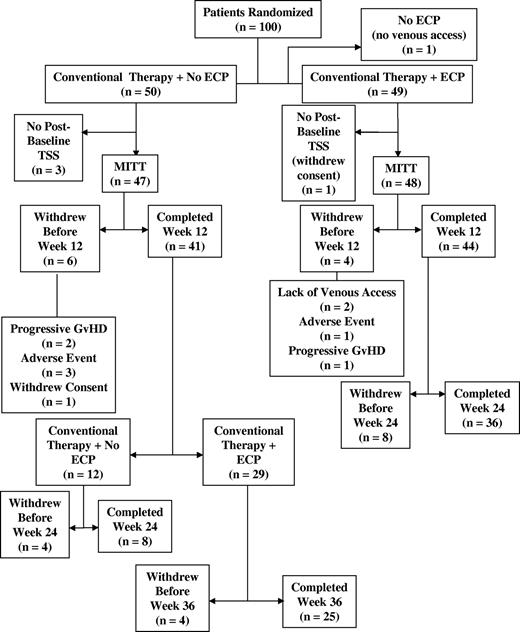
The disposition of patients enrolled in this study is summarized in Figure 1. One hundred patients were randomized into the study (50 in each treatment group). One patient randomized into the ECP group did not have satisfactory venous access and did not receive ECP treatments. One patient randomized to the ECP treatment arm and 3 patients in the control arm did not have a postbaseline TSS evaluation. Ten patients (4 in the ECP arm and 6 in the control arm) withdrew before week 12, the primary end point assessment week. Eighty-five patients (44 in the ECP arm and 41 in the control arm) completed the week 12 evaluation. Of the 44 patients in the ECP arm who completed week 12, 41 elected to receive an additional 12 weeks of ECP treatments and 36 completed the week 24 assessment. Of the 41 patients in the control arm who completed week 12, 8 completed the week 24 assessment without crossing over to receive open-label ECP. Twenty-nine of the 41 control arm patients crossed over to receive open-label ECP. The large number of patients who discontinued in the control arm precluded any statistical comparisons for the week 24 time point. The open-label data from this latter cohort are not described in this report.
Results
Demographics and transplant characteristics
Table 1 summarizes the demographic and transplant characteristics of the 95 patients included in the MITT dataset. Both ECP and control arms were well-balanced at baseline with respect to age, sex, stem cell source, donor characteristics, and primary underlying disease leading to transplantation. Most patients had related HLA-identical donors. Ten percent of ECP-treated patients (n = 5) and 6% of the control arm patients (n = 3) had a 1-locus mismatch, whereas 0% of ECP-treated patients and 6% (n = 3) of the control arm patients had 2-locus mismatch donors.
Patient and transplant characteristics
| Characteristic . | ECP, n = 48 . | Control, n = 47 . |
|---|---|---|
| Median age, y (range) | 41 (16-67) | 43 (13-67) |
| White race, n (%) | 45 (94) | 41 (87) |
| Males, n (%) | 30 (63) | 26 (55) |
| Primary disease, n (%) | ||
| Acute myeloid leukemia | 12 (25) | 9 (19) |
| Acute lymphoid leukemia | 6 (13) | 6 (13) |
| Chronic myeloid leukemia | 13 (27) | 16 (34) |
| Non-Hodgkin lymphoma | 2 (4) | 2 (4) |
| Others | 15 (31) | 14 (30) |
| Transplant cell source, n (%) | ||
| Bone marrow | 17 (35) | 15 (32) |
| Peripheral blood | 31 (65) | 32 (68) |
| HLA mismatch, n (%) | ||
| One locus | 5 (10) | 3 (6) |
| Two loci | 0 (0) | 3 (6) |
| Stem cell donors, n (%) | ||
| Related | 31 (65) | 34 (72) |
| Unrelated | 17 (35) | 13 (28) |
| Characteristic . | ECP, n = 48 . | Control, n = 47 . |
|---|---|---|
| Median age, y (range) | 41 (16-67) | 43 (13-67) |
| White race, n (%) | 45 (94) | 41 (87) |
| Males, n (%) | 30 (63) | 26 (55) |
| Primary disease, n (%) | ||
| Acute myeloid leukemia | 12 (25) | 9 (19) |
| Acute lymphoid leukemia | 6 (13) | 6 (13) |
| Chronic myeloid leukemia | 13 (27) | 16 (34) |
| Non-Hodgkin lymphoma | 2 (4) | 2 (4) |
| Others | 15 (31) | 14 (30) |
| Transplant cell source, n (%) | ||
| Bone marrow | 17 (35) | 15 (32) |
| Peripheral blood | 31 (65) | 32 (68) |
| HLA mismatch, n (%) | ||
| One locus | 5 (10) | 3 (6) |
| Two loci | 0 (0) | 3 (6) |
| Stem cell donors, n (%) | ||
| Related | 31 (65) | 34 (72) |
| Unrelated | 17 (35) | 13 (28) |
cGVHD characteristics
cGVHD characteristics are summarized in Table 2. Median time from transplantation to the onset of cGVHD was 140 days in the ECP arm and 129 days in the control treatment arms (P = .86). The median duration of prestudy corticosteroid treatment was 50 weeks in the ECP arm compared with 55 weeks in the control arm (P = .88). The median time from onset of cGVHD to randomization was 569 days in the ECP arm compared with 630 days in the control arm (P = .83). Selected baseline characteristics according to corticosteroid status (refractory, intolerant, dependent) are shown in Table S1.
Chronic GVHD characteristics
| Characteristic . | ECP, n = 48 . | Control, n = 47 . |
|---|---|---|
| Corticosteroid status, n (%)* | ||
| Corticosteroid-dependent | 28 (58) | 25 (53) |
| Corticosteroid-refractory | 7 (15) | 5 (11) |
| Corticosteroid-intolerant | 13 (27) | 17 (36) |
| Median days from transplantation to cGVHD (range) | 140 (69-637) | 129 (70-1389) |
| Median days from cGVHD to randomization (range)† | 569 (35-2743) | 630 (1-2253) |
| Onset type of cGVHD, n (%) | ||
| Progressive | 28 (58) | 25 (53) |
| Quiescent | 5 (10) | 6 (13) |
| de novo | 15 (32) | 16 (34) |
| Severity of cGVHD, n (%) | ||
| Limited | 3 (6) | 3 (6) |
| Extensive | 45 (94) | 44 (94) |
| Total Skin Score (TSS) at baseline, median (range) | 9.4 (0.6-23.6) | 9.2 (1.0-20.7) |
| Extracutaneous GVHD involvement, n (%) | ||
| Ocular | 27 (56.3) | 28 (59.6) |
| Gastrointestinal | 2 (4.2) | 9 (19.1) |
| Liver | 14 (29.2) | 14 (29.8) |
| Lung | 9 (18.8) | 7 (14.9) |
| Oral mucosa | 30 (62.5) | 30 (63.8) |
| Joints | 18 (37.5) | 16 (34) |
| Median duration (wks) of corticosteroid usage for cGVHD before study entry (range) | 50 (2.7-426) | 55 (1.9-319) |
| Median daily total oral corticosteroid dose (mg) at baseline (range) | 16 (4-64) | 20 (3.3-60) |
| Concurrent immunosuppressive medication at baseline, n (%) | ||
| Mycophenolate mofetil | 26 (54) | 26 (55) |
| Cyclosporin A | 24 (50) | 28 (60) |
| FK-506 | 11 (23) | 6 (13) |
| Baseline platelet count < 100 000/μL, n (%) | 5 (10.4) | 6 (12.8) |
| Characteristic . | ECP, n = 48 . | Control, n = 47 . |
|---|---|---|
| Corticosteroid status, n (%)* | ||
| Corticosteroid-dependent | 28 (58) | 25 (53) |
| Corticosteroid-refractory | 7 (15) | 5 (11) |
| Corticosteroid-intolerant | 13 (27) | 17 (36) |
| Median days from transplantation to cGVHD (range) | 140 (69-637) | 129 (70-1389) |
| Median days from cGVHD to randomization (range)† | 569 (35-2743) | 630 (1-2253) |
| Onset type of cGVHD, n (%) | ||
| Progressive | 28 (58) | 25 (53) |
| Quiescent | 5 (10) | 6 (13) |
| de novo | 15 (32) | 16 (34) |
| Severity of cGVHD, n (%) | ||
| Limited | 3 (6) | 3 (6) |
| Extensive | 45 (94) | 44 (94) |
| Total Skin Score (TSS) at baseline, median (range) | 9.4 (0.6-23.6) | 9.2 (1.0-20.7) |
| Extracutaneous GVHD involvement, n (%) | ||
| Ocular | 27 (56.3) | 28 (59.6) |
| Gastrointestinal | 2 (4.2) | 9 (19.1) |
| Liver | 14 (29.2) | 14 (29.8) |
| Lung | 9 (18.8) | 7 (14.9) |
| Oral mucosa | 30 (62.5) | 30 (63.8) |
| Joints | 18 (37.5) | 16 (34) |
| Median duration (wks) of corticosteroid usage for cGVHD before study entry (range) | 50 (2.7-426) | 55 (1.9-319) |
| Median daily total oral corticosteroid dose (mg) at baseline (range) | 16 (4-64) | 20 (3.3-60) |
| Concurrent immunosuppressive medication at baseline, n (%) | ||
| Mycophenolate mofetil | 26 (54) | 26 (55) |
| Cyclosporin A | 24 (50) | 28 (60) |
| FK-506 | 11 (23) | 6 (13) |
| Baseline platelet count < 100 000/μL, n (%) | 5 (10.4) | 6 (12.8) |
One patient in the ECP arm and 3 in the control arm were corticosteroid-dependent and -intolerant; these 4 patients are categorized as corticosteroid-intolerant.
Subjects were allowed to enter the study if they had a history of severe steroid intolerance before the onset of cGVHD.
Most patients had extensive cGVHD by the criteria of Lee et al.5 Both arms were well balanced with respect to cGVHD characteristics except that gastrointestinal involvement was less frequent in the ECP arm (4%, 2 patients) than in the control arm (19%, 9 patients; P = .02).
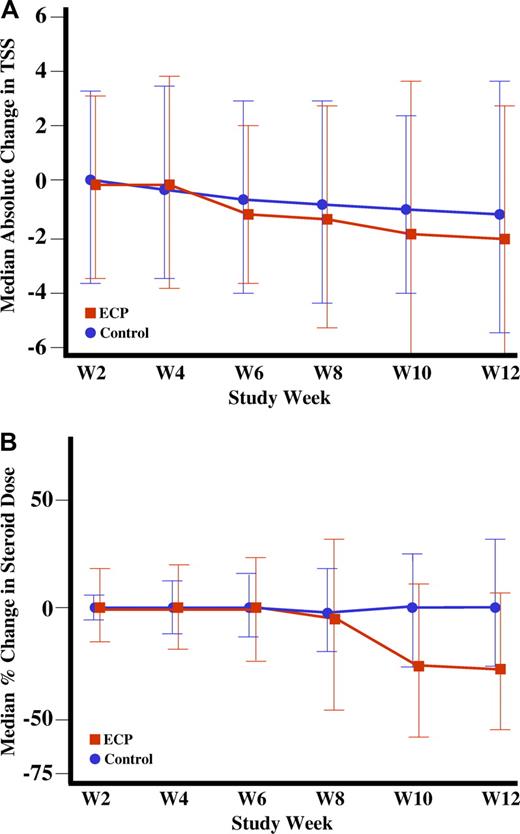
Cutaneous response to ECP treatment and steroid-sparing effects
The changes in TSS from baseline until week 12 between the ECP (−14.5%) and control (−8.5%) arms were not statistically different (Table 3). Figure 2A depicts the absolute change in TSS for both groups during the first 12 weeks of the study. Several of the secondary endpoints assessed a reduction in daily corticosteroid dose and parallel improvement in the cutaneous manifestations of cGVHD. By week 12, 25.0% (n = 12) of ECP-treated patients and 12.8% (n = 6) of control-treated patients had a 50% or greater reduction in the total daily dose of corticosteroids (P = .13). At week 12, the percentage of patients experiencing both a 50% or greater reduction in daily corticosteroid dose and a 25% or greater improvement in the TSS was higher in the ECP group than the control group (8.3%; 4 patients vs 0%; 0 patients; P = .04; Table 3). In a post hoc analysis, we observed that by week 12, 20.8% of the ECP-treated patients and 6.4% of the control-treated patients had both a 50% or greater reduction in steroid dose and a daily dose of less than 10 mg/day (P = .04). Figure 2B depicts the percentage change in corticosteroid dose over time. The beneficial effects of ECP on improvement in the TSS and reduction of daily corticosteroids dose persisted in exploratory analyses that excluded corticosteroid-refractory patients in the ECP group (Table S2a) or the combined ECP and control groups (Table S2b). Total daily corticosteroid doses of 10 mg/day or less are generally considered to be a desirable therapeutic goal in order to minimize steroid-induced metabolic complications, because doses in excess of 10 mg prednisone equivalent per day have been clinically associated with an increased incidence of steroid-induced complications.28
Total Skin Score (TSS) and corticosteroid response to ECP treatment
| Parameter . | Week 12 . | P . | Week 24 . | ||
|---|---|---|---|---|---|
| ECP, n = 48 . | Control, n = 47 . | ECP, n = 48 . | Control, n = 47* . | ||
| Median percent change from baseline in TSS | −14.5 | −8.5 | .48 | −31.4 | N/A |
| > 50% reduction in corticosteroid dose, n (%)† | 12 (25) | 6 (12.8) | .13 | 19 (39.6) | N/A |
| > 50% reduction in corticosteroid dose and > 25% improvement in TSS, n (%) | 4 (8.3) | 0 (0.0) | .04 | 11 (22.9) | N/A |
| > 50% reduction in corticosteroid dose and final corticosteroid dose of < 10 mg/day, n (%)† | 10 (20.8) | 3 (6.4) | .04 | 17 (35.4) | N/A |
| Parameter . | Week 12 . | P . | Week 24 . | ||
|---|---|---|---|---|---|
| ECP, n = 48 . | Control, n = 47 . | ECP, n = 48 . | Control, n = 47* . | ||
| Median percent change from baseline in TSS | −14.5 | −8.5 | .48 | −31.4 | N/A |
| > 50% reduction in corticosteroid dose, n (%)† | 12 (25) | 6 (12.8) | .13 | 19 (39.6) | N/A |
| > 50% reduction in corticosteroid dose and > 25% improvement in TSS, n (%) | 4 (8.3) | 0 (0.0) | .04 | 11 (22.9) | N/A |
| > 50% reduction in corticosteroid dose and final corticosteroid dose of < 10 mg/day, n (%)† | 10 (20.8) | 3 (6.4) | .04 | 17 (35.4) | N/A |
Results based on MITT population, defined as all randomized patients who received at least 1 study treatment and who had at least 1 postbaseline TSS. The last available TSS was used for patients who did not have a TSS at week 12.
The large numbers of patients who discontinued the study in the control arm precluded statistical comparison for week 24.
In the ECP group, 41 patients were receiving treatment with corticosteroids at baseline and 38 had doses recorded at week 12. In the control group, 43 patients were receiving treatment with corticosteroids at baseline and 39 had doses recorded at week 12.
In both groups, the last known dose of corticosteroids was used when the week 12 dose was missing.
Improvement in Total Skin Score (TSS) and reduction in steroid dose through week 12. (A) Absolute median change in the TSS through week 12. (B) Median percentage change in steroid dose through week 12.
Improvement in Total Skin Score (TSS) and reduction in steroid dose through week 12. (A) Absolute median change in the TSS through week 12. (B) Median percentage change in steroid dose through week 12.
The median absolute change in TSS in the ECP arm during the entire 24 weeks of ECP treatment is presented in Figure 3. In the ECP arm at week 24, improvement continued as determined by the progressive decrease in TSS (−31.4% from baseline) that was also mirrored by a reduction in corticosteroid usage. The proportion of patients in the ECP arm with a 50% or greater reduction in steroid dose increased from 25% at week 12 to 39.6% at week 24, also mirroring the continued improvement in TSS. The proportion of patients in the ECP arm with both a 50% or greater reduction in steroid dose and a daily dose of less than 10 mg/day also increased, from 20.8% to 35.4%. However, interpretation of the findings at week 24 is limited due to inadequate numbers of controls for comparison.
Median absolute change in TSS through week 24. Median absolute change in TSS in patients receiving ECP treatment for 24 weeks.
Median absolute change in TSS through week 24. Median absolute change in TSS in patients receiving ECP treatment for 24 weeks.
Investigator assessment of skin response to treatment at week 12
An unblinded assessment of skin involvement was also performed by the experienced clinical investigator who was aware of the treatment assignment. At week 12, 40% (n = 17) of the patients in the ECP arm had a complete or partial skin response as assessed by the investigator, compared with 10% (n = 4) of the patients in the control arm (P = .002). Figure 4 displays the cumulative incidence of a complete or partial cutaneous response from baseline to week 12 (P < .001).
Response in extracutaneous organs involved by cGVHD
For the more commonly involved extracutaneous organ systems at week 12, improvement favored ECP therapy for eye involvement (30% resolved or improved, compared with 7% in the control arm; P = .04). Improvement in oral involvement was experienced in 53% and 27% of the patients in the ECP and control arms, respectively, (P = .06). For joint symptoms, improvement was noted in 22% versus 12% (ECP vs control; P = .66). Response in extracutaneous organ systems at week 12 is shown in Table S3. Response in extracutaneous organ systems at week 24 for ECP-treated subjects is summarized in TableS 4.
Targeted Symptom Assessment quality of life self-evaluation
Baseline Targeted Symptom Assessment (TSA) scores were similar between the ECP and control groups (see Table S5). At week 12, the median TSA score in the ECP arm improved by 19% compared with 2.5% improvement in the control arm (P = .01).
Safety
During the 12-week comparative period, serious adverse events were reported for 28.6% (n = 14) and 26.0% (n = 13) of subjects in the ECP and control arms, respectively (P = .78). Infections were the most common serious adverse event occurring in 18.4% (n = 9) and 16.0% (n = 8) of subjects in the ECP and control arms, respectively. The most common infection was pneumonia, occurring in 4.1% (n = 2) of ECP-treated patients and 6.0% (n = 3) of standard therapy patients. Bacterial sepsis occurred in 2 (4.1%) patients in the ECP arm and in 1 (2%) control patient. No serious adverse events were judged by the investigators to be related to ECP treatment.
During the initial 12-week treatment period, 90% and 92% of the patients in the ECP and control arms, respectively, experienced an adverse event (P = .74). Diarrhea occurred in 20.4% (n = 10) of ECP patients and 20.0% (n = 10) of control patients (P = 1.0), anemia occurred in 24.5% of ECP patients (n = 12) and 6.0% of control patients (n = 3; P = .02), and nausea occurred in 18.4% ECP patients (n = 9) and 12.0% control patients (n = 6; P = .41). The incidence of infection was 53.1% in the ECP arm versus 44% in the control arm (P = .42). Adverse events that led to withdrawal from the ECP arm included thrombocytopenia, hypoglycemic coma, tremor, mental status changes, progressive GVHD, Pseudomonas sp lung infection, and catheter-related complications (1 patient each). In the control arm, patients discontinued participation in the study because of progressive tendinous contracture, bacterial (Pseudomonas sp) pneumonia, or fungal pneumonia (1 patient each).
Mortality
Two percent (n = 1) of patients in the ECP arm and 6% (n = 3) of patients in the control arm died during the 12-week observation period. The cause of death and interval times from randomization to death in the 1 patient treated in the ECP arm was an infection (multiple organ system failure due to Shigella sp sepsis occurring 86 days after randomization). The causes of death in the 3 control arm patients were multiple organ failure (95 days after randomization), cardiac failure (51 days after randomization), and infection (fatal pneumonia caused by Pseudomonas sp occurring 138 days after randomization).
Discussion
This study represents the first prospective randomized controlled clinical trial of ECP in the treatment of chronic GVHD. In this trial, we used the TSS, a new skin scoring tool, as the primary end point in an attempt to quantify and document the skin changes associated with conventional and ECP treatment. We used the skin as the primary end point in our study because this organ is frequently involved, manifestations are for the most part diagnostic for cGVHD and cutaneous involvement can be assessed quantitatively. A limitation of using skin as the primary endpoint to assess response is that many organs can be affected by cGVHD.
We found that the percentage reduction in TSS from baseline until week 12 was numerically greater for the ECP arm (−14.5% vs −8.5%) but did not reach statistical significance (P = .48). The lack of significant difference in TSS between the ECP and control arms at week 12 may be explained by the short duration of treatment. This is supported by the continued improvement in the TSS plus concomitant decrease in corticosteroids use at week 24 observed in the ECP group (Table 3). This is also in agreement with experts' opinion that improvement of more advanced forms of cGVHD such as sclerotic manifestation often requires at least 6 to 12 months to be observed. Assessment of skin involvement by the unblinded experienced clinical investigators revealed a significantly higher complete and partial resolution of cGVHD in the ECP arm compared with the control arm by week 12. In addition, more patients in the ECP arm compared with the control arm (25.0% vs 12.8%) were able to reduce their steroid doses by at least 50% in the 12-week study period. Significantly more patients in the ECP arm (20.8% vs 6.4%) achieved reduction of at least 50% of their steroid dose and had a final steroid dose less than 10 mg/day by week 12. Thus, our results demonstrated the steroid-sparing effect of ECP in a cohort in which most patients were steroid-dependent and not steroid-refractory. Although progressive improvement in TSS and continued reduction of steroid dose was noted in patients in the ECP arm by week 24, comparison at this time point is not possible because most patients in the control arms discontinued participation in the study or had crossed over to receive open label ECP after week 12.
Using assessments by an unblinded treating physician, other authors have reported promising responses and a steroid-sparing effect in patients with cutaneous GVHD treated with ECP in retrospective or prospective nonrandomized clinical studies. Apisarnthanarax16 conducted a retrospective review of ECP in 32 steroid-dependent or steroid-refractory cGVHD patients and reported complete and partial responses in 56% after a median of 36 cycles of ECP. In addition, 64% of patients could reduce their steroid dose by at least 50%. Foss17 observed a 64% response rate in a prospective study on 25 patients with extensive steroid-refractory or steroid-intolerant cGVHD and in 80% a reduction or discontinuation of immunosuppressive medication was possible. Couriel,25 in a retrospective review, analyzed 71 patients with chronic refractory GVHD given ECP and reported a complete and partial response rate of 61%. One year after the initiation of ECP, 22% of patients had discontinued steroids and 10% had discontinued all immunosuppressive medications. Similarly, Greinix27 treated 15 patients with extensive cGVHD who were failing steroids and observed complete responses in 12 of 15 with skin disease and in all patients with involvement of oral mucosa. In addition, a steroid-sparing effect was observed in responding patients with no increase in infectious complications.
The results of the above retrospective and prospective single-arm studies are in accordance with our observations, although several investigators reported higher response rates and a more prominent steroid-sparing effect of ECP. The discrepancies can be explained by several factors, including the differences in ECP schedule investigated and, most of all, the duration of therapy. In the literature, ECP was administered to steroid-refractory patients up to 24 months.16-20,27 The continued apparent improvement in patients in the ECP arm during weeks 12 to 24 suggests that longer treatment may be necessary to obtain optimal response to ECP, particularly in patients with more advanced forms of cGVHD. Of note, most patients in our study had long-standing skin involvement and thus, a comparison of treatment efficacy by week 12 may have been too early to observe statistically significant improvement in TSS. In addition to skin improvement, we observed a trend toward differential improvement of oral mucosa and gut involvement in the ECP treatment arm. Complete response in oral mucosa, liver, and gut GVHD has been reported in retrospective studies with ECP.11,18,20,21,26
Improvement in quality of life was noted in patients in the ECP treatment arm compared with the control arm, consistent with the work of Lee and colleagues.29 The improvement in quality of life may relate to an amelioration of disease manifestation as well as the reduction of corticosteroid dose administered. Nonetheless, because patients were aware of the treatment assignment, improvement in quality of life could be related to a placebo effect. As surrogate marker for quality of life, an improvement of Karnofsky performance scores from between 50% and 60% before ECP to at least 90% after ECP has been reported.18,21
ECP was well tolerated with few major complications reported. The adverse event profile observed in the ECP arm of this study was consistent with the underlying cGVHD process and was not different from that described by other authors.10,20,25 Importantly, there was no indication that ECP induced generalized immunosuppression, because the incidence of infections was not significantly higher in the ECP arm compared with the control arm. Moreover, the ability of ECP therapy to exhibit a steroid-sparing effect in this study can lead to a reduction in the long-term adverse sequelae of steroid treatment (eg, increased transplantation-related mortality, avascular necrosis, hypertension, diabetes mellitus, osteoporosis, cataracts).1,2
This study also demonstrates the challenges encountered in designing and conducting a trial in a disease state which does not have established and validated research methodologies. The interpretation of the results of our study is therefore influenced by the limitations of the study design itself. This study was conducted as an open-label, single-blind, randomized clinical trial. A “sham-pheresis” treatment arm was judged to be unethical based on the fact that ECP is an invasive procedure that lasts for approximately 3 hours and the expectation of red blood cell transfusion of sham-treated patients to achieve a minimum pretreatment hematocrit of 28% would have obvious ethical implications. A 12-week treatment period was judged to be the maximal time that one could ethically keep patients in the control arm in the trial without offering alternative therapies. There were plans for additional statistical comparisons at week 24 but the unexpectedly high discontinuation rate in the control arm precluded such analyses. As indicated earlier, the 3-month treatment period may not have been long enough to capture the full benefits of ECP, in particular because chronic cutaneous GVHD has a fibrotic component requiring extensive time for resolution.
This trial also demonstrates some of the methodologic challenges associated with the design and conduct of a clinical study of a new therapeutic modality in patients with cGVHD, as has been noted previously in clinical trial working groups charged with the development of standards for the serial evaluation of new therapeutic agents.30 The schedule for tapering corticosteroid doses was at the discretion of the investigator. Adjustment of corticosteroid dosing by the investigator was based on the overall activity of cGVHD in both cutaneous and extracutaneous organs or on treatment toxicity following standard of care guidelines. Adjustments in corticosteroid dosing may not necessarily have been made exclusively based on improvements or deterioration in the cutaneous manifestations of cGVHD alone. The doses of steroids were kept constant for the first 6 weeks of the trial to avoid the potential confounding factor of initiating a new therapy, ECP, and decreasing the steroid dose simultaneously. This left only weeks 7 to 12 during which to decrease the steroid dose, and this likely accounts for the inability to demonstrate a statistical difference in attaining a 50% or greater reduction in steroid dose by week 12. Because this was an open-label study, investigator bias cannot be excluded as an explanation for the more rapid steroid tapering in the ECP group. The paradox and pitfalls of attempting to standardize a steroid tapering regimen have been noted in the National Institutes of Health Consensus Conference31 and should be addressed a priori in future protocols. Decisions regarding who should determine steroid dosing, the blinded assessor or the unblinded investigator, should also be addressed a priori in future protocols.
The results of this study suggest that ECP may have a steroid-sparing effect in the treatment of chronic GVHD, as evidenced by reduction in corticosteroids concomitant with improvement in skin disease assessed by a blinded observer.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank John D. Klein, PhD, and Mary M. Horowitz, MS, MD, for the conduct and interpretation of additional analyses for this manuscript. The authors are grateful to all patients who participated in this clinical trial.
This work was supported by a grant from Therakos, Inc. (Exton, PA).
Authorship
Contribution: M.E.D.F. and H.T.G. designed the study, analyzed data, and wrote the paper; J.F.A. and A.B. analyzed the data; K.v.B. analyzed data and wrote the paper; A.E., A.G., V.R., H.-J.K., L.B., M.M., and H.M.P. recruited subjects and collected the data for the study; M.M. recruited subjects, collected and analyzed the data for the study; R.K. designed the study and analyzed the data; D.P. analyzed the data; and J.G. performed statistical analysis.
Conflict-of-interest disclosure: D.P. and J.G. are employees of Therakos, Inc.; R.K. and H.T.G. have served as consultants to Therakos; and H.T.G participated as lecturer in the speakers bureau for Therakos. The remaining authors declare no competing financial interests.
Correspondence: Mary E.D. Flowers, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue North, D5-290, Seattle, WA 98109-1024; e-mail: mflowers@fhcrc.org.
Appendix
Institutions and Principal Investigators and Steering Committee Advisors:
Karl Gustav Carus University, Dresden, Germany (Christina Holig)
Rotherham General Hospital, Rotherham, United Kingdom (Peter Taylor)
Ankara University Medical School, Ankara, Turkey (Osman Ilhan)
Kantonsspital Basel, Switzerland (Alois Gratwol)
Instituto Portuges de Oncologia de Fransisco Gentil (IPOFG), Lisbon, Portugal (Alexandria Machado)
Medizinische Universitätsklinik, Freiberg, Germany (Jürgen Finke)
Hospital de Clinicas da USP, Sao Paulo, Brazil (Frederico Luiz Dulley)
Royal Brisbane and Women's Hospital, Brisbane, Australia (Simon Durrant)
Mount Sinai Medical Center, New York, NY (Patricia Shi)
St Vincents Hospital, Darlinghurst, Australia (Tony Dodds)
Peter MacCallum Cancer Center, Melbourne, Australia (H. Miles Prince)
Clinical Hematology and Medical Oncology, Parkville, Australia (Andrew Grigg)
The Alfred Hospital, Melbourne, Australia (Tony Schwarer)
Klinika Hematologie a Transfuziologie, Bratslavia, Slovakia (Martin Mistrik)
UZ Leuven Gasthuisberg, Leuven, Belgium (Koen Theunissen)
Universitäts-Klinikum Essen, Germany (Ahmet Elmaagacli and Uwe Hillen)
Hospital Edouard Herriot, Lyon, France (Mauricette Michallet)
University of Chicago, Chicago, IL (Koen van Besien)
Ospedale S. Martino, Genova, Italy (Andrea Bacigalupo)
University of Florida, Gainesville, FL (Vijay Reddy)
Royal Hammersmith Hospital, London, United Kingdom (Jane Apperley)
Fred Hutchinson Cancer Research Center, University of Washington School of Medicine, Seattle, WA (Mary Flowers)
University of Munich, Munich, Germany (Hans-Jochem Kolb)
National Cancer Institute (INCA), Rio de Janeiro, Brazil (Luis Bouzas)
Medical University of Vienna, Vienna, Austria (Hildegard Greinix, Robert Knobler)
References
Author notes
*M.E.D.F. and H.T.G. contributed equally to the preparation of this manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal