Abstract
We have recently demonstrated that nuclear expression of BCL10 predicts Helicobacter pylori (HP) independence of early-stage gastric diffuse large B-cell lymphoma (DLBCL) with histologic evidence of mucosa-associated lymphoid tissue (MALT). In this study, we examined the role of B cell–activating factor of TNF family (BAFF) in mediating BCL10 nuclear translocation and HP independence of gastric DLBCL (MALT). We used immunohistochemistry and immunoblotting to measure the expression of BAFF, pAKT, BCL3, BCL10, and NF-κB. Transactivity of NF-κB was measured by electromobility shift assay. In lymphoma samples from 26 patients with gastric DLBCL (MALT), we detected aberrant expression of BAFF in 7 of 10 (70%) HP-independent and in 3 of 16 (18.8%) HP-dependent cases (P = .015). BAFF overexpression was associated with pAKT expression (P = .032), and nuclear expression of BCL3 (P = .014), BCL10 (P = .015), and NF-κB (P = .004). In B-cell lymphoma Pfeiffer cells, BAFF activated NF-κB and AKT; the activated NF-κB up-regulated BCL10, and the activated AKT caused formation of BCL10/BCL3 complexes that translocated to the nucleus. Inhibition of AKT by LY294002 (a PI3K inhibitor) blocked BCL10 nuclear translocation, NF-κB transactivity, and BAFF expression. Our results indicate that autocrine BAFF signal transduction pathways may contribute to HP-independent growth of gastric DLBCL (MALT).
Introduction
We recently showed that early-stage gastric diffuse large B-cell lymphoma (DLBCL) with histologic evidence of mucosa-associated lymphoid tissue (MALT) lymphoma is as responsive to Helicobacter pylori eradication therapy (HPET) as low-grade MALT lymphoma.1 Furthermore, we demonstrated that nuclear translocation of BCL10 is closely associated with the H pylori–independent status of DLBCL (MALT) and MALT lymphoma of the stomach, regardless of the presence of chromosomal translocations characteristic of gastrointestinal lymphomas, t(11;18)(q21;q21) and t(1;14)(p22;q32).2,3 Given that these translocations are rare in gastric DLBCL (MALT),4 the biologic significance and molecular mechanisms responsible for the aberrant nuclear translocation of BCL10 in these tumors are intriguing, but heretofore uncharacterized.
BCL10 was originally identified and cloned from MALT lymphoma cells with the chromosomal translocation t(1;14)(p22:q32).5 Subsequently, BCL10 was found to be expressed in the cytoplasm of normal T and B cells where it relays antigen receptor-mediated signals, which culminate in the activation of NF-κB.6-8 Once NF-κB is activated, it translocates to the nucleus where it induces the production of cytokines and growth factors that are important for cellular activation, proliferation, and survival of malignant B cells.9 Therefore, activation of NF-κB in gastric DLBCL (MALT) may be involved in mediating the translocation of BCL10. This hypothesis is supported by our recent observation that tumor necrosis factor (TNF)–α up-regulates the expression of BCL10 and induces a fraction of BCL10 nuclear translocation in carcinoma cells.10 We also showed that AKT, activated by TNF-α, phosphorylates BCL10 on Ser218 and Ser231, and the phosphorylated BCL10 subsequently complexes with BCL3, which contains a nuclear localization signal, to enter the nucleus.10 These findings characterize a molecular linkage that directs BCL10 nuclear translocation in response to TNF-α treatment.
Recent studies indicate that alternative pathways triggered by stimulation of B cell–activating factor belonging to the TNF family (BAFF) for activation of NF-κB may contribute to the development, survival, and attenuation of apoptosis of B lymphoma cells.11-13 A recent study of B-cell lymphomas showed that high serum BAFF levels are associated with a poor survival rate of patients with nodal DLBCL.14 Another recent study demonstrated that constitutive activation of NF-κB and nuclear factor of activated T cells (NFAT) in aggressive B-cell lymphoma cells can lead to aberrant BAFF expression through one NF-κB and 2 NFAT binding sites in the BAFF promoter.15 Constitutive activation of NF-κB and BAFF can form a positive feedback loop that promotes the survival and proliferation of these lymphoma cells.15 Because NF-κB mediates inflammation and tumor formation, activation of NF-κB by BAFF may contribute to the H pylori–independent transformation of gastric DLBCL (MALT).
In this study, we examined the role of BAFF in mediating BCL10 nuclear translocation and H pylori–independence of gastric DLBCL (MALT). We found that overexpression of BAFF was closely associated with phosphorylated AKT expression, nuclear translocation of BCL3, BCL10, and NF-κB, and H pylori–independent status of these tumors. Further exploration of the biologic significance of these observations in Pfeiffer cells (DLBCL cell line) revealed that BAFF activated NF-κB and AKT; the activated NF-κB up-regulated BCL10, and the activated AKT caused formation of BCL10/BCL3 complexes that translocated to the nucleus. Our results indicate that BAFF production by tumor cells can result in long-term NF-κB activation and BCL10 nuclear translocation by forming a positive feedback loop, thereby contributing to the H pylori–independent growth of gastric DLBCL (MALT).
Methods
Patients, treatment, and tissue samples
We studied 26 patients who had participated in a previous prospective study of HPET for stage IE gastric DLBCL (MALT) and for whom had pre-HPET endoscopic biopsies specimens. The clinicopathologic features of these patients have been previously reported.2,16 In this study, the diagnosis of DLBCL (MALT) was according to the histologic criteria described by Chan et al17 and de Jong et al,18 based on the presence of a diffuse increase of large cells resembling centroblasts or lymphoblasts to between 1% and 10% of the total tumor cells within predominantly low-grade centrocyte-like cell infiltrates (MALT lymphoma predominant), or the predominance of a high-grade large cell lymphoma with only a small residue, low-grade foci, and/or the presence of lymphoepithelial lesions (DLBCL-predominant). Occasional clusters of transformed blast cells or sheets of transformed blast cells (up to 20 cells without the formation of larger sheets) confined within the colonized follicles were considered as an immune response to H pylori stimulation rather than DLBCL (MALT). Patients with primary pure large cell lymphoma, without evidence of a low-grade component of the stomach, were excluded from this study. All specimens were immunohistochemically stained by CD20, CD5, CD3, and CD43 for routine diagnostic purposes. Additional immunohistochemical studies with anticytokeratin (1:50, clone AE1/AE3; Ylem, Rome, Italy) were performed to identify lymphoepithelial lesions in the context of the minimal MALT lymphoma components in DLBCL. The histopathologic characteristics of all tumor specimens were independently reviewed by 2 expert hematopathologists. The clinicopathologic features of these patients are listed in Table 1.
Clinicopathologic features of the patients with gastric DLBCL (MALT)
| Patient no. . | Sex . | Age, y . | Depth of tumor invasion* . | Tumor response to HP eradication . | Current status . | Histology† . | t(11;18)‡ . |
|---|---|---|---|---|---|---|---|
| 1 | F | 48 | Muscularis propria | CR | DLBCL-P | − | |
| 2 | M | 21 | Muscularis propria | PD | Chemotherapy | DLBCL-P | − |
| 3 | F | 63 | SD | Chemotherapy | MALToma-P | + | |
| 4 | F | 68 | CR | MALToma-P | − | ||
| 5 | F | 52 | Submucosa | PD | Chemotherapy | MALToma-P | − |
| 6 | F | 42 | Serosa | CR | MALToma-P | − | |
| 7 | F | 66 | Serosa | CR | DLBCL-P | − | |
| 8 | F | 71 | Muscularis propria | CR | MALToma-P | − | |
| 9 | F | 54 | CR | MALToma-P | − | ||
| 10 | M | 83 | Muscularis propria | PD | Chemotherapy | MALToma-P | − |
| 11 | F | 52 | Submucosa | CR | MALToma-P | − | |
| 12 | F | 73 | Muscularis propria | CR | DLBCL-P | − | |
| 13 | M | 46 | Submucosa | CR | DLBCL-P | − | |
| 14 | F | 38 | CR | MALToma-P | − | ||
| 15 | M | 73 | Serosa | PD | Chemotherapy | DLBCL-P | − |
| 16 | F | 45 | Muscularis propria | PD | Chemotherapy | MALToma-P | − |
| 17 | F | 56 | Submucosa | CR | MALToma-P | − | |
| 18 | F | 59 | Serosa | PD | Chemotherapy + gastrectomy | DLBCL-P | − |
| 19 | F | 65 | CR | DLBCL-P | − | ||
| 20 | M | 35 | Submucosa | CR | DLBCL-P | − | |
| 21 | F | 73 | CR | MALToma-P | − | ||
| 22 | M | 45 | Serosa | SD | Chemotherapy | DLBCL-P | − |
| 23 | F | 53 | Submucosa | SD | Chemotherapy | DLBCL-P | − |
| 24 | F | 80 | Muscularis propria | PD | Chemotherapy | MALToma-P | − |
| 25 | M | 70 | Submucosa | CR | DLBCL-P | − | |
| 26 | F | 84 | Submucosa | CR | DLBCL-P | − |
| Patient no. . | Sex . | Age, y . | Depth of tumor invasion* . | Tumor response to HP eradication . | Current status . | Histology† . | t(11;18)‡ . |
|---|---|---|---|---|---|---|---|
| 1 | F | 48 | Muscularis propria | CR | DLBCL-P | − | |
| 2 | M | 21 | Muscularis propria | PD | Chemotherapy | DLBCL-P | − |
| 3 | F | 63 | SD | Chemotherapy | MALToma-P | + | |
| 4 | F | 68 | CR | MALToma-P | − | ||
| 5 | F | 52 | Submucosa | PD | Chemotherapy | MALToma-P | − |
| 6 | F | 42 | Serosa | CR | MALToma-P | − | |
| 7 | F | 66 | Serosa | CR | DLBCL-P | − | |
| 8 | F | 71 | Muscularis propria | CR | MALToma-P | − | |
| 9 | F | 54 | CR | MALToma-P | − | ||
| 10 | M | 83 | Muscularis propria | PD | Chemotherapy | MALToma-P | − |
| 11 | F | 52 | Submucosa | CR | MALToma-P | − | |
| 12 | F | 73 | Muscularis propria | CR | DLBCL-P | − | |
| 13 | M | 46 | Submucosa | CR | DLBCL-P | − | |
| 14 | F | 38 | CR | MALToma-P | − | ||
| 15 | M | 73 | Serosa | PD | Chemotherapy | DLBCL-P | − |
| 16 | F | 45 | Muscularis propria | PD | Chemotherapy | MALToma-P | − |
| 17 | F | 56 | Submucosa | CR | MALToma-P | − | |
| 18 | F | 59 | Serosa | PD | Chemotherapy + gastrectomy | DLBCL-P | − |
| 19 | F | 65 | CR | DLBCL-P | − | ||
| 20 | M | 35 | Submucosa | CR | DLBCL-P | − | |
| 21 | F | 73 | CR | MALToma-P | − | ||
| 22 | M | 45 | Serosa | SD | Chemotherapy | DLBCL-P | − |
| 23 | F | 53 | Submucosa | SD | Chemotherapy | DLBCL-P | − |
| 24 | F | 80 | Muscularis propria | PD | Chemotherapy | MALToma-P | − |
| 25 | M | 70 | Submucosa | CR | DLBCL-P | − | |
| 26 | F | 84 | Submucosa | CR | DLBCL-P | − |
HP indicates H pylori; CR, complete remission; PD, progressive disease; SD, stable disease; +, positive; and −, negative.
Evaluated by endoscopic ultrasonography (20 cases) and histologic examination of surgical specimens (1 case).
Histology: DLBCL predominant (DLBCL-P), MALT lymphoma predominant (MALToma-P).
t(11;18) was identified by a multiplex RT-PCR of the API2-MALT1 chimeric transcript or by FISH.
We histologically evaluated tumor regression after HPET according to the criteria of Wotherspoon et al.19 Histologic complete remission was defined as tumor resolution to Wotherspoon grade 2 or less. Tumors were considered H pylori–dependent if they showed histologic and endoscopic complete remission. Tumors showing stable or progressive disease on follow-up endoscopic examination and a persistent or increasing proportion of large cells on microscopic examination were considered H pylori–independent. The Institutional Review Board at National Taiwan University Hospital approved the prospective clinical trial, the pathologic review, and the genetic studies of archived tumor tissues, all in accordance with the Declaration of Helsinki.
Immunohistochemistry
Immunohistochemistry for BCL10 (sc-9560; Santa Cruz Biotechnology, Santa Cruz, CA), BCL3 (IE8; Novocastra, Newcastle, United Kingdom), phosphorylated AKT (pAKT; Thr308; sc-16 646-R, Santa Cruz Biotechnology), TNF-α (AF-410-NA; R&D Systems, Minneapolis, MN), NF-κB (p65; sc-109; Santa Cruz Biotechnology), and BAFF (AF124; R&D Systems) was performed on paraffin-embedded sections of serial endoscopic biopsies specimens, including pre-HPET and post-HPET endoscopic biopsies, using an indirect immunoperoxidase method according to the manufacturer's instructions. In the present study, BCL3 (IE8; Novocastra), pAKT (Thr308; sc-16 646-R; Santa Cruz Biotechnology), TNF-α (AF-410-NA; R&D Systems), and BAFF (AF124; R&D Systems) have been used for immunohistochemistry to detect BCL3 in classic Hodgkin, anaplastic large-cell lymphoma, and other peripheral T-cell lymphomas,20 pAKT in breast cancer,21 TNF-α in hepatocytes of chronic hepatitis type C,22 and BAFF in bone marrow specimens of Waldenstrom macroglobulinemia,23 respectively.
In the present study, cell blocks of a human HL60 myelomonocytic cell line (known to express BAFF) were used as a positive control for BAFF.24 Cell blocks of a human Karpas 299 anaplastic large cell line (known to express pAKT and BCL3) was used as positive controls for pAKT and BCL3.20,25 Paraffin-embedded, formalin-fixed liver biopsies from 2 chronic type C hepatitis patients with known expression of TNF-α were used as positive controls for TNF-α.22
Immunohistochemical scoring
In the present study, the immunohistochemical stainings of BAFF, pAKT, BCL3, TNF-α, and CD20 were analyzed together with hematoxylin and eosin staining of serial pre-HPET endoscopic paraffin-sections of each patient. Although double staining was not done in all cases, the pathologists were able to score only malignant B cells, which met the criteria by both histomorphology and CD20 positivity. To further confirm that only malignant B cells were scored for these molecules, double immunohistochemical staining using a DAKO Envision Doublestain System was performed in selected cases for CD20, BAFF, pAKT, or BCL3, as described previously. To verify the specificity for the staining, paraffin sections with omission of the first, second, or both primary antibodies were used as negative controls.
In the present study, the percentages of positive cells were averaged to yield an immunohistologic score of 0% to 100%. Staining was considered positive for BCL3, BCL10, or NF-κB when the protein was detected in more than 10% of the tumor cells with nuclear staining according to the criteria described by Mathas et al,20 Ye et al,26 and Ohshima et al.27 Staining was considered positive for pAKT, BAFF, or TNF-α when the protein was detected in tumor cells with readily appreciable brown staining distinctly marking the tumor cell cytoplasm and/or nucleus.14,28 In the present study, the majority of H pylori–dependent tumors were either negative for these markers or had positive cells far less than 10%; and most H pylori–independent tumors had positive cells more than 20%, and in some cases, more than 50%. In doubtful cases, immunostains were performed twice. All slides were observed with light microscopy. An Olympus BX40 microscope equipped with 10×/0.25 and 40×/0.65 objective lenses (Olympus, Tokyo, Japan) was used to visualize images. Pictures were taken with an Olympus DP11 camera, and Adobe Photoshop 6.0 was used to enlarge the images to their present magnification levels (Adobe Systems, Mountain View, CA).
Cell lines and chemicals
Pfeiffer cell line was established from a patient in the leukemic phase of diffuse large cell lymphoma (DLBCL). Importantly, Pfeiffer cells are negative for Epstein-Barr virus and contain somatic hypermutations in the rearranged immunoglobulin variable genes.29 Recent studies have shown that gastric DLBCL, as well as gastric MALT lymphomas, have somatic hypermutations in the immunoglobulin heavy-chain variable genes.30,31 Therefore, the Pfeiffer (DLBCL) cell line was selected to mimic the in vitro environment of gastric DLBCL (MALT). The cells were maintained in RPMI 1640 medium (with 10% fetal bovine serum, 1% penicillin, and streptomycin) at 37°C, 5% CO2. BAFF was from Chemicon International (Temecula, CA), and other chemicals were from Sigma-Aldrich (St Louis, MO).
Immunoblotting and immunoprecipitation
Cell fractionation protocols were used as previously described.32 Cytosolic and nuclear lysates were prepared, and the protein concentration was determined using the Bio-Rad determination kit (Hercules, CA). Aliquots (15 μg) of lysates were subjected to immunoblotting. For reprobing, the membrane was washed with a stripping buffer (2% sodium dodecyl sulfate, 7 μL β-mercaptoethanol in 100 mL of Tris-buffered saline containing 0.2% Tween 20) for 30 minutes at room temperature and then subjected to another immunoblotting. Antibodies, including anti-TNF-α, anti-AKT, anti-pAKT (Ser473), anti-pAKT (Thr308), anti-BAFF, anti-BCL3, anti-p52, anti-BCL10, and anti-p65, were purchased from Santa Cruz Biotechnology.10
Electromobility shift assay
The activation of NF-κB was determined by the LightShift chemiluminescent electromobility shift assay (EMSA) kit (Pierce Chemical, Rockford, IL) with a biotin end-labeled NF-κB oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) as previously described.33 Equal amounts of nuclear protein extracts of lymphoma cell lines after treatment with various doses of TNF-α or BAFF were incubated with biotin end-labeled NF-κB oligonucleotide for 20 minutes at room temperature to allow DNA/protein binding. The DNA/protein complexes were then analyzed by 6% native polyacrylamide gel electrophoresis and transferred to a nylon membrane (Biodyne B nylon membrane; Pierce Chemical). After transfer to the membrane, the biotin end-labeled DNA was detected with streptavidin-linked horseradish peroxidase and the LightShift chemiluminescent EMSA kit (Pierce Chemical) according to the manufacturer's instructions, and the membranes were exposed to x-ray films for 2 to 5 minutes before developing.
Statistical analysis
Fisher exact test and χ2 test were used to analyze the correlation between (1) the H pylori–independent status of gastric DLBCL (MALT) and the expression patterns of BAFF, BCL3, pAKT, and TNF-α; and (2) the expression of BAFF and the expression patterns of BCL3, BCL10, pAKT, TNF-α, and NF-κB.
Results
Correlation of pAKT, BCL3, BCL10, NF-κB, and BAFF expression with tumor response to H pylori eradication therapy
Aberrant expression of BAFF was detected in 7 of 10 (70%) H pylori–independent and in 3 of 16 (18.8%) H pylori–dependent lymphoma samples of gastric DLBCL (MALT) (P = .015, Figure 1; Table 2). The correlation between aberrant expression of BAFF and disease extent was studied further. BAFF expression was detected in 6 of 15 (40%) tumors that were confined to the mucosa or submucosa, and in 3 of 6 (50%) tumors that invaded the muscular layer or serosa (P = .67, Table 2). The expression of BAFF had a sensitivity of 70% in predicting the H pylori independence of gastric DLBCL (MALT). The specificity of aberrant expression of BAFF for predicting H pylori independence was 82.3%.
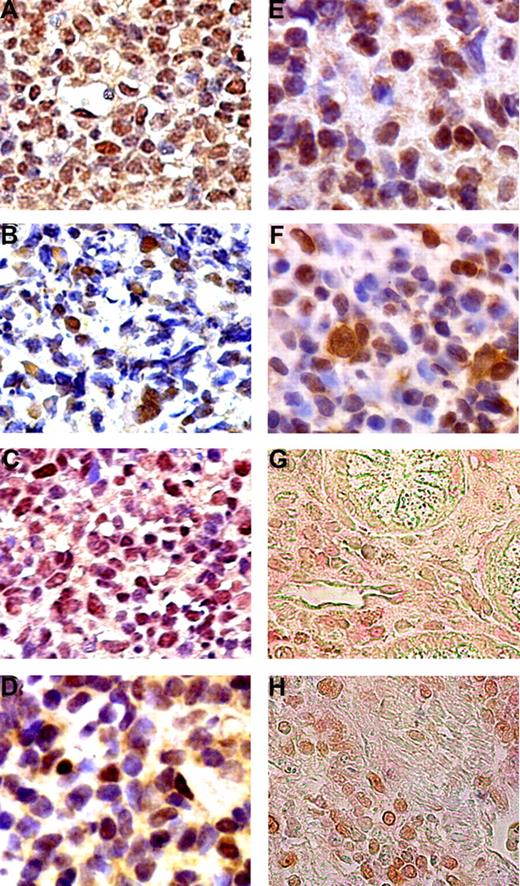
Expression of TNF-α, pAKT, BAFF, BCL3, BCL10, and NF-κB in gastric DLBCL (MALT) tumors. Aberrant expression of TNF-α (A), pAKT (B), and BAFF (C), and nuclear expression of BCL3 (D), BCL10 (E), and NF-κB (F) in the tumor cells of H pylori–independent gastric DLBCL (MALT) lymphoma patients. Double immunohistochemical staining showed that (G) tumor cells with BAFF nuclear staining (brown) are also CD20+ (red). (H) Tumor cells expressing pAKT staining (brown) are also CD20+ (red).
Expression of TNF-α, pAKT, BAFF, BCL3, BCL10, and NF-κB in gastric DLBCL (MALT) tumors. Aberrant expression of TNF-α (A), pAKT (B), and BAFF (C), and nuclear expression of BCL3 (D), BCL10 (E), and NF-κB (F) in the tumor cells of H pylori–independent gastric DLBCL (MALT) lymphoma patients. Double immunohistochemical staining showed that (G) tumor cells with BAFF nuclear staining (brown) are also CD20+ (red). (H) Tumor cells expressing pAKT staining (brown) are also CD20+ (red).
Clinicopathologic features of patients and their tumor response to H pylori eradication
| Characteristic . | H pylori–dependent, no. (%) . | H pylori–independent, no. (%) . | P . |
|---|---|---|---|
| No. (%) of patients | 16 (61.6) | 10 (38.4) | NS |
| Median age, y (range) | 60.5 (35-84) | 56 (21-83) | NS |
| Sex | |||
| Male | 3 (18.8) | 4 (40) | NS |
| Female | 13 (81.3) | 6 (60) | |
| Depth of tumor invasion* | |||
| Mucosa/submucosa | 9 (81.8) | 6 (60) | NS |
| Muscularis propria/serosa | 2 (18.2) | 4 (40) | |
| BAFF expression | |||
| Positive | 3 (18.7) | 7 (70) | .015 |
| Negative | 13 (82.3) | 3 (30) | |
| pAKT expression | |||
| Positive | 4 (25.0) | 7 (70) | .043 |
| Negative | 12 (75.0) | 3 (30) | |
| BCL3 expression | |||
| Nuclear/cytoplasmic | 4 (25.0) | 8 (80) | .014 |
| Cytoplasmic | 12 (75.0) | 2 (20) | |
| BCL10 expression | |||
| Nuclear/cytoplasmic | 1 (6.3) | 9 (90) | <.001 |
| Cytoplasmic | 15 (93.7) | 1 (10) | |
| NF-κB expression | |||
| Nuclear/cytoplasmic | 3 (18.8) | 9 (90) | .001 |
| Cytoplasmic | 13 (81.2) | 1 (10) | |
| Characteristic . | H pylori–dependent, no. (%) . | H pylori–independent, no. (%) . | P . |
|---|---|---|---|
| No. (%) of patients | 16 (61.6) | 10 (38.4) | NS |
| Median age, y (range) | 60.5 (35-84) | 56 (21-83) | NS |
| Sex | |||
| Male | 3 (18.8) | 4 (40) | NS |
| Female | 13 (81.3) | 6 (60) | |
| Depth of tumor invasion* | |||
| Mucosa/submucosa | 9 (81.8) | 6 (60) | NS |
| Muscularis propria/serosa | 2 (18.2) | 4 (40) | |
| BAFF expression | |||
| Positive | 3 (18.7) | 7 (70) | .015 |
| Negative | 13 (82.3) | 3 (30) | |
| pAKT expression | |||
| Positive | 4 (25.0) | 7 (70) | .043 |
| Negative | 12 (75.0) | 3 (30) | |
| BCL3 expression | |||
| Nuclear/cytoplasmic | 4 (25.0) | 8 (80) | .014 |
| Cytoplasmic | 12 (75.0) | 2 (20) | |
| BCL10 expression | |||
| Nuclear/cytoplasmic | 1 (6.3) | 9 (90) | <.001 |
| Cytoplasmic | 15 (93.7) | 1 (10) | |
| NF-κB expression | |||
| Nuclear/cytoplasmic | 3 (18.8) | 9 (90) | .001 |
| Cytoplasmic | 13 (81.2) | 1 (10) | |
NS indicates not significant; and EUS, endoscopic ultrasonography.
Evaluated by EUS (20 patients) and histologic examination of surgical specimens (1 patient).
The expression of pAKT was detected in 7 (70%) of 10 H pylori–independent and in 4 (25%) of 16 H pylori–dependent gastric DLBCL (MALT) (P = .043; Table 2). Similarly, nuclear expression of BCL3 was detected in 8 (80%) of 10 H pylori–independent and in 4 (25%) of 16 H pylori–dependent gastric DLBCL (MALT) (P = .014; Table 2). In addition, nuclear expression of BCL3 was significantly associated with nuclear expression of BCL10 (P = .004); and overexpression of pAKT was significantly associated with nuclear expression of NF-κB (P = .014; Table 2). Expression of BAFF was associated with the expression of pAKT (P = .032), nuclear expression of BCL3 (P = .014), nuclear expression of BCL10 (P = .015), and nuclear expression of NF-κB (P = .004).
In the present study, hematoxylin and eosin and immunohistochemical stainings showed that the majority of BAFF-positive, pAKT-positive, and BCL3-positive cells were morphologically abnormal and expressing CD20, indicating that these molecules were mostly expressed on neoplastic cells (Figure 1). In addition to the immunohistochemical approach, we used reverse-transcription polymerase chain reaction to examine the mRNA status of BAFF in selected cases of gastric DLBCL (MALT; supplemental data available on the Blood website; see the Supplemental Materials link at the top of the online article). We found that the expression of BAFF mRNA correlated well with BAFF immunohistochemistry staining. Raji cells served as negative controls (no mRNA of BAFF; Figure S1). These findings suggest that overexpression of BAFF might be involved in the molecular mechanisms responsible for the H pylori–independent growth of gastric DLBCL (MALT) via the AKT/BCL10/BCL3 signaling transduction pathway.
Up-regulation and nuclear translocation of BCL10 by BAFF-induced NF-κB activation
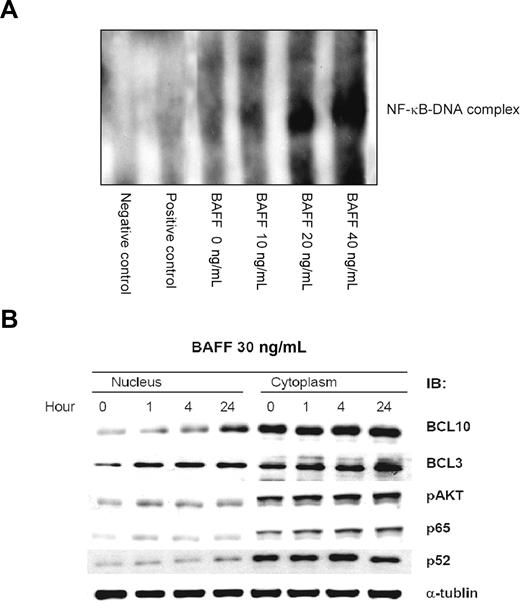
To further explore the biologic significance of these observations, we investigated whether BAFF can induce BCL10 nuclear translocation in the Pfeiffer lymphoma cell line. As indicated by the results of EMSA, BAFF treatment induced NF-κB DNA binding activity (Figure 2A). Parallel measurements of the protein level of pAKT, BCL10, and BCL3 by Western blotting revealed a sustained increase in the level of cytoplasmic pAKT, nuclear BCL10, and nuclear BCL3 during the course of BAFF treatment (Figure 2B). As a control, we characterized the typically dynamic change in the levels of nuclear NF-κB p65 and NF-κB p52 induced by BAFF.
BAFF induces NF-κB activation and results in up-regulation and nuclear translocation of BCL10. (A) BAFF induces NF-κB DNA-binding activity. Pfeiffer cells were treated with BAFF at the indicated concentration. The NF-κB DNA-binding activity was determined by the LightShift chemiluminescent EMSA kit. (B) BAFF induces a time course–dependent pAKT expression and nuclear translocation of BCL10 and BCL3. After BAFF (20 ng/mL) treatment, the protein level of pAKT, BCL10, and BCL3 of Pfeiffer lymphoma cells was determined by immunoblotting (IB). Staining with NF-κB p65, NF-κB p52, and α-tubulin served as loading control.
BAFF induces NF-κB activation and results in up-regulation and nuclear translocation of BCL10. (A) BAFF induces NF-κB DNA-binding activity. Pfeiffer cells were treated with BAFF at the indicated concentration. The NF-κB DNA-binding activity was determined by the LightShift chemiluminescent EMSA kit. (B) BAFF induces a time course–dependent pAKT expression and nuclear translocation of BCL10 and BCL3. After BAFF (20 ng/mL) treatment, the protein level of pAKT, BCL10, and BCL3 of Pfeiffer lymphoma cells was determined by immunoblotting (IB). Staining with NF-κB p65, NF-κB p52, and α-tubulin served as loading control.
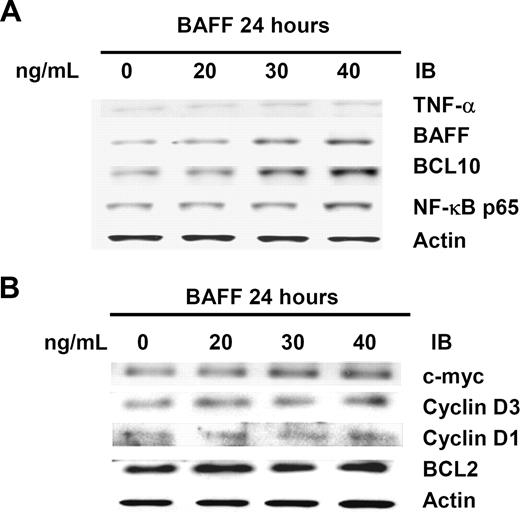
In subsequent experiments, we studied the effect of dose-scheduled BAFF on induction of the activation of the AKT/BCL10/ NF-κB signaling in Pfeiffer cells. The results show that an elevated level of pAKT was associated with an elevated level of BCL10 and BCL3 in the BCL10-immunopreciptated complex. Further measurements of the protein level of TNF-α, BAFF, c-myc, cyclin D1, cyclin D3, and BCL2 by Western blotting revealed a sustained increase in the level of TNF-α as well as BAFF during the course of BAFF treatment (Figure 3A). Parallel sustained increases were also observed in c-myc and cyclin D3 (the downstream genes of NF-κB), but not cyclin D1 and BCL2, during the time course of BAFF treatment (Figure 3B). Notably, in BAFF-treated lymphoma cells, the increased expression of pAKT and the increased nuclear translocation of BCL10 were significantly greater than in TNF-α-treated cells (data not shown).
BAFF activates the AKT/BCL10/BCL3 signaling transduction pathway. (A) BAFF treatment increases levels of TNF-α, BCL10, NF-κB p65, and BAFF. Whole-cell lysates of BAFF-treated Pfeiffer cells were subjected to immunoblotting (IB) using anti–TNF-α, anti–BCL10, anti–NF-κB p65, and anti-BAFF antibodies to show the total amount of protein. (B) BAFF increases levels of c-myc and cyclin D3 (the downstream genes of NF-κB), but not cyclin D1 and BCL-2. Actin stain was used as a loading control.
BAFF activates the AKT/BCL10/BCL3 signaling transduction pathway. (A) BAFF treatment increases levels of TNF-α, BCL10, NF-κB p65, and BAFF. Whole-cell lysates of BAFF-treated Pfeiffer cells were subjected to immunoblotting (IB) using anti–TNF-α, anti–BCL10, anti–NF-κB p65, and anti-BAFF antibodies to show the total amount of protein. (B) BAFF increases levels of c-myc and cyclin D3 (the downstream genes of NF-κB), but not cyclin D1 and BCL-2. Actin stain was used as a loading control.
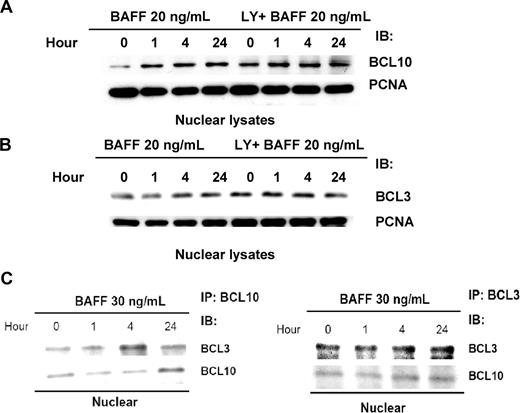
Next, we studied the effect of BAFF on induction of BCL3 nuclear translocation. The Western blotting results clearly showed that BAFF induced nuclear translocation of BCL10 and BCL3 (Figure 4A,B). Western blotting of the BCL10-coimmunoprecipitated complex from whole-cell lysates showed that BAFF treatment increased the level of BCL3 as well as the level of BCL10 (Figure 4C). Reciprocal coimmunoprecipitation followed by Western blotting demonstrated that BAFF treatment led to the formation of a BCL10/BCL3 complex in the nucleus (Figure 4C).
AKT activity is involved in BAFF-induced BCL10 and BCL3 nuclear translocation. (A) AKT activity is involved in BAFF-induced BCL10 nuclear translocation. Pfeiffer cells were treated with 20 ng/mL of BAFF for 0 to 24 hours with or without 10 μM of LY294002 (1- to 24-hour pretreatment as indicated). The nuclear lysates were subjected to immunoblotting with anti-BCL10 antibody. Immunoblotting of PCNA served as a loading control. (B) AKT activity is involved in BAFF-induced BCL3 nuclear translocation. Pfeiffer cells were treated as indicated. The nuclear lysates were subjected to immunoblotting with anti-BCL3 antibody. (C) BAFF triggers BCL10/BCL3 complex. The BCL10/BCL3 complex coexists in the nucleus. BCL3- or BCL10-coimmunprecipitated complex was isolated from the nuclear lysates prepared from Pfeiffer cells with 30-ng/mL BAFF treatments. Immunoblotting (IB) of BCL3 and BCL10 in the complex was performed.
AKT activity is involved in BAFF-induced BCL10 and BCL3 nuclear translocation. (A) AKT activity is involved in BAFF-induced BCL10 nuclear translocation. Pfeiffer cells were treated with 20 ng/mL of BAFF for 0 to 24 hours with or without 10 μM of LY294002 (1- to 24-hour pretreatment as indicated). The nuclear lysates were subjected to immunoblotting with anti-BCL10 antibody. Immunoblotting of PCNA served as a loading control. (B) AKT activity is involved in BAFF-induced BCL3 nuclear translocation. Pfeiffer cells were treated as indicated. The nuclear lysates were subjected to immunoblotting with anti-BCL3 antibody. (C) BAFF triggers BCL10/BCL3 complex. The BCL10/BCL3 complex coexists in the nucleus. BCL3- or BCL10-coimmunprecipitated complex was isolated from the nuclear lysates prepared from Pfeiffer cells with 30-ng/mL BAFF treatments. Immunoblotting (IB) of BCL3 and BCL10 in the complex was performed.
To further confirm the biologic significance of our observation in Pfeiffer cells, we examined the expression pattern of pAKT, BCL3, BCL10, NF-κB, and TNF-α by immunofluorescence microscopy and immunohistochemical staining. Notably, in BAFF-treated lymphoma cells, it can be seen that the BCL3, BCL10, and NF-κB were expressed in the nucleus and cytoplasm of these cells; and the expression of pAKT and TNF-α increased in a dose- and schedule-dependent manner (Figure S2).
Inhibition of NF-κB activity reduced BAFF expression, AKT activation, and BCL10 nuclear translocation
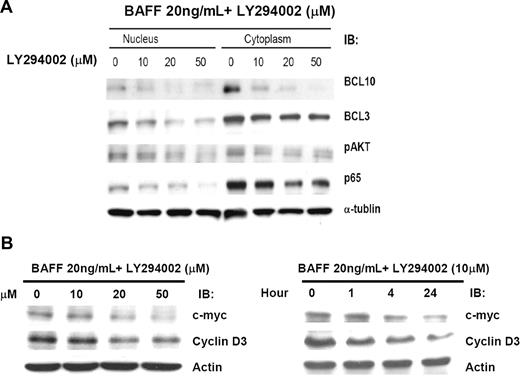
Because constitutive activation of NF-κB can result in aberrant BAFF expression, we studied the effect of down-regulation of NF-κB on BAFF expression and subsequent inhibition of BCL10 nuclear translocation. The results show that LY294002 (a PI3 kinase inhibitor) inhibited BAFF-induced AKT activation and concurrently blocked BAFF-induced BCL10 nuclear translocation (Figure 5A). These findings suggest that the BAFF/PI3K/AKT signaling pathway is necessary for BCL10 nuclear translocation. As shown in Figures 4A, 4B, and 5A, BAFF induced nuclear translocation of BCL3 and BCL10 was suppressed by pretreatment with LY294002. Furthermore, LY294002 treatment substantially decreased the expression of BAFF. Western blot analysis indicated that LY294002 treatment also dose- and schedule-dependently decreased the expression of c-myc and cyclin D3 during the course of LY294002 treatment (Figure 5B). Taken together, these findings indicate that BAFF expression is involved in cell-cycle control and NF-κB activation and may be important for H pylori–independent growth of gastric lymphoma cells.
Inhibition of NF-κB activity reduces BAFF expression and the AKT/BCL10/BCL3 signaling transduction pathway. (A) Inhibition of NF-κB activation decreases AKT activation, BCL3 nuclear translocation, and BCL10 nuclear translocation. Pfeiffer cells were treated with 20 ng/mL of BAFF for 24 hours with or without LY294002 (1-hour pretreatment as indicated). The whole-cell lysates were subjected to immunoblotting with anti-pAKT antibody, anti-BCL3 antibody, anti-BCL10 antibody, and anti–NF-κB p65 antibody. The membrane was reprobed with anti–α-tubulin antibody as a loading control. (B) Inhibition of NF-κB and BAFF activation down-regulates c-myc and cyclin D3 expression. Pfeiffer cells were treated with 20 ng/mL of BAFF for 24 hours with or without LY294002 (0-50 μM, 1-hour pretreatment as indicated). Pfeiffer cells were also treated with 20 ng/mL of BAFF for 24 hours with or without 10 μM LY294002 (1- to 24-hour pretreatment as indicated). The protein levels of c-myc and cyclin D3 were determined by immunoblotting (IB). Immunoblotting of actin served as a loading control.
Inhibition of NF-κB activity reduces BAFF expression and the AKT/BCL10/BCL3 signaling transduction pathway. (A) Inhibition of NF-κB activation decreases AKT activation, BCL3 nuclear translocation, and BCL10 nuclear translocation. Pfeiffer cells were treated with 20 ng/mL of BAFF for 24 hours with or without LY294002 (1-hour pretreatment as indicated). The whole-cell lysates were subjected to immunoblotting with anti-pAKT antibody, anti-BCL3 antibody, anti-BCL10 antibody, and anti–NF-κB p65 antibody. The membrane was reprobed with anti–α-tubulin antibody as a loading control. (B) Inhibition of NF-κB and BAFF activation down-regulates c-myc and cyclin D3 expression. Pfeiffer cells were treated with 20 ng/mL of BAFF for 24 hours with or without LY294002 (0-50 μM, 1-hour pretreatment as indicated). Pfeiffer cells were also treated with 20 ng/mL of BAFF for 24 hours with or without 10 μM LY294002 (1- to 24-hour pretreatment as indicated). The protein levels of c-myc and cyclin D3 were determined by immunoblotting (IB). Immunoblotting of actin served as a loading control.
Discussion
In this study, we demonstrated that overexpression of BAFF is closely associated with pAKT expression, nuclear translocation of BCL3, BCL10, and NF-κB, and the H pylori–independent status of gastric DLBCL (MALT). Further exploration of the biologic significance of these observations in Pfeiffer cells revealed that BAFF activates NF-κB and BCL10 nuclear translocation via the classic pathway as well as an alternative pathway. The classic pathway up-regulates the expression of BCL10, leading to its nuclear translocation. The alternative pathway, in conjunction with the classic pathway, induces NF-κB activation and BAFF expression. These findings indicate that autocrine BAFF signal transduction pathways may contribute to H pylori–independent growth of gastric DLBCL (MALT).
The classic pathway for activation of NF-κB is regarded as important for tumor development and progression. Activation of an alternative NF-κB pathway, via processing and cleavage of NF-κB2/p100 precursor to p52 (triggered by stimulation of BAFF), was reported to be necessary for the survival of B lymphocytes and the development of secondary lymphoid organs.9,34-36 In addition, several studies found that BAFF can be expressed by malignant B cells of chronic lymphocytic leukemia and multiple myeloma and that BAFF can up-regulate anti–apoptosis-related protein expression and promote the proliferation of lymphoma cells.37,38 Additional studies indicated that constitutive activation of BAFF is crucial for maintaining endogenous activation of the classic- and alternative-signaling pathways of NF-κB in DLBCL cells.15,39 These findings support our observation that activation of BAFF induced NF-κB activation and BCL10 nuclear translocation and that inhibition of NF-κB by a PI3K inhibitor decreased BAFF expression and BCL10 nuclear translocation. Our data also show that overexpression of BAFF was associated with nuclear translocation of BCL3, BCL10, and NF-κB and that coexpression of these markers was significantly associated with H pylori independence of gastric DLBCL (MALT).
Our recent study suggested that up-regulation of BCL10 (nuclear translocation of BCL10) may be involved in the TNF-α–activated NF-κB signaling transduction pathway via an NF-κB binding site in the BCL10 5′-untranslated region.10 Furthermore, we found that depletion of BCL10 did not affect TNF-α–induced NF-κB DNA-binding activity but suppressed TNF-α–induced NF-κB transcriptional activity.10 These findings are consistent with recent observations that nuclear BCL10 translocation can act as a transcriptional activator in mammalian cells through its interaction with a component of the basal transcription machinery TFIIB.40 The present study revealed that overexpression of BAFF is associated with nuclear translocation of BCL10, and activation of BAFF results in unremitting NF-κB activation and BCL10 nuclear translocation. Taken together, these findings suggest that autocrine BAFF signal transduction pathways promote BCL10 nuclear translocation and that BCL10 nuclear translocation could increase the activation of NF-κB–regulated genes and BCL10-transcripted genes and therefore contributes to the H pylori independence of gastric DLBCL (MALT).
Recently, we demonstrated that expression of TNF-α and NF-κB did not occur in postsubtotal gastrectomy biopsies of 2 H pylori–independent gastric MALT lymphoma patients who remained disease-free at 9.2 to 9.6 years after subtotal gastrectomy.41 These cases suggest that surgical excision of the gastric antrum and body (gastric microenvironments) may have been curative by eradicating unknown sources of the NF-κB signaling pathway.41 In the present study, we examined whether the expression of BAFF can exist in post-HPET biopsies of H pylori–independent early-stage gastric DLBCL (MALT) patients. Interestingly, aberrant BAFF expression persisted in tumor cells of post-HPET biopsies of 6 H pylori–independent case in this series. Our data also show that activation of BAFF in lymphoma cells can lead to up-regulation of c-myc, an NF-κB downstream gene that is overexpressed in H pylori–independent gastric DLBCL (MALT). Indeed, BAFF is produced by monocytes, macrophages, dendritic cells, and some T cells, and its expression can be up-regulated by inflammatory cytokines that are present in the microenvironment of the stomach.42,43 We also found that nuclear expression of BCL3 was closely associated with the aberrant expression of BAFF and that nuclear expression of BCL10 and NF-κB was present in H pylori–independent cases of gastric DLBCL (MALT). These findings agree with recent observations that the concentration of BCL3 may determine the transcriptional activity of NF-κB.44-46 Taken together, these results suggest that BAFF may be a mediator that links inflammation and tumor formation of gastric DLBCL (MALT) and that elevated BAFF activity may be responsible for inflammation-associated tumor development and extracellular stimuli-independent progression of this tumor (Figure 6).
BAFF activates NF-κB in gastric DLBCL (MALT) lymphoma cells via the classic pathway as well as an alternative pathway. The classic pathway (I) up-regulates BCL10, and AKT phosphorylates BCL10, leading to the formation of BCL10/BCL3 complexes that translocate to the nucleus. The alternate pathway (II), in conjunction with the classic pathway, induces BAFF expression and NF-κB activation. BAFF is also produced by monocytes, macrophages, dendritic cells, and some T cells, and its expression can be up-regulated by inflammatory cytokines that are present in the microenvironment of the stomach (pathway III). These findings suggest that BAFF production by tumor cells or gastric microenvironments can result in long-term NF-κB activation by forming a positive feedback loop, thereby contributing to the H pylori–independent growth of gastric DLBCL (MALT).
BAFF activates NF-κB in gastric DLBCL (MALT) lymphoma cells via the classic pathway as well as an alternative pathway. The classic pathway (I) up-regulates BCL10, and AKT phosphorylates BCL10, leading to the formation of BCL10/BCL3 complexes that translocate to the nucleus. The alternate pathway (II), in conjunction with the classic pathway, induces BAFF expression and NF-κB activation. BAFF is also produced by monocytes, macrophages, dendritic cells, and some T cells, and its expression can be up-regulated by inflammatory cytokines that are present in the microenvironment of the stomach (pathway III). These findings suggest that BAFF production by tumor cells or gastric microenvironments can result in long-term NF-κB activation by forming a positive feedback loop, thereby contributing to the H pylori–independent growth of gastric DLBCL (MALT).
Except in one case, overexpression of BAFF, pAKT expression, and BCL3 nuclear expression were found in both low-grade MALT lymphoma and DLBCL components of H pylori–independent cases. Indeed, in t(11;18)(q21;q21)-negative, H pylori–independent, low-grade gastric MALT lymphomas, BAFF overexpression was associated with pAKT expression (P = .044), nuclear expression of BCL3 (P = .013), nuclear expression of BCL10 (P = .02), and nuclear expression of NF-κB (P = .011; S.-H.K., K.-H.Y., L.-T.C., M.-S.W., C.-W.L., P.-N.H., and A.-L.C., unpublished data, June 2008). These findings suggest that H pylori–independent transformation is related to the BAFF-induced signals, whereas large-cell transformation is not the cause of BAFF expression in most cases. The only exceptional case (case 22) in this series had BAFF overexpression in H pylori–independent DLBCL components but not in H pylori–dependent MALT lymphoma components. The causal relationship between high-grade transformation and BAFF expression of the large-cell component in this particular case remains to be clarified.
In conclusion, our results indicate that BAFF-induced inflammation-related signal transduction, produced by tumor cells or tumor microenvironments, can lead to BCL10 nuclear translocation and NF-κB activation. This appears to be caused by a positive feedback loop and thereby contributes to H pylori–independent growth of gastric DLBCL (MALT).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the National Science Council, Taiwan (research grants NSC96-2321-B-002-013, NSC96-2321-B-002-014, and NSC96-2314-B-002-164MY3), Department of Health, Taiwan (DOH96-DT-B-111-001), and National Taiwan University Hospital, Taiwan (NTUH 96-S626).
Authorship
Contribution: S.-H.K. designed the research, analyzed data, and wrote the paper; P.-Y.Y. and L.-T.C. performed research and analyzed data; M.-S.W., C.-W.L., and K.-H.Y. provided tissue samples and discussed data; Y.-S.T. and J.-Y.C. performed research; P.-N.H. and J.-T.L. analyzed data; and A.-L.C. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ann-Lii Cheng, Department of Internal Medicine and Department of Oncology, National Taiwan University Hospital, No. 7, Chung-Shan S Road, Taipei, Taiwan; e-mail: alcheng@ntu.edu.tw; or Li-Tzong Chen, National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan; e-mail: leochen@nhri.org.tw.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal